Abstract
Background and aims Rye supernumerary (B) chromosomes have an accumulation mechanism involving the B subtelomeric domain highly enriched in D1100- and E3900-related sequences. In this work, the effects of heat stress during the early stages of male meiosis in 0B and +B plants were studied.
Methods In-depth cytological analyses of chromatin structure and behaviour were performed on staged rye meiocytes utilizing DAPI, fluorescence in situ hybridization and 5-methylcytosine immune labelling. Quantitative real-time PCR was used to measure heat effects on the expression of the Hsp101 gene as well as the 3·9- and 2·7-kb E3900 forms in various tissues and meiotic stages.
Key Results and Conclusions Quantitative real-time PCR established that heat induced equal up-regulation of the Hsp101 gene in 0B and 2B plants, with a marked peak in anthers with meiocytes staged at pachytene. Heat also resulted in significant up-regulation of E3900-related transcripts, especially at pachytene and for the truncated 2·7-kb form of E3900. Cytological heat-induced anomalies in prophase I, measured as the frequency of anomalous meiocytes, were significantly greater in 0B plants. Whereas telomeric sequences were widely distributed in a manner close to normal in the majority of 2B pachytene cells, most 0B meiocytes displayed abnormally clustered telomeres after chromosome pairing had occurred. Relevantly, bioinformatic analysis revealed a significantly high-density heat responsive cis regulatory sequence on E3900, clearly supporting stress-induced response of transcription for the truncated variant. Taken together, these results are the first indication that rye B chromosomes have implications on heat tolerance and may protect meiocytes against heat stress-induced damage.
Keywords: Rye B chromosomes, heat stress, meiotic chromosome dynamics, E3900 sequence family, prophase I, transcription regulation, heat responsive cis-regulatory elements
INTRODUCTION
B chromosomes (Bs) were initially identified as supernumerary chromosomes in the plant bug Metapodius by Wilson (1907), and later designated as additional or extra chromosomes (Jones and Rees, 1982; Jones et al., 2008a). Since their discovery, these enigmatic chromosomes have been identified in all major groups of outcrossing plants, animals and fungi. Interestingly, B chromosome accumulation mechanisms involving non-Mendelian modes of transmission have been described in approx. 60 % of the organisms that carry Bs (Jones, 2012). In rye (Secale cereale), these chromosomes are non-essential for growth and development and genes with specific phenotypic functions have not been described on Bs (Jones et al., 2008b; Jones, 2012). However, the presence of Bs in most natural rye populations is believed to be due to a rye B-specific accumulation mechanism, or drive (Jones et al., 2008a; Jones, 2012). This drive involves non-disjunction of B sister chromatids in the first mitotic division of the gametophyte, and is controlled by the Bs themselves. In combination with the asymmetric geometry of the first pollen mitosis, B non-disjunction leads to the preferential accumulation of Bs in the generative nucleus (Jones, 1991).
The cytogenetic and molecular structure of rye Bs has been extensively studied and its approx. 580-Mbp sequence reveals their origin as a mosaic of nuclear and organellar DNA (Martis et al., 2012). In common with maize Bs (Page et al., 2001; Cheng and Lin, 2003), rye Bs have functional domains including centromeric and telomeric sequences homologous to those found on the As (Jones, 2012). Their cytological morphology is virtually invariant throughout geographical regions (Jones and Puertas, 1993), being roughly half the length of the As and with a subterminal centromere that defines a short and a long arm. Lima-de-Faria (1962) initially proposed that the rye B drive mechanism is controlled by the subtelomeric domain of the B long arm, where two sequence families, D1100 (Sandery et al., 1990) and E3900 (Blunden et al., 1993), accumulate (Langdon et al., 2000). Later, analysis of chromosome behaviour of wheat lines with introgressed fragments of rye Bs established that non-disjunction is in fact dependent on the D1100 and E3900 sequence families (Endo et al., 2008). More recently, a B-specific chromatid adhesion site involving (peri)centromeric repeat ScCI11 has also been implicated in the delay of sister chromatid separation (Banaei-Moghaddam et al., 2012). It has been previously suggested that Bs have a monophyletic origin in Secale (Niwa and Sakamoto, 1995; Marques et al., 2013), and the organization of D1100 and E3900 is highly conserved. B contains various repetitive sequences, which are also present on As. However, the complex organization of E3900 and D1100 sequence families as high-copy repetitive DNA is specific to Bs, where their organization and behaviour are highly conserved (Klemme et al., 2013). Fluorescence in situ hybridization (FISH) shows that D1100 accumulates in two zones in the subtelomeric region physically separated by an interstitial less labelled space, while E3900 has a more homogeneous and distal signal that overlaps with the D1100 domain closer to the telomere (Wilkes et al., 1995). Besides the 3·9-kb form of E3900, a shorter 2·7-kb E3900-related sequence has been identified which also accumulates on the B-specific domain (Pereira et al., 2009). Estimates of copy number have shown that E3900 sequences are highly conserved and are present in 100–150 copies on Bs and in single or low copy numbers in rye and other cereal A genomes (Pereira et al., 2009). Importantly, E3900 sequences are differentially expressed in a tissue- and developmental-specific manner in plants with and without B chromosomes (Pereira et al., 2009). Whereas the expression levels of E3900 do not vary in leaves from plants with and without Bs, they are significantly up-regulated during meiosis exclusively in plants with Bs, maintaining a high level of transcription in the gametophyte (Pereira et al., 2009).
A significant amount of information is available regarding the effects of rye Bs upon sporophyte and gametophyte fitness and viability, from seed germination to seed set (Jones and Rees, 1982). As the number of Bs increases, negative effects on fertility (Müntzing, 1943), delays in seed germination (Moss, 1966) and loss of vigour (Müntzing, 1963) have been described. Bs also induce nuclear physiology effects in proportion to their number, such as increased cell cycle length (Evans et al., 1972) and decreased nuclear protein and RNA levels (Kirk and Jones, 1970). Relevantly, these extra chromosomes have various effects on mitotic and meiotic A chromosome behaviour. For example, Bs alter rDNA condensation patterns in mitotic cells (Morais-Cecílio et al., 1997; Delgado et al., 2004) and induce alterations in the frequency and distribution of A chromosome chiasmata at meiosis (Jones and Rees, 1982). Interestingly, these effects are genotype specific, as shown through their differential effects on a set of pure rye breeding lines (Jiménez et al., 1994). More direct evidence that Bs affect A chromosome behaviour is observed as a dosage-dependent increase in the frequency of anomalous adherences between sister chromatids at anaphase and metaphase cells in the first mitosis of pollen grains (Pereira et al., 2009).
The existence and evolution of B chromosomes has been a topic of considerable discussion and speculation for decades, in particular their persistence in natural populations and whether they are ‘selfish’ and/or ‘adaptive’ in nature (Jones, 1975, 2012; Jones and Rees, 1982; Jones et al., 2008a). B equilibrium models depend on their effects on fitness as well as their transmission ratio (accumulation mechanism) in comparison to the regular chromosome complement, which together dictate their evolutionary significance (Camacho et al., 2000). A rare example of B mutualism has been demonstrated in natural chive (Allium schoenoprasum) populations, where plants carrying B chromosomes display boosted germination under drought conditions (Holmes and Bougourd, 1989). In rye, although B numbers vary from zero to eight per plant and natural populations cover a broad range of geographical regions (Jones and Puertas, 1993), there is so far no solid indication of Bs providing positive fitness effects. Density experiments in pots using rye from experimental populations (zero to four Bs per plant) show a decrease in B frequency under conditions of high sowing density (Rees and Hutchison, 1974). However, field experiments to estimate the effect of selection on B frequency in experimental populations have been confounded due to difficulties in controlling environmental variables (Jones and Rees, 1982). Although Teoh and Jones (1978) found superior survival of 0B plants under density stress, the overall frequency of B chromosomes was found to have increased, indicating that both transmission and selection have to be taken into account. In general, although favourable environmental conditions have higher frequencies of Bs (Jones and Rees, 1982), it is yet unknown if they confer selective advantage to survival or fitness under specific environmental conditions.
Rye exhibits excellent tolerance against many biotic and abiotic stresses, and similarly to wheat and barley is a cool-season cereal with optimum growth temperatures ranging from 18 to 24 °C (Oelke et al., 1990). Elevated temperatures have been shown to be one of the most significant factors affecting cereal growth and development, ultimately leading to significant losses in fertility and yield (Stone, 2001; Barnabás et al., 2008; De Storme and Geelen, 2014). Temperatures above 30 °C from flower initiation (early meiosis) to pollen maturity have been reported to have damaging effects on pollen grain viability in wheat (Saini and Aspinall, 1982). In barley, heat-hypersensitive phases of reproductive development include early panicle differentiation, pre-meiosis and meiosis, with heat exposure resulting in cellular abnormalities, and absence or sterility of pollen grains, respectively (Sakata et al., 2000). In this species, heat induces premature progression of anther development associated with cell-proliferation arrest, degradation of anther cell walls, disruption of nuclear membranes in pre-meiotic cells and premature synapsis of meiotic prophase chromosomes (Oshino et al., 2007). Relevantly, these heat-induced anomalies are found to be accompanied by premature up-regulation of key meiotic and anther-specific genes (Oshino et al., 2007).
A series of specific genetic and epigenetic regulatory mechanisms have been implicated in plant responses to heat stress (Xu et al., 2011). In maize and wheat, a heat-induced increase in heat shock protein Hsp101 transcript level has been shown to be accompanied by a corresponding increase in the amount of Hsp101 protein in the vegetative and floral meristematic regions (Campbell et al., 2001; Young et al., 2001). Epigenetic response mechanisms putatively contributing to safeguarding genome integrity in Arabidopsis thaliana include heat stress-mediated release of gene silencing of a reporter gene correlated with pronounced alterations in histone occupancy and histone H3 acetylation (Lang-Mladek et al., 2010). Prolonged heat stress has also been shown to activate transcription of several repetitive elements of A. thaliana that are normally under epigenetic regulation by transcriptional gene silencing (Cavrak et al., 2014). This activation appears to occur without loss of DNA methylation and with only minor changes in histone modifications (Pecinka et al., 2010).
The present study provides detailed cytogenetic and molecular insight into the effects of heat stress during reproductive development on meiosis in rye plants with 0 and 2B chromosomes. In-depth cytological analyses of chromatin structure and behaviour are performed on staged meiocytes utilizing DAPI, FISH as well as 5-mC immune labelling. In association with the cytogenetic data, quantitative real-time PCR is used to measure heat effects on the expression of the Hsp101 gene as well as the 3·9- and 2·7-kb E3900 forms in various tissues and meiotic stages. Taken together, the results provided here represent the first indication that rye B chromosomes have implications for heat tolerance and protection against heat stress-induced damage at early stages of meiosis.
MATERIALS AND METHODS
Plant material and heat stress conditions
Seeds from Experimental Population (EP) rye plants carrying Bs (Secale cereale L., 2n = 2x = 14 + 2Bs; experimental population established in Aberystwyth by Professor N. R. Jones) were germinated and grown in controlled conditions. The number of Bs was determined for each plant by inducing c-metaphases and counting Bs, as previously described (Delgado et al., 2004). 0B and 2B plants were allowed to grow for approx. 2 months, at which time they were divided to produce between two and four clones per original plant. This allowed for comparative molecular and cytological analysis between heat-stressed and control plants to be performed on paired treated and control clones originating from the same seed/plant.
Meiocyte developmental stages were determined prior to heat stress regimes and anthers labelled as undergoing premeiosis, prophase I of meiosis or pollen grain. Since meiocytes are generally synchronous in anthers, and spikelet initiation begins in the middle of the spike and proceeds toward the tip and base (Oelke et al., 1990), flowers from the middle of the spike were collected for staging. A single anther was excised from a flower bud and immediately dissected onto a slide in a drop of 45 % acetic-carmine for determination of meiocyte developmental stages. Immediately after staging, plants were maintained in control conditions (24 °C) or exposed to heat stress 42 °C for 4 h. The heat stress temperature was chosen based on agronomically relevant temperatures shown to have a significant effect in cool season grasses, such as rye (Xu et al., 2011). After 4 h, heat-shocked or control anthers were collected and fixed for cytological examination, as described below. For transcriptional analysis, entire plants in vases were exposed to the heat regime or maintained in control conditions and leaves, roots and staged anthers were collected, frozen in liquid nitrogen and kept at − 80 °C until use.
cDNA isolation and quantitative PCR
Plant material collected from heat-stressed or control 0B and 2B plants in vases were utilized for quantitative real-time PCR (qRT-PCR) with sequence-specific primers, as shown in Table 1. To examine the effects of our heat stress regime on Hsp101, a gene which encodes a heat shock protein involved in thermotolerance in plants (Tonsor et al., 2008), primers specific for a highly conserved region of Hsp101 mRNA encoding this protein family were designed based on nucleotide sequence homology between rice, wheat and maize. The correct sized fragments were obtained after PCR (259 bp) with unique melting peaks and the expected Aegilops umbellulata melting temperature of 86 °C. To further verify correspondence to rye Hsp101, PCR products obtained with cDNA from leaves and anthers staged at meiosis with and without B chromosomes were utilized for nucleotide sequencing. Consensus sequences based on results obtained from at least three genomes of each tissue type and B presence were uploaded into GenBank, with accession numbers KX578038 (2B leaf), KX578039 (0B leaf), KX578040 (2B anthers at meiosis) and KX578041 (0B anthers at meiosis). The four 259-bp sequences were 99·6 % identical to each other and 96 and 97 % identical at the nucleotide level with Hsp101 mRNA from Triticum turgidum and Aegilops umbellulata, respectively. To analyse the transcript levels of E3900-related sequences, the primer combination tE3900-F/tE3900-R was utilized to amplify the truncated 2·7-kb form of E3900 and E3900-F/E3900-R the longer 3·9-kb variant (Pereira et al., 2009).
Table 1.
Primers utilized for Hsp101 and E3900 analysis
| Primer (position in bp*) | Primer sequence |
|---|---|
| Hsp101-F | 5′-TTTGGCCCGCGTGGCTGATCTCAGA |
| Hsp101-R | 5′-TCCGACTGCATTGACGGCCTCATA |
| tE3900-F (1973)* | 5′-attagtcacgtgtgatcaacgtgg |
| tE3900-R (3497)* | 5′-TGACTCCAGTCGTTGCCCAGCTG |
| E3900-F (2181)* | 5′-TAAAGGCATCGTCCTGGGTCTTGT |
| E3900-R (2582)* | 5′-ATGCATCTGCATGTGCGTCCTTTG |
| Actin2-F | 5′-gctggattctggtgatggtgtgag |
| Actin2-R | 5′-caatgagagatggctggaagaggac |
Position on E3900 published sequence (accession number AF222021).
The effects of heat stress on Hsp101 and E3900 transcriptional activity were quantified by comparing specific transcript levels between heat-stressed and control plants with 0B or 2Bs. Since plants from one seed were cloned into multiple individuals, it was possible to perform pairwise comparisons of heat-stressed and control plants derived from the same original plant. Comparative transcriptional analysis was performed on leaves, roots or anthers staged at pre-meiotic interphase, leptotene/zygotene, pachytene or pollen grain. Total RNA was isolated with the Qiagen RNeasy Mini Kit (cat. no. 74904; Valencia, CA, USA), following the manufacturer’s instructions. After verifying concentration and integrity, 3 μg of total RNA was utilized for RNase-free DNase digestion. To ensure that genomic DNA was completely absent prior to cDNA synthesis, PCRs were performed with Actin2 primers and 250 ng of DNase-digested RNA. Control PCRs were also carried out without template for all primer combinations.
After DNase digestion, total RNA was utilized for first-strand cDNA synthesis with reverse transcriptase Superscript II and random primers (dN9), following the manufacturer’s instructions (Invitrogen, cat. no. 18064-014). For transcript quantification, cDNA from leaves and staged anthers from 0B and 2B plants was analysed by qRT-PCR with the Bio-Rad IQ SYBR Green Supermix (cat. no. 170-8880S). PCRs were conducted as in Pereira et al. (2009) with gene- or sequence-specific primers. At least three biological replicates were utilized for all qRT-PCR experiments, resulting in RNA being extracted from at least three plants at each meiotic stage for each treatment and genotype and experiments were repeated three times per genotype, treatment and primer combination. Comparisons of expression levels were performed on identical cDNA dilutions. Melt curves were observed to ensure single amplification products with correct dissociation temperatures. Hsp101 and E3900 threshold cycles (Ct) were equilibrated with mean actin Ct to calculate ΔCt (ΔCt = Ct of interest – mean actin2 Ct). Truncated (tE3900) as well as complete E3900 sequences or Hsp101 transcript levels were compared between heat-stressed and control plants with ΔΔCt (ΔΔCt = ΔCt heat stressed – mean ΔCt control), which in turn was used to determine mean fold change (2−ΔΔCt) ± standard deviation in heat-stressed roots, leaves, or anthers with meiocytes at various developmental stages from 0B or 2B plants. Student’s t-test was utilized for statistical analysis.
FISH and 5-methyl cytosine immunofluorescence
Cytological analysis of meiocytes from 0B and 2B plants was carried out on staged anthers from heat-stressed or control single spikes. After collection, anthers were fixed in ethanol/acetic acid (3 : 1, v/v) and prepared for chromosome squashes as in Caperta et al. (2008). Briefly, fixed anthers were digested with cytohelicase, pectolyase and pectinase at 3 % each (Sigma, St Louis, MO, USA). FISH was performed as previously described (Carchilan et al., 2007). Probes for E3900-related sequences (tE3900 and E3900 labelling the short or long E3900 variant, respectively) and D1100 were utilized for 2B plants whereas probes for rDNA and rye subtelomeric sequence pSc200 were used on 0B plants. E3900-related sequences were labelled with digoxigenin- or biotin–dUTP (deoxyuridine triphosphate) (Roche, Gipf-Oberfrick, Switzerland) by PCR following the reaction conditions described by Pereira et al. (2009). B sequence D1100 (Sandery et al., 1990) was labelled with either digoxigenin- or biotin–dUTP using a nick translation kit (Roche, Indianapolis, IN, USA). For rDNA labelling, the pTa71 probe (containing the Triticum aestivum 18S, 5·8S, 25S rDNA repetitive unit; Gerlach and Bedbrook, 1979) was labelled using a nick-translation kit (Roche). Rye subtelomeric repetitive sequence pSc200 (Vershinin et al., 1995) was labelled with with digoxigenin by PCR as described by Tomás et al. (2013).
For 5-methylcytosine (5-mC) immunofluorescence, chromosome squashes of cells were collected from staged anthers of control and heat-stressed plants with 2Bs and 0Bs. Good quality squashes were subsequently washed with 1× PBS (3 × 20 min) and utilized for immunofluorescence with a monoclonal antibody against 5-mC (1 : 200, AB10805-50; Abcam, Cambridge, MA, USA) and detected with anti-mouse-Cy3 IGG (1 : 100, Sigma) according to Carvalho et al. (2010).
After FISH or immunofluorescence, slides were counterstained with 4′,6-diamidino-2-phenylindole hydrochloride (DAPI) (1 mg mL−1) in Citifluor antifade mounting medium (AF1, Agar Scientific). Samples were examined using a Zeiss Axioskop 2 epifluorescence microscope, images obtained using a Zeiss AxioCam digital camera and digital images processed using Photoshop (Adobe Systems). Approximately 100 cells were examined from at least three control or heat-stressed plants with and without Bs. Pairwise comparisons of heat-stressed and control plants derived from the same original plant were performed for analysis of chromatin structure and behaviour at each meiotic stage, and Student’s t-test was utilized for statistical analysis.
E3900 bioinformatics analysis
The location and frequency of plant heat-responsive cis-acting regulatory elements on E3900 (accession number AF222021) were determined by querying the entire 3984-nt sequence (accession number AF222021) against PLANTCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/), PLACE (http://www.dna.affrc.go.jp/PLACE/) and new PLACE (https://sogo.dna.affrc.go.jp/cgi-bin/sogo.cgi?sid= &lang =en &pj=640&action=page&page=newplace). Expected frequencies of each class of cis-regulatory elements were calculated by estimating the probability of the cis element occurring on the entire E3900: 1/[(number of nucleotides in sequence)4 × (number of possible sequences)] × [3984 − (number of nucleotides in sequence+1)], or upstream of the putative translation initiation site at nucleotide position 2023 in tE3900: 1/[(number of nucleotides in sequence)4 × (number of possible sequences)] × [2023 − (number of nucleotides in sequence+1)]. Chi-square goodness of fitness tests were utilized for statistical analysis
RESULTS
All molecular and cytological analysis of heat stress on transcript levels and meiotic chromosome behaviour/structure were performed on at least three biological replicates of cloned plants either exposed to heat stress or maintained in control conditions. This allowed for pairwise comparisons of the effects of heat on plants with the same genetic background.
Heat stress induces Hsp101 up-regulation equally in 0B and 2B plants
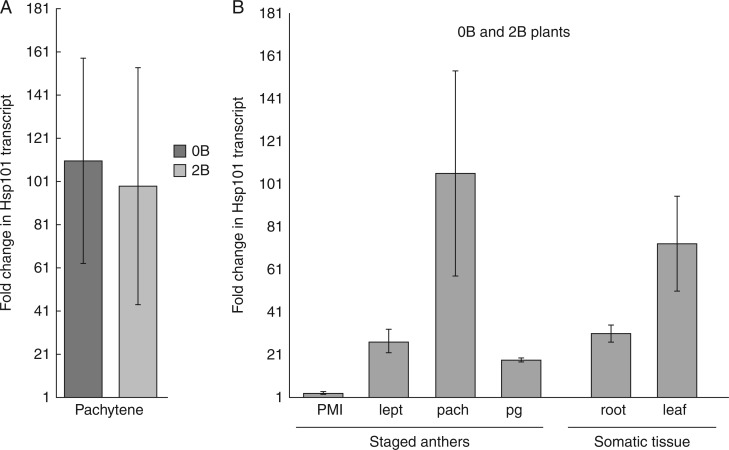
Hsp101 transcript levels in plants exposed to heat treatment were compared to those maintained in control conditions by qRT-PCR. PCR products were sequenced and were 96 and 97 % identical at the nucleotide level to Triticum turgidum and Aegilops umbellulata, respectively. Rye Hsp101 mRNA (cDNA) nucleotide sequences for 0B and 2B leaves as well as 0B and 2B anthers staged at meiosis were uploaded into GenBank with accession numbers KX578038–KX578041. As shown in Fig. 1A, heat stress induced a significant increase in Hsp101 transcript in anthers staged at pachytene immediately after exposure to heat, and this response was considerably variable between plants as demonstrated by the large error bars. This was evident as fold increases in Hsp101 (means ± s.d.) of 111 ± 48 and 99 ± 55 for 0B and 2B plants, respectively. As observed in pachytene, data showed there were no differences in heat-induced increases in Hsp101 transcript levels between 0B and 2B plants for other meiotic developmental stages (Student’s t-tests, P > 0·1) or for somatic tissues, permitting for pooling results of plants with and without Bs (Fig. 1B).
Fig. 1.
Quantitative real-time PCR of Hsp101 transcript levels in heat-stressed plants in relation to plants grown in control conditions shown as relative fold changes in transcription levels (2−ΔΔCt) ± s.d. (A) Hsp101 transcript levels are highly increased equally in 0B and 2B heat-stressed anthers with meiocytes staged at pachytene. (B) Differential heat-induced up-regulation of Hsp101 anthers with meiocytes at various developmental stages (PMI, pre-meiotic interphase; lept, leptotene; pach, pachytene; pg, pollen grain) and in somatic root and leaf tissues. No significant differences were detected between 0B and 2B plants; pooled results show an up-regulation of Hsp101 for all organs analysed with a marked peak in anthers with meiocytes staged at pachytene.
Pooled results for 0B and 2B plants showed that Hsp101 up-regulation varied significantly in anthers depending on the meiocyte developmental stage. Whereas there was a moderate 3 ± 1 increase in Hsp101 transcript in heat-shocked anthers at pre-meiotic interphase (PMI), this fold change increased significantly to 27 ± 6 at leptotene (Student’s t-test, P = 0·00002). As shown above for 0B and 2B plants separately, a peak of Hsp101 transcription in anthers staged at pachytene was evident as fold increases of 106 ± 48 in Hsp101 transcript. This fold change was significantly greater than that observed at leptotene (Student’s t-test, P = 0·0005) and was highly variable between plants, ranging between a minimum of 58 ± 7 and a maximum of 166 ± 15 per plant. Hsp101 transcription also increased in anthers with meiocytes staged at pollen grains, where a fold change increase of 18 ± 1 was observed in heat-stressed plants in comparison to controls.
Up-regulation of Hsp101 transcript levels was also observed in somatic tissue, with fold changes of 31 ± 4 and 73 ± 22 in heat-stressed roots and leaves, respectively. Interestingly, the difference in Hsp101 up-regulation was only slightly significantly greater in leaves in comparison to roots (Student’s t-test, P = 0·04), probably due to the high variability between plants in Hsp101 up-regulation in leaves. Also of interest was the effect of heat on Hsp101 in roots, since these remain submersed in moist soil throughout treatments.
Taken together, the qRT-PCR results establish that our heat shock regime induces up-regulation of Hsp101 in roots, leaves and anthers identically in 0B and 2B plants. Furthermore, up-regulation in anthers appears to be specific to the stage of reproductive development.
Differential heat-induced up-regulation in transcript levels of the two E3900 variants in 0B and 2B anthers
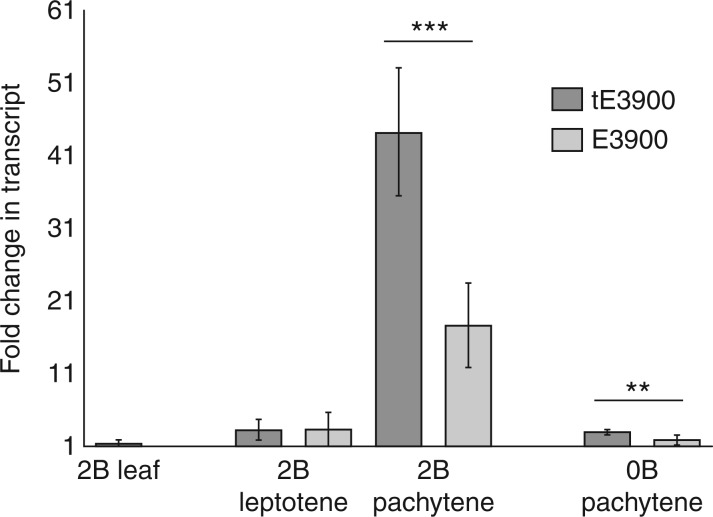
E3900 is an interesting sequence in which to study the effects of heat due to its high degree of sequence conservation, tissue and developmental transcription regulation, and the presence of a truncated variant with a putative open reading frame (t3900). Heat-induced effects on the transcript levels of the complete 3·9-kb (E3900) and truncated 2·7-kb (tE3900) E3900 variants are shown in Fig. 2. As has been previously shown for leaf somatic tissue (Pereira et al., 2009), the E3900 and tE3900 transcript levels were extremely low in roots and indistinguishable between 0B and 2B control plants (data not shown). Our heat stress regime did not significantly affect the levels of tE3900 and E3900 transcripts in leaves, evident as fold changes of < 2 between heat-stressed control and 2B leaves and Student’s t-tests results of P > 0·1 (Fig. 2).
Fig. 2.
Comparative analysis of the complete E3900 and truncated tE3900 transcript levels between heat-stressed and control plants by quantitative real-time PCR. Results are shown as relative fold changes (2−ΔΔCt) ± s.d. in relation to plants grown in control conditions. Due to the extremely low amount of transcript present in leaves, both in control and in heat-treated plants, it was not possible to perform qRT-PCR measurements for the complete E3900 sequence in leaves from 2B plants. The transcription of both E3900 variants shows a marked peak in anthers from 2B with meiocytes staged at pachytene. This heat-induced up-regulation is significantly greater for the truncated tE3900 variant in comparison with the complete E3900 (***P < 0·0001). In 0B plants, there is also a heat-induced increase in E3900 transcript levels, which are significantly greater for the truncated tE3900 variant than for the complete E3900 sequence (**P < 0·001).
In contrast, the expression of the two E3900 variants was noticeably differentially regulated in a reproductive developmental manner in heat-stressed anthers from 2B plants (Fig. 2). Whereas E3900 expression was extremely low in anthers staged at PMI, probably due to low overall transcription, there was a marked up-regulation of E3900 expression in heat-stressed 2B anthers at pachytene. Furthermore, there were obviously significant differences in heat-induced up-regulation of the two variants at pachytene (Student’s t-test, P = 0·0001), with the complete E3900 form increasing from 3 ± 2 to 18 ± 6 in comparison to tE3900 increasing from 3 ± 1 to 44 ± 9 from leptotene to pachytene, respectively. Although it was not possible to test anthers staged at all meiotic stages in 0B plants due to extremely low transcript levels of E3900-related sequences, the increase in the level of the 2·7-kb tE3900 transcript (fold change of 3 ± 0·3) is greater than that observed for the complete E3900 (fold change of 1·8 ± 0·7) in 0B anthers staged at pachytene (Student’s t-test, P = 0·001). In conclusion, although there were no heat effects on E3900 transcription in somatic tissue, the two E3900 variants were up-regulated in a developmental manner in heat-stressed anthers and this effect was significantly greater for the truncated 2·7-kb E3900 form.
E3900 is highly dynamic throughout meiosis in 2B plants
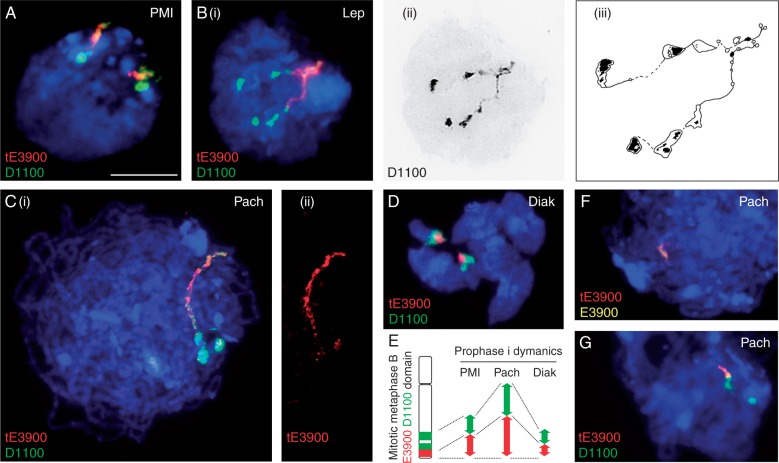
To further explore the marked peak of Hsp101 expression as well as the differential up-regulation of E3900 variants in heat-stressed anthers staged at pachytene, we focused cytological studies on PMI and prophase I. An in-depth analysis utilizing DAPI and FISH with probes for tE3900 and D1100 on 2B plants grown in control conditions showed that 2B meiocytes exhibit characteristic PMI and prophase I rye chromatin arrangement (Fig. 3). At PMI, pericentromeric and sub-telomeric domains adopted the typical Rabl orientation (Mikhailova et al., 2001) with visible partially decondensed D1100 and E3900 domains completely localizing to the heterochromatic telomeric pole (Fig. 3A). At leptotene, where the meiotic bouquet is evident (Carlton and Cande, 2002; Bass, 2003), D1100 signals showed highly condensed blocks with interstitial gaps of variable sizes and distal E3900 labels were more homogeneously decondensed (Fig. 3B). Strikingly, there was a marked difference in the condensation state E3900/D1100 B sub-telomeric domains between PMI and leptotene (compare Fig. 3A and 3B), where well-resolved chromosome fibres corresponding to D1100 and E3900 are visible spanning from the telomeric heterochromatic cluster towards the opposite cell pole [Fig. 3B(i) and (ii)]. Although D100/E3900 condensation state is somewhat variable between cells as well as between B chromosomes within the same cell, these B sub-telomeric sequences behave consistently and noticeably differently from the highly condensed cluster of A sub-telomeric domains. The fact that D1100 and E3900 domains were visibly unpaired at this meiotic stage establishes that their unexpected extension is not related to precocious chromosome pairing.
Fig. 3.
Dynamics of tE3900 (red) and D1100 (green) throughout pre-meiotic interphase and prophase I in 2B plants grown in control conditions. (A) PMI showing E3900 and D1100 domains partially decondensed in Rabl configuration. (B) (i) Leptotene with D1100 and E3900 B sub-telomeric domains associated with clustered telomeric heterochromatin, (ii) black and white image and (iii) schematic representation showing a striking extension of well-resolved chromosome fibres corresponding to B regions enriched in D1100 and E3900 from the telomeric heterochromatic cluster towards the opposite cell pole. (C) (i) Pachytene with paired B D1100/E3900-enriched regions evident as single extended FISH signals containing two distinct D1100 domains. (ii) High-resolution images of paired pachytene B chromosomes show tE3900 overlapping with the condensed blocks of the proximal D1100 domain and its complete absence in D1100 gaps. The distal D1100 region is highly enriched in both 2·7- and 3·9-kb E3900 variants and is consistently decondensed in a homogeneous manner. (D) Diakinesis with highly condensed D1100 and E3900 signals visible on commonly early resolved B chromosomes. (E) Schematic representation of dynamics in the condensation state of D1100- and E3900-enriched regions in early prophase I. D1100-rich/E3900-poor region is represented in green and D1100/E3900-rich region in red. Condensed mitotic metaphase B chromosome is presented as a reference. (F,G) Pachytene nuclei from a plant with two B chromosomes with a large deletion in the distal extended domain (2B-del). (F) Nucleus showing overlapping of tE3900 (red) and E3900 (yellow). (G) Nucleus showing the proximal condensed D1100 blocks (green) and interstitial regions, which appear intact, together with tE3900 (red). Scale bar = 10 μm.
At pachytene, paired B chromosomes D1100/E3900-enriched regions were evident as single extended FISH signals with two highly distinct D1100 domains [Fig. 3C(i)]. The proximal D1100 domain consisted of a large condensed block followed by a gap and another block, which often presented split signals [Fig. 3C(i)]. High-resolution images permitted the detection of E3900 signal overlapping with D1100 in the condensed blocks of the proximal D1100 domain and its complete absence in D1100 gaps. The distal D1100 domain, highly enriched in 2·7- and 3·9-kb E3900 variants, was consistently decondensed in a homogeneous manner [Fig. 3C(i) and (ii)]. B chromosome D1100-rich/E3900-poor regions are highly condensed whereas regions enriched in both D1100 and E3900 are vastly decondensed, suggesting that the condensation state of D1100 is correlated with the quantity of E3900 interspersed sequences. As meiocytes reached diakinesis, highly condensed D1100 and E3900 signals were visible on commonly early resolved B chromosomes (Jiménez et al., 1997) (Fig. 3D).
Taken together, these results provide the first detailed analysis of D1100 and E3900 B domains throughout prophase I. Of particular interest is the striking dynamics in the condensation state of D1100- and E3900-enriched regions throughout meiosis (Fig. 3E) as well as maintenance in the organization of the heterochromatic domains. Interestingly, E3900 FISH signals on extended pachytene chromosomes identified one plant (2B-del) carrying two B chromosomes with a large deletion mapping to the homogeneously decondensed E3900 extended B chromosome region (Fig. 3F). Comparative analysis of D1100 and tE3900 signals in 2B-del demonstrated that at least two-thirds of the distal E3900/D1100-rich domain was absent in this plant, although the more proximal condensed D1100 blocks and interstitial regions appeared intact (Fig. 3G).
Reduced heat-induced meiotic chromatin abnormalities in 2B plants
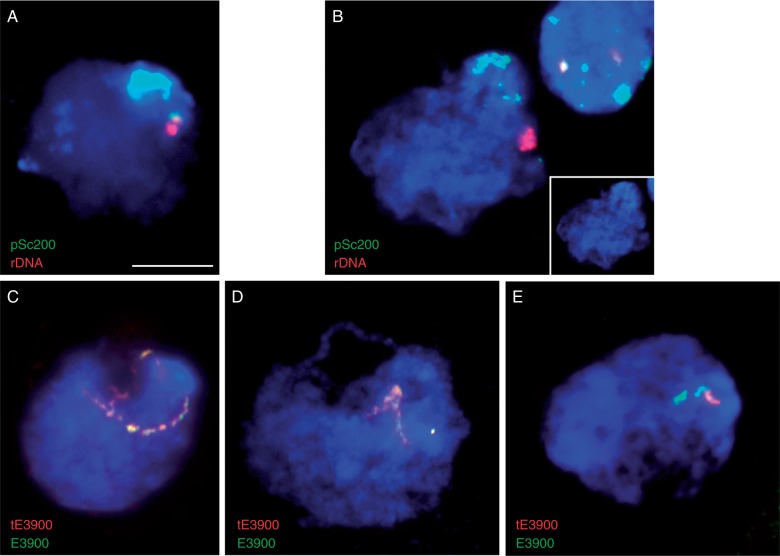
Heat effects on PMI and early prophase I chromosome behaviour and methylation status were analysed by DAPI, FISH and 5-mC immunofluorescence on meiocytes from 0B and 2B plants immediately after heat exposure and compared to those from clone plants grown in control conditions. No detectable differences are observed between heat-stressed and control plants at PMI, where typical Rabl configuration is observed (data not shown). However, heat exposure causes obvious anomalies in chromatin structure and behaviour differentially in 0B and 2B plants at subsequent meiotic stages (Fig. 4 and Table 2). At leptotene, the reorganization of sub-telomeric regions to form the typical tight polar bouquet cluster occurs normally. This is evident in heat-stressed leptotene cells with the typical bouquet configuration (Mikhailova et al., 2001) with sub-telomeric domains labelled by pSc200 signals clearly visible in 0B plants (Fig. 4A) and unpaired subtelomeric E3900 signals associated with clustered sub-telomeric blocks in 2B plants (Fig. 4C). However, although bouquet occurs normally, there are clear heat-induced alterations in chromatin structure, visible as euchromatin, which is nebulous in appearance, and a lack of resolution between euchromatic and heterochromatic regions (compare Fig. 3B to Fig. 4C for DAPI staining euchromatin/heterochromatin discrimination).
Fig. 4.
Heat-induced anomalies in 0B (A and B), 2B (C and D) and 2B-del (E) meiocytes. (A) Heat-stressed 0B leptotene with typical bouquet formation with clustered sub-telomeric domains (pSc200-green) and precociously paired rDNA (red). (B) 0B pachytene with unresolved sub-telomeric domains (pSc200-green) and paired rDNA (red). Inset shows DAPI staining with visible clustering of heterochromatic regions. (C) 2B leptotene with unpaired E3900-enriched domains (tE3900 in red and complete E3900 in green) associated with the sub-telomeric heterochromatic cluster. (D) 2B pachytene with paired E3900 domains (tE3900 in red and complete E3900 in green) and resolved sub-telomeric cluster. (E) 2B-del pachytene cell with clustered sub-telomeric regions showing paired D1100 (green) and tE3900 (red) with a visible large deletion in E3900-enriched domain. Scale bar = 10 μm.
Table 2.
Frequency of pachytene cells with heat-induced chromatin anomalies in plants with 0 and B chromosomes
| Percentage abnormal cells, mean ± s.d. (n) | ||
|---|---|---|
| 0B | 2B | 2B-del |
| 94 ± 9 (108) | 29 ± 19 (90) | 55 ± 17 (30) |
| t-test (P) | 0·01 | 0·04 |
Heat-induced chromatin alterations become exceedingly obvious and quantifiable at pachytene. In control conditions, sub-telomeric clusters are re-organized and become distributed throughout the nucleus as chromosomes pair through zygotene and pachytene (Mikhailova et al., 2001). It is precisely this re-organization of sub-telomeric chromosome regions that is highly disrupted by heat stress in a differential manner between 0B (Fig. 4A and B) and 2B (Fig. 4C and D) plants (Table 2). Heat-damaged pachytene cells display easily recognizable paired chromosome fibres and a single amorphous and heterochromatic mass closely associated with the nuclear periphery. In 0B plants, 94 ± 9 % of pachytene cells with completely paired rDNA loci evident as a single pTa71 signal maintain one visible non-resolved pSc200-positive heterochromatic block (Fig. 4B, Table 2). In contrast, in 2B plants the frequency of anomalous pachytene cells is significantly reduced to 29 ± 19 % (Fig. 4D, Table 2) (Student’s t test, P = 0·01). Interestingly, in plant 2B-del, which contains a large deletion in the E3900 domain, an intermediate value of 55 ± 17 % anomalous pachytene cells were scored (Table 2, Fig. 4E). This frequency is slightly less than what is observed in 0B plants (Student’s t-test, P = 0·04) and is not significantly different from that scored in plants with 2B chromosomes (Student’s t-test, P = 0·09).
To further explore putative epigenetic effects of the observed heat-induced changes in chromatin such as lack of resolution between euchromatin and heterochromatin as well as altered chromatin behaviour, we performed 5-mC immunodetection. The nuclear distribution of methylated cytosines on pachytene chromosomes does not appear to be altered by heat stress (Fig. 5). Immunofluorescence 5-mC signal is equally distributed throughout extended pachytene chromosome arms in control and heat-stressed 0B (compare Fig. 5A and B) and 2B (Fig. 5C) pachytene cells, with enrichment of 5-mC label in several but not all condensed regions as seen by stronger DAPI staining. Therefore, it is important to note that although there is a large frequency of anomalous pachytene cells with a single unresolved sub-telomeric heterochromatic cluster in 0B plants (Table 2), there is no evidence of heat-induced alterations on chromosome pairing or the distribution of methylated cytosines.
Fig. 5.
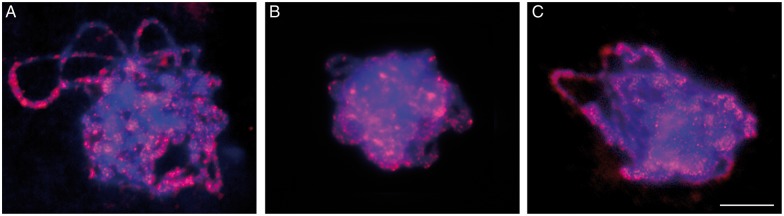
5-Methyl cytosine immunofluorescence with 5-mC signal equally distributed throughout extended pachytene chromosome arms in (A) 0B control (B) 0B heat-treated and (C) 2B heat-treated plants. Scale bar = 10 μm.
Distribution of heat-responsive cis-regulatory elements on E3900 suggests a functional role in heat tolerance
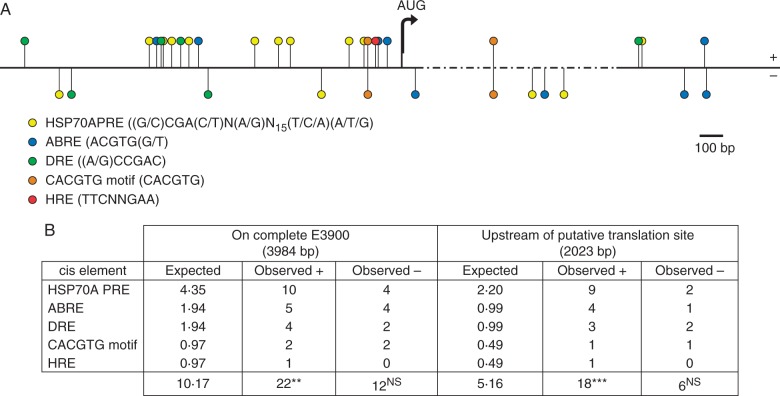
The bioinformatic results of PLANTCARE, PLACE and NewPLACE databases are shown in Fig. 6. PLANTCARE indicates the presence of an open reading frame in tE3900 with translation initiation at nucleotide position 2024, reinforcing previously published results suggesting that the 2·7-kb tE3900 contains a putative gene product (Pereira et al., 2009). PLACE and NewPLACE uncover a number of consensus sequences for cis elements implicated in the complex transcriptional response to heat stress (Sun et al., 2010). Figure 6A shows five relevant cis elements on the + and – strands of E3900, the putative translation initiation site at nucleotide position 2024 as well as the deletion uncovered by tE3900. The five cis regulatory elements shown are: HSP70A plastid response element (HSP70A PRE), which acts as an enhancer mediating the nuclear heat shock gene HSP70A in Chlamydomonas (von Gromoff et al., 2006); ABRE and DRE elements with nucleotide sequences ACGTG(G/T) and (A/G)CCGAC, respectively (Mishra et al., 2014); and CACGTG motif and heat responsive element (HRE) with nucleotide sequence TTCNNGAA (Cavrak et al., 2014). As is evident in Fig. 6A, regulatory elements accumulate on two noteworthy clusters upstream of the putative translation initiation site at nucleotide position 2024 on the + strand of E3900. Figure 6B shows the expected and observed frequencies of each cis element on the + and – strand of the entire E3900 as well as upstream of the putative translation site at nucleotide position 2024. The most represented element is HSP70A PRE, which is present ten times on the + strand of E3900, nine of which are upstream of the putative AUG start codon. ABRE and DRE are also present nine times on the + strand, seven of which are upstream of AUG. Also upstream of position 2024 there is one CACGTG motif and one HRE. Taken together, although the frequency of cis elements on the – strand of E3900 is not significantly different to what is expected, there is a significant increase in the frequency of cis elements on the + strand in comparison to the expected frequency of 10·17 (χ24 = 15·45, P < 0·004). More striking is the highly significant difference between the five expected and 18 observed cis elements upstream of the putative translation initiation site at nucleotide position 2024 on the + strand (χ24 = 35·31, P < 4 × 10−7).
Fig. 6.
Putative plant heat responsive cis regulatory elements on E3900 (accession number AF222021). (A) The complete E3900 sequence is shown with the putative translation start site (AUG) and the 1132-bp deletion present in tE3900 shown by dashed line. The distribution of five cis elements on E3900 reveals two clusters approx. 1 kb and immediately upstream of the putative translation initiation site on the + strand of E3900. (B) Observed and expected frequencies of each cis regulatory element on the + and – strands of E3900 indicate that these are more frequent than expected on the + strand of the entire sequence (**χ2, P < 0·004), and not on the – strand (NSχ2 , P > 0·05). Strikingly, 18 of the 22 observed elements on the + strand are upstream of nucleotide 2024, which is highly significantly greater than expected (***χ2 , P < 4 × 10−7).
DISCUSSION
In this work we demonstrate, for the first time, that rye supernumerary (B) chromosomes may have a role on heat stress tolerance during male sporogenesis (meiosis), considered a critically stress-sensitive stage of development (De Storme and Geelen, 2014). Due to its large genome and diploid number of 2n = 14, rye is an excellent model for cytological studies of meiosis, where the formation of stable homologous chromosome pairs requires major reorganization events (Tiang et al., 2012). Chromosome condensation changes provide the main criterion for classifying PMI and early prophase I stages leptotene, zygotene and pachytene. In this study, rye prophase I stages were classified as in Mikhailova et al. (2001), where leptotene typically displays subtelomeric regions clustered into bouquet conformation. The full bouquet is a relatively short stage, representing a transient intermediate arrangement which is completely absent by pachytene via progressive dispersal of telomeres around the inner periphery of the nuclear envelope at zygotene (Zickler and Kleckner, 1998). The cytological data shown in this work demonstrate that post-leptotene dispersal of telomeric sequences is affected in rye meiocytes that have undergone heat stress during early meiosis. Remarkably, this heat-induced meiotic anomaly is less frequent in plants carrying supernumerary (B) chromosomes, which are present in most natural rye populations.
The in-depth cytological analysis of meiosis in 2B plants presented here reiterates previous studies showing that meiotic prophase I occurs regularly under control conditions (Santos et al., 1993), with Bs forming normal bivalents at pachytene and segregating early at met/anaphase I. At PMI, the organization of B-specific domains is reminiscent of what has been observed in somatic interphase cells, where the distal portion enriched in E3900 as well as D1100 shows greater decondensation in comparison to the proximal region enriched in D1100 alone (Carchilan et al., 2007). However, in contrast to the highly condensed cluster of A sub-telomeric domains observed at PMI and leptotene, the condensation state of the D1100 and E3900 enriched B sub-telomeric domains is strikingly dynamic. B domains which completely localize to the heterochromatic telomeric cluster at PMI become extensively decondensed and span from the clustered telomeric heterochromatin to the opposite cell pole at leptotene. As has been previously demonstrated, the optimal resolution provided by extended pachytene chromosomes clearly shows two distinct D1100 domains (Klemme et al., 2013). Interestingly, our FISH results indicate that E3900-related sequences are also present in low copy number in the condensed blocks of the proximal D1100 domain. The highly decondensed state of D1100 and E3900 from leptotene to pachytene may be associated with previously described up-regulation in transcription of these sequences during meiosis in +B plants, where increased RNA levels of E3900 and D1100 are maintained in the gametophyte (Carchilan et al., 2007; Pereira et al., 2009).
Heat and drought stresses are highly damaging to plant reproductive development, causing various structural and functional abnormalities from meiosis to seed establishment (Saini, 1997; Zinn et al., 2010). Remarkably, the heat stress regime utilized here does not affect the complex processes involved in leptotene bouquet chromosome arrangement, although there are obvious anomalies in telomere dispersal as meiosis proceeds through zygotene and pachytene, as discussed in detail below. Relevant processes such as DNA condensation and chromosome pairing also proceed normally in heat-stressed plants, evident as typically highly decondensed and paired rDNA loci or E3900/D1100-rich B sub-telomeric regions in 0B or 2B plants, respectively.
Surprisingly, the frequency of meiocytes with heat-induced pachytene retention of telomeric clustering is significantly different between plants with and without Bs. Whereas most pachytene cells (94 %) from 0B plants show this abnormal chromosome arrangement, sub-telomeric regions are well resolved and distributed throughout the nucleus in the majority (approx. 70 %) of pachytene cells from heat-stressed 2B plants. Particularly interesting is the intermediate value of 55 % anomalous pachytenes scored in a heat-stressed plant which contains one B with a large deletion in the distal D1100 domain enriched in E3900 (2B-del). B deletion variants occur naturally (Müntzing, 1948; Marques et al., 2012), and often do not have accumulating capacity due to the loss of the B-specific non-disjunction control region (Jones, 1991; Marques et al., 2012). The functional role of telomere clustering and the multi-protein and DNA interactions involved in bouquet are not fully understood. Although chromosome pairing appears to initiate at the telomeres in plants, synaptic mutants in cereals have established that sub-telomeric clustering is neither essential nor sufficient for homologous pairing (Mikhailova et al., 2001; Lee et al., 2012; Tiang et al., 2012). To date, none of the several mutants showing defects in bouquet formation identified in wheat, maize and rye (Bozza and Pawlowski, 2008, and references therein) result in the heat-induced phenotype we observe in this work.
In relation to other biotic stresses such as cold and drought, gene expression responses to heat are considered to be particularly complex (Sun et al., 2010). Heat-induced transcriptional responses involve many genes including those encoding various heat shock proteins (HSPs) (Xu et al., 2011). As expected, our heat stress regime resulted in strong induction of heat shock protein Hsp101 transcript. This HSP is a member of the Hsp100/ClpB family of proteins and is believed to have a role in the disaggregation of misfolded proteins and their by-products for either refolding by Hsp90 or Hsp70 or degradation (Tonsor et al., 2008). It is considered to be essential for thermal tolerance in both Arabidopsis and maize (Tonsor et al., 2008), where Hsp101 transcript and protein production increases are observed immediately when plants are exposed to temperatures above optimal (Young et al., 2001). Our qRT-PCR results show that Hsp101 transcripts are present at low copy numbers in both somatic tissues and anthers grown in control conditions. In contrast, there is significant up-regulation of Hsp101 transcription immediately after heat shock in all organs analysed equally in 0B and 2B plants. Interestingly, heat-induced Hsp101 up-regulation is variable between tissues, presenting a marked peak in anthers staged at pachytene. These results are in accordance with previous studies in wheat showing that heat-induced HSP101 transcript and protein expression regulation occurs in an organ-specific manner (Young et al., 2001; Xu et al., 2011). In lily, heat shock induced expression of small heat shock protein LimHSP16.45 almost specifically in anther pollen mother cells and tapetal cells with a marked peak at late zygotene to pachytene stages (Mu et al., 2011). Taken together, our results establish that Hsp101 is highly responsive to our heat stress regime in rye and its transcriptional response to heat is not influenced by the presence of Bs.
Contrary to indistinguishable effects of heat on Hsp101 transcription in 0B and 2B plants, this work shows that the level of E3900 up-regulation is significantly greater in heat-stressed anthers from 2B plants. Here, it is important to note that both E3900 and tE3900 are present in approx. 100–150 copies on Bs and in single or low copy number on As, and that these sequences are transcriptionally regulated in a tissue- and developmental-dependent manner (Pereira et al., 2009). Of particular interest is the marked heat-induced up-regulation of the truncated 2·7-kb form tE3900 at pachytene, although this sequence occupies the same chromosomal region as the complete 3·9-kb variant on Bs (Pereira et al., 2009; Klemme et al., 2013). It has been previously shown that although all E3900-related sequences are up-regulated in meiocytes with B chromosomes, the truncated form is relatively more up-regulated and occupies a wider distribution on pachytene chromosomes (Pereira et al., 2009). Accordingly, our results show that although heat shock causes a marked up-regulation of E3900, this is significantly more marked for the tE3900 truncated variant. This interesting variant has been shown to be highly conserved between A and B rye chromosomes and other cereal species and contains an open reading frame for a gag retrotransposon domain with a nucleotide binding motif (Pereira et al., 2009). Interestingly, heat-induced up-regulation of tE3900 is also obvious in heat-stressed anthers without Bs. Considering the difference in the number of copies of E3900 between plants carrying Bs and those that do not, an up-regulation of tE3900 by three-fold in 0B plants which have close to a single copy of the sequence is remarkable compared to the 40-four fold upregulation in 2B plants where there are 100–150 copies. The heat responsive transcriptional behaviour of E3900 and the greater response of the truncated tE3900 is further supported by bioinformatics results showing the presence of a significant number of heat responsive cis regulatory elements on the + E3900 strand of the sequence. It is important to note that the distribution of these elements shows that 18 of the 22 map in two clusters approx. 1 kb and immediately upstream of the putative translation initiation nucleotide of the tE3900 at position 2024. Of particular interest in these two clusters is the presence of nine motifs for the plastid-responsive element (PRE) with consensus sequence (G/C)CGA(C/T)N(A/G)N15(T/C/A)(A/TG) responsive to the intermediate of chlorophyll biosynthesis Mg-protoporphyrin IX (MgProto) (von Gromoff et al., 2006). Initially identified within the Chlamydomonas HSP70A promoter (von Gromoff et al., 2006), this cis element has been found upstream of a stress-regulated TSPO-related protein in the Arabidopsis endoplasmic reticulum–Golgi membrane (Guillaumot et al., 2009). MgProto has also been implicated in the chloroplast retrograde control of nuclear gene transcription (Zhang et al., 2011).
Gene expression profiles of pollen mother cells at prophase I are highly specific, as has been shown in maize am1 meiotic mutants (Wang et al., 2010; Nan et al., 2011). This is evident as estimates of between 800 and 1500 genes being specifically or preferentially expressed in Arabidopsis and rice meiocytes under normal non-stress conditions (Chen et al., 2010; Tang et al., 2010), which is believed to be an underestimate of the number of genes expressed in prophase I male meiocytes (Ma et al., 2008; Nan et al., 2011). In addition to gene coding regions, approx. 1000 transposable element (TE)-related sequences were found to be up-regulated and fewer than 100 TE sequences down-regulated in meiocytes in comparison to seedlings (Chen et al., 2010). Considering that B chromosome E3900-related sequences have a partial gag motif from a Ty3/gypsy-type retrotransposon with a DNA binding motif, the increased E3900 transcriptional activity in 2B meiocytes may be associated with the substantial TE activity at meiosis (Carchilan et al., 2007; Pereira et al., 2009).
The complexity of transcriptional regulation of TE-rich regions such as the E3900 domain (Langdon et al., 2000; Carchilan et al., 2007; Pereira et al., 2009) is believed to increase greatly under heat stress conditions. Generally, retrotransposon transcriptional activity is believed to be epigenetically silenced by repressive chromatin modifications installed and maintained by RNA-directed DNA methylation. Silenced mobile elements are therefore typically associated with high levels of DNA methylation at cytosines in every sequence context (mCG, mCHG, mCHH, where H stands for A, T or C). Elevated temperatures were found to induce activation of heterochromatic transcription in a genome-wide manner in Arabidopsis (Tittel-Elmer et al., 2010), including retrotransposon-rich regions. Furthermore, heat-induced transcriptional activation of a Ty1/copia-type long terminal repeat retrotransposon family (ATCOPIA78) named ONSEN was found to be accompanied by the presence of double stranded DNA copies of this element in Arabidopsis (Cavrak et al., 2014; Matsunaga et al., 2015). Although stress-mediated release of retroelement transcriptional silencing has been repeatedly related to pronounced histone alterations, no adjustments in DNA methylation were detected (Pecinka et al., 2010). This is in accordance with the results presented here, where no differences in the nuclear distribution of methylated cytosines between heat-stressed and control plants were detected in 0B or 2B plants. The lack of accumulation of methylated cytosines on B chromosomes is in accordance with previous results on rye somatic metaphase chromosome spreads, where methylated DNA residues display a punctuated and uniform pattern along both the As and the Bs, without any particular sites of accumulation (Carchilan et al., 2007). The results presented here on extended pachytene chromosome arms show enrichment of methylated cytosines in several but not all heterochromatic regions, regardless of the presence or absence of Bs.
Until now, the perseverance of B chromosomes in natural rye populations remained a mystery, considering that Bs have various negative effects in high numbers and were not considered to confer any advantages (Jones, 2012). A functional role for rye B chromosomes has been suggested due to their similar molecular structure across cultivated and weedy rye species from various populations (Marques et al., 2013). This work shows, for the first time, that there is a reduction in heat-induced abnormalities in meiocytes of +B plants, which is associated with a significant up-regulation of E3900-related sequences accumulated on the non-disjunction control region of Bs. Further exploration of these results is important from scientific as well as practical perspectives. From a scientific point of view, our results may shed insight into the mechanisms involved in the interaction between telomeres and the rest of the cell at interphase and meiotic prophase (Cowan et al., 2001). Considering the serious impacts heat stress has on reproductive processes, understanding stress tolerance is undoubtedly a limiting factor influencing crop productivity (Zinn et al., 2010). Rye is a cool-season grass species and one of the most important crops in Eastern and Northern Europe, and its genome is highly related to that of bread and durum wheat. Despite the numerous beneficial features of rye, its annual production is continuously decreasing, probably due to the lack of progress in breeding and biotechnology in this species compared with other cereals (Targonska et al., 2013). Perhaps B chromosomes, which until now have been selected against in rye breeding programmes, actually hold some interesting answers for practical applications.
ACKNOWLEDGEMENTS
The Portuguese Research Foundation (FCT) under projects Pest-OE/AGR/UI0240/2011, and LEAF Unit (UID/AGR/04129/2013 provided financial support for this work. The authors declare that they have no competing interests. HSP, ADC and WV designed and coordinated the study. HSP, JMR, ADC and AB performed work. HSP, ADC, MD and WV drafted the manuscript. All authors read and approved the final manuscript.
LITERATURE CITED
- Banaei-Moghaddam AM, Schubert V, Kumke K, et al. 2012. Nondisjunction in favor of a chromosome: the mechanism of rye B chromosome drive during pollen mitosis. The Plant Cell 24: 4124–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabás B, Jäger K, Fechér A. 2008. The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell & Environment 31: 11–38. [DOI] [PubMed] [Google Scholar]
- Bass HW. 2003. Telomere dynamics unique to meiotic prophase: formation and significance of the bouquet. Cellular and Molecular Life Sciences 60: 2319–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunden R, Wilkes TJ, Forster JW, et al. 1993. Identification of the E3900 family, a second family of rye B chromosome specific repeated sequences. Genome 36: 706–711. [DOI] [PubMed] [Google Scholar]
- Bozza CG, Pawlowski WP. 2008. The cytogenetics of homologous chromosome pairing in meiosis in plants. Cytogenetic and Genome Research 120: 313–319. [DOI] [PubMed] [Google Scholar]
- Camacho JP, Sharbel TF, Beukeboom LW. 2000. B-chromosome evolution. Philosophical Transactions of the Royal Society of London. Series B Biological Sciences 355: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Klueva NY, Zheng HG, Nieto-Sotelo J, Ho TH, Nguyen H T. 2001. Cloning of new members of heat shock protein HSP101 gene family in wheat (Triticum aestivum (L.) Moench) inducible by heat, dehydration, and ABA. Biochimica et Biophysica Acta 1517: 270– 277. [DOI] [PubMed] [Google Scholar]
- Caperta AD, Rosa M, Delgado M, et al. 2008. Distribution patterns of phosphorylated Thr 3 and Thr 32 of histone H3 in plant mitosis and meiosis. Cytogenetic and Genome Research 122: 73–79. [DOI] [PubMed] [Google Scholar]
- Carchilan M, Delgado M, Ribeiro T, et al. 2007. Transcriptionally active heterochromatin in rye B chromosomes. The Plant Cell 19: 1738–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton PM, Cande WZ. 2002. Telomeres act autonomously in maize to organize the meiotic bouquet from a semipolarized chromosome orientation. Journal of Cell Biology 157: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Delgado M, Barão A, et al. 2010. Chromosome and DNA methylation dynamics during meiosis in the autotetraploid Arabidopsis arenosa. Sexual Plant Reproduction 23: 29–37. [DOI] [PubMed] [Google Scholar]
- Cavrak VV, Lettner N, Jamge S, Kosarewicz A, Bayer LM, Scheid OM. 2014. How a retrotransposon exploits the plant’s heat stress response for its activation. PLoS Genetics 10: e1004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Farmer AD, Langley RJ, et al. 2010. Meiosis-specific gene discovery in plants: RNA-Seq applied to isolated Arabidopsis male meiocytes. BMC Plant Biology 10: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YM, Lin BY. 2003. Cloning and characterization of maize B chromosome sequences derived from microdissection. Genetics 164: 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CR, Carlton MP, Cande WZ. 2001. The polar arrangement of telomeres in interphase and meiosis. Rabl organization and the bouquet. Plant Physiology 125: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D. 2014. The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant, Cell & Environment 37: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Caperta A, Ribeiro T, Viegas W, Jones RN, Morais-Cecílio L. 2004. Different numbers of rye B chromosomes induce identical compaction changes in distinct A chromosome domains. Cytogenetic and Genome Research 106: 320–324. [DOI] [PubMed] [Google Scholar]
- Endo TR, Nasuda S, Jones N, et al. 2008. Dissection of rye B chromosomes, and nondisjunction properties of the dissected segments in a common wheat background. Genes & Genetic Systems 83: 23–30. [DOI] [PubMed] [Google Scholar]
- Evans GM, Rees H, Snell CL, Sun S. 1972. The relationship between nuclear DNA amount and the duration of the mitotic cycle. Chromosomes Today 3: 24–31. [Google Scholar]
- Gerlach WL, Bedbrook JR. 1979. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Research 7: 1869–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaumot D, Guillon S, Déplanque T, et al. 2009. The Arabidopsis TSPO-related protein is a stress and abscisic acid-regulated, endoplasmic reticulum–Golgi-localized membrane protein. The Plant Journal 60: 242–256. [DOI] [PubMed] [Google Scholar]
- Holmes DS, Bougourd SM. 1989. B-chromosome selection in Allium schoenoprasum L. Natural populations. Heredity 63: 83–87. [Google Scholar]
- Jiménez MM, Romera F, Puertas MJ, Jones RN. 1994. B chromosomes in inbred lines of rye (Secale cereale L.) I. Vigour and fertility. Genetica 92: 149–154. [Google Scholar]
- Jiménez MM, Romera F, González-Sánchez M, Puertas MJ. 1997. Genetic control of the rate of transmission of rye B chromosomes. III. Male meiosis and gametogenesis. Heredity 78: 636–644. [Google Scholar]
- Jones RN. 1975. B-chromosome systems in flowering plants and animal species. International Review Cytology 40: 1–100. [DOI] [PubMed] [Google Scholar]
- Jones RN. 1991. B-chromosome drive. The American Naturalist 137: 430–442. [Google Scholar]
- Jones N. 2012. B chromosomes in plants. Plant Biosystems 146: 727–737. [Google Scholar]
- Jones RN, Puertas MJ. 1993. The B chromosomes of rye (Secale cereale L.) In: Dhir KK, Sareen TS, eds. Frontiers in plant science research. Delhi: Bhagwati Enterprises, 81–112. [Google Scholar]
- Jones RN, Rees H. 1982. B Chromosomes. London: Academic Press. [Google Scholar]
- Jones RN, González-Sánchez M, González-García M, Vega JM, Puertas MJ. 2008a. Chromosomes with a life of their own. Cytogenetic and Genome Research 120: 265–280. [DOI] [PubMed] [Google Scholar]
- Jones RN, Viegas W, Houben A. 2008b. A century of B chromosomes in plants: so what? Annals of Botany 101: 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D, Jones RN. 1970. Nuclear genetic activity in B chromosome rye, in terms of quantitative interrelationships between nuclear protein, nuclear RNA and histone. Chromosoma 31: 241–254. [Google Scholar]
- Klemme S, Banaei-Moghaddam AM, Macas J, Wicker T, Novák P, Houben A. 2013. High-copy sequences reveal distinct evolution of the rye B chromosome. New Phytologist 199: 550–558. [DOI] [PubMed] [Google Scholar]
- Lang-Mladek C, Popova O, Kiok K, et al. 2010. Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis. Molecular Plant 3: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon T, Seago C, Jones RN, et al. 2000. De novo evolution of satellite DNA on the rye B chromosome. Genetics 154: 869–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Conrad MN, Dresser ME. 2012. Meiotic chromosome pairing is promoted by telomere-led chromosome movements independent of bouquet formation. PLoS Genetics 8: e1002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-de-Faria A. 1962. Genetic interaction in rye expressed at the chromosomes phenotype. Genetics 47: 1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Skibbe DS, Fernandes J, Walbot V. 2008. Male reproductive development: gene expression profiling of maize anther and pollen ontogeny. Genome Biology 9: R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A, Klemme S, Guerra M, Houben A. 2012. Cytomolecular characterization of de novo formed rye B chromosome variants. Molecular Cytogenetics 5: 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A, Banaei-Moghaddam AM, Klemme S, et al. 2013. B chromosomes of rye are highly conserved and accompanied the development of early agriculture. Annals of Botany 112: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martis MM, Klemme S, Banaei-Moghaddam AM, et al. 2012. Selfish supernumerary chromosome reveals its origin as a mosaic of host genome and organellar sequences. Proceedings of the National Academy of Sciences of the United States of America 109: 13343–13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga W, Ohama N, Tanabe N, et al. 2015. A small RNA mediated regulation of a stress-activated retrotransposon and the tissue specific transposition during the reproductive period in Arabidopsis. Frontiers in Plant Science 6: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailova EI, Sosnikhina SP, Kirillova GA, et al. 2001. Nuclear dispositions of subtelomeric and pericentromeric chromosomal domains during meiosis in asynaptic mutants of rye (Secale cereale L.). Journal of Cell Science 114: 1875–1882. [DOI] [PubMed] [Google Scholar]
- Mishra S, Shukla A, Upadhyay S, et al. 2014. Identification, occurrence, and validation of DRE and ABRE cis-regulatory motifs in the promoter regions of genes of Arabidopsis thaliana. Journal of Integrative Biology 56: 388–399. [DOI] [PubMed] [Google Scholar]
- Morais-Cecílio L, Delgado M, Jones RN, Viegas W. 1997. Interphase arrangement of rye B chromosomes in rye and wheat. Chromosome Research 5: 177–181. [DOI] [PubMed] [Google Scholar]
- Moss JP. 1966. The adaptive significance of B chromosomes in rye. Chromosomes Today 1: 15–23. [Google Scholar]
- Mu C, Wang S, Zhang S, et al. 2011. Small heat shock protein LimHSP16.45 protects pollen mother cells and tapetal cells against extreme temperatures during late zygotene to pachytene stages of meiotic prophase I in David Lily. Plant Cell Reports 30: 1981–1989. [DOI] [PubMed] [Google Scholar]
- Müntzing A. 1943. Genetical effects of duplicated fragment chromosomes in rye. Hereditas 29: 91–112. [Google Scholar]
- Müntzing A. 1948. Cytological studies of extra fragment chromosomes in rye. V. A new fragment type arisen by deletion. Hereditas 34: 435–442. [Google Scholar]
- Müntzing A. 1963. Effects of accessory chromosomes in diploid and tetraploid rye. Hereditas 66: 279–286. [Google Scholar]
- Nan G-L, Ronceret A, Wang RC, Fernandes JF, Cande WZ, Walbo V. 2011. Global transcriptome analysis of two ameiotic1 alleles in maize anthers: defining steps in meiotic entry and progression through prophase I. BMC Plant Biology 11: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Sakamoto S. 1995. Origin of B-chromosomes in cultivated rye. Genome 38: 307–312. [DOI] [PubMed] [Google Scholar]
- Oelke EA, Oplinger ES, Bahri H, et al. 1990. Alternative Field Crops Manual University of Wisconsin Cooperative Extension Service, the University of Minnesota Extension Service and the Center for Alternative Plant and Animal Products. www.hort.purdue.edu/newcrop/afcm/rye.html.
- Oshino T, Abiko M, Saito R, et al. 2007. Premature progression of anther early developmental programs accompanied by comprehensive alterations in transcription during high-temperature injury in barley plants. Molecular Genetics and Genomics 278: 31–42. [DOI] [PubMed] [Google Scholar]
- Page BT, Wanous MK, Birchler JA. 2001. Characterization of a maize chromosome 4 centromeric sequence: evidence for an evolutionary relationship with the B chromosome centromere. Genetics 159: 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecinka A, Dihn HK, Baubec T, Rosa M, Lettner N, Sheid OM. 2010. Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. The Plant Cell 22: 3118–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira HS, Barão A, Caperta A, Rocha J, Viegas W, Delgado M. 2009. Rye Bs disclose ancestral sequences in cereal genomes with a potential role in gametophyte chromatid segregation. Molecular Biology and Evolution 26: 1683–1697. [DOI] [PubMed] [Google Scholar]
- Rees H, Hutchinson J. 1974. Nuclear DNA variation due to B chromosomes. Cold Spring Harbor Symposia on Quantitative Biology 38: 175–182. [DOI] [PubMed] [Google Scholar]
- Sakata T, Takahashi H, Nishiyama I, Higashitani A. 2000. Effects of high temperature on the development of pollen mother cells and microspores in barley Hordeum vulgare L. Journal of Plant Research 113: 395–402. [Google Scholar]
- Saini HS. 1997. Effects of water stress on male gametophyte development in plants. Sexual Plant Reproduction 10: 67–73. [Google Scholar]
- Sandery MJ, Forster JW, Blunden R, Jones RN. 1990. Identification of a family of repeated sequences on the rye B chromosome. Genome 33: 908–913. [DOI] [PubMed] [Google Scholar]
- Saini HS, Aspinall D. 1982. Abnormal sporogenesis in wheat (Triticum aestivum L.) induced by short periods of high-temperature. Annals of Botany 49: 835–846. [Google Scholar]
- Santos JL, Jiménez MM, Díez M. 1993. Synaptic patterns of rye B chromosomes. I: The standard type. Chromosome Research 1: 145–152. [DOI] [PubMed] [Google Scholar]
- Stone P. 2001. The effects of heat stress on cereal yield and quality In Basra AS, ed. Crop Responses and Adaptations to Temperature. Stress. Binghamton, NY: Food Products Press, 243–291. [Google Scholar]
- Sun X, Zou Y, Nikiforova V, Kurths J, Walther D. 2010. The complexity of gene expression dynamics revealed by permutation entropy. BMC Bioinformatics 11: 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Zhang ZY, Zhang WJ, et al. 2010. Global gene profiling of laser-captured pollen mother cells indicates molecular pathways and gene subfamilies involved in rice meiosis. Plant Physiology 154: 1855–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targoñska M, Hromada-Judycka A, Bolibok-Brągoszewska H, Rakoczy-Trojanowska M. 2013. The specificity and genetic background of the rye (Secale cereale L.) tissue culture response. Plant Cell Reports 32(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiang C-L, He Y, Pawlowski WP. 2012. Chromosome organization and dynamics during interphase, mitosis, and meiosis in plants. Plant Physiology 158(1): 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh S, Jones RN. 1978. B-chromosome fitness and selection in rye. Heredity 41: 35–48. [Google Scholar]
- Tittel-Elmer M, Bucher E, Broger L, Mathieu O, Paszkowski J, Vaillant I. 2010. Stress-induced activation of heterochromatic transcription. PLoS Genetics 6: e1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás D, Brazão J, Viegas W, Silva M. 2013. Temperature stress on nuclear topology and transcription of repetitive noncoding and coding rye sequences. Cytogenetic and Genome Research 139: 119–127. [DOI] [PubMed] [Google Scholar]
- Tonsor SJ, Scott C, Boumaza I, Liss TR, Brodsky JL, Vierling E. 2008. Heat shock protein 101 effects in A. thaliana: genetic variation, fitness and pleiotropy in controlled temperature conditions. Molecular Ecology 17: 1614–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vershinin AV, Schwarzacher T, Heslop-Harrison JS. 1995. The large-scale genomic organization of repetitive DNA families at the telomeres of rye chromosomes. The Plant Cell 7(11): 1823–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gromoff ED, Schroda M, Oster U, Beck CF. 2006. Identification of a plastid response element that acts as an enhancer within the Chlamydomonas HSP70A promoter. Nucleic Acids Research 34: 4767–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Oses-Prieto JA, Li KH, Fernandes JF, Burlingame AL, Walbot V. 2010. The male sterile 8 mutant of maize disrupts the temporal progression of the transcriptome and results in the mis-regulation of metabolic functions. The Plant Journal 63: 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes TM, Francki MG, Langridge P, Karp A, Jones RN, Forster JW. 1995. Analysis of rye B-chromosome structure using fluorescence in situ hybridization (FISH). Chromosome Research 3: 466–472. [DOI] [PubMed] [Google Scholar]
- Wilson EB. 1907. The supernumerary chromosomes of Hemiptera. Science 26: 870–871. [Google Scholar]
- Xu Y, Zhan C, Huang B. 2011. Heat shock proteins in association with heat tolerance in grasses. International Journal of Proteomics 2011: 529648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TE, Ling J, Geisler-Lee CJ, Tanguay RL, Caldwell C, Gallie D. 2001. Developmental and thermal regulation of the maize heat shock protein, HSP101. Plant Physiology 127: 777–791. [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Yuan S, Feng H, et al. 2011. Transient accumulation of Mg-protoporphyrin IX regulates expression of PhANGs – New evidence for the signaling role of tetrapyrroles in mature Arabidopsis plants. Journal of Plant Physiology 168: 714–721. [DOI] [PubMed] [Google Scholar]
- Zickler D, Kleckner N. 1998. The leptotene-zygotene transition of meiosis. Annual Review of Genetics 32: 619–97. [DOI] [PubMed] [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF. 2010. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany 61: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]