Abstract
Background:
Overactivation of aldose reductase (AR) enzyme has been implicated in the development of various diabetic complications. In the present study, the inhibitory effect of thymol was investigated on AR enzyme and its anti-cataract activity was also examined on isolated goat lens.
Materials and Methods:
Various concentrations of thymol were incubated with AR enzyme prepared from isolated goat lens. Molecular docking studies were carried out using Schrodinger software to verify the binding of thymol with AR as well as to understand their binding pattern. Further, thymol was evaluated for its anti-cataract activity in high-glucose-induced cataract in isolated goat lens in vitro. Quercetin was maintained as standard (positive control) throughout the study.
Results:
Thymol showed potent inhibitory activity against goat lens AR enzyme with an IC50 value of 0.65 μg/ml. Docking studies revealed that thymol binds with AR in similar binding pattern as that of quercetin. The high–glucose-induced cataract in isolated goat lens was also improved by thymol treatment. Thymol was also able to significantly (P < 0.001) reduce the oxidative stress associated with cataract.
Conclusion:
The results suggest that thymol may be a potential therapeutic approach in the prevention of diabetic complications through its AR inhibitory and antioxidant activities.
KEY WORDS: Aldose reductase, cataract, diabetes, docking, goat lens, thymol
Diabetes mellitus is a chronic and complex metabolic disorder characterized by chronic hyperglycemia and is one of the most prevalent metabolic syndromes worldwide. About 35% of the population worldwide is increasingly becoming diabetic annually. The high mortality rate is a result of micro- and macro-vascular complications which causes irreversible end-organ damage.[1] Under chronic hyperglycemic conditions, increased glucose flux occurs in tissues/organs, which are independent of insulin regulation. This enhanced glucose entry causes saturation of the glycolysis pathway and leads to the activation of alternate stress signaling pathways. The stimulation of these stress signaling pathways initiates the diabetic complications cascade via rise in oxidative stress and inflammation.[2]
The polyol pathway is one such stress signaling pathway activated by increased glucose flux. Under nondiabetic conditions, about 5% of glucose enters this pathway. However, under diabetic conditions, 30% of glucose gains access into the polyol system, thus potentiating the toxic effects of this pathway.[3] Aldose reductase (AR) is the rate-limiting enzyme of the polyol pathway and in normal physiology acts as an extrahepatic detoxifying enzyme against endogenous and xenobiotic aldehydes. The activity of this enzyme is found to be increased in diabetic patients.[4] AR catalyzes the conversion of glucose (aldehyde) to sorbitol (alcohol) with concomitant consumption of NADPH. Sorbitol is further metabolized to fructose by sorbitol dehydrogenase enzyme with simultaneous conversion of NAD+ to NADH.[5]
Hyperactive polyol pathway is believed to contribute to the etiology of diabetic complications by eliciting a wide array of harmful effects. Accumulation of sorbitol causes osmotic imbalance owing to its minimal permeability through membranes. The redox balance is altered due to decreased NADPH/NADP+ ratio, which subsequently affects nitric oxide synthase and depletion of glutathione stores and subsequent accumulation of lipid peroxidation end products, leading to oxidative stress.[6] Fructose, formed as a byproduct in this reaction, acts as a glycating agent initiating the formation of advanced glycation end products, thus aggravating the complications.[7]
Cataract is highly prevalent among diabetes mellitus patients and is a major cause of blindness worldwide.[8] Duration of diabetes and quality of glycemic control are the major risk factors associated with diabetic cataract formation.[7] Overactive AR is an important contributor in the development of diabetic cataracts. Thus, inhibition of AR acts as a vital strategy in controlling the development of diabetic cataract. Although AR inhibitors (ARIs) have proved to be beneficial in diabetic complications, many of them were withdrawn from the market owing to their toxic nature, for example, sorbinil developed hypersensitivity reactions and tolrestat was associated with liver toxicity and death.[5] Therefore, it is essential to investigate more effective and nontoxic ARIs that could be used for the treatment of diabetic cataract or other diabetes-associated complications.
In recent times, research on therapeutic interventions in diabetes has been diverted toward natural sources. Several plant extracts as well as isolated phytoconstituents have effectively inhibited AR enzyme and subsequently prevented the development of diabetic complications, for example, trans-anethole[9] and Garcinia mangostana.[10] Thymol is a dietary monoterpene phenol essentially found in thymus species. It has displayed anti-inflammatory activity in isoproterenol-induced inflammation in myocardial infracted rats,[10] collagenase-induced osteoarthritis,[11] and allergic airway inflammation in ovalbumin-induced mouse asthma.[12] Thymol also exhibited neuroprotective activities by attenuating β-amyloid or scopolamine-induced cognitive impairment in rats.[13] It is reported to be a strong antioxidant and has shown hepatoprotective activity against carbon tetrachloride-induced mice liver injury[14] and maintained the antioxidant status in the brain.[15] Thymol demonstrated anti-convulsant, anti-epileptogenic, antibacterial, antifungal, antioxidant, free radical scavenging, and anti-lipid peroxidative activities.[16,17] The aqueous extract of seed of Trachyspermum ammi inhibited rat lens AR enzyme and the IC50 was found to be 0.65 mg/ml.[18] Thymol being an active constituent of T. ammi,[19] we decided to examine the inhibitory effect of thymol on AR enzyme, high-glucose-induced cataract in goat lens, and related oxidative stress.
Materials and Methods
Chemical
Thymol was purchased from Sigma Chemicals, India. NADPH was procured from HiMedia laboratories, Mumbai. All the chemicals used were of analytical grade.
Collection of goat eyeballs
Fresh goat eyeballs were procured from slaughter house in Govandi, Mumbai, immediately after slaughter. They were transported to the laboratory within 2 h at 0–4°C conditions. The lenses were isolated through extracapsular extraction.
In vitro aldose reductase inhibitory activity
In vitro aldose reductase inhibitory activity was determined as per the method described by Dongare et al., 2012.[9]
Preparation of aldose reductase enzyme
The isolated lenses were washed in saline and weighed. They were homogenized in sodium phosphate buffer pH 6.2 containing 10 mM β-mercaptoethanol to obtain a 1:3 w/v homogenate. The resulting homogenate was then centrifuged at 10000 rpm at 4°C for 20 min, and the clear supernatant was separated. This supernatant was subjected to 40% ammonium sulfate precipitation. After centrifugation at 10000 rpm for 15 min, the supernatant was collected. ARI activity was determined in this supernatant. Protein estimation was carried out by Bradford method using bovine serum albumin as standard.
Aldose reductase inhibitory activity
The ARI assay was carried out as per the method described by Somani and Sathaye, 2015.[20] The reaction mixture comprised freshly prepared enzyme, different concentrations of thymol, 0.067 M buffer (pH 6.2), 0.125 mM NADPH, 400 mM lithium sulfate, and 40 mM xylose. The reaction was performed in a 96-well plate, and the final reaction volume was maintained as 200 µl. The formation of NADP was observed as decrease in absorbance at 340 nm recorded for 3 min. Quercetin was maintained as standard. The assay was performed in triplicates. The results were expressed as percentage inhibition and were calculated as follows:
% Inhibition = ([Absorbance of control/min – absorbance of sample/min]/absorbance of control/min) × 100
Docking studies of thymol on human reductase inhibitory enzyme
Docking studies were carried out using standard grid-based ligand docking with energetics molecular docking protocol[21] implemented within maestro molecular modeling suite by Schrodinger, LLC, New York, USA, 2010, installed on AMD Athlon™ workstation (Glide, version 5.6). The protein structure used in the present study is a three-dimensional (3D) crystal structure of human AR in complex with NADP+ and the inhibitor IDD594 with a resolution of 0.81Å and was downloaded from protein data bank code2I16. All water molecules were deleted except for the active site. Structure of thymol was constructed using build option within Maestro using standard geometries and standard bond lengths. With the help of LigPrep facility, appropriate hydrogen was added, and low energy conformers were produced by energy minimization using molecular mechanics force fields (Schrödinger, LLC, New York, USA, 2008). The low energy conformation of thymol was selected and was docked into the grid generated from protein structures using a standard precision docking mode. The final evaluation was done by comparing interactions, glide score (docking score), and binding energy.
In vitro high–glucose-induced cataract in goat lens
In vitro high-glucose induced cataract in goat lens was performed as per the method described by Somani and Sathaye, 2015.[20] The isolated goat lenses were incubated in artificial aqueous humor. Cataract was induced by adding high concentration of glucose (55 mM) in the artificial aqueous humor pH of 7.8 (NaCl 140 mM, KCl 5 mM, MgCl2 2 mM, NaHCO3 0.5 mM, NaH (PO4)2 0.5 mM, CaCl2 0.4 mM, and glucose 5.5 mM). Penicillin 32 mg % and streptomycin 250 mg% were added to the artificial aqueous humor to prevent bacterial contamination. The lenses were divided into the following groups:
Group I - Normal control with glucose 5.5 mM (normal concentration); n = 4
Group II - Negative control with glucose 55 mM (high concentration); n = 4
Group III - Thymol 20 µg/ml with glucose 55 mM; n = 4
Group IV - Thymol 40 µg/ml with glucose 55 mM; n = 4
Group V - Thymol 60 µg/ml with glucose 55 mM; n = 4
Group VI - Quercetin 500 µg/ml with glucose 55 mM; n = 4.
The lenses were incubated at 37°C for 72 h. At the end of the incubation period, the lenses were visually evaluated for the development of opacification by placing them on a graph paper. The number of squares clearly visible through the lens was counted as a measure of lens opacity. Then, they were homogenized in Tris buffer (0.23 M; pH 7.8) containing 0.25 × 10−3 M ethylenediaminetetraacetic acid to obtain a 10% w/v homogenate. The homogenate was further centrifuged at 10000 g at 4°C and the supernatant was separated. The supernatant was used for the analysis of biochemical parameters such as reduced glutathione and lipid peroxidation. Total protein was determined by Bradford's method.
Reduced glutathione
Reduced glutathione was estimated as per the method described by Patil et al.[22] Reduced glutathione (GSH) content was measured with 5,5-Dithiobis (2-nitrobenzoic acid) (DTNB) reagent which binds to the thiol group (-SH) to form a chromophore detected at 412 nm. Each reaction mixture contained 200 µl of DTNB prepared in potassium phosphate buffer (0.1 M, pH 7.4) and 10 µl of lens homogenate. This mixture was incubated for 15 min at 37 °C and absorbance was recorded at 412 nm. The results were expressed in GSH/mg protein.
Lipid peroxidation
Lipid peroxidation was determined as per the method described by Patil et al.[22] Lipid peroxidation was determined spectrophotometrically by measuring the malondialdehyde (MDA) content at the end of reaction. The reaction mixture consisted of 0.2 ml lens homogenate, 0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid adjusted to pH 3.5 with sodium hydroxide, and 1.5 ml of 0.8% of thiobarbituric acid. The reaction mixture was made up to 4 ml with distilled water and heated at 95°C for 60 min in a water bath. After allowing to cool to room temperature, 1 ml of distilled water and 5.0 ml of n-butanol-pyridine mixture (15:1 v/v) were added and samples were shaken vigorously. The samples were centrifuged at 4000 rpm for 10 min and the absorbance of the organic layer was measured at 532 nm. The results were expressed as nmol of MDA/mg protein.
Statistical analysis
The statistical analysis was conducted with the help of analysis of variance followed by Dunnett's test. *P < 0.05, **P < 0.01, and ***P < 0.001 were considered to be statistically significant when compared with negative control group. ###P < 0.001 was considered to be statistically significant when compared to control group.
Results
Aldose reductase inhibition
In the present study, thymol significantly inhibited goat lens AR enzyme in a linear manner in concentrations ranging from 0.25 to 2 µg/ml. The highest percent inhibition exhibited by thymol was 80% at 2 µg/ml and the IC50 was found to be 0.65 µg/ml. Quercetin, used as standard, inhibited 64.64% of AR enzyme at a concentration of 4.5 µg/ml. The percentage inhibition of AR by thymol and quercetin is shown in Table 1.
Table 1.
Percentage inhibition of aldose reductase enzyme by various concentrations of thymol and standard quercetin

Docking studies and glide score
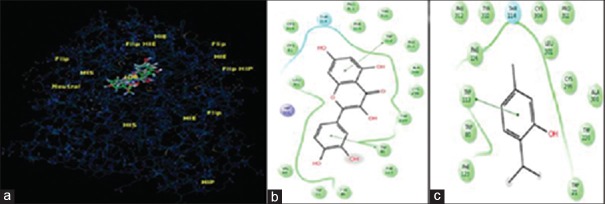
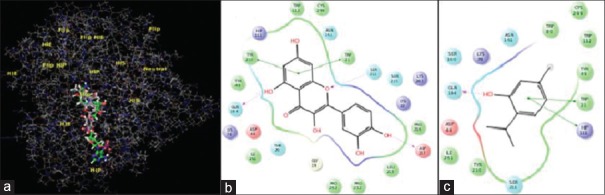
Molecular docking was conducted to validate the binding pattern and selective inhibition of AR enzyme by thymol. It was observed that thymol and quercetin interacted with two sites in the AR enzyme, namely the active site and cofactor NADPH-binding site. At the active site, quercetin exhibited Π–Π stacking interactions with Trp 90 and Trp 112 aminoacid residues, thymol due to the presence of a single aromatic ring exhibited a single Π–Π interaction with Trp 112 [Figure 1]. At the NADPH-binding site, quercetin showed Π–Π stacking interactions with Trp 21 and Try 210. It formed hydrogen bonding with Gln 184, Ser 211, and Asp 217. It was obvious that thymol with its one ring system mimicked the interactions of one of the ring systems of quercetin showing hydrogen bonding with Gln 184 and Π–Π stacking interactions with Trp21 and Hip 111 [Figure 2]. The docking score and binding energy of thymol and quercetin are shown in Table 2.
Figure 1.
Overlapping interaction of thymol and quercetin on site 1 of aldose reductase enzyme (a); binding pattern of quercetin with different aminoacids at site 1 (b); binding pattern of thymol with aminoacids at site 1 (c)
Figure 2.
Overlapping interaction of thymol and quercetin on site 2 of aldose reductase enzyme (a); binding pattern of quercetin with different aminoacids at site 2 (b); binding pattern of thymol with aminoacids at site 2 (c)
Table 2.
Docking score and binding energy of thymol and quercetin

High-glucose-induced cataract
Visual examination and number of squares
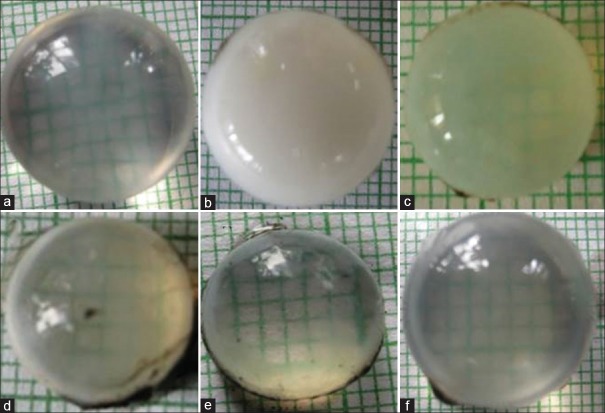
The negative control group developed dense opacities as compared to the normal control group which remained clear at the end of the incubation period. Initially, the opacification started at the periphery of the lens and later progressively increased toward the center. Thymol demonstrated a dose-dependent decrease in opacification with highest concentration, 60 µg/ml, showing clear lens. Quercetin (standard) also exhibited clear lens [Figure 3].
Figure 3.
Representative illustrations of anti-cataract activity of thymol; lens incubated with 5.5 mM (a), high-glucose 55 mm (b), 20 μg/ml thymol (c), 40 μg/ml thymol (d), 60 μg/ml thymol, and (e) 500 μg/ml quercetin (f)
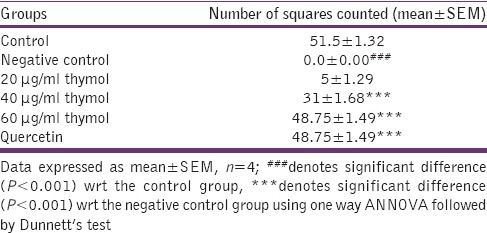
The above results were also correlated with the number of squares counted wherein dose-dependent inhibition of opacification of lens was observed [Table 3].
Table 3.
Number of squares counted on high glucose-induced cataract in isolated goat lens
Reduced glutathione
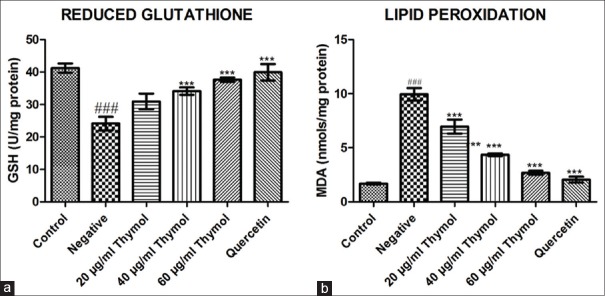
The negative control group demonstrated a significant (P < 0.001) decrease in reduced glutathione level as compared to normal control group. Treatment with thymol significantly (P < 0.001) restored GSH levels in a dose-dependent manner, especially at 40 and 60 µg/ml as compared to negative control group. Quercetin was also able to significantly (P < 0.001) increase the reduced GSH compared to negative control group [Figure 4].
Figure 4.
Effect of thymol on reduced glutathione (a) and lipid peroxidation levels (b) on high-glucose-induced cataract in isolated goat lens. Data expressed as mean ± standard error of the mean, n = 4; ### denotes significant difference (P < 0.001) with respect to the control group, *** denotes significant difference (P < 0.001) with respect to the negative control group using one way ANNOVA followed by Dunnett's test
Lipid peroxidation
Lipid peroxidation levels were found to be significantly (P < 0.001) high in negative control group as compared to normal control group. Lens treated with thymol showed significantly (P < 0.001) reduced lipid peroxidation level at all dose levels compared with negative control group [Figure 4].
Discussion
It is a well-established fact that maintaining a strict glycemic control has a beneficial role in the prevention of diabetic complications. Conventional anti-diabetic drugs have failed to achieve consistent euglycemia in diabetic patients. As a result, they do not prevent the development or progression of diabetic complications. Thus, alternative strategies that are able to prevent the onset and/or delay progression of diabetic complications would offer several advantages, irrespective of the glycemic control. Inhibition of AR enzyme is one such mechanism.[23]
Several plants and natural products have shown promising outcomes in inhibiting this enzyme. To determine whether thymol produced a dose-dependent inhibitory effect, it was evaluated at various graded concentrations. Thymol inhibited AR reductase in a dose-dependent manner with IC50 value of 0.65 µg/ml. It was found to be more potent than quercetin, a naturally occurring flavonoid, which has been demonstrated to be a potent AR reductase inhibitor in vitro with an IC50 of 4.5 µg/ml. Molecular docking is a commonly used tool to understand the binding nature of small molecules to a target protein with known 3D structure. The binding energies are generated and the position of the ligand in the enzyme binding site can be visualized.[24] To substantiate the binding of thymol and quercetin with AR reductase enzyme, molecular docking studies were conducted. It was found that thymol and quercetin interacted with AR reductase enzyme in a similar manner at the active site as well as NADPH-binding site. The difference in the glide score and low binding energy could be attributed to the single ring structure of thymol as compared to the multiple ring structure of quercetin.
Quercetin has been used as a positive control in many AR reductase inhibitory and diabetic cataractogenesis studies. Molecular docking studies showed better interaction of quercetin with AR reductase than thymol. According to literature, quercetin has poor aqueous solubility (1 µg/ml water) and is unstable in physiological medium which results in poor bioavailability along with poor permeability.[25] Contrary to this, the aqueous solubility of thymol is higher than quercetin (1 mg/ml in water).[26] These differences in the physio-chemical properties of thymol and quercetin may have resulted in higher enzyme inhibitory activity of thymol in spite of higher binding energies of quercetin.
ARIs have been shown to attenuate or prevent the development of experimental diabetic cataracts in animals as well as functional anatomic abnormalities of diabetic retinopathy and nephropathy.[9] The high–glucose-induced cataract study in vitro has been conducted on lens culture obtained from various sources such as goat, chicken, and rats. All these models enable us to study and evaluate the anti-cataract activity of test compounds. Goat lenses are easily available, economic, and convenient to use. The large size of these lenses as compared to other models facilitates easy and accurate visualization of opacification and cataract formation in lens, thus enabling screening of various doses of test compounds effectively and with ease.[27] Hence, goat lenses were used in the present study to evaluate the potential of thymol in preventing high–glucose-induced cataract in isolated goat lens.
Visual examination of the lens as well as the number of squares observed revealed that though the negative control group showed opacification at the end of 72 h, no such effect was observed with thymol at all dose levels and significantly at 40 and 60 µg/ml. The results that were compared with quercetin at 1 mg/ml and thymol were found to be more potent than the standard.
Oxidative stress is cited as one of the major reasons in the development of cataracts due to the altered redox status and diminished pool of GSH. Oxidation of lens proteins by reactive oxygen species plays an important role in the process leading to lens opacification. Glutathione is a major endogenous antioxidant, present in unusually high concentration, in the lens that prevents protein oxidation. It plays a vital role in maintaining lens transparency.[28] Under high-glucose condition, the triggering of the polyol pathway causes depletion of GSH levels which results in oxidation of the protein thiols to form disulphide cross-linking. This disulfide formation is accompanied by protein cross-linking and solubility loss, high molecular protein aggregation, and culminates in lens opacification.[7,27] Thymol was able to restore GSH to normal levels at both 40 and 60 µg/ml, which was better than quercetin which restored the GSH levels at 500 µg/ml.
Oxidative stress is manifested by the accumulation of lipid peroxidation products such as MDA.[28,29] Lipid peroxidation has been recognized as a key factor in the pathogenesis of cataract. Oxidation of unsaturated fatty acids in lens epithelial layer has been identified as an initial step in the development of cataract. Accumulation of lipid oxidation products as well as its migration to lens fiber cells causes alteration in fiber cell structure and increased opacity, ultimately leading to cataract formation.[30] The negative control group demonstrated oxidative stress as indicated by enhanced lipid peroxidation levels. Thymol effectively reduced the lipid peroxidation as compared to negative control group, thus exhibiting its antioxidant activity. Thus, thymol was able to inhibit the progression of cataract through its anti-lipid peroxidative activity.
Conclusion
Diabetic cataract occurs as a consequence of various insults to the lens, primarily hyperactive AR enzyme and oxidative stress. Thus, along with ARIs, antioxidants are also important to prevent cataract formation.[7] According to our present study, thymol was able to curb the progression of high–glucose-induced cataract through its AR reductase inhibitory and antioxidant activities. Thus, these multiple properties of thymol support its utility for controlling diabetic complications.
Financial support and sponsorship
This study was supported by DST-INSPIRE fellowship.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge DST-INSPIRE for providing financial assistance for the above study.
References
- 1.Fatmawati S, Ersam T, Shimizu K. The inhibitory activity of aldose reductase in vitro by constituents of Garcinia mangostana Linn. Phytomedicine. 2015;22:49–51. doi: 10.1016/j.phymed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar A, Srivastava SK. Aldose reductase: Congenial and injurious profiles of an enigmatic enzyme. Biochem Med Metab Biol. 1992;48:91–121. doi: 10.1016/0885-4505(92)90055-4. [DOI] [PubMed] [Google Scholar]
- 4.Djoubissie PO, Snirc V, Sotnikova R, Zurova J, Kyselova Z, Skalska S, et al. In vitro inhibition of lens aldose reductase by (2-benzyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b] indole-8-yl)-acetic acid in enzyme preparations isolated from diabetic rats. Gen Physiol Biophys. 2006;25:415–25. [PubMed] [Google Scholar]
- 5.Zhu C. Aldose reductase inhibitors as potential therapeutic drugs of diabetic complications. In: Oguntibeju O, editor. Diabetes Mellitus #; Insights and Perspectives. [Book on Internet] InTech. 2013. [Last updated on 2013 Jan 23; Last cited on 2016 Jan 02]. Available from: http://www.intechopen.com/books/diabetes-mellitus-insights-andperspectives/aldose-reductase-inhibitors-as-potentialtherapeutic-drugs-of-diabetic-complications . [Google Scholar]
- 6.Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Obrosova IG, Chung SS, Kador PF. Diabetic cataracts: Mechanisms and management. Diabetes Metab Res Rev. 2010;26:172–80. doi: 10.1002/dmrr.1075. [DOI] [PubMed] [Google Scholar]
- 8.Young SK, Nan HK, Nam HY, Yun ML, Il-Ha J, Jin SK. Quercitrin gallate inhibits aldose reductase activity and xylose-induced lens opacity and oxidation. Biomed Aging Pathol. 2011;1:123–7. [Google Scholar]
- 9.Dongare V, Kulkarni C, Kondawar M, Magdum C, Haldavnekar V, Arvindekar A. Inhibition of aldose reductase and anti-cataract action of trans-anethole isolated from Foeniculum vulgare Mill. Fruits. Food Chem. 2012;132:385–90. doi: 10.1016/j.foodchem.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Nagoor Meeran MF, Jagadeesh GS, Selvaraj P. Thymol attenuates inflammation in isoproterenol induced myocardial infarcted rats by inhibiting the release of lysosomal enzymes and downregulating the expressions of proinflammatory cytokines. Eur J Pharmacol. 2015;754:153–61. doi: 10.1016/j.ejphar.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Patil D, Dhaneshwar S, Kadam P. Diacerein-thymol prodrug: In vivo release and pharmacological screening in experimental models of osteoarthritis in Wistar rats. Inflamm Allergy Drug Targets. 2015;13:393–405. doi: 10.2174/1871528114666150212125600. [DOI] [PubMed] [Google Scholar]
- 12.Zhou E, Fu Y, Wei Z, Yu Y, Zhang X, Yang Z. Thymol attenuates allergic airway inflammation in ovalbumin (OVA)-induced mouse asthma. Fitoterapia. 2014;96:131–7. doi: 10.1016/j.fitote.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Azizi Z, Ebrahimi S, Saadatfar E, Kamalinejad M, Majlessi N. Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav Pharmacol. 2012;23:241–9. doi: 10.1097/FBP.0b013e3283534301. [DOI] [PubMed] [Google Scholar]
- 14.Al-Malki AL. Antioxidant properties of thymol and butylated hydroxytoluene in carbon tetrachloride – Induced mice liver injury. J King Abdulaziz Univ Sci. 2010;22:239–48. [Google Scholar]
- 15.Youdim KA, Deans SG. Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fatty acid composition of the ageing rat brain. Br J Nutr. 2000;83:87–93. [PubMed] [Google Scholar]
- 16.Sancheti J, Shaikh MF, Chaudhari R, Somani G, Patil S, Jain P, et al. Characterization of anticonvulsant and antiepileptogenic potential of thymol in various experimental models. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:59–66. doi: 10.1007/s00210-013-0917-5. [DOI] [PubMed] [Google Scholar]
- 17.Sanjay SB, Mahaveer PK. To evaluate anti-anxiety activity of thymol. J Acute Dis. 2014;3:136–40. [Google Scholar]
- 18.Saraswat M, Muthenna P, Suryanarayana P, Petrash JM, Reddy GB. Dietary sources of aldose reductase inhibitors: Prospects for alleviating diabetic complications. Asia Pac J Clin Nutr. 2008;17:558–65. [PubMed] [Google Scholar]
- 19.Tsimidou M, Boskou D. Charalambous G. Spices, Herbs and Edible Fungi. Vol. 34. Amsterdam: Elsevier; 1994. Antioxidant activities of essential oils from the plants of the Lamiaceae family; pp. 273–84. [Google Scholar]
- 20.Somani G, Sathaye S. Bioactive fraction of Saraca indica prevents diabetes induced cataractogenesis: An aldose reductase inhibitory activity. Pharmacogn Mag. 2015;11:102–10. doi: 10.4103/0973-1296.149722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somani G, Kulkarni C, Shinde P, Shelke R, Laddha K, Sathaye S. In vitro acetylcholinesterase inhibition by psoralen using molecular docking and enzymatic studies. J Pharm Bioallied Sci. 2015;7:32–6. doi: 10.4103/0975-7406.148775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil SP, Jain PD, Sancheti JS, Ghumatkar PJ, Tambe R, Sathaye S. Neuroprotective and neurotrophic effects of apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology. 2014;86:192–202. doi: 10.1016/j.neuropharm.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Bhanuprakash RG, Muthenna P, Akileshwari C, Megha S, Mark PJ. Inhibition of aldose reductase and sorbitol accumulation by dietary rutin. Curr Sci. 2011;101:1191–7. [Google Scholar]
- 24.Arumugam M, Muthuswamy U, Kuppusamy A, Thirumalaisamy S, Varadharajan S, Puliyath J. Discovery of potential aldose reductase inhibitors using in silico docking studies. Orient Pharm Exp Med. 2012;12:157–61. [Google Scholar]
- 25.Cai X, Fang Z, Dou J, Yu A, Zhai G. Bioavailability of quercetin: Problems and promises. Curr Med Chem. 2013;20:2572–82. doi: 10.2174/09298673113209990120. [DOI] [PubMed] [Google Scholar]
- 26.O’Neil MJ, editor. The Merck Index – An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry; 2013. [Google Scholar]
- 27.Ganeshpurkar A, Bhadoriya SS, Pardhi P, Jain AP, Rai G. In vitro prevention of cataract by oyster mushroom Pleurotus florida extract on isolated goat eye lens. Indian J Pharmacol. 2011;43:667–70. doi: 10.4103/0253-7613.89823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giblin FJ. Glutathione: A vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16:121–35. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 29.Kisic B, Miric D, Zoric L, Ilic A, Dragojevic I. Antioxidant capacity of lenses with age-related cataract. Oxid Med Cell Longev 2012. 2012:467130. doi: 10.1155/2012/467130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bojana K, Dijana M, Lepsa Z, Aleksandra I. Catala A, editor. Role of lipid peroxidation in the pathogenesis of age-related cataract. Lipid Peroxidation. [Book on Internet] InTech. 2012. [Last updated on 2012 Aug 29; Last cited on 2016 Jan 02]. Available from: http://www.intechopen.com/books/lipid-peroxidation/role-of-lipid-peroxidation-in-the-pathogenesis-of-age-related-cataract .