Abstract
Background Dormancy in higher plants is an adaptive response enabling plant survival during the harshest seasons and has been more explored in woody species than in herbaceous species. Nevertheless, winter and summer shoot meristem dormancy are adaptive strategies that could play a major role in enhancing seasonal stress tolerance and resilience of widespread herbaceous plant communities.
Scope This review outlines the symmetrical aspects of winter and summer dormancy in order to better understand plant adaptation to severe stress, and highlight research priorities in a changing climate. Seasonal dormancy is a good model to explore the growth–stress survival trade-off and unravel the relationships between growth potential and stress hardiness. Although photoperiod and temperature are known to play a crucial, though reversed, role in the induction and release of both types of dormancy, the thresholds and combined effects of these environmental factors remain to be identified. The biochemical compounds involved in induction or release in winter dormancy (abscisic acid, ethylene, sugars, cytokinins and gibberellins) could be a priority research focus for summer dormancy. To address these research priorities, herbaceous species, being more tractable than woody species, are excellent model plants for which both summer and winter dormancy have been clearly identified.
Conclusions Summer and winter dormancy, although responding to inverse conditions, share many characteristics. This analogous nature can facilitate research as well as lead to insight into plant adaptations to extreme conditions and the evolution of phenological patterns of species and communities under climate change. The development of phenotypes showing reduced winter and/or enhanced summer dormancy may be expected and could improve adaptation to less predictable environmental stresses correlated with future climates. To this end, it is suggested to explore the inter- and intraspecific genotypic variability of dormancy and its plasticity according to environmental conditions to contribute to predicting and mitigating global warming.
Keywords: Summer dormancy, winter dormancy, perennial herbaceous species, drought, frost, dehydration tolerance, survival, hormones, induction, climate change
INTRODUCTION
Importance of seasonal dormancy
Dormancy in higher plants is an adaptive response, evolved in the species’ origin environment, enabling survival during seasons when environmental conditions are most threatening (Vegis, 1964). Among these adaptations, seed dormancy, which provides a mechanism for plants to delay germination until conditions are optimal for the survival of the next generation, has been extensively reviewed (Finch-Savage and Leubner-Metzger, 2006; Finkelstein et al., 2008; Graeber et al., 2012). With regard to dormancy in vegetative tissues, this ability to cease meristem activity and render it insensitive to growth-promoting signals is a feature of the perennial lifestyle in plants (Rohde and Bhalerao, 2007). Most of the research on this issue has dealt with bud dormancy since the activity–dormancy cycle is an important adaptive trait ensuring survival for most tree species native to temperate and boreal regions (van der Schoot et al., 2014) and it reflects adaptation to prevailing climatic conditions (Cooke et al., 2012).
In contrast, dormancy responses in herbaceous species, key adaptations to survive the harshest seasonal stresses, have been less explored. Winter dormancy (also referred to as autumn dormancy) and summer dormancy are both adaptive strategies of herbaceous perennials allowing them to persist under seasonal, severe stress through the tolerance of key organs to dehydration induced by water deficit, heat or frost (Castonguay et al., 2006; Volaire and Norton, 2006; Anderson et al., 2010). These adaptive responses during the periods of intense stress contribute to the persistence and thus to the agricultural importance of many species, e.g. potato (Solanum tuberosum L.) (Muthoni et al., 2014), alfalfa syn. lucerne (Medicago sativa L.) (Castonguay et al., 2006) or phalaris (Phalaris aquatica L.) (Culvenor, 2009). In the context of climate change, it is timely to question the nature and role of these adaptive strategies since plant phenology is affected by temperature increase and climate change patterns (Hansen et al., 2006; Campoy et al., 2011; IPCC, 2014). The earlier onset of leaf unfolding and flowering has already been documented in dormancy-expressing tree species in Europe (Menzel et al., 2006; Campoy et al., 2011), indicating earlier breaking of winter dormancy. Warming winter temperatures (Cayan et al., 2001) may inhibit winter dormancy as the required low temperatures for dormancy induction may no longer be reached. Similarly, even if dormancy induction is achieved, the shortened winters may impede or alter dormancy breaking because there may be insufficient chilling requirement for release (Campoy et al., 2011; Pagter et al., 2015; Chuine et al., 2016). This effect on the phenology of winter dormant plants may lead to a decline in the expression of the winter dormancy trait associated with reduced overwintering, i.e. lower ability to survive winter conditions (Charrier et al., 2015). Likewise, in herbaceous species, future climate projections suggest that cold acclimation will occur later (Rapacz et al., 2014) and, although higher temperatures reduce the risk of plant exposure to frost, the effects of a warmer winter may jeopardize freezing tolerance and overwintering ability especially in the case of extreme events (such as spring frosts), which are predicted to increase in frequency and amplitude (Rapacz et al., 2014). Conversely, summer dormancy may become a progressively valuable trait to survive higher overall temperatures, temperature extremes and droughts (Meehl and Tebaldi, 2004; IPCC, 2014; Balachowski et al., 2016).
To face these new challenges and help predict species responses to a changing climate, this review aims to outline and compare the basic aspects of winter and summer dormancy in herbaceous perennials which, in contrast to woody species, have been reported to exhibit both types of dormancy. It will focus on the eco-physiology of dormancy characteristics under stresses imposed by seasonal conditions, induction and release factors, and the relationships between dormancy and stress tolerance. The biochemical regulation of winter dormancy has already been reviewed in woody species (Arora et al., 2003; Olsen, 2006, 2010; Aragoncillo et al., 2008) and in herbaceous species (Horvath et al., 2003; Anderson et al., 2010). However, summer dormancy is still little explored, and this review aims to identify the known generic biochemical drivers in winter dormancy as a research priority to better understand summer dormancy. Although overwintering in many ornamental herbaceous species (Orchidaceae, Gentianeae, Chrysanthemum spp. L., etc.) has been extensively explored, the research goal is often to modulate bud break for flower production in controlled conditions and thus to analyse the effects of vernalization on the subsequent flowering stage (Sumitomo et al., 2008; Takahashi et al., 2014; Samarakoon et al., 2015). Moreover, for these species, the research aim is rarely to understand the role of winter dormancy for winter frost survival in situ. These model plants will therefore not be extensively reviewed here. Overall, this comparative study aims to identify the extent of which summer and winter dormancy are analogous strategies to climatically symmetrical conditions in order to explore and underline generic ecophysiological issues applicable to both seasonal dormancy types. Moreover, neither genetic nor epigenetic control will be considered since we are still lacking the basis of biochemical regulation in summer dormant species. Finally, this review also aims to contribute to the identification of future research directions that are required to understand the mechanisms driving phenological shifts and their consequence on ecosystem structure and functions (Wolkovich et al., 2014).
Types of dormancy
Dormancy is generally defined as ‘a temporary suspension of visible growth of any plant structure containing a meristem’ (Lang et al., 1987), and is subsequently divided it into three sub-groups endo-, para- and ecodormancy, with endodormancy, inhibition of growth by internal bud signals; paradormancy, inhibition of growth by signals from distal organs; and ecodormancy, inhibition of growth by temporary unfavourable environmental conditions. However, this typology is controversial since growth within meristems is not readily visible and dormancy is the inability to resume growth not exclusively the absence of growth, which led to a more precise definition of endodormancy as ‘the inability to initiate growth from meristems under favourable conditions’ (Rohde and Bhalerao, 2007). It was also underlined that any meristem waiting for the right sprouting conditions (e.g. rain or temperature) would be categorized as eco-dormant although ready and able to grow (Volaire and Norton, 2006). In the case of summer dormancy, these authors then proposed a more stringent categorization according to which, under full summer irrigation, complete summer dormancy is associated with growth cessation and induced dehydration of meristems, while incomplete dormancy is detected when meristem (and then leaf) growth rate is partially reduced. Likewise, it was shown that reducing the signals causing paradormancy could also result in incomplete growth inhibition (Horvath, 1999; Horvath and Anderson, 2002). In agreement with Rohde and Bhalerao (2007), under endo- or complete dormancy, shoot meristem growth should be inhibited even under favourable growing conditions. However, despite these shortcomings, the aforementioned definition and categorization remain the standard in the literature. Both categorizations (endo-/para- and complete/incomplete dormancy) will be used in this review. Eco-dormancy (or quiescence, according to Considine and Considine, 2016), will not be addressed due to the specified drawback. In agreement with the terminology developed to characterize seed dormancy, the notions of facultative or obligate dormancy associated with the intensity of response to environmental cues, although poorly defined, are starting to be applied to winter and summer dormancy of vegetative tissues in herbaceous species (Vaughton and Ramsey, 2001; Newell et al., 2015). Conversely, the notion of dormancy depth, which has not been reported for summer dormant species so far, will not be discussed in this review.
Winter dormancy
In areas with harsh winters, winter dormancy is a common persistence strategy for both herbaceous and woody plants. Notable examples of species that express winter dormancy for survival are alfalfa, Medicago sativa L. (Castonguay et al., 2006); leafy spurge, Euphorbia esula L. (Chao et al., 2007); potato, Solanum tuberosum L. (Muthoni et al., 2014); lily, Lilium spp. L. (Kim et al., 1994); tulip, Tulipa spp. L. (Kamenetsky et al., 2003); strawberry, Fragaria spp. L. (Zhang et al., 2012); and onion, Allium cepa L. (Benkeblia and Selselet-Attou, 1999). However, the expression of dormancy varies greatly between and within species, and exhibits considerable genotype by environment interaction (Brummer et al., 2000; Muthoni et al., 2014).
In regions where plants express winter dormancy, the predominant stresses include freezing temperatures, soil heaving and ice encasement (Castonguay et al., 2006; Muthoni et al., 2014; Pembleton and Sathish, 2014). Freezing temperatures induce dehydration, which disrupts the plant’s water status and causes potentially lethal osmotic and oxidative stress (Pembleton and Sathish, 2014). Soil heaving, the uplift of soil caused by repetitive freezing and thawing, can lead to the exposure of resting organs to cold temperatures with little or no insulation from the soil (Castonguay et al., 2006) as well as damage to organs, e.g. roots, subjected to the physical stresses of soil heaving. Ice encasement can hinder or halt gas exchange, creating anaerobic conditions and injurious levels of CO2 (Castonguay et al., 2006; Muthoni et al., 2014). These stresses can lead to tissue damage and potentially plant death.
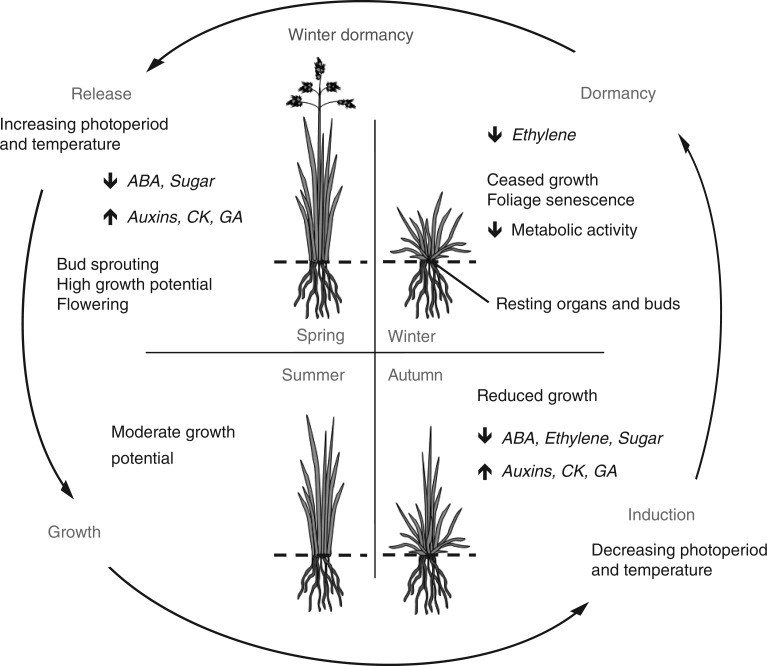
Winter dormancy in herbaceous perennials (Fig. 1) is marked by (1) the decrease or cessation of growth (Castonguay et al., 2006; Horvath, 2009); (2) senescence of above-ground foliage (Horvath et al., 2003; Anderson et al., 2005; Muthoni et al., 2014); (3) possible preceding development of resting organs (also referred to as dormant or storage organs, e.g. crown, tuber, bulb and corm) (Vegis, 1964; Kim et al., 1994); and (4) reduction of metabolic activity (Anderson et al., 2005; Aksenova et al., 2013). As a result, a major effect of winter dormancy is the inhibition against precocious sprouting when unseasonal favourable conditions occur, which could be detrimental to the plant’s survival following the return of seasonal unfavourable conditions (Chao et al., 2007). The enhancement of frost and dehydration resistance was also suggested to be associated with winter dormancy (Havranek and Tranquillini, 1995) since it is correlated with greater winter survival (Weishaar et al., 2005). However, other studies suggest that this superior survival is possibly due to higher winter hardiness and not winter dormancy both in alfalfa and in switchgrass (Castonguay et al., 2006; Sarath et al., 2014). In alfalfa, dormancy is often associated with slower regrowth and less growth through late summer and early autumn (Castonguay et al., 2006). Conversely, winter dormant cultivars have been found to be more summer drought resistant, which, during water-limiting conditions, leads to higher forage yields than non-dormant cultivars (Pembleton and Sathish, 2014). Winter dormant cultivars are also more tolerant of ice encasement (lower metabolic rates slowing CO2 production and accumulation) and have deeper-set crowns serving to protect against ice heaving (Castonguay et al., 2006; Aksenova et al., 2013).
Fig. 1.
Diagram of the annual induction and release cycle of winter dormancy in a herbaceous perennial species and the associated variations in phytohormone and molecule concentrations. ABA, abscisic acid; CK, cytokinin; GA, gibberellin.
In addition, winter dormancy in tuber and bulb species confers greater protection against pathogens (Aksenova et al., 2013). Indeed, the benefits of dormancy are variable depending on the species and environmental conditions experienced, but would be most valuable for plants subjected to predictably cold, dry winter climates.
Summer dormancy
Summer dormancy in herbaceous perennials has mainly been observed in plant geophytes from semi-arid Mediterranean-type climatic regions with mild winters and predictably long, dry and hot summers (Norton et al., 2009). Among the few Poaceae species known to express summer dormancy, bulbous bluegrass, Poa bulbosa L. (Ofir, 1986); bulbous barley, Hordeum bulbosum L. (Ofir, 1976); cocksfoot, Dactylis glomerata L. (Volaire et al., 2009) as well as ornamental species such as Hyacinthus spp. Tourn. (Nowak and Rudnicki, 1993) or Gladiolus spp. L. (Cohat, 1993) have been the most studied species. Yet, as with winter dormancy, the expression of dormancy varies greatly between and within species, and exhibits considerable genotype by environment interaction (Vaughton and Ramsey, 2001; Volaire and Norton, 2006; Norton et al., 2009). In addition, the grass Southern rush, Lyginia barbata R.Br., is the only species reported to exhibit summer dormancy in root apices (Shane et al., 2009, 2010). Presently, and to the best of our knowledge, no woody species have been clearly reported to be summer dormant apart from blackbrush (Coleoyne ramosissima Torr.), a shrub species from the Nevada desert, USA that was shown to exhibit either complete (obligatory) or facultative summer dormancy according to the ecotype’s origin site (Lei, 2005). This lack of known summer dormant woody plants may be due to use of different terminology. The abscission of leaves during summer by deciduous woody plants is generally seen as a drought stress response (Kozlowski, 1976), although this may be a potential indicator of summer dormancy in species that abscise annually during the summer months. These species warrant further research for the expression of summer dormancy-like characteristics such as the inability to initiate growth under favourable conditions. However, the summer dormancy trait cannot be expected when plants arrest their development to survive but regrow opportunistically after any pulse of rainfall in arid areas (Pugnaire et al., 1996).
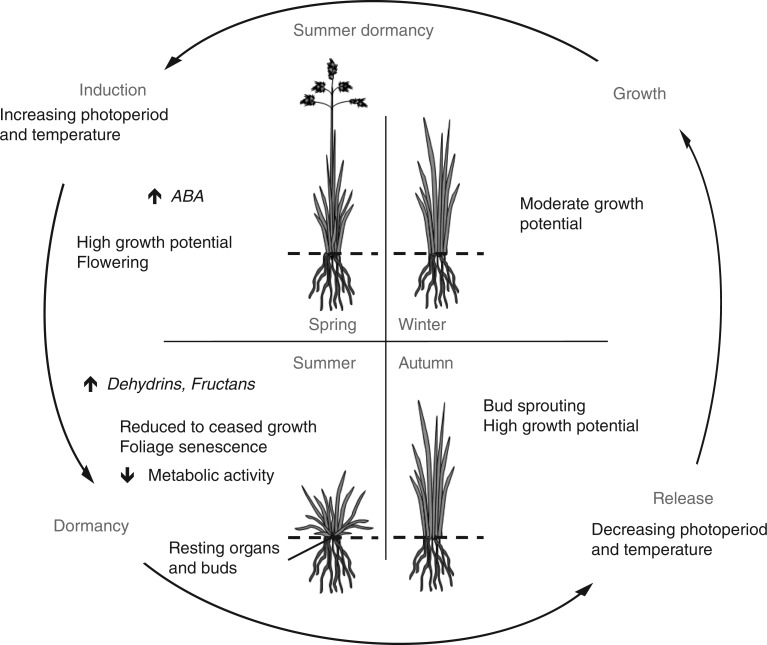
As summarized by Volaire and Norton (2006), summer dormancy in herbaceous perennials (Fig. 2) is characterized by: (1) cessation or reduction of leaf meristem growth; (2) senescence of most or all above-ground herbage; (3) possible dehydration of the bases of the youngest leaves at the base of vegetative tillers; and (4) possible preceding formation of resting organs in the form of swollen basal internodes (corm/tuber) or swollen leaf bases (such as bulbs).
Fig. 2.
Diagram of the annual induction and release cycle of summer dormancy in a herbaceous perennial species and the associated variations in phytohormone and molecule concentrations. ABA, abscisic acid.
Summer dormancy is correlated with improving survival during long and severe summer dry conditions typical of semi-arid and arid Mediterranean climates where the dry season is typically 4 months or longer (Norton et al., 2006a, b, 2012). In contrast, obligate dormancy (complete or endodormancy) was found disadvantageous when environmental conditions were unpredictable (Vaughton and Ramsey, 2001). However, dehydration avoidance, dehydration tolerance and summer dormancy are drought resistance traits that are often confused (Norton et al., 2009) and, although frequently expressed together, they are independent phenomena (Volaire and Norton, 2006). In addition, dormant plants require continuous access to water, even if only in small amounts typically accessed via a deep root system, otherwise desiccation and death invariably occur (Nie and Norton, 2009).
As with winter dormancy, this adaptation protects against premature sprouting when unseasonal favourable conditions occur. The decreased metabolic activity during dormancy reduces water consumption, thus ensuring dehydration avoidance (Volaire, 2005). Senescence of mature foliage also allows for better water conservation by reducing moisture loss though leaves. Furthermore, it allows nutrients from older leaves to be reused in developing leaves (Volaire, 2005). Summer dormant plants have elevated levels of dehydrins (proteins that are also found in desiccated embryos) and fructans (polymers of fructose), which help maintain cell membrane integrity during a drought and facilitate autumn regrowth (Volaire et al., 2001; Volaire, 2002).
FACTORS IN MERISTEM DORMANCY INDUCTION
Environmental drivers
In winter dormant plants, photoperiod and temperature are crucial environmental signals for flowering and dormancy regulation, which were proposed to be linked based on multiple shared genes between these two processes in herbaceous perennials (Horvath, 2009; Sarath et al., 2014). The perception of changing daylength and temperatures signals the production of various phytohormones (Sorce et al., 2000; Horvath et al., 2003; Chao et al., 2007; Aksenova et al., 2013), which in turn act as growth inhibitors, growth stimulators or inhibitors to one another. The detection of a short photoperiod involves phytochrome A (PHYA), which appears to then interact with or through signalling pathways of abscisic acid (ABA) and ethylene (Chao et al., 2007), two phytohormones involved in dormancy induction. As with dormancy depth and length, the extent to which these environmental signals regulate dormancy induction, maintenance and release varies greatly between and within species, and exhibits considerable genotype by environment interaction (Brummer et al., 2000; Chao et al., 2007; Zhang et al., 2012). In general, short daylengths and/or decreasing, low temperatures are required for herbaceous perennials to enter winter dormancy (Heide, 2001; Suttle, 2004b; Zhang et al., 2012). For instance, in leafy spurge, endodormancy was initiated by a combination of a photoperiod decrease from 16 to 8 h and a reduction of temperatures from 27 to 10 °C over 12 weeks (Doğramacı et al., 2015). In this species, the exposure to dehydration also induced a transition from para- to endodormancy in buds (Doğramacı et al., 2011).
Photoperiod and temperature are also the main abiotic factors that control summer dormancy induction (Laude, 1953). Conversely and symmetrically with winter dormancy, summer dormancy in vegetative organs develops under increasing daylength and temperature at the end of spring (Volaire et al., 2009). The critical photoperiod for summer dormancy induction at an optimal temperature (22/17 °C day/night) was between 11 and 12 h for P. bulbosa (Ofir and Kigel, 1999), a 13 h 30 min photoperiod for D. glomerata (Volaire et al., 2009) and a 16 h photoperiod for H. bulbosum, while an 8 h photoperiod provided a non-inductive environment in most studies. Moreover, induction is potentiated during early winter since it is enhanced by pre-exposure to short days alone or in combination with low temperatures in P. bulbosa (Ofir and Dorenfield, 1992; Volaire et al., 2009; Norton et al., 2012). As in winter dormancy, vernalization is another potential species-dependent induction factor since vernalization was required for flowering and enhanced dormancy induction in H. bulbosum L. (Ofir and Koller, 1974). Other species-dependent factors may be required or involved in induction. Intraspecific variations in the onset of summer dormancy were associated with origin site aridity in different populations of P. bulbosa (Ofir and Kigel, 2003). Moreover, water deficit was shown to be an induction factor for P. bulbosa L. (Ofir and Kigel, 2006), though this is not consistently found to be the case (Volaire et al., 2009). Volaire et al. (2009) suggested that variance in plants (age, level of selection, i.e. landrace or bred, local climate genetic adaptations, etc.) might have influenced these contradictory findings. These environmental factors signal phytohormonal and chemical signals within the plant.
General biochemical regulation
Abscisic acid.
In winter dormant species, ABA is a stress response plant hormone that is the principal dormancy-inducing and maintaining agent in perennial buds and seeds (Horvath et al., 2003; Chao et al., 2007; Campoy et al., 2011; Muthoni et al., 2014). In winter dormant, herbaceous perennials, elevated levels of ABA at the end of spring or early autumn induce the suppression of meristem growth and initiate senescence (Horvath et al., 2003). Exogenously applied ABA was shown to promote tuber formation in potatoes, though this is believed to be a secondary effect arising from its inhibition of stolon elongation (Xu, 1998). Moreover, no link was found between ABA and resting organ growth in lilies (Kim et al., 1994). Following endodormancy induction, sustained synthesis and action of endogenous ABA is necessary for winter dormancy maintenance, though synthesis, and thus ABA concentration, declines with dormancy progression (Horvath et al., 2008; Aksenova et al., 2013). Exogenous ABA was found to restore dormancy in potato tubers and lily bulbs treated with fluridone to break dormancy (Kim et al., 1994; Suttle and Hultstrand, 1994). Yet, sensitivity to ABA was found to be dependent on temperature and resting organ maturity, indicating that the correct environmental signals, not just hormone presence, are required for dormancy induction.
Regarding summer dormancy, ABA was shown to play a role in dormancy induction in P. bulbosa L. (Ofir and Kigel, 1998). ABA was applied under non-inductive photoperiodic conditions, resulting in bulb development and cessation of shoot activity, which in turn caused the termination of leaf emergence and tillering, started leaf senescence and finally induced bulb dormancy. However, a later study showed no clear association between dormancy induction and ABA in P. bulbosa L., D. glomerata L. ‘Kasbah’ and Lolium arundinaceum Schreb. cv. ‘Flecha’. Poa bulbosa L., a desiccation-tolerant species, did, however, show higher concentrations of ABA in summer than D. glomerata L. and L. arundinaceum Schreb. (Volaire et al., 2009). Although more summer dormant than L. arundinaceum Schreb., D. glomerata L. ‘Kasbah’ had similar concentrations of ABA. These results suggest a closer relationship between ABA concentration and the level of desiccation tolerance than to the level of summer dormancy in these species.
Ethylene.
Ethylene is another plant hormone involved in winter dormancy induction in leafy spurge and potato (Horvath et al., 2003; Suttle, 2004b; Chao et al., 2007; Aksenova et al., 2013; Muthoni et al., 2014). However, studies show contradictory results concerning its role in dormancy maintenance and release (Aksenova et al., 2013). In potato, ethylene has been shown either to hasten or to delay sprouting: short exposure resulted in premature termination of tuber dormancy, while long or continuous exposure inhibited sprout growth (Suttle, 2004b). This incoherence between results may be attributed to different species, genotypes or ethylene levels (Aksenova et al., 2013). Studies showed that ethylene levels were highest at endodormancy induction and dropped rapidly afterwards (Horvath et al., 2008; Aksenova et al., 2013); this spike is thought to be required for the induction of ABA, thus indirectly prolonging dormancy in herbaceous species (Chao et al., 2007; Horvath, 2009). However, this does not indicate a direct connection between ethylene and dormancy maintenance or length. Ethylene, along with ABA, also plays a role in plant senescence (Horvath et al., 2003; Chao et al., 2007). Moreover, ethylene is known to reduce the level of the growth promoter gibberellin (GA), thus antagonizing growth (Doğramacı et al., 2010). Overall, the role ethylene plays in winter dormancy is not fully understood (Chao et al., 2007; Aksenova et al., 2013), and whether it plays a role in summer dormancy has yet to be studied.
Gibberellins.
In summer dormant plants, GAs enhanced dormancy induction only in vernalized plants exposed to photoinductive conditions (long days) in H. bulbosum L., but did not appear to be a driving factor in dormancy induction (Ofir, 1976). It was proposed that another factor other than GAs initiated induction while GAs might stimulate the sensitivity to this factor and bulb initiation. Gibberellin though, is generally considered a growth stimulator and therefore would not be likely to be involved in dormancy induction. Conversely, GA is associated with dormancy release in winter dormant plants. This will be further discussed below.
Sugars.
In leafy spurge, sugar signalling influences the maintenance of winter paradormancy, and potentially the transition between para- and endodormancy (Chao et al., 2007) via sugar and phytohormone ‘cross-talking’ (Anderson et al., 2005). Sugars, such as glucose and sucrose, act as growth inhibitors by antagonizing the perception of GA, an ABA antagonist (Horvath, 1999; Chao et al., 2006), and increasing ABA perception (Horvath et al., 2003). Sugars from photosynthesizing leaves show direct growth inhibition in paradormant, underground buds (Horvath et al., 2003). The conversion of starch to sucrose also coincides with the transition between para- and endodormancy (Chao et al., 2007), although the direct link between this transition and sugar levels has not been fully studied. Moreover, winter dormant plants accumulate higher sugar concentrations in their roots and crowns, with higher concentrations in more dormant plants [e.g. alfalfa (Haagenson et al., 2003; Weishaar et al., 2005) and lily (Jásik and Klerk, 2006)]. In potato, increased sugar concentrations saw a decrease in GA levels, a decrease in stolon length and an increase in tuber growth (Xu, 1998). No change in ABA levels was detected, but sugar concentrations may influence ABA sensitivity as seen with leafy spurge. This evidence suggests that sugar concentration plays an important role in growth inhibition and tuber development through its influence on phytohormones, potentially acting as a nutrient and a signal molecule at the same time (Sonnewald and Sonnewald, 2014). It might also be noted that higher sugar concentrations may also be the result of dehydration/cold stress, and the subsequent impact on ABA and GA signalling may be part of the signalling mechanism for dormancy induction.
As in winter dormancy, the increase in sugars with the decline of carbohydrates is an early indicator of summer dormancy induction (Volaire, 2005). However, no studies have been conducted to investigate whether sugar levels play a role in the induction, maintenance or release of dormancy.
Other compounds
Brassinosteroids (BRs), steroid hormones and growth regulators, are cited in multiple reviews as factors in winter dormancy (Horvath et al., 2003; Chao et al., 2007; Aksenova et al., 2013), yet there are few studies on BRs, and most concern potato tubers (Suttle, 2004b; Aksenova et al., 2013). They have been attributed to extending endodormancy in potato tubers (Korableva et al., 2002), but breaking dormancy and germination in seeds (Koornneef et al., 2002; Finkelstein et al., 2008). There have also been no studies conducted on BRs in summer dormant plants.
Auxins are cited as growth inhibitors that influence para- and endodormancy (Horvath et al., 2003; Anderson et al., 2005; Chao et al., 2007; Baba et al., 2011). Conversely, other papers report that experiments do not indicate a role for auxins in dormancy, though these hormones probably influence the subsequent sprout growth (Wiltshire and Cobb, 1996; Sorce et al., 2000; Suttle, 2004b; Aksenova et al., 2013; Muthoni et al., 2014). Result incoherence may be due to different species cited: potato tubers (Wiltshire and Cobb, 1996; Sorce et al., 2000; Muthoni et al. 2014), pea (Pisum sativum L.) (Horvath et al., 2003) and arabidopsis (Horvath et al., 2003). Potato tubers appear to respond differently to auxins compared with other herbaceous species such as strawberry, Fragaria × ananassa Duch. (Zhang et al., 2012). Although auxins have been implicated in the dormancy process in woody species (Chao et al., 2007; Baba et al., 2011), whether they act as a growth stimulator, inhibitor or a dormancy driver in winter dormant, herbaceous plants has yet to be elucidated (Sonnewald and Sonnewald, 2014). The role of auxins in summer dormancy, as with BR and ethylene, has yet to be studied.
Jasmonic acid (JA) and associated compounds are phytohormones and growth regulators previously implicated as factors in winter dormancy regulation (Del Pozo et al., 2005; Horvath et al., 2008; Ladyzhenskaya and Korablyova, 2011); however, existing research regarding their role in winter dormancy is contradictory (Aksenova et al., 2013). For summer dormancy, JA positively influenced tuber formation in Pterostylis sanguinea D.L. Jones & M. Clements, an Australian summer dormant orchid, and a symbiotic relationship between JA and sugar was proposed (Debeljak et al., 2002). Nonetheless, this does not necessarily link them to summer dormancy induction. The role of JA in both winter and summer dormancy needs further research.
FACTORS IN MERISTEM DORMANCY RELEASE
Dormancy release, or the resumption of growth competence following dormancy, is less studied than dormancy induction (Considine and Considine, 2016).
Environmental drivers
In winter dormancy, low temperatures enhance the initiation of endodormancy, but extended periods of cold result in its release (Benkeblia and Selselet-Attou, 1999; Chao et al., 2007; Horvath, 2009). Increasing photoperiods have also been implicated as influencing dormancy release in herbaceous, temperate perennials (Heide, 2001; Sønsteby and Heide, 2006). In potato tubers, other genotype-dependent factors have been shown to hasten or break dormancy such as extreme temperatures (>35 °C), greater diurnal temperature variation and high CO2 concentrations or low oxygen levels (Muthoni et al., 2014). Intraspecific variability between genotypes, such as different chilling requirements among Calopogon tuberosus Britton, Sterns & Poggenb. (Orchidaceaes) populations (Kauth et al., 2011), often results from varying environmental conditions at origin sites.
Symmetrically with winter dormancy and as extended periods of extreme temperatures are generally required to release buds from endodormancy (Anderson et al., 2010), high temperatures facilitate the release of summer dormancy at the end of summer (Volaire and Norton, 2006). In P. bulbosa L., the gradual release of dormancy is facilitated by relatively high temperatures (decreased inhibition by these high temperatures) at the end of the summer, while sprouting of the dormant buds is accelerated once the lower temperatures characteristic of early autumn recommence (Ofir, 1986). Mirroring winter dormancy, this pattern may be a classic transition between endodormancy and ecodormancy where sufficient heat units need to accumulate to bring about release of endodormancy, but growth of these competent meristems is still inhibited by temporary unfavourable environmental conditions.
General biochemical regulation
Cytokinins.
Cytokinins (CKs) are phytohormone regulators that control the transition between winter dormancy release, onset of bud growth and organ senescence (Wiltshire and Cobb, 1996; Chao et al., 2007; Aksenova et al., 2013; Suttle et al., 2014). CK inactivity results in dormancy extension, while enhanced CK activity results in dormancy shortening (Aksenova et al., 2013). During winter dormancy, CK levels and sensitivity to CKs is low, but it increases as dormancy declines (Suttle, 2004b; Aksenova et al., 2013; Muthoni et al., 2014). However, CK metabolism does not consistently change with this increase of sensitivity, which suggests a change in CK perception and/or the signal transduction pathway with dormancy state (Suttle, 2004b). CK has been well studied in winter dormant plants and has been demonstrated as a primary regulator for dormancy release. The effects of CK on summer dormant plants have yet to be explored.
Gibberellins.
Studies on the role of GAs in winter dormancy show contradictory results. Discrepancies appear to be between exogenous and endogenous GA as well as between species. Exogenous GA has long been used to break dormancy and promote sprouting both experimentally and as an approved commercial treatment in both herbaceous (Claassens and Vreugdenhil, 2000; Suttle, 2004b) and woody species, described as a ‘potent inducer of bud burst of fully dormant buds’ in Populus (Rinne et al., 2011). However, results are highly species dependent. Application of exogenous GA was able to break dormancy and induce sprouting in dormant potato and leafy spurge buds, but could not do so in ironweed (Vernonia baldwini Torr.) (Shafer and Monson, 1958; Suttle, 2004b; Rentzsch et al., 2012). Endogenous GA levels change with the dormancy cycle: rapidly decreasing upon endodormancy induction, remaining low during dormancy and increasing nearing dormancy release and onset of sprouting (Claassens and Vreugdenhil, 2000; Horvath et al., 2006). Yet, this is probably a result, not a driver, of dormancy. The study of Suttle (2004a) did not support a role for endogenous GA in potato tuber dormancy release, suggesting that it was more correlated with successive bud growth. Similarly, Hartmann et al. (2011) found that GA was not sufficient to break dormancy in the absence of CK, but was able to induce sprouting. Both endogenous and exogenous GA have been shown to block tuber formation under tuberizing conditions (e.g. short photoperiods), but under non-tuberizing conditions (e.g. long photoperiods) tuber formation could be induced by blocking GA synthesis (Claassens and Vreugdenhil, 2000). After tuber formation, however, GA had no effect on growth (Vreugdenhil and Sergeeva, 1999). In potato and leafy spurge, studies showed that GAs reduced sucrose content and overcame sugar inhibition of growth (Vreugdenhil and Sergeeva, 1999; Anderson et al., 2005; Chao et al., 2006), but whether this is related to dormancy release remains unclear. These results support a role for both endogenous and exogenous GA in antagonizing tuber formation and promoting sprout growth, but show that the role of exogenous GA in dormancy release varies with species.
PERSPECTIVES
The annual, phenological cycles of winter and summer dormant perennial herbaceous species are remarkably similar, with the succession of three seasons of growth followed by a season of dormancy and reduced metabolic activity in specific organs. In both cases, this process is mediated by symmetrical, inductive daylength and temperatures (Figs 1 and 2). In parallel with bud and seed dormancy, which are similar but not identical phenomena (Dennis, 1996), winter and summer dormancy of meristems in perennial, herbaceous, temperate species are similarly driven by seasonal cues in order to enhance whole-plant survival during the harshest season.
This similar pattern allows the identification of a few main research priorities that are relevant to both types of dormancy (Fig. 3; Table 1). These research priorities could be efficiently addressed in herbaceous species since (1) they are more tractable than woody species to carry out experiments (transplantation of adult plants, controlled conditions, etc.) and (2) in contrast to woody species for which summer dormancy has not been clearly reported, they represent the only known model plants for which both summer and winter dormancy have been clearly identified. Potential model systems for the parallel study of both types of dormancy should be identified. Ubiquitous herbaceous species with a large biogeographical distribution could provide ecotypes originating from hot and dry summer climates as well as cold winter climates, and thus potentially exhibiting both seasonal dormancy types. Dactylis glomerata is a good candidate for the study of dormancy since its ecotypes are broadly distributed in Europe, North Africa and Asia (Borrill, 1978; Lumaret, 1988), and exhibit major differences in growth patterns in response to seasonal variations of photoperiod and temperatures (Eagles, 1967; Treharne and Eagles, 1970; Eagles and Ostgard, 1971). Hence, along this strong climatic gradient, ecotypes exhibit contrasting strategies, from frost tolerance and winter dormancy in northern ecotypes to drought tolerance and summer dormancy in Mediterranean ecotypes (Cooper, 1964).
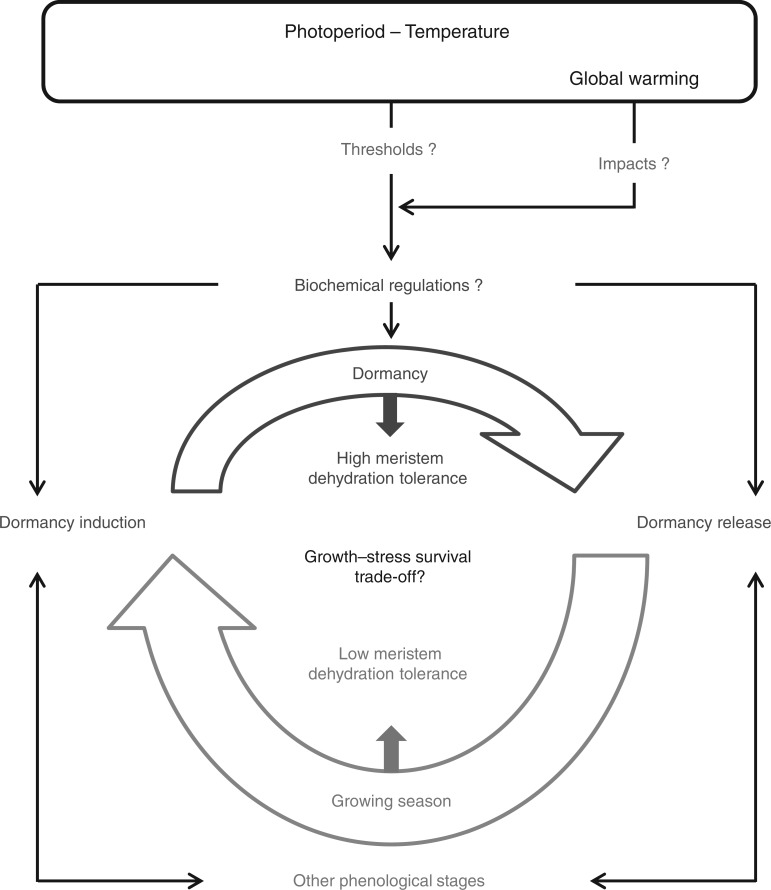
Fig. 3.
Diagram of the annual phenology of herbaceous, temperate, perennial plants exhibiting either winter or summer dormancy. Research priorities on the eco-physiology of both types of dormancy in these model species: (1) to identify the thresholds of seasonal cues for induction/release; (2) to better understand biochemical regulations of dormancy; and (3) to explore the growth–stress survival trade-off.
Table 1.
Research status of different dormancy drivers and regulators in herbaceous, temperate, perennial plants
| Winter dormancy |
Summer dormancy |
||||
|---|---|---|---|---|---|
| Induction | Release | Induction | Release | ||
| Environmental drivers | Photoperiod | SD | LD | LD | ? |
| Temperature | Decreasing | Extended cold | Increasing | High | |
| General biochemical regulation | Abscisic acid (ABA) | X | ? | ||
| Ethylene | X | ? | ? | ||
| Sugars | X | ? | |||
| Cytokinins (CKs) | X | ? | |||
| Gibberellins (GAs) | ? | ? | |||
| Brassinosteroids (BRs) | ? | ? | ? | ||
| Auxins | ? | ? | ? | ||
| Jasmonic acid (JA) | ? | ? | ? | ||
The environmental drivers influencing the induction and release of dormancy are reasonably well studied, including short-day (SD) and long-day (LD) photoperiod and temperature changes or extended durations.
Well-established biochemical regulators influencing the dormancy cycle in a number of model species are indicated with an ‘X’. Drivers and regulators lacking conclusive research are indicated with a ‘?’ under either induction or release depending on their putative role; if unknown, both columns are marked.
For suitable model plants, the major research priorities are presented below.
Identification of thresholds of seasonal cues for induction/release of both dormancy types
Photoperiod and temperature are known to play a crucial, though reversed, role for the induction and release of both types of dormancy. However, the thresholds and combined effects of these environmental factors remain little explored (Norton et al., 2016). Shifts in plant phenology, at least in woody plants (Fu et al., 2015; Chuine et al., 2016), are already visible under the effects of climate change. To understand and predict their impacts requires the current and potential range of seasonal cues for dormancy induction and release to be defined at the inter- and intraspecific levels (Fig. 3). Applying these environmental threshold parameters to existing models of plant growth and development could provide insights for a future environmental range of adaptations of species and populations. It can be hypothesized that for perennial herbaceous species in the most northern and southern ranges of temperate environments, global warming could tend to decrease the occurrence of winter dormancy while, in parallel, summer dormancy could become a more widespread strategy. Long-term field monitoring and common garden experiments comparing a species such as D. glomerata L., with populations exhibiting winter dormancy (Scandinavia) and summer dormancy (Morocco), could provide interesting insights into potential shifts in plant phenological adaptations.
Such studies would require monitoring the other phenological stages (flowering, length of growing season, etc.) in parallel, since many pre- and post-dormancy effects have been identified involving, for instance, a trade-off between the maximization of the growth period and the minimization of the dormancy stage in woody plants (van der Schoot et al., 2014; Körner et al., 2016).
Analysis of biochemical regulation of dormancy in herbaceous plants
The comparison of hormonal and biochemical factors associated with induction and release between summer and winter dormancy is challenging due to the paucity of research on summer dormancy (Volaire and Norton, 2006). The induction of winter dormant species is driven or aided by ABA, ethylene and possibly influenced by sugars, JA, auxins and BRs depending on species and concentration, and dormancy release is primarily driven by CKs. Summer dormancy induction and release, in contrast, are little understood. ABA was shown to influence summer dormancy imposition (Ofir and Kigel, 1998), yet other results dispute this (Volaire et al., 2009). Research on GAs also shows contrasting effects. In winter dormant plants, GA was associated with dormancy breaking or subsequent growth; in summer dormant plants, GA was correlated with dormancy induction. It raises the need to explore further the role of GA in both dormancy types. Summer dormancy research can target aspects of winter dormancy already studied. For example, the factors shown or thought to influence induction or release in winter dormancy in herbaceous, temperate, perennial plants (ABA, ethylene, sugars, CKs and GAs) could be a priority research focus for summer dormancy. In addition, deciphering the hormonal and genetic factors involved in summer and winter dormancy would be an important step in understanding dormancy dynamics. To this end, as summer and winter dormant populations of D. glomerata can be crossed, a forward genetic approach might be fruitful. To complement genetic and genomic studies, biochemical and functional approaches are crucial to understand dormancy regulation (Ríos et al., 2014).
Exploration of the growth–stress survival trade-off
Since the timing of dormancy enables optimal resource reallocation to maintain the balance between stress adaptation and growth (Bjornson et al., 2016), the study of adaptive responses associated with seasonal dormancy illustrates the classical growth–stress survival trade-off in both seasons (Sibly and Calow, 1989; Wright et al., 2010). However, the strength of this trade-off is unknown since the extent to which dormancy and stress tolerance can be uncoupled remains little explored (Wisniewski et al., 2014). The distinction between dormancy and stress hardiness remains poorly delineated and, in particular, separating dormancy and acclimation remains difficult, notably in woody perennials (Considine and Considine, 2016). Moreover, the role of thermo-period in dormancy is somewhat confounded with acclimation, particularly to water stress such as freezing or dehydration (Vitasse et al., 2014). Summer dormancy could be an interesting model to address these issues since it is measured under full irrigation allowing differentiation of endodormancy and ecodormancy (or quiescence), which is triggered by lack of water and is only an opportunistic avoidance state (Considine and Considine, 2016). Conversely, winter dormancy in a species such as alfalfa is measured through autumn growth reduction, and no clear assessment of dormancy/quiescence has been proposed during the winter. In any case, parallel to summer dormancy, testing periods of high temperature in the middle of the winter could be promising to clarify the dormancy status. This would allow the identification of the trade-off limits between growth potential during a season and stress survival ability. Notably in alfalfa, it was suggested that considerable improvement in both autumn growth and winter hardiness can be achieved simultaneously (Brummer et al., 2000).
To investigate this trade-off further, it would be interesting to explore the occurrence of crossed stress resistance, such as a higher drought tolerance for winter dormant plants as suggested with alfalfa (Pembleton and Sathish, 2014) or a possible greater survival of a counter season (winter) drought on populations that exhibit dormancy only in summer. Consequently, testing the potential decoupling between dormancy and stress survival could also cast a light on the role of seasonal acclimation in dehydration tolerance. Finally, unravelling the covariation of plant productivity and stress resistance can have important implications for the ecological management of plant communities and the enhancement of breeding programmes for species of agronomical interest such as D. glomerata.
CONCLUSION
The parallels between summer and winter dormancy confirm their symmetrical nature as seasonal adaptive strategies and allow the proposition of research priorities common to both plant strategies. The comparison of both types of dormancy in herbaceous plants has limitations since (1) there is an overall lack of research in these species compared with woody plants; and (2) the different model plants studied are mainly dicotyledons for winter dormancy and mainly monocotyledons for summer dormancy. It raises the need to investigate the occurrence of summer dormancy in a larger range of species, including woody species. Moreover, phylogenetic information on the co-occurrence of dormancy, other life history and physiological traits could provide exciting directions for further research in plant evolutionary ecology (Shefferson, 2009). In addition, this review did not include the genetic and molecular processes that drive the cycle of dormant buds in synchrony with the seasons since they are largely elusive (van der Schoot et al., 2014). However, at ecological and ecophysiological levels, the understanding of dormancy can help predict the evolution of phenological patterns of species and communities under climate change (Volaire et al., 2014). Furthermore, a better knowledge of the dormancy trait would certainly be useful for ecological and agricultural roles in locations of increasingly severe, seasonal conditions. Under low and high latitudes, in areas with strong seasonal contrasting environmental conditions, it may be worth exploring the inter- and intraspecific genotypic variability of plant dormancy and its plasticity across environmental gradients. It would assist the development of phenotypes with enhanced dormancy or opportunistic dormancy adaptations that could improve adaptation to less predictable environmental stresses associated with future climates (Vaughton and Ramsey, 2001; Newell et al., 2015).
ACKNOWLEDGEMENTS
We thank Dr Mark Norton for his valuable comments on this manuscript. We also thank the editor and the anonymous reviewers for their helpful insights and suggestions. This study was supported by the European Research Council (ERC) Starting Grant Project ‘Ecophysiological and biophysical constraints on domestication in crop plants’ (Grant ERC-StG-2014-639706-CONSTRAINTS).
LITERATURE CITED
- Aksenova NP, Sergeeva LI, Konstantinova TN, Golyanovskaya SA, Kolachevskaya OO, Romanov GA. 2013. Regulation of potato tuber dormancy and sprouting. Russian Journal of Plant Physiology 60: 301–312. [Google Scholar]
- Anderson J, Gesch R, Jia Y, Chao WS, Horvath DP. 2005. Seasonal shifts in dormancy status, carbohydrate metabolism, and related gene expression in crown buds of leafy spurge. Plant, Cell & Environment 28: 1567–1578. [Google Scholar]
- Anderson JV, Horvath DP, Chao WS, Foley ME. 2010. Bud dormancy in perennial plants: a mechanism for survival In: Lubzens E, Cerda J, Clark M, eds. Dormancy and resistance in harsh environments. Berlin: Springer, 69–90. [Google Scholar]
- Aragoncillo C, Allona I, Ramos A, Casado R, Ibáñez C, Contreras A. 2008. Review. Molecular control of winter dormancy establishment in trees. Spanish Journal of Agricultural Research 6: 201–210. [Google Scholar]
- Arora R, Rowland L, Tanino K. 2003. Induction and release of bud dormancy in woody perennials: a science comes of age. HortScience 38: 911–921. [Google Scholar]
- Baba K, Karlberg A, Schmidt J, et al. 2011. Activity–dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proceedings of the National Academy of Sciences, USA 108: 3418–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachowski JA, Bristiel PM, Volaire FA. 2016. Summer dormancy, drought survival and functional resource acquisition strategies in California perennial grasses . Annals of Botany 118: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkeblia N, Selselet-Attou G. 1999. Role of ethylene on sprouting of onion bulbs (Allium cepa L.). Acta Agriculturae Scandinavica. Section B, Soil and Plant Science 49: 122–124. [Google Scholar]
- Bjornson M, Dandekar A, Dehesh K. 2016. Determinants of timing and amplitude in the plant general stress response. Journal of Integrative Plant Biology 58: 119–126. [DOI] [PubMed] [Google Scholar]
- Borrill M. 1978. Evolution and genetic resources in cocksfoot. Annual Report of the Welsh Plant Breeding Station. Wales, UK: University College, 190–209. [Google Scholar]
- Brummer E, Shah M, Luth D. 2000. Reexamining the relationship between fall dormancy and winter hardiness in alfalfa. Crop Science 40: 971–977. [Google Scholar]
- Campoy J, Ruiz D, Egea J. 2011. Dormancy in temperate fruit trees in a global warming context: a review. Scientia Horticulturae 130: 357–372. [Google Scholar]
- Castonguay Y, Laberge S, Brummer EC, Volenec JJ. 2006. Alfalfa winter hardiness: a research retrospective and integrated perspective. Advances in Agronomy 90: 203–265. [Google Scholar]
- Cayan D, Kammerdiener SA, Dettinger MD, Caprio JM, Peterson DH. 2001. Changes in the onset of spring in the western United States. Bulletin of the American Meteorological Society 82: 399–415. [Google Scholar]
- Chao WS, Foley ME, Horvath DP, Anderson JV. 2007. Signals regulating dormancy in vegetative buds. International Journal of Plant Developmental Biology 1: 49–56. [Google Scholar]
- Chao W, Serpe M, Anderson J, Gesch RW, Horvath DP. 2006. Sugars, hormones, and environment affect the dormancy status in underground adventitious buds of leafy spurge (Euphorbia esula). Weed Science 54: 59–68. [Google Scholar]
- Charrier G, Ngao J, Saudreau M, Améglio T. 2015. Effects of environmental factors and management practices on microclimate, winter physiology, and frost resistance in trees. Frontiers in Plant Science 6: 259. doi:10.3389/fpls.2015.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuine I, Bonhomme M, Legave J-M, et al. 2016. Can phenological models predict tree phenology accurately in the future? The unrevealed hurdle of endodormancy break. Global Change Biology 22: 3444–3460. [DOI] [PubMed] [Google Scholar]
- Claassens M, Vreugdenhil D. 2000. Is dormancy breaking of potato tubers the reverse of tuber initiation? Potato Research 43: 347–369. [Google Scholar]
- Cohat J. 1993. Gladiolus. The physiology of flower bulbs. Amsterdam: Elsevier, 297–320. [Google Scholar]
- Considine MJ, Considine JA. 2016. On the language and physiology of dormancy and quiescence in plants. Journal of Experimental Botany 67: 3189–3203. [DOI] [PubMed] [Google Scholar]
- Cooke JEK, Eriksson ME, Junttila O. 2012. The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant, Cell & Environment 35: 1707–1728. [DOI] [PubMed] [Google Scholar]
- Cooper JP. 1964. Climatic variation in forage grasses. I. Leaf development in climatic races of Lolium and Dactylis. Journal of Applied Ecology 1: 45. [Google Scholar]
- Culvenor R. 2009. Breeding and use of summer-dormant grasses in southern Australia, with special reference to phalaris. Crop Science 49: 2335–2346. [Google Scholar]
- Debeljak N, Regvar M, Dixon KW, Sivasithamparam K. 2002. Induction of tuberisation in vitro with jasmonic acid and sucrose in an Australian terrestrial orchid, Pterostylis sanguinea. Plant Growth Regulation 36: 253–260. [Google Scholar]
- Del Pozo J, Lopez-Matas MA, Ramirez-Parra E, Gutierrez C. 2005. Hormonal control of the plant cell cycle. Physiologia Plantarum 123: 173–183. [Google Scholar]
- Dennis F. 1996. A physiological comparison of seed and bud dormancy In: Lang G. ed. Plant dormancy: physiology, biochemistry and molecular biology. Wallingford, UK: CAB International, 47–56. [Google Scholar]
- Doğramacı M, Horvath DP, Anderson J V. 2015. Meta-analysis identifies potential molecular markers for endodormancy in crown buds of leafy spurge; a herbaceous perennial In: Anderson JV, ed. Advances in plant dormancy. Cham: Springer International Publishing, 197–219. [Google Scholar]
- Doğramacı M, Horvath D, Chao W, Foley ME, Christoffers MJ, Anderson JV. 2010. Low temperatures impact dormancy status, flowering competence, and transcript profiles in crown buds of leafy spurge. Plant Molecular Bology 73: 207–226. [DOI] [PubMed] [Google Scholar]
- Doğramacı M, Horvath DP, Christoffers MJ, Anderson JV. 2011. Dehydration and vernalization treatments identify overlapping molecular networks impacting endodormancy maintenance in leafy spurge crown buds. Functional and Integrative Genomics 11: 611–626. [DOI] [PubMed] [Google Scholar]
- Eagles CF. 1967. The effect of temperature on vegetative growth in climatic races of Dactylis glomerata in controlled environments. Annals of Botany 31: 31–39. [Google Scholar]
- Eagles CF, Ostgard O. 1971. Variation in growth and development in natural populations of Dactylis glomerata from Norway and Portugal. I – Growth analysis. Journal of Applied Ecology 8: 367–381. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. 2008. Molecular aspects of seed dormancy. Plant Biology 59: 387–415. [DOI] [PubMed] [Google Scholar]
- Fu YH, Zhao H, Piao S, et al. 2015. Declining global warming effects on the phenology of spring leaf unfolding. Nature 526: 104–107. [DOI] [PubMed] [Google Scholar]
- Graeber K, Nakabayashi K, Miatton E, Leubner-Metzger G, Soppe WJJ. 2012. Molecular mechanisms of seed dormancy. Plant, Cell & Environment 35: 1769–1786. [DOI] [PubMed] [Google Scholar]
- Haagenson D, Cunningham S, Joern BC, Volenec JJ. 2003. Autumn defoliation effects on alfalfa winter survival, root physiology, and gene expression. Crop Science 43: 1340–1348. [Google Scholar]
- Hansen J, Sato M, Ruedy R, Lo K, Lea DW, Medina-Elizade M. 2006. Global temperature change. Proceedings of the National Academy of Sciences, USA 103: 14288–14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Senning M, Hedden P, Sonnewald U, Sonnewald S. 2011. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiology 155: 776–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek W, Tranquillini W. 1995. Physiological processes during winter dormancy and their ecological significance In: Workshop on physiological ecology of coniferous forests, Laramie, WY, USA, 16–19 September 1991. San Diego: Academic Press, 95–124. [Google Scholar]
- Heide OM. 2001. Photoperiodic control of dormancy in Sedum telephium and some other herbaceous perennial plants. Physiologia Plantarum 113: 332–337. [DOI] [PubMed] [Google Scholar]
- Horvath DP. 1999. Role of mature leaves in inhibition of root bud growth in Euphorbia esula L. Weed Science 47: 544–550. [Google Scholar]
- Horvath D. 2009. Common mechanisms regulate flowering and dormancy. Plant Science 177: 523–531. [Google Scholar]
- Horvath DP, Anderson JV. 2002. A molecular approach to understanding root bud dormancy in leafy spurge. Weed Science 50: 227–231. [Google Scholar]
- Horvath D, Anderson J, Chao W, Foley M. 2003. Knowing when to grow: signals regulating bud dormancy. Trends in Plant Science 8: 534–540. [DOI] [PubMed] [Google Scholar]
- Horvath D, Anderson J, Soto-Suárez M, Chao WS. 2006. Transcriptome analysis of leafy spurge (Euphorbia esula) crown buds during shifts in well-defined phases of dormancy. Weed Science 5: 821–827. [Google Scholar]
- Horvath DP, Chao WS, Suttle JC, Thimmapuram J, Anderson JV. 2008. Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). BMC Genomics 9: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. 2014. Climate change 2014 synthesis report. Contribution of working groups I, II, and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Jásik J, de Klerk G. 2006. Effect of methyl jasmonate on morphology and dormancy development in lily bulblets regenerated in vitro. Journal of Plant Growth Regulation 25: 45–51. [Google Scholar]
- Kamenetsky R, Zemah H, Ranwala AP, et al. 2003. Water status and carbohydrate pools in tulip bulbs during dormancy release. New Phytologist 158: 109–118. [Google Scholar]
- Kauth PJ, Kane ME, Vendrame WA. 2011. Chilling relieves corm dormancy in Calopogon tuberosus (Orchidaceae) from geographically distant populations. Environmental and Experimental Botany 70: 283–288. [Google Scholar]
- Kim K, Davelaar E, Klerk G. 1994. Abscisic acid controls dormancy development and bulb formation in lily plantlets regenerated in vitro. Physiologia Plantarum 90: 59–64. [Google Scholar]
- Koornneef M, Bentsink L, Hilhorst H. 2002. Seed dormancy and germination. Current Opinion in Plant Biology 5: 33–36. [DOI] [PubMed] [Google Scholar]
- Korableva N, Platonova T, Dogonadze MZ, Evsunina AS. 2002. Brassinolide effect on growth of apical meristems, ethylene production, and abscisic acid content in potato tubers. Biologia Plantarum 45: 39–43. [Google Scholar]
- Körner C, Basler D, Hoch G, et al. 2016. Where, why and how? Explaining the low-temperature range limits of temperate tree species. Journal of Ecology 104: 1076–1088. [Google Scholar]
- Kozlowski T. 1976. Water supply and leaf shedding Water deficits and plant growth: soil water measurements, plant responses, and breeding for drought resistance. Madison, WI: Department of Forestry & University of Wisconsin, 191–231. [Google Scholar]
- Ladyzhenskaya E, Korablyova N. 2011. Effect of salicylic acid on the proton translocation activity of plasmalemma of potato tuber cells. Applied Biochemistry and Microbiology 47: 435–439. [PubMed] [Google Scholar]
- Lang G, Early J, Martin G, Darnell R. 1987. Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. HortScience 22: 371–377. [Google Scholar]
- Laude HM. 1953. The nature of summer dormancy in perennial grasses. Botanical Gazette 114: 282–292. [Google Scholar]
- Lei SA. 2005. Ecotypic variation in summer dormancy of blackbrush (Coleogyne ramosissima) and its ecological significance. Journal of the Arizona-Nevada Academy of Science 38: 1–5. [Google Scholar]
- Lumaret R. 1988. Cytology, genetics, and evolution in the genus Dactylis. CRC Critical Reviews in Plant Sciences 7: 55–91. [Google Scholar]
- Meehl G, Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305: 994–997. [DOI] [PubMed] [Google Scholar]
- Menzel A, Sparks TH, Estrella N, et al. 2006. European phenological response to climate change matches the warming pattern. Global Change Biology 12: 1969–1976. [Google Scholar]
- Muthoni J, Kabira J, Shimelis H, Melis R. 2014. Regulation of potato tuber dormancy: a review. Australian Journal of Crop Science 8: 754–759. [Google Scholar]
- Newell MT, Hayes RC, Virgona JM, Larkin PJ. 2015. Summer dormancy in Elymus scaber and its hybridity with wheat. Euphytica 204: 535–556. [Google Scholar]
- Nie Z, Norton MR. 2009. Stress tolerance and persistence of perennial grasses: the role of the summer dormancy trait in temperate Australia. Crop Science 49: 2405. [Google Scholar]
- Norton MR, Lelièvre F, Volaire F. 2006a. Summer dormancy in Dactylis glomerata L.: the influence of season of sowing and a simulated mid-summer storm on two contrasting cultivars. Australian Journal of Agricultural Research 57: 565. [Google Scholar]
- Norton MR, Volaire F, Leliévre F. 2006b. Summer dormancy in Festuca arundinacea Schreb.; the influence of season of sowing and a simulated mid-summer storm on two contrasting cultivars. Australian Journal of Agricultural Research 57: 1267. [Google Scholar]
- Norton MR, Volaire F, Lelièvre F, Fukai S. 2009. Identification and measurement of summer dormancy in temperate perennial grasses. Crop Science 49: 2347. [Google Scholar]
- Norton MR, Lelièvre F, Volaire F. 2012. Summer dormancy in Phalaris aquatica L., the influence of season of sowing and summer moisture regime on two contrasting cultivars. Journal of Agronomy and Crop Science 198: 1–13. [Google Scholar]
- Norton MR, Malinowski DP, Volaire F. 2016. Plant drought survival under climate change and strategies to improve perennial grasses. A review. Agronomy for Sustainable Development 36: 29. [Google Scholar]
- Nowak J, Rudnicki RM. 1993. Hyacinthus. The physiology of flower bulbs. Amsterdam: Elsevier, 335–348. [Google Scholar]
- Ofir M. 1976. Interaction of gibberellin with photoinduction in the initiation of the dormant phase in vernalized Hordeum bulbosum L. Australian Journal of Plant Physiology 3: 827. [Google Scholar]
- Ofir M. 1986. . Seasonal changes in the response to temperature of summer-dormant Poa bulbosa L. bulbs. Annals of Botany 58: 81–89. [Google Scholar]
- Ofir M, Dorenfield Y. 1992. Induction of summer-dormancy in Poa bulbosa L. under natural environment and subsequent controlled photo-thermal conditions. Israel Journal of Botany 41: 265–277. [Google Scholar]
- Ofir M, Kigel J. 1998. Abscisic acid involvement in the induction of summer-dormancy in Poa bulbosa, a grass geophyte. Physiologia Plantarum 102: 163–170. [Google Scholar]
- Ofir M, Kigel J. 1999. Photothermal control of the imposition of summer dormancy in Poa bulbosa, a perennial grass geophyte. Physiologia Plantarum 105: 633–640. [Google Scholar]
- Ofir M, Kigel J. 2003. Variation in onset of summer dormancy and flowering capacity along an aridity gradient in Poa bulbosa L., a geophytic perennial grass. Annals of Botany 91: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir M, Kigel J. 2006. Regulation of summer dormancy by water deficit and ABA in Poa bulbosa ecotypes. Annals of Botany 99: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir M, Koller D. 1974. Relationship between thermoinduction and photoinduction of flowering and dormancy in Hordeum bulbosum L., a perennial grass. Australian Journal of Plant Physiology 1: 259. [Google Scholar]
- Olsen JE. 2006. Mechanisms of dormancy regulation. Acta Horticulturae 727: 157–166. [Google Scholar]
- Olsen JE. 2010. Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant Molecular Biology 73: 37–47. [DOI] [PubMed] [Google Scholar]
- Pagter M, Andersen UB, Andersen L. 2015. Winter warming delays dormancy release, advances budburst, alters carbohydrate metabolism and reduces yield in a temperate shrub. AoB PLANTS 7: plv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembleton KG, Sathish P. 2014. Giving drought the cold shoulder: a relationship between drought tolerance and fall dormancy in an agriculturally important crop. AoB PLANTS 6: plu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugnaire FI, Haase P, Incoll LD, Clark SC. 1996. Response of the tussock grass Stipa tenacissima to watering in a semi-arid environment. Functional Ecology 10: 265. [Google Scholar]
- Rapacz M, Ergon Å, Höglind M, et al. 2014. Overwintering of herbaceous plants in a changing climate. Still more questions than answers. Plant Science 225: 34–44. [DOI] [PubMed] [Google Scholar]
- Rentzsch S, Podzimska D, Voegele A, et al. 2012. Dose- and tissue-specific interaction of monoterpenes with the gibberellin-mediated release of potato tuber bud dormancy, sprout growth and induction of α-amylases and β-amylases. Planta 235: 137–151. [DOI] [PubMed] [Google Scholar]
- Rinne PLH, Welling A, Vahala J, et al. 2011. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-β-glucanases to reopen signal conduits and release dormancy in Populus. The Plant Cell 23: 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos G, Leida C, Conejero A, Badenes ML. 2014. Epigenetic regulation of bud dormancy events in perennial plants. Frontiers in Plant Science 5: 247. doi: 10.3389/fpls.2014.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde A, Bhalerao RP. 2007. Plant dormancy in the perennial context. Trends in Plant Science 12: 217–223. [DOI] [PubMed] [Google Scholar]
- Samarakoon UC, Funnell KA, Woolley DJ, Morgan ER. 2015. Influence of photoperiod regime and exogenous plant growth regulators on crown bud formation in gentian. Scientia Horticulturae 182: 56–64. [Google Scholar]
- Sarath G, Baird LM, Mitchell RB. 2014. Senescence, dormancy and tillering in perennial C4 grasses. Plant Science 217–218: 140–151. [DOI] [PubMed] [Google Scholar]
- van der Schoot C, Paul LK, Rinne PLH. 2014. The embryonic shoot: a lifeline through winter. Journal of Experimental Botany 65: 1699–1712. [DOI] [PubMed] [Google Scholar]
- Shafer NE, Monson WG. 1958. The role of gibberellic acid in overcoming bud dormancy in perennial weeds. I. Leafy spurge (Euphorbia esula L.) and ironweed (Vernonia baldwini Torr.). Weeds 6: 172–178. [Google Scholar]
- Shane MW, McCully ME, Canny MJ, et al. 2009. Summer dormancy and winter growth: root survival strategy in a perennial monocotyledon. New Phytologist 183: 1085–1096. [DOI] [PubMed] [Google Scholar]
- Shane MW, McCully ME, Canny MJ, et al. 2010. Seasonal water relations of Lyginia barbata (Southern rush) in relation to root xylem development and summer dormancy of root apices. New Phytologist 185: 1025–1037. [DOI] [PubMed] [Google Scholar]
- Shefferson RP. 2009. The evolutionary ecology of vegetative dormancy in mature herbaceous perennial plants. Journal of Ecology 97: 1000–1009. [Google Scholar]
- Sibly RM, Calow P. 1989. A life-cycle theory of responses to stress. Biological Journal of the Linnean Society 37: 101–116. [Google Scholar]
- Sonnewald S, Sonnewald U. 2014. Regulation of potato tuber sprouting. Planta 239: 27–38. [DOI] [PubMed] [Google Scholar]
- Sønsteby A, Heide OM. 2006. Dormancy relations and flowering of the strawberry cultivars Korona and Elsanta as influenced by photoperiod and temperature. Scientia Horticulturae 110: 57–67. [Google Scholar]
- Sorce C, Lorenzi R, Ceccarelli N, Ranalli P. 2000. Changes in free and conjugated IAA during dormancy and sprouting of potato tubers. Australian Journal of Plant Physiology 27: 371. [Google Scholar]
- Sumitomo K, Narumi T, Satoh S, Hisamatsu T. 2008. Involvement of the ethylene response pathway in dormancy induction in chrysanthemum. Journal of Experimental Botany 59: 4075–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle JC. 2004a. Involvement of endogenous gibberellins in potato tuber dormancy and early sprout growth: a critical assessment. Journal of Plant Physiology 161: 157–164. [DOI] [PubMed] [Google Scholar]
- Suttle JC. 2004b. Physiological regulation of potato tuber dormancy. American Journal of Potato Research 81: 253–262. [Google Scholar]
- Suttle J, Hultstrand J. 1994. Role of endogenous abscisic acid in potato microtuber dormancy. Plant Physiology 105: 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle JC, Huckle LL, Lu S, Knauber DC. 2014. Potato tuber cytokinin oxidase/dehydrogenase genes: biochemical properties, activity, and expression during tuber dormancy progression. Journal of Plant Physiology 171: 448–457. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Imamura T, Konno N, et al. 2014. The gentio-oligosaccharide gentiobiose functions in the modulation of bud dormancy in the herbaceous perennial Gentiana. The Plant Cell 26: 3949–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne KJ, Eagles CF. 1970. Effect of temperature on photosynthetic activity of climatic races of Dactylis glomerata L. Photosynthetica 4: 107–117. [Google Scholar]
- Vaughton G, Ramsey M. 2001. Variation in summer dormancy in the lilioid geophyte Burchardia umbellata (Colchicaceae). American Journal of Botany 88: 1223–1229. [PubMed] [Google Scholar]
- Vegis A. 1964. Dormancy in higher plants. Annual Review of Plant Physiology 15: 185–224. [Google Scholar]
- Vitasse Y, Lenz A, Körner C. 2014. The interaction between freezing tolerance and phenology in temperate deciduous trees. Frontiers in Plant Science 5: 541. doi:10.3389/fpls.2014.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F. 2002. Drought survival, summer dormancy and dehydrin accumulation in contrasting cultivars of Dactylis glomerata. Physiologia Plantarum 116: 42–51. [DOI] [PubMed] [Google Scholar]
- Volaire F. 2005. Seasonal patterns of growth, dehydrins and water-soluble carbohydrates in genotypes of Dactylis glomerata varying in summer dormancy. Annals of Botany 95: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Norton M. 2006. Summer dormancy in perennial temperate grasses. Annals of Botany 98: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Conéjero G, Lelièvre F. 2001. Drought survival and dehydration tolerance in Dactylis glomerata and Poa bulbosa. Functional Plant Biology 28: 743. [Google Scholar]
- Volaire F, Seddaiu G, Ledda L, Lelievre F. 2009. Water deficit and induction of summer dormancy in perennial Mediterranean grasses. Annals of Botany 103: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Barkaoui K, Norton M. 2014. Designing resilient and sustainable grasslands for a drier future: adaptive strategies, functional traits and biotic interactions. European Journal of Agronomy 52: 81–89. [Google Scholar]
- Vreugdenhil D, Sergeeva LI. 1999. Gibberellins and tuberization in potato. Potato Research 42: 471–481. [Google Scholar]
- Weishaar MA, Brummer EC, Volenec JJ, Moore KJ, Cunningham S. 2005. Improving winter hardiness in nondormant alfalfa germplasm. Crop Science 45: 60. [Google Scholar]
- Wiltshire JJJ, Cobb AH. 1996. A review of the physiology of potato tuber dormancy. Annals of Applied Biology 129: 553–569. [Google Scholar]
- Wisniewski M, Nassuth A, Teulières C, et al. 2014. Genomics of cold hardiness in woody plants. Critical Reviews in Plant Sciences 33: 92–124. [Google Scholar]
- Wolkovich EM, Cook BI, Davies TJ. 2014. Progress towards an interdisciplinary science of plant phenology: building predictions across space, time and species diversity. New Phytologist 201: 1156–1162. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Kitajima K, Kraft NJB, et al. 2010. Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91: 3664–3674. [DOI] [PubMed] [Google Scholar]
- Xu X. 1998. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiology 117: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang Y, Zhang X, et al. 2012. Dynamics of phytohormone and DNA methylation patterns changes during dormancy induction in strawberry (Fragaria × ananassa Duch.). Plant Cell Reports 31: 155–165. [DOI] [PubMed] [Google Scholar]