Abstract
Background and Aims Recent developments in DNA sequencing, so-called next-generation sequencing (NGS) methods, can help the study of rare lineages that are known from museum specimens. Here, the taxonomy and evolution of the Malagasy grass lineage Chasechloa was investigated with the aid of NGS.
Methods Full chloroplast genome data and some nuclear sequences were produced by NGS from old herbarium specimens, while some selected markers were generated from recently collected Malagasy grasses. In addition, a scanning electron microscopy analysis of the upper floret and cross-sections of the rachilla appendages followed by staining with Sudan IV were performed on Chasechloa to examine the morphology of the upper floret and the presence of oils in the appendages.
Key Results Chasechloa was recovered within tribe Paniceae, sub-tribe Boivinellinae, contrary to its previous placement as a member of the New World genus Echinolaena (tribe Paspaleae). Chasechloa originated in Madagascar between the Upper Miocene and the Pliocene. It comprises two species, one of them collected only once in 1851. The genus is restricted to north-western seasonally dry deciduous forests. The appendages at the base of the upper floret of Chasechloa have been confirmed as elaiosomes, an evolutionary adaptation for myrmecochory.
Conclusions Chasechloa is reinstated at the generic level and a taxonomic treatment is presented, including conservation assessments of its species. Our study also highlights the power of NGS technology to analyse relictual or probably extinct groups.
Keywords: Chasechloa, convergent evolution, Echinolaena, extinct species, Forest Shade Clade, genome skimming, herbarium, microendemic, myrmecochory, next-generation sequencing, plastome, Poaceae
INTRODUCTION
Madagascar is home to one of the most diverse, and at the same time one of the most threatened biotas of the world. This island was elected as the hottest hotspot (Myers et al., 2000), and considered to be one of the world’s highest biodiversity conservation priorities (Mittermeier et al., 2005). According to Callmander et al. (2011), the Malagasy flora is composed of 10 319 native species of angiosperms, of which 8621 (80 %) are endemic. Despite the high levels of diversity and endemism, only approx. 10 % of the natural habitats originally present in the 594 150 km2 surface area of Madagascar still remain (Goodman and Benstead, 2005). Since the arrival of humans, approx. 4000 years ago (Gommery et al., 2011), many species of animals and plants have seen their populations considerably decline and some have become extinct (e.g. Burney et al., 1997, 2004). Numerous species are only known from sub-fossil or museum specimens collected in the field within the last two centuries. Assessment of this diversity is challenging due to the scarcity of material and its poor quality for molecular analyses, but next-generation sequencing (NGS) methods have recently revolutionized the analysis of such specimens (e.g. Staats et al., 2013; Bakker et al., 2016). Organelle genomes have been reconstructed from herbarium DNA samples, allowing a reassessment of the taxonomy and biogeography of recently extinct lineages within a phylogenetic framework, for instance in Poaceae, Lamiaceae and Oleaceae (Besnard et al., 2014; Welch et al., 2016; Zedane et al., 2016).
Poaceae is among the ten families with the largest number of endemic species in Madagascar (271 species; Callmander et al., 2011). Its level of endemism (approx. 40 %, Vorontsova et al., 2016) is relatively low compared with approx. 80 % average endemism for other families of angiosperms in Madagascar (Callmander et al., 2011). The grasses are dominant and ecologically important particularly in the grasslands and savannas, which cover up to 65 % of the island (Moat and Smith, 2007). They are usually considered to be weeds and cattle feed, and are frequently overlooked by scientists (Vorontsova and Rakotoarisoa, 2014). Yet, grasses are also diverse in dry forests as well as rainforests (e.g. bamboos and Forest Shade clade; Dransfield, 2000; Vorontsova and Rakotoarisoa, 2014).
The taxonomy and systematics of Poaceae in Madagascar is incomplete and represents a significant gap in the global knowledge of the family (Vorontsova and Rakotoarisoa, 2014). A research programme is currently underway, with recent studies based on systematic and phylogenetic data (e.g. Besnard et al., 2013; Vorontsova, 2013; Vorontsova et al., 2013, 2015, 2016; Vorontsova and Rakotoarisoa, 2014). However, numerous rare taxa remain poorly known, including the likely endemic genus Chasechloa A. Camus (Vorontsova and Rakotoarisoa, 2014).
The genus Chasechloa includes few species [i.e. C. egregia (Mez) A. Camus, C. humbertiana A. Camus and C. madagascariensis (Baker) A. Camus] (Camus, 1949, 1954) and has been placed in synonymy under Echinolaena Desv., a genus particularly diverse in the New World (e.g. Clayton and Renvoize, 1986; Soreng et al., 2015). The South American Echinolaena were showed to be polyphyletic and thus the genus was recircumscribed (Silva et al., 2015). A new genus named Oedochloa C. Silva & R.P. Oliveira was proposed to accommodate part of its species, and some other species previously placed in Ichnanthus P. Beauv., which was also shown to be polyphyletic and was recircumscribed, erecting another new genus named Hildaea C. Silva & R.P. Oliveira (Silva et al., 2015). The phylogenetic placement of the Malagasy taxa of Echinolaena (hereafter named ‘Chasechloa’) was not assessed by Silva et al. (2015), but the authors examined the morphology of these taxa and stated that Chasechloa is possibly not related to the American taxa.
The South American species of Echinolaena, Hildaea, Ichnanthus, Oedochloa and some other panicoid genera possess rachilla appendages at the base of the upper floret. These appendages were hypothesized to be elaiosomes following observation of oils in the cells of these structures (Berg, 1985; Davidse, 1987). They represent an evolutionary adaptation to myrmecochorous dispersal of the caryopsis that arose several times independently within the subfamily Panicoideae (Morrone et al., 2012; Silva et al., 2015). The species of Chasechloa present similar structures at the base of the upper floret, but to date there is no evidence of oils within them.
In this study, we investigated the phylogenetic placement of Chasechloa within Poaceae for the first time. Full chloroplast genome data and some nuclear markers were produced by NGS from old herbarium specimens, while some selected markers were generated from recently collected Malagasy grasses. A scanning electron microscopy (SEM) analysis of the upper floret and cross-sections of the rachilla appendages followed by staining with Sudan IV were performed to examine the morphology of the upper floret and the presence of oils in the appendages. We carried out a taxonomic revision in order to provide more detailed descriptions, means for identification and reference data for the Malagasy taxa.
MATERIALS AND METHODS
Taxon sampling
The samples used in this study were either from old herbarium specimens or gathered during field trips in Madagascar between 2011 and 2014. Leaf fragments were obtained from specimens housed in the herbaria K, MO and P (acronyms according to Thiers, 2016), or from silica-dried specimens. The sampling includes three Malagasy accessions of Chasechloa [one of C. egregia from Nosy Be, and two of C. madagascariensis from the Ankarana (north) and Ankarafantsika (north-west)] and other selected endemic and non-endemic Malagasy taxa, which were added to the plastid and nuclear data sets of Besnard et al. (2013) and Silva et al. (2015). These data sets include representatives of the different lineages within Poaceae, with a broader sampling on Panicoideae for the ndhF gene. Lists of taxa, voucher information and GenBank accession numbers for the sequences used in this study are given in Supplementary Data Table S1.
DNA extraction, amplification and sequencing
Fragments of dried tissue (20–50 mg) were ground using mortars and pestles or in a 2 mL tube containing three tungsten balls with a TissueLyser (Qiagen Inc., TX, USA), and then DNA was extracted using the DNeasy Plant Mini Kit (Qiagen GmbH, Hilden, Germany) or the BioSprint 15 DNA Plant Kit (Qiagen Inc.).
First, two markers, the plastid gene ndhF and the nuclear ribosomal internal transcribed spacer (ITS), were selected because of their broad use in phylogenetic studies in Poaceae (e.g. Hsiao et al., 1999; Grass Phylogeny Working Group II, 2012), and their previous use in phylogenies of related taxa (Silva et al., 2015). ndhF and ITS were amplified and sequenced following the procedures described by Silva et al. (2015), and others were obtained from GenBank.
Next-generation sequencing and read assembly
The amplification of ndhF was not successful for C. egregia [L.H. Boivin s.n. (LHB), collected in 1851; P00710482], C. madagascariensis [H. Perrier de la Bâthie 11217 (HPB11217), collected in 1910; P02351581] and another rare Malagasy panicoid of the dry forest understorey with an uncertain phylogenetic placement: Brachiaria fragrans A. Camus [J.M. Bosser 19160 (JMB19160), collected in 1964; P06769493]. We consequently analysed these three old herbarium accessions with the shotgun sequencing method recently reported by Besnard et al. (2013, 2014). DNA concentration was estimated using PicoGreen. A 200 ng aliquot of double-stranded DNA was used for HPB11217 and LHB, while only 27 ng were available for JMB19160. The libraries were constructed using the Illumina TruSeq DNA Sample Prep v.2 kit following the instructions of the supplier, but without prior DNA sonication because the DNA was supposedly moderately too highly degraded. Purified fragments were A-tailed and ligated to sequencing indexed adaptors. Fragments with an insert size of approx. 50–300 bp were gel-extracted and enriched with ten cycles of PCR (with a high-fidelity Taq polymerase provided in the Illumina TruSeq Nano DNA LT Sample Prep kit) before library quantification and validation. The three libraries were multiplexed with 21 other libraries (generated in other projects). The pool of libraries was then hybridized to the HiSeq 2000 flow cell using the Illumina TruSeq PE Cluster Kit v.3. Bridge amplification was performed to generate clusters, and paired-end reads of 100 nucleotides were collected on the HiSeq 2000 sequencer using the Illumina TruSeq SBS Kit v.3 (200 cycles).
Duplicate paired-end reads were removed before the assembly of genomic regions in each sample using FastUniq (Xu et al., 2012). Partial or complete chloroplast sequences (cpDNA) and nuclear ribosomal units (nrDNA) were assembled using the approach described by Besnard et al. (2013). Annotation of the consensus plastid sequence was done with Geneious v6.0.5 (Kearse et al., 2012). In addition to these multiple-copy genomic regions, three low-copy nuclear genes were investigated as in Besnard et al. (2013). Gene regions for which sequences were already available for C3 and C4 panicoid grasses were targeted. This included two ppc genes encoding phosphoenolpyruvate carboxylase (PEPC) and phyB (phytochrome B). The ppc fragments cover exons 8–10 of two different paralogues (i.e. ppc-aL2 and ppc-aR). Gene sequences from Cyrtococcum Stapf (sub-tribe Boivinellinae, tribe Paniceae, subfamily Panicoideae) that were available in GenBank were used as the references. Illumina reads corresponding to ppc and phyB were assembled as described in Besnard et al. (2013).
Data analyses
The data sets were analysed separately because of the different taxon sampling and sequence length variation among the markers. The new sequences for ndhF, ITS, phyB, ppc-aL2 and ppc-aR were added to the ‘ndhF extended’ and ITS data sets from Silva et al. (2015) (plus Lecomtella madagascariensis A. Camus), and to the phyB, ppc-aL2 and ppc-aR data sets from Besnard et al. (2013). The matrices (available upon request) were then aligned using MUSCLE (Edgar, 2004) at the EMBL-EBI website (http://www.ebi.ac.uk/Tools/msa/muscle/), and subsequently manually refined using Geneious v6.0.5 (Kearse et al., 2012).
The ndhF, ITS, phyB, ppc-aL2 and ppc-aR data sets were analysed through Bayesian inference (BI) using MrBayes v.3.2.3 (Ronquist and Huelsenbeck, 2003) under the best-fitting nucleotide substitution model, GTR + G + I, in the CIPRES Science Gateway v.3.3 (Miller et al., 2010). Two parallel analyses were run for 10 000 000 generations using the Metropolis-coupled Markov Chain Monte Carlo (MCMC) algorithm with four random-initiated chains (Huelsenbeck et al., 2001), sampling trees every 1000 generations. The convergence of the runs was monitored using Tracer v.1.6 (Rambaut and Drummond, 2013). Trees generated before the standard deviation of split frequencies reached a value below 0·01 were discarded as burn-in (ndhF, 7500 trees; ITS, 2000 trees; phyB and ppc-aL2, 2500 trees; and ppc-aR, 6000 trees). A majority-rule (50 %) consensus tree was computed on the remaining trees with the posterior probabilities as branch support estimates. We also performed maximum likelihood (ML) analyses under a GTR + G + I model and 100 bootstrap replicates using RAxML v.8 (Stamatakis, 2006) in the CIPRES Science Gateway v.3.3 (Miller et al., 2010).
A dated phylogenetic tree was inferred from the nhdF data set using BEAST v1.8.2 (Drummond et al., 2012) in the CIPRES Science Gateway v.3.3 (Miller et al., 2010) under a GTR + G + I substitution model, an uncorrelated lognormal relaxed clock model, a tree prior with a Yule speciation model and a random starting tree. For this analysis, the ndhF data set was downsized to Gynerieae and core panicoids (Lecomtelleae, Andropogoneae, Paniceae, and Paspaleae) to avoid the possibility that under-representation of other subfamilies could affect the outcome of the analysis. Clades were preset according to the results of the ML analysis. Monophyly was enforced only for the core panicoids clade. Four calibration points were used based on the mean ages and standard deviations obtained by Christin et al. (2013), one for the crown node of the clade including tribes Gynerieae, Paspaleae, Lecomtelleae, Andropogoneae and Paniceae (normal prior with a mean of 27·6 and s.d. of 4·3), one for the crown node of Andropogoneae (normal prior with a mean of 19·96 and s.d. of 4·5), one for the crown node of Paniceae (normal prior with a mean of 22·41 and s.d. of 4) and one for the crown node of Paspaleae (normal prior with a mean of 21·6 and s.d. of 3·9). Ten independent runs of MCMC chains were run for 15 million generations, sampling trees every 10 000 generations. Convergence of the runs and effective sample size (ESS) values (only ESS >200 were accepted) were assessed using Tracer v.1.6 (Rambaut and Drummond, 2013). The first 1500 trees from each run were discarded as burn-in and tree files were combined using LogCombiner v.1.8.2 (Drummond et al., 2012). Results were summarized in a maximum clade credibility tree using TreeAnnotator v.1.8.2 (Drummond et al., 2012). Mean ages and 95 % confidence intervals of ages are reported on the nodes. The chronogram was edited using FigTree v.1.3.1 (Rambaut, 2009).
Scanning electron microscopy (SEM)
Upper anthecia were gathered for C. madagascariensis from the herbarium specimen Vorontsova et al. 1822 (K), mounted on stubs without pre-treatment and covered with gold. The photomicrographs were taken with the JEOL 6390LV scanning electron microscope. The terminology is in accordance with Shaw and Webster (1983) and the results are compared with data from the literature and our database on upper floret micromorphology (including some unpublished data).
Sudan IV test
The presence of oils in the rachilla appendages was investigated through a histochemical test with Sudan IV (Pearse, 1985). Mature (fertile) florets were gathered for C. madagascariensis from the herbarium specimen Vorontsova et al. 1822 (K), and their appendages were sectioned using a Leica CM1850 cryostat, followed by staining with Sudan IV. Photomicrographs were taken using an Olympus microscope (model BX51).
Taxonomic survey
Based on examination of the specimens housed in the herbaria B, BR, G, K, MA, MO, P, S, TAN, W and WAG, data gathered during the field trip and reassessment of the taxonomy of Chasechloa, we provide detailed morphological descriptions, a key to the species, photographs of the living plants, distribution and habitat data, and conservation assessments. All specimens cited were seen by M.S.V.
RESULTS
DNA data sets generated in the present study
Based on NGS data, we assembled a complete chloroplast genome, a nearly complete ribosomal unit and partial gene sequences (phyB, ppc-aR and ppc-aL2) for Chasechloa egregia, C. madagascariensis and Brachiaria fragrans. Gene order on chloroplast genomes was identical to that reported in other panicoid grasses (see EMBL accessions: KX663836–KX663838). The sequencing depth of plastomes and ribosomal units is superior to 140× and 850×, respectively, indicative of a high quality sequence (Supplementary Data Table S2). In contrast, the mean sequencing depth of low-copy genes (phyB, ppc-aR and ppc-aL2) was about 3× and thus insufficient for a complete assembly of these regions (Supplementary Data Table S3). New ndhF sequences were generated for ten Paniceae accessions, and one additional ITS sequence was obtained for C. madagascariensis (Table S1).
Phylogenetic reconstructions and dating analysis
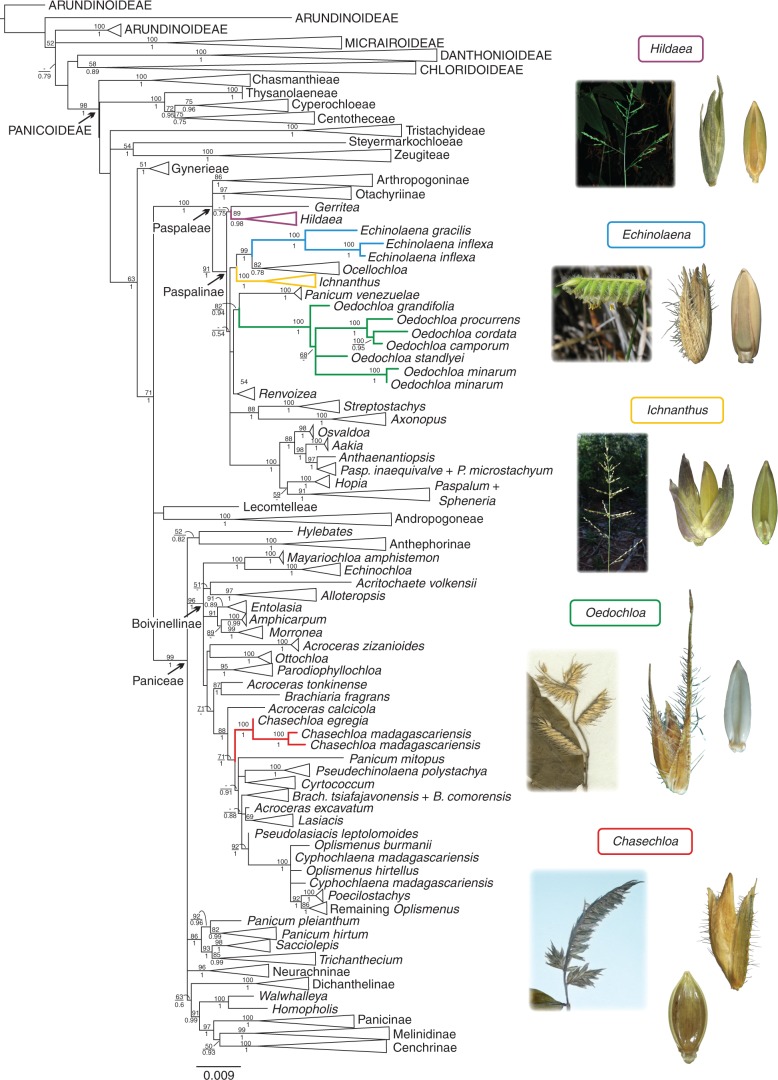
Results of the analyses are shown and discussed based on the ndhF marker since it was the most comprehensive sampled marker (Fig. 1; Supplementary Data Fig. S1). Trees resulting from the analyses of the remaining data sets are shown in Supplementary Data Figs S2–S5).
Fig. 1.
Maximum likelihood (ML) phylogram from the analysis of the plastid marker ndhF showing the phylogenetic placement of Chasechloa. ML bootstrap support values are indicated above branches and Bayesian support values below the branches. Some branches were collapsed to reduce the length of the tree. The full tree is available in Fig. S1.
All accessions of Chasechloa were recovered in a clade with high support within sub-tribe Boivinellinae, tribe Paniceae (Fig. 1). The Chasechloa clade is sister to a clade comprising Panicum mitopus K. Schum., Pseudechinolaena polystachya (Kunth) Stapf, Cyrtococcum, Brachiaria tsiafajavonensis A. Camus, B. comorensis (Mez) A. Camus, Acroceras excavatum (Henrard) Zuloaga & Morrone, Lasiacis (Griseb.) Hitchc., Pseudolasiacis leptolomoides (A. Camus) A. Camus, Cyphochlaena madagascariensis Hack., Oplismenus P. Beauv. and Poecilostachys Hack. (Fig. 1). This whole group is sister to Acroceras calcicola A. Camus (Fig. 1). Analyses of the nuclear ITS and low-copy genes also recovered Chasechloa as a monophyletic lineage within Paniceae, consistently associated with other Boivinellinae (Figs S2–S5). Echinolaena, Hildaea, Ichnanthus and Oedochloa were also recovered as monophyletic within sub-tribe Paspalinae, tribe Paspaleae (Fig. 1).
The origin of the Chasechloa lineage was estimated to have occurred in the transition between the Upper Miocene and the Pliocene, with a mean stem age of 8·9 Mya (with a 95 % confidence interval of 11·7–6·1 Mya) and a mean crown age of 4·8 Mya (7·8–2·1 Mya; Supplementary Data Fig. S6).
Microscopic observations
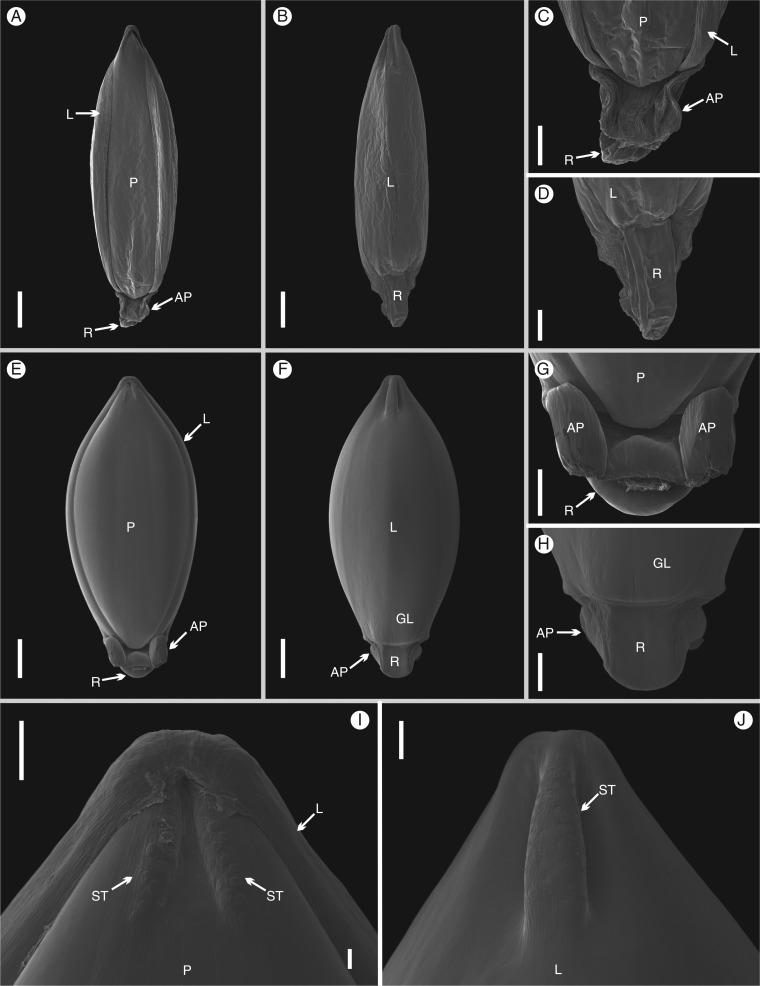
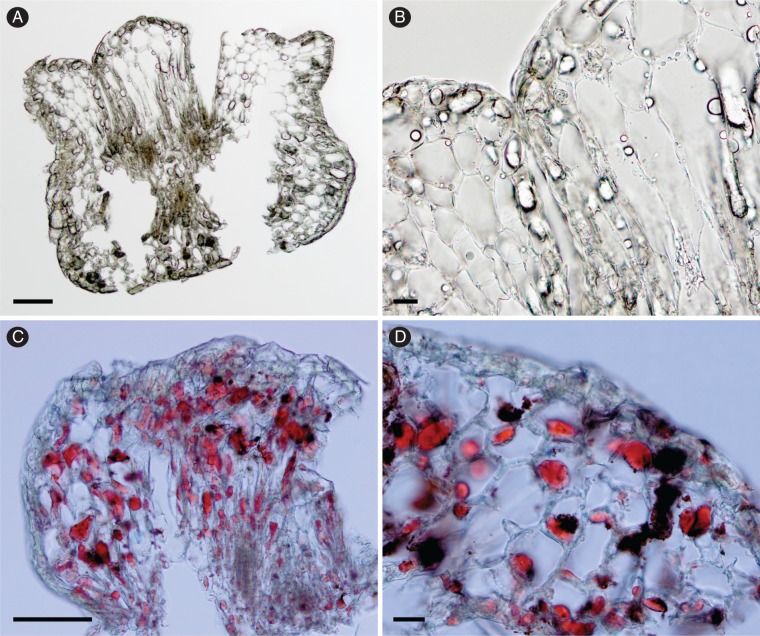
The upper floret is elliptical, white and somewhat soft when young (Fig. 2A, B). It is slightly obovate, brown, inflated and hardened after full development of the caryopsis, at maturity (Fig. 2E, F). The surface of the lemma and the palea is smooth (Fig. 2A–F) and a little wrinkly when young due to dehydration (Fig. 2A, B). At the base, there is a dorsal germination lid (Fig. 2F, H) and a rachillar stipe subtending the floret, from which the appendages arise (Fig. 2A–H). Both rachilla and appendages also become large and inflated at maturity (Fig. 2G, H). At the apex, there is a dorsal crest and two ventral folds, both covered with stomata (Fig. 2I, J). Staining with Sudan IV demonstrated the presence of oils in the appendages at maturity (Fig. 3A–D).
Fig. 2.
Scanning electron microscopy (SEM) images of the upper floret of Chasechloa madagascariensis (Vorontsova et al. 1822). (A–D) Young floret; (A) ventral view; (B) dorsal view; (C) base, ventral view; (D) base, dorsal view. (E–J) Mature floret; (E) ventral view; (F) dorsal view; (G) base, ventral view; (H) base, dorsal view; (I) apex, ventral view; (J) apex, dorsal view. Scale bars = 500 μm (A, B, E, F); 200 μm (C, D, G, H); 100 μm (I, J). AP, appendages; GL, germination lid; L, lemma; P, palea; R, rachilla; ST, stomata.
Fig. 3.
Cross-sections of the rachilla appendages at the base of the mature (fertile, i.e. with caryopsis) upper floret of Chasechloa madagascariensis (Vorontsova et al. 1822). (A and B) without staining; (C and D) staining with Sudan IV. Scale bars = 100 μm (A, C); 20 μm (B, D).
DISCUSSION
During our recent field work (in north-western and south-western Madagascar in 2015 and 2014, respectively), we were not successful in locating either Chasechloa egregia or Brachiaria fragrans in areas where they have been previously sampled. Due to the lack of recent collections, the only viable analysis method to evaluate the evolutionary affinities of these taxa was the NGS shotgun approach (so-called ‘genome skimming’; Straub et al., 2012) from museum specimens. NGS allowed us to retrieve complete plastomes, nrDNA and some selected single-copy genes for these rare Malagasy grass species (Table S1). We in fact generated more data from herbarium samples than from material analysed with PCR and Sanger sequencing, demonstrating the power of NGS to generate genomic data even from poor quality DNA extracts (e.g. Staats et al., 2013; Besnard et al., 2014; Welch et al., 2016; Zedane et al., 2016). In particular, we obtained unique data for C. egregia, which is probably extinct (see ‘Taxonomic Treatment’) and has been collected only once in 1851.
Phylogenetic placement of Chasechloa and novelties in Boivinellinae
We place the Chasechloa lineage within subfamily Panicoideae, tribe Paniceae, sub-tribe Boivinellinae. This was unexpected since Chasechloa was previously placed in synonymy under Echinolaena, a member of tribe Paspaleae, sub-tribe Paspalinae, to which Hildaea, Ichnanthus and Oedochloa also belong. Our results corroborate the distinction between these genera suggested by Silva et al. (2015) based on morphological attributes and geographical distribution.
As pointed out by Silva et al. (2015), Chasechloa is similar to the species of Oedochloa that were previously placed in Echinolaena, but it can be easily distinguished from both Echinolaena and Oedochloa by the morphology of its inflorescences, which are composed of 1–6 one-sided racemes, with the proximal racemes shorter than the terminal raceme, which is erect and continuous with the main axis. In Echinolaena, inflorescences are composed of a single one-sided raceme and main axis ending in a sterile prolongation, whereas in Oedochloa they are composed of 3–7 one-sided racemes, with the proximal racemes longer than the others, which become shorter towards the apex of the inflorescence, ending in a single spikelet (Silva et al., 2015). The comparison with other genera of Panicoideae with rachilla appendages is addressed in Silva et al. (2015).
The sub-tribe Boivinellinae, also informally known as the ‘Forest Shade Clade’ (Giussani et al., 2001), is a poorly known pantropical group, particularly diverse in Madagascar, that until now included 13–15 genera and 113–135 species of largely sprawling or trailing broad-leaved C3 grasses usually found in shady or damp conditions, also comprising C3–C4 intermediates and C4 NADP-ME taxa (i.e. Alloteropsis J. Presl and Echinochloa P. Beauv.; Grass Phylogeny Working Group II, 2012; Morrone et al., 2012; Kellogg, 2015; Soreng et al., 2015; Lundgren et al., 2016). Chasechloa and Pseudolasiacis (A. Camus) A. Camus were analysed here for the first time and placed within the Boivinellinae, as well as previously unplaced members of Malagasy Brachiaria (Trin.) Griseb. sensu lato (B. comorensis, B. fragrans and B. tsiafajavonensis) and Panicum L. sensu lato (Panicum mitopus), raising the number of genera in the sub-tribe to 19 (including Microcalamus, not sampled by us, and excluding Brachiaria and Panicum sensu lato).
Origin and diversification of Chasechloa, current distribution and implications for conservation
Most taxa belonging to the Chasechloa sister group (except for the New World Acroceras excavatum and Lasiacis) are endemic or partly endemic to Madagascar, as well as Acroceras calcicola, sister to the whole clade. All species of this lineage occur in forest shade environments. As Madagascar has been a separate land mass for >80 Mya (Scotese, 2000; Yoder and Novak, 2006), the high endemicity of this clade suggests that Chasechloa diversified on the island. The age of Chasechloa, dated to the transition between the Miocene and the Pliocene, is temporally congruent with the origin of some angiosperm genera endemic to north and west Madagascar (e.g. Chadsia Bojer, Colvillea Bojer, Pongamiopsis R. Vig., Humbertioturraea J.-F. Leroy; Buerki et al., 2013). Its species richness also fits in with patterns typical for Madagascar, where most endemic genera are monotypic or have only two or three species (Callmander et al., 2011).
Our inferred divergence time estimates also indicate that the origin of Chasechloa could be linked to environmental changes associated with a worldwide aridification (Zachos et al., 2001), the establishment of the Indian monsoons (e.g. Yoder amd Novak, 2006; Buerki et al., 2013) and/or the uplift of eastern Malagasy mountains (e.g. Vences et al., 2009; Roberts et al., 2012). In particular, Chasechloa is present in the Sambirano region (north-western Madagascar), which is characterized by heavy seasonal rains. Sub-humid forests have expanded in this area during the Late Miocene (e.g. Yoder and Novak, 2006), and a few lineages have diversified in this area during this period (e.g. Tina Schult.; Buerki et al., 2011). The genus currently inhabits a restricted area of the island, in north-western wet and seasonally dry forests. It is likely that C. egregia is now extinct at its only known location on Nosy Be (see ‘Taxonomic Treatment’). Madagascar is largely covered by grasslands and the forests represent around 20 % of the area (Moat and Smith, 2007). These forested areas are severely impacted by human activity (Mittermeier et al., 2005) and global climate change (Ingram and Dawson, 2005), threatening this lineage among many others. Thus, we re-emphasize the importance of increasing the protected area coverage on the island pointed out by Mittermeier et al. (2005).
Morphology of the upper floret and convergent evolution for myrmecochory
Overall morphology of the upper floret of Chasechloa resembles most Paniceae and Paspaleae genera. Change in shape, colour and consistency after fertilization was also observed for Ichnanthus by Silva et al. (2013), and we also have seen it in Echinolaena, Hildaea, Oedochloa and other genera within Poaceae, including outside Panicoideae. It is probably related to protection of the seeds and attraction of dispersal agents.
The dorsal crested apex is a feature of unknown function also found in other genera of Boivinellinae: Acroceras, Cyrtococcum, Entolasia Stapf, Mayariochloa Salariato, Morrone & Zuloaga, Microcalamus Franch., Oplismenus (Kellogg, 2015) and Ottochloa Dandy (C. Silva, pers. obs.). In Chasechloa, the crest surface is fully covered with stomata, but we do not know whether stomata also occur in these other genera. This apex may have been inherited by some genera from the common ancestor of the sub-tribe, or may have originated independently several times. The possibility of multiple independent origins is made more likely by the presence of this character outside Boivinellinae, in Homopholis C.E. Hubb., Rupichloa Salariato & Morrone and Urochloa P. Beauv. (Kellogg, 2015). Further research is required to understand its function and evolution within Paniceae. The structures we describe as ventral folds also have stomata on the surface. We found no reports about similar features or their proposed functions in other genera of Boivinellinae.
The presence of oils in the appendages at the base of the upper floret is here established for Chasechloa. Although we have no direct evidence of dispersal by ants, the presence of elaiosomes is a sign of adaptation for myrmecochory (Lengyel et al., 2009). Myrmecochory, i.e. seed dispersal mediated by ants, is a globally important plant–animal mutualism consistently associated with higher rates of diversification in angiosperms, and considered to be one of the best examples of convergent evolution (Lengyel et al., 2009, 2010). This seed dispersal syndrome is estimated to occur in at least 11 000 species of angiosperms and to have independently evolved 101–147 times, mostly from the Eocene when ants became numerically dominant (Dunn et al., 2007; Lengyel et al., 2010).
The studies mentioned above may have underestimated the origins of myrmecochory in grasses because elaiosomes are also known to be present in a series of other genera such as Chasechloa (this study), Echinolaena, Hildaea, Ichnanthus, Oedochloa, Yakirra Lazarides & R.D. Webster, among others (Berg, 1985; Davidse, 1987; Morrone et al., 2012; Silva et al., 2015). In Boivinellinae, only Chasechloa possesses elaiosomes in its upper floret. The outer bracts of the spikelets of Lasiacis accumulate oils and become shiny and black when they are pollinated and fertilized, but fruit dispersal is bird mediated in this genus (Davidse and Morton, 1973). Myrmecochory and its evolutionary consequences are still poorly known within Poaceae and deserve further attention.
In Madagascar, only one species of ant has been recorded as attracted to seeds: Aphaenogaster swammerdami Forel, which disperses the seeds of Commiphora guillauminii H. Perrier (Böhning-Gaese et al., 1996). These ants carry the seeds into their colony, remove the arils and discard the seeds undamaged on the refuse (Böhning-Gaese et al., 1996). A similar ant behaviour was observed in Australia for Yakirra (Berg, 1985), a grass genus similar to Chasechloa in its upper floret with oil-accumulating rachilla appendages. Ants of the genera Aphaenogaster Mayr, Pheidole Westwood or Tetramorium Mayr could be those attracted to the elaiosomes of Chasechloa (B. Fisher, pers. comm.); no data are currently available.
Reinstatement of Chasechloa
We accept Chasechloa at the generic level, and present a taxonomic revision of this genus endemic to north-western Madagascar. An analysis of the type collections and herbarium specimens indicated that C. humbertiana is within the range of morphological variation of C. madagascariensis. Thus we place C. humbertiana in synonymy under C. madagascariensis. The condensed racemose inflorescence units resembling toothbrushes are quite unique within the Boivinellinae. The pollination biology and dispersal of Chasechloa are in need of further study.
TAXONOMIC TREATMENT
Chasechloa A. Camus, Bull. Soc. Bot. France 95: 330 (1949).
Type: C. madagascariensis A. Camus
Erect, loosely tufted, rhizomatous perennial 0·2–1 m tall. Culms branching near the base or not branching. Leaf blades membranous to chartaceous, ovate to lanceolate or linear–lanceolate. Flowering culms with a terminal one-sided raceme, continuous with the main axis, and 2–5 lateral one-sided racemes. Rachis narrowly winged, finely pubescent or more rarely glabrous, with a tendency to develop a covering of tubercle-based hairs, apex ending in a single spikelet. Spikelets paired, two-flowered, laterally compressed. Glumes keeled, two-thirds to as long as the florets or slightly exceeding the florets, sub-equal with the upper somewhat longer or shorter, with 5–9 partly visible veins. Lower floret male, with a fully developed palea. Lower lemma with 9–11 veins, apically dentate. Upper floret with a basal swollen stipe (rachilla) provided with appendages, and a dorsal crest and two ventral folds at the apex. Upper lemma and upper palea smooth, shiny, white becoming brown at maturity.
Key to species
Culm internodes 1–10 cm long; 1–3 racemes per flowering culm............................................Chasechloa madagascariensis
Culm internodes 10–15 cm long; 4–6 racemes per flowering culm..............................................................Chasechloa egregia
Chasechloa madagascariensis (Baker) A. Camus, Bull. Soc. Bot. France 95: 331 (1949). Echinolaena madagascariensis Baker, J. Linn. Soc. Bot. 21: 452 (1885).
Type: Madagascar, Diego Suarez (Antsiranana), bois sablonneux derrière le village d’Ambanihala, 1846, Bernier 2278 (holotype K, isotype P02441420); Fig. 4.
Fig. 4.
Chasechloa madagascariensis in the Réserve Spéciale de l’Ankarana, Madagascar, Vorontsova et al. 1822. (A) Habitat in the understorey of semi-deciduous forest. (B) Perennial shrubby habit. (C) Flowering habit.
Chasechloa humbertiana A. Camus, Mém. Inst. Sci. Madagascar, Sér. B, Biol. Vég. 5: 203 (1954), synon. nov.
Type: Madagascar, Antsiranana, plateau calcaire de l’Ankarana du Nord entre Ambilobe et Anivorano, 200–350 m, 4–9 March 1951, H.M. Humbert and R. Capuron 25536 (Lectotype selected here, P02441421; isolectotypes, B100162303, BR0000009428614, G00007816, G00074080, K000979165, MA729055, MO2160599, P02441422, P02441423, P02441424, TAN, WAG0102738).
Rhizomatous perennial 20–50 cm tall, the rhizomes short, knotty, woody, 1·5–2·5 mm in diameter. Culms wiry, 0·4–1·8 mm in diameter, sometimes rooting at the lower nodes, the nodes finely pubescent, sometimes glabrescent or villous, the internodes 1–10 cm long, finely pubescent, sometimes glabrescent or villous. Leaf sheaths glabrous to pubescent or villous, usually with cilia along the margin. Ligule a truncate ciliate membrane 0·2–0·5 mm long. Leaf blades membranous to chartaceous, ovate to lanceolate, 5–15 × 0·8–4 cm, yellow-green to green or red-brown, discolorous or concolorous, with cross-veins visible when dry, basally unequal and usually clasping the culm, apically acute to acuminate, glabrous to pubescent or villous, the margin often ciliate with tubercle-based hairs at the base. Racemes 1–5 cm long, 1–3 per flowering culm, with 0·2–1(–3) cm between the racemes, the rachis 0·6–1·2 mm wide, finely pubescent, often with tubercle-based hairs along the margins. Spikelets 5–13 mm long, green to white at the apex or purple when fresh, yellowish green to yellow-brown when dry, glabrous to pubescent or villous with tubercle-based hairs. Glumes 4–13 mm long, two-thirds to as long as the florets or slightly exceeding the florets, acute to caudate. Lower lemma 6–12 mm long. Upper lemma 4–7 mm long.
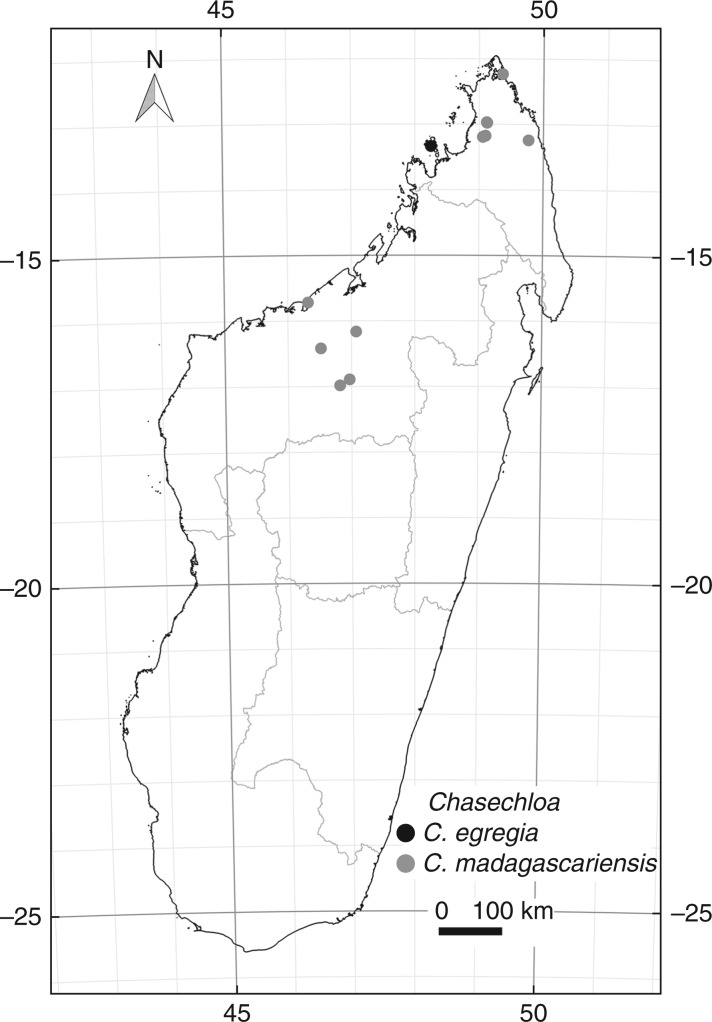
Distribution: Northern and north-western Madagascar (Fig. 5).
Fig. 5.
Geographical distribution of the two species of Chasechloa in Madagascar (see records in ‘Taxonomic Treatment’).
Habitat: Understorey of seasonally dry deciduous forest on limestone or sand, 30–350 m elevation.
IUCN conservation assessment: We assess this species as Vulnerable (VU) as the extent of occurrence is estimated to be < 20 000 km2, the species is known from fewer than ten locations and the populations have been observed to be in decline (IUCN, 2012). A field study carried out in February 2015 searched for Chasechloa in several locations outside protected areas where it has been previously recorded, noted reduction in forest canopy, and failed to locate Chasechloa. The only location where a successful collection was made was the Réserve Spéciale d’Ankarana which is under threat from sapphire mining in spite of its successful management programme (Cardiff and Befourouack, 2003).
Specimens examined: Madagascar. Antsiranana: Ambondromifehy, Réserve Spéciale d’Ankarana, Mahoro, sentier botanique partie sud, forêt sur calcaire, 12°52'19”S, 49°13'52''E, 300 m, 23 January 2003, M. Bardot-Vaucoulon, O. Andrianantoanina, A. Toly and J. Manesy 1281 (K, MO5966882, P00455583, TAN); Anivorano du Nord, PK 88, May 1970, J.M. Bosser 20230 (P02351590); collines et plateaux calcaires de l’Ankarana du nord, Ouest de la grande route, Mt Ambatopiraka, 24 January 1959, H.M. Humbert and M.G. Cours 32670 (P02329354); Ankarana National Park, path to Ambohimalaza viewpoint, 1 km from the road leading to ‘Campement des Princes’, 28 February 2015, M.S. Vorontsova, F. Randriatsara and J. Razanatsoa 1822 (K, MO, P, TAN); Ankarana du Nord, PK 103, May 1970, J.M. Bosser 20216 (P02351586); Daraina, forêt de Bekaraoka, partie sud, ca. 1 km au Nord Ouest, à 250 m à l’E du point coté 329, 15 March 2003, L. Gautier, S. Wohlhauser and L. Nusbaumer 4387 (G00007173, MO5966423); Montagne d’Andavkafanihy, calcaires de l’Ankarana, KM 105 de la route d’Ambilobe, 3 February 1960, M.G. Cours and H.M. Humbert 5600 (P02329355); dans un bois derrière le village d’Ambanihala à Diego Suarez, Bernier 48 (P02351584, P02351585, P02351587, P02351588); Orangea, 16 April 1970, J.M. Bosser 20199 (MO4844119, P02351577); commune rurale de Daraina, forêt de Bobankora, partie sud, 2 February 2005, L. Nusbaumer and P. Ranirison 1452 (G00019257, MO5966420, P06770648). Mahajanga: Ankarafantsika près de Marovoay, June 1910, H. Perrier de la Bâthie 11217 (P02351581); Belambo, près de Maevatanana, April 1922, H. Perrier de la Bâthie 14649 (P02351580); Haut Bemarivo, Boina, February 1907, H. Perrier de la Bâthie 11305 (P02351578); bois, Morataiha, Rive gauche de la Betsiboka, March 1899, H. Perrier de la Bâthie 909 bis (P02351579); environs de Majunga, March 1926, H. Perrier de la Bâthie 17623 (P02351583); près de Majunga, February 1926, H. Perrier de la Bâthie 17624 (P02351582). Province unknown: J. Goudot s.n. (P02351576).
Notes: This species encompasses a range of variation in indumentum, lower glume length, and shape of the lower glume apex; variabilities in these characters do not correlate with each other or with geographical location. The number of racemes per flowering culm, the length of racemes, the length of the spikelets, and the size of the leaves appear to vary with the local environmental conditions. This species was observed and collected in the Réserve Spéciale de l’Ankarana in February 2015.
The lectotype for Chasechloa humbertiana was selected from the original material annotated by Aimée Camus at P and constitutes the best quality flowering material.
Chasechloa egregia (Mez) A. Camus, Bull. Soc. Bot. France 95: 331 (1949). Panicum egregium Mez, Bot. Jahrb. Syst. 56: 5 (1921). Echinolaena boiviniana A. Camus, Bull. Soc. Bot. France 75: 912 (1928 publ. 1929), nom. illeg. superfl.
Type: Madagascar. Antsiranana, Nosy Be, crête de Loucoubé, près de la vigie, March 1851, L.H. Boivin s.n. (holotype P00710482, isotypes B100394106, G00007815, G00386796, K000979166, P00710482, P00710483, S11-6499, W0027576, W0027577).
A likely rhizomatous perennial 1 m tall; the rhizome morphology is not known. Culms wiry, 2–3 mm in diameter, sometimes rooting at the lower nodes, the nodes finely pubescent, the internodes 10–15 cm long, glabrous. Leaf sheaths glabrous or with some tubercle-based hairs, often with long cilia along the margin. Ligule a truncate membrane approx. 0·5 mm long. Leaf blades chartaceous, linear–lanceolate to lanceolate, 11–18 × 1–1·5 cm, green, concolorous, with cross-veins visible when dry, basally equal and never clasping the culm, apically acuminate, glabrous to pubescent, the margin often ciliate with tubercle-based hairs at the base. Racemes 1–8 cm long, 4–6 per flowering culm, 0·5–3 cm between the racemes, the rachis approx. 1 mm wide, finely pubescent. Spikelets 5–6 mm long, yellow-brown when dry, glabrous to finely pubescent, often with some tubercle-based hairs. Glumes 3·5–5 mm long, two-thirds to three-quarters, to as long as the florets, apiculate. Lower lemma 5–6 mm long. Upper lemma approx. 3·5 mm long.
Distribution: Known only from the highest point of the Réserve Naturelle Intégrale de Lokobe, Nosy Be, Madagascar (Fig. 5).
Habitat: Under wet forest canopy at 300 m elevation.
IUCN conservation assessment: The only collection of this species was made in 1851. Chasechloa egregia is assessed here as probably ‘Extinct in the Wild’ [EW] following IUCN categories and criteria (IUCN, 2012). The island has been subject to extensive development and environmental degradation. An expedition by M. S. Vorontsova and colleagues to Nosy Be in February 2015 visited the Réserve Naturelle Intégrale de Lokobe, the locality cited in the isotype herbarium sheet P00710483. The search did not reveal any populations of Chasechloa. Clear conclusions may not be drawn at this stage but we would like to highlight its extreme rarity and the recent lack of records for this species.
Specimens examined: Known from the type collection only.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: voucher information and GenBank accession numbers for the sequences used in this study. Table S2: sequence characteristics of nuclear ribosomal DNA units and complete chloroplast genomes generated in the present study by NGS. Table S3: number of paired-end reads, size of the assembled fragments and mean sequencing depth for the three low-copy genes analysed in this study. Figure S1: full maximum likelihood phylogram from the analysis of the plastid marker ndhF showing the phylogenetic placement of Chasechloa. Figure S2: maximum likelihood phylogram from the analysis of the nuclear marker ITS showing the phylogenetic placement of Chasechloa. Figure S3: maximum likelihood phylogram from the analysis of the nuclear marker phyB showing the phylogenetic placement of Chasechloa. Figure S4: maximum likelihood phylogram from the analysis of the nuclear marker ppc-aL2 showing the phylogenetic placement of Chasechloa. Figure S5: maximum likelihood phylogram from the analysis of the nuclear marker ppc-aR showing the phylogenetic placement of Chasechloa. Figure S6: maximum clade credibility chronogram of the core Panicoideae resulting from the BEAST analysis based on the ndhF data set.
ACKNOWLEDGEMENTS
We would like to thank Madagascar National Parks for granting research permits and for their collaboration, colleagues at Kew Madagascar Conservation Centre and Parc Botanique et Zoologique de Tsimbazaza for collaboration and support, staff of Réserve Spéciale de l’Ankarana and the Réserve Naturelle Intégrale de Lokobe for sharing their knowledge and for their assistance in the field, FIOCRUZ-CPqGM, Laboratório de Microscopia Eletrônica de Varredura (LABMEV-UEFS) and Luis F. P. Gusmão (LAMIC-UEFS) for making their facilities and equipment available for SEM analysis, curators of the cited herbaria for loans, samples, support during the visits, and for providing images of the specimens, and Instituto Estadual de Florestas (IEF) and Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for collecting permits. Stuart Cable (Royal Botanic Gardens, Kew) and staff at the Kew Madagascar Conservation Centre provided project support. We are grateful to Thomas Haevermans and the Muséum National d’Histoire Naturelle Paris for access to their collections and for DNA sampling of specimens, and to Ana Angélica S. Mascarenhas and Cristiane Snak for their support in the histochemical test and dating analysis, respectively. We are grateful to Brian Fisher (California Academy of Sciences) for data on myrmecochory. G.B. is member of the Laboratoire Evolution & Diversité Biologique (EDB) part of the LABEX entitled TULIP managed by Agence Nationale de la Recherche (ANR-10-LABX-0041). Hélène Holota (EDB) and Olivier Bouchez (Genopole of Toulouse) helped with the Illumina sequencing. C.S. thanks the Missouri Botanical Garden for the 2013 Shirley A. Graham Fellowship in Systematic Botany and Biogeography. We thank the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil, grants 562349/2010-3, 563558/2010-5 and 552589/2011-0), FAPESB (Fundação de Amparo à Pesquisa do Estado da Bahia, Brazil, grants PNX0014/2009) and Programa de Pesquisa em Biodiversidade do Semi-Árido (PPBio, grants 558317/2009-0 and 457427/2012-4) for financial support. This paper is part of the first author’s PhD thesis, developed in the Programa de Pós-Graduação em Botânica of the Universidade Estadual de Feira de Santana, and supported by a CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil) PhD scholarship. R.P.O. also thanks the CNPq for a productivity fellowship (PQ1-D).
LITERATURE CITED
- Bakker FT, Lei D, Yu J, et al. 2016. Herbarium genomics: plastome sequence assembly from a range of herbarium specimens using an Iterative Organelle Genome Assembly pipeline. Biological Journal of the Linnean Society 117: 33–43. [Google Scholar]
- Berg RY. 1985. Spikelet structure in Panicum australiense (Poaceae): taxonomic and ecological implications. Australian Journal of Botany 33: 579–583. [Google Scholar]
- Besnard G, Christin PA, Malé PJG, Coissac E, Ralimanana H, Vorontsova MS. 2013. Phylogenomics and taxonomy of Lecomtelleae (Poaceae), an isolated panicoid lineage from Madagascar. Annals of Botany 112: 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard G, Christin PA, Malé PJG, et al. 2014. From museums to genomics: old herbarium specimens shed light on a C3 to C4 transition. Journal of Experimental Botany 65: 6711–6721. [DOI] [PubMed] [Google Scholar]
- Böhning-Gaese K, Burkhardt JF, Schmid J. 1996. Seed dispersal in the tree Commiphora guillaumini: a combination of ornithocory and myrmecochory in a dry tropical forest in western Madagascar In: Ganzhorn JU, Sorg J-P, eds. Ecology and economy of a tropical dry forest in Madagascar. Göttingen, Germany: Primatenzentrum, 305–310. [Google Scholar]
- Buerki S, Devey DS, Callmander MW, Phillipson PB, Forest F. 2013. Spatio-temporal history of the endemic genera of Madagascar. Botanical Journal of the Linnean Society 171: 304–329. [Google Scholar]
- Buerki S, Forest F, Alvarez N, Nylander JAA, Arrigo N, Sanmartín I. 2011. An evaluation of new parsimony-based versus parametric inference methods in biogeography: a case study using the globally distributed plant family Sapindaceae. Journal of Biogeography 38: 531–550. [Google Scholar]
- Burney DA, James HF, Grady FV, et al. 1997. Environmental change, extinction and human activity: evidence from caves in NW Madagascar. Journal of Biogeography 24: 755–767. [Google Scholar]
- Burney DA, Burney LP, Godfrey LR, et al. 2004. A chronology for late Prehistoric Madagascar. Journal of Human Evolution 47: 25–63. [DOI] [PubMed] [Google Scholar]
- Callmander MW, Phillipson PB, Schatz GE, et al. 2011. The endemic and non-endemic vascular flora of Madagascar updated. Plant Ecology and Evolution 144: 121–125. [Google Scholar]
- Camus A. 1949. Chasechloa A. Camus (Graminées), genre nouveau de Madagascar et de Nossi-Bé. Bulletin de la Société Botanique de France 95: 329–331. [Google Scholar]
- Camus A. 1954. Chasechloa A. Camus, genre de Graminées Malgaches. Mémoires de l’Institut Scientifique de Madagascar, Série B, Biologie Végétale 5: 201–204. [Google Scholar]
- Cardiff SG, Befourouack J. 2003. The Réserve Spéciale d’Ankarana In: Goodman SM, Benstead JP, eds. The natural history of Madagascar, University of Chicago Press, 1501–1507. [Google Scholar]
- Christin PA, Osborne CP, Chatelet DS, et al. 2013. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proceedings of the National Academy of Sciences, USA 110: 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton WD, Renvoize SA. 1986. Genera graminum: grasses of the world. Kew Bulletin Additional Series 13: 1–389. [Google Scholar]
- Davidse G. 1987. Fruit dispersal in the Poaceae In: Soderstrom TR, Hilu KW, Campbell SC, Barkworth ME, eds. Grass systematics and evolution. Washington, DC: Smithsonian Institution Press, 143–155. [Google Scholar]
- Davidse G, Morton E. 1973. Bird-mediated fruit dispersal in the tropical grass genus Lasiacis (Gramineae: Paniceae). Biotropica 5: 162–167. [Google Scholar]
- Dransfield S. 2000. Woody bamboos (Gramineae-Bambusoideae) of Madagascar In: Jacobs SWL, Everett J, eds. Grasses: systematics and evolution. Melbourne, Australia: CSIRO, 43–50. [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn RR, Gove AD, Barraclough TG, Givinish TJ, Majer JD. 2007. Convergent evolution of an ant–plant mutualism across plant families, continents, and time. Evolutionary Ecology Research 9: 1349–1362. [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani LM, Cota-Sánchez JH, Zuloaga FO, Kellogg EA. 2001. A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of C4 photosynthesis. American Journal of Botany 88: 1993–2012. [PubMed] [Google Scholar]
- Gommery D, Ramanivosoa B, Faure M, et al. 2011. Les plus anciennes traces d’activités anthropiques de Madagascar sur des ossements d’hippopotames subfossiles d’Anjohibe (Province de Mahajanga). Comptes Rendus Palevol 10: 271–278. [Google Scholar]
- Goodman SM, Benstead JP. 2005. Updated estimates of biotic diversity and endemism for Madagascar. Oryx 39: 73–77. [Google Scholar]
- Grass Phylogeny Working Group II . 2012. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytologist 193: 304–312. [DOI] [PubMed] [Google Scholar]
- Hsiao C, Jacobs SWL, Chatterton NJ, Asay KH. 1999. A molecular phylogeny of the grass family (Poaceae) based on the sequences of nuclear ribosomal DNA (ITS). Australian Systematic Botany 11: 667–688. [Google Scholar]
- Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. 2001. Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294: 2310–2314. [DOI] [PubMed] [Google Scholar]
- Ingram JC, Dawson TP. 2005. Climate change impacts and vegetation response on the island of Madagascar. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 363: 55–59. [DOI] [PubMed] [Google Scholar]
- IUCN . 2012. Guidelines for application of IUCN Red List criteria at regional and national levels: version 4.0. Gland, Switzerland and Cambridge, UK: IUCN. [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA. 2015. Flowering plants. monocots: Poaceae The families and genera of vascular plants, Vol. 13 Heidelberg: Springer. [Google Scholar]
- Lengyel S, Gove AD, Latimer AM, Majer JD, Dunn RR. 2009. Ants sow the seeds of global diversification in flowering plants. PLoS One 4: e5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel S, Gove AD, Latimer AM, Majer JD, Dunn RR. 2010. Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: a global survey. Perspectives in Plant Ecology, Evolution and Systematics 12: 43–55. [Google Scholar]
- Lundgren M, Christin PA, Gonzalez Escobar E, et al. 2016. Evolutionary implications of C3–C4 intermediates in the grass Alloteropsis semialata. Plant, Cell and Environment 39: 1874–1885. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees In: Proceedings of the Gateway Computing Environments Workshop (GCE), November 2010. New Orleans, 1–8. [Google Scholar]
- Mittermeier RA, Hawkins F, Rajaobelina S, Langrand O. 2005. Wilderness conservation in a biodiversity hotspot. International Journal of Wilderness 11: 42–45. [Google Scholar]
- Moat J, Smith P. 2007. Atlas of the vegetation of Madagascar. Kew, London, UK: Royal Botanic Gardens. [Google Scholar]
- Morrone O, Aagesen L, Scataglini MA, et al. 2012. Phylogeny of the Paniceae (Poaceae: Panicoideae): integrating plastid DNA sequences and morphology into a new classification. Cladistics 28: 333–356. [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. 1985. Histochemistry: theoretical and applied Vol. 2. Analytical technology, 4th edn. Edinburgh: Churchill Livingstone. [Google Scholar]
- Rambaut A. 2009. FigTree version 1.3.1: tree figure drawing tool. Computer program and documentation distributed by the author. http://tree.bio.ed.ac.uk/software/figtree.
- Rambaut A, Drummond AJ. 2013. Tracer version 1.6. Computer program and documentation distributed by the author. http://beast.bio.ed.ac.uk/Tracer.
- Roberts GG, Paul JD, White N, Winterbourne J. 2012. Temporal and spatial evolution of dynamic support from river profiles: a framework for Madagascar. Geochemistry, Geophysics, Geosystems 13: 4. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Scotese CR. 2000. PALEOMAP Project: earth history (paleogeographicmaps). Department of Geology, University of Texas, Arlington. [Google Scholar]
- Shaw RB, Webster RD. 1983. Characteristics of the upper anthecia of Ichnanthus (Poaceae: Paniceae). Botanical Gazette 144: 363–370. [Google Scholar]
- Silva C, Ferreira FM, Viana PL, Oliveira RP. 2013. A new species of Ichnanthus (Poaceae, Paspaleae) endemic to Southern Minas Gerais, Brazil. Phytotaxa 104: 21–29. [Google Scholar]
- Silva C, Snak C, Schnadelbach AS, van den Berg C, Oliveira RP. 2015. Phylogenetic relationships of Echinolaena and Ichnanthus within Panicoideae (Poaceae) reveal two new genera of tropical grasses. Molecular Phylogenetics and Evolution 93: 212–233. [DOI] [PubMed] [Google Scholar]
- Soreng RJ, Peterson PM, Romaschenko K, et al. 2015. A worldwide phylogenetic classification of the Poaceae (Gramineae). Journal of Systematics and Evolution 53: 117–137. [Google Scholar]
- Staats M, Erkens RHJ, van de Vossenberg B, et al. 2013. Genomic treasure troves: complete genome sequencing of herbarium and insect museum specimens. PLoS One 8: e69189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Straub SCK, Parks M, Weitemier K, Fishbein M, Cronn RC, Liston A. 2012. Navigating the tip of the genomic iceberg: next-generation sequencing for plant systematics. American Journal of Botany 99: 349−364. [DOI] [PubMed] [Google Scholar]
- Thiers B. 2016. [continuously updated]. Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium; http://sweetgum.nybg.org/science/ih/ (last accessed 21 June 2016). [Google Scholar]
- Vences M, Wollenberg KC, Vieites DR, Lees DC. 2009. Madagascar as a model region of species diversification. Trends in Ecology and Evolution 24: 456–465. [DOI] [PubMed] [Google Scholar]
- Vorontsova MS. 2013. Variable morphology of the Madagascar endemic Aristida tenuissima (Poaceae: Aristidoideae) and the absence of Stipa (Poaceae: Pooideae, Stipeae) from Madagascar. Phytotaxa 92: 55–58. [Google Scholar]
- Vorontsova MS, Rakotoarisoa SE. 2014. Endemic non-bambusoid genera of grasses (Poaceae) in Madagascar: review of current knowledge. Malagasy Nature 8: 14–34. [Google Scholar]
- Vorontsova MS, Ratovonirina G, Randriamboavonjy T. 2013. Revision of Andropogon and Diectomis (Poaceae: Sacchareae) in Madagascar and the new Andropogon itremoensis from the Itremo Massif. Kew Bulletin 68: 193–207. [Google Scholar]
- Vorontsova MS, Haevermans T, Haevermans A, Razanatsoa J, Lundgren MR, Besnard G. 2015. The genus Sartidia (Poaceae: Aristidoideae) in Madagascar. Systematic Botany 40: 448–453. [Google Scholar]
- Vorontsova MS, Besnard G, Forest F, et al. 2016. Madagascar’s grasses and grasslands: anthropogenic or natural? Proceedings of the Royal Society B: Biological Sciences 283: 20152262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch AJ, Collins K, Ratan A, Drautz-Moses DI, Schuster SC, Lindqvist C. 2016. The quest to resolve recent radiations: plastid phylogenomics of extinct and endangered Hawaiian endemic mints (Lamiaceae). Molecular Phylogenetics and Evolution 99: 16–33. [DOI] [PubMed] [Google Scholar]
- Xu H, Luo X, Qian J, et al. 2012. FastUniq: a fast de novo duplicates removal tool for paired short reads. PLoS One 7: e52249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder AD, Novak MD. 2006. Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell. Annual Review of Ecology Evolution and Systematics 37: 405–431. [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292: 686–693. [DOI] [PubMed] [Google Scholar]
- Zedane Z, Hong-Wa C, Murienne J, Jeziorski C, Baldwin BG, Besnard G. 2016. Museomics illuminate the history of an extinct, paleoendemic plant lineage (Hesperelaea, Oleaceae) known from an 1875 collection on Guadalupe Island, Mexico. Biological Journal of the Linnean Society 117: 44–57. [Google Scholar]