Abstract
Objective:
Alpha-ketoglutarate (α-KG) is a cellular intermediary metabolite of Krebs cycle, involved in energy metabolism, amino acid synthesis, and nitrogen transport. It is available over-the-counter and marketed as a nutritional supplement. There is a growing body of evidence to suggest that dietary α-KG has the potential to maintain cellular redox status and thus can protect various oxidative stress induced disease states. The aim of the present study was to investigate the hepatoprotective role of α-KG in acetaminophen (APAP) induced toxicity in rats.
Materials and Methods:
Animals were divided into three groups of six animals each. Group I (Vehicle control): Normal Saline, Group II (APAP): A single intraperitoneal injection of 0.6 g/kg, Group III (APAP + α-KG): APAP as in Group II with α-KG treatment at a dose of 2 g/kg, orally for 5 days. Then the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) with oxidative stress markers including malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and histopathology were analyzed.
Results:
The results indicate that APAP caused significant elevations in ALT, AST, ALP, and MDA levels, while GSH, SOD, and CAT were significantly depleted while co-administration of α-KG showed a significant (P < 0.05) reduction in the severity of these damages. Histologically, the liver showed inflammation and necrosis after APAP treatment, which were significantly restored with co-administration of α-KG.
Conclusion:
These results indicate the possible therapeutic potential of α-KG in protecting liver damage by APAP in rats.
KEY WORDS: Acetaminophen, alpha-ketoglutarate, hepatotoxicity, oxidative stress
Acetaminophen (N-acetyl-p-aminophenol; APAP), commonly known as Paracetamol, is one of the most successful analgesic and antipyretic drug. As an OTC drug, it can be readily obtained without prescription. Within recommended dosages (4 g/day), APAP is considered to be a safe drug whereas a dose greater than this can lead to severe liver damage.[1] APAP-induced hepatotoxicity results from saturation of normal metabolic pathways, cytochrome P-450, which leads to the formation of reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI). NAPQI depletes natural antioxidant such as reduced intracellular glutathione (GSH) and enhanced lipid peroxidation (LPO) which results in cellular oxidative stress.[2]
Alpha-ketoglutarate (α-KG), a citric acid cycle intermediate metabolite and precursor to glutamine which in turn is a precursor to GSH, has been marketed as nutritional supplement since a long time.[3] In vitro and in vivo studies have shown that exogenously administered α-KG has the potential to improve redox homeostasis and can protect cell from oxidative stress caused by free radicals.[4] The experimental findings suggest that exogenous α-KG may have the potential to prevent various oxidative stress induced diseased liver states. This study was therefore taken up to assess the protective role of α-KG in APAP-induced hepatotoxicity in an established rat model.
Materials and Methods
Chemicals and reagents
Disodium α-KG was purchased from Fluka Chemika, Buchs, Switzerland and APAP was purchased from Lambert Pvt. Ltd., India. All other chemicals were of analytical grade.
Experimental animals
Eighteen male Sprague-Dawley rats weighing (200–250 g) were obtained from the Experimental Animal facility of Institute of Nuclear Medicine and Allied Sciences, Delhi. The study protocol was approved by the Institutional Animal Ethics Committee (IAEC) of the institute (INM/IAEC/2009/06/009).
Acetaminophen-induced hepatotoxicity
Animals were divided into three groups of 6 animals each. Group I (vehicle control): Normal saline, Group II (APAP): a single intraperitoneal injection of 0.6 g/kg, Group III (APAP+ α-KG): APAP as in Group II with α-KG treatment at a dose of 2 g/kg, orally for 5 days. The doses for α-KG and APAP were selected on the basis of previous works reported in the literature from our laboratory and elsewhere.[5,6] The animals were sacrificed after 24 h of the treatment period using diethyl ether. Blood and liver tissue samples were collected. Serum was separated by centrifugation at 3000 rpm for 10 min for liver profile test.
Measurement of liver function test and antioxidant activities
Intracellular enzymes - alanine aminotransferase (ALT), aspartate aminotransferase (AST), and ALP were determined using commercially available kits.
The homogenate of frozen liver tissue (stored at −80°C) was used for evaluating antioxidant activities. LPO, superoxide dismutase (SOD) activity, GSH, catalase (CAT), and protein content were determined by the method of Mihara and Uchiyama,[7] Robak and Gryglewski,[8] Ellman,[9] Sinha,[10] and Lowry et al.[11] respectively.
Statistical analysis
The statistical significance was determined using one-way analysis of variance. Statistical significance value was set at P < 0.05.
Results
Effect of alpha-ketoglutarate on acetaminophen-induced hepatotoxicity
Results on hepatoprotective effect of α-KG against APAP-induced liver damage in rats are shown in Figure 1. The effect on antioxidant status (levels of GSH, CAT, SOD, and malondialdehyde (MDA) in liver tissues) against APAP-induced hepatotoxicity is shown in Figures 2–5. Histopathology of control group shows normal hepatic cells while in APAP-treated animals the section of liver biopsy showed encapsulated hepatic tissues comprising of cords of hepatocytes, portal tract and part of central vein. Supplementation of α-KG significantly reversed the adverse effects of APAP as shown by regenerating hepatocytes and milder inflammation in the portal area [Figure 6].
Figure 1.
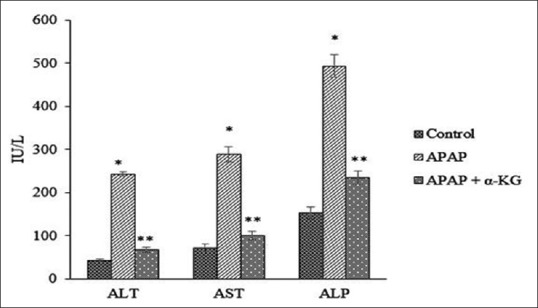
Effect of alpha-ketoglutarate on liver function status against acetaminophen-induced toxicity in rats *P < 0.001, when compared to control, **P < 0.05, when compared to acetaminophen group
Figure 2.
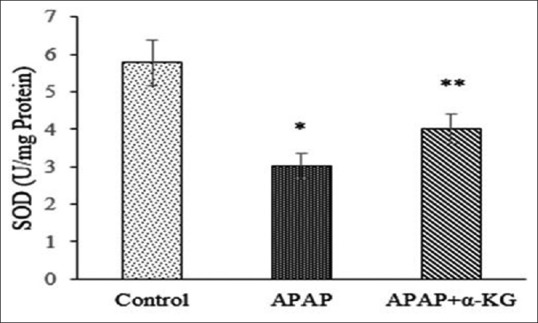
Effect of alpha-ketoglutarate on superoxide dismutase (50% inhibition of nitroblue tetrazolium reaction/min/mg protein) in acetaminophen-induced liver damage in rats. Mean ± standard deviation; n = 6, *P < 0.05, when compared to control, **P < 0.05, when compared to acetaminophen group
Figure 5.
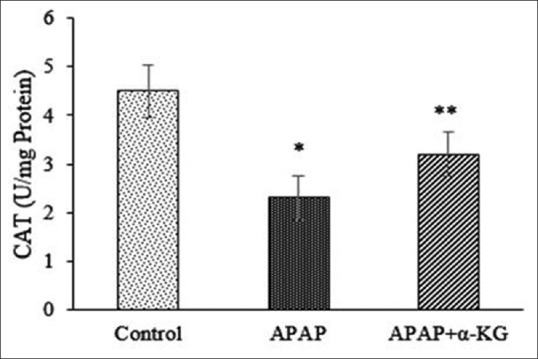
Effect alpha-ketoglutarate on catalase in acetaminophen induced liver damage in rats. Mean ± standard deviation; n = 6, *P < 0.05, when compared to control, **P < 0.05, when compared to acetaminophen group
Figure 6.
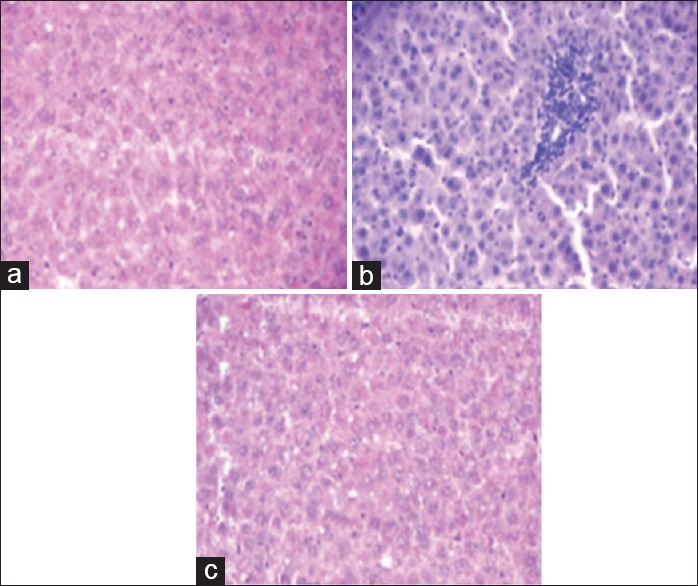
Photomicrograph of liver tissue (a) control rats showing normal hepatic cells with central vein and Sinusoidal dilation, (b) acetaminophen treated rats showing nonspecific inflammation of hepatic tissue with prominent features of vasculitis (c) rats supplemented with alpha-ketoglutarate along with acetaminophen showing milder degree of inflammation as compared to acetaminophen alone
Figure 3.
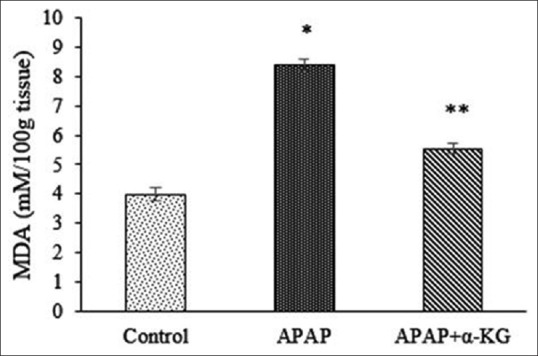
Effect of alpha-ketoglutarate on malondialdehyde in acetaminophen-induced liver damage in rats. Mean ± standard deviation; n = 6, *P < 0.05, when compared to control, **P < 0.05, when compared to acetaminophen group
Figure 4.
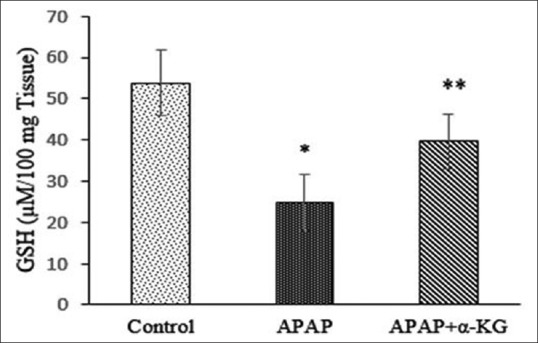
Effect of alpha-ketoglutarate on reduced glutathione in acetaminophen-induced liver damage in rats. Mean ± standard deviation; n = 6, *P < 0.05, when compared to control, **P < 0.05, when compared to acetaminophen group
Discussion
APAP is a widely used nonprescription analgesic and anti-pyretic medication for mild to moderate pain and fever. When APAP is taken in at toxic doses, large proportions of NAPQI are generated, which are highly reactive and toxic electrophilic intermediate metabolites.[12] Presence of excessive NAPQI leads to substantial depletion of intracellular GSH, resulting in mitochondrial dysfunction and hepatocellular death.[13] In addition, NAPQI can increase the formation of reactive oxygen species and reactive nitrogen species, which leads to LPO and depletion of the antioxidant enzymes (SOD, CAT, and GSH).[14]
Oral administration of α-KG prevented hepatotoxic effects of APAP by restoring the activities of antioxidant enzymes (SOD, CAT) and significantly preserving the levels of reduced GSH. This can be attributed to the documented observations that exogenous α-KG can scavenge free radicals, restore, or enhance intracellular GSH pools and chelate pro-oxidants such as iron and phosphate.[15,16] Moreover, being a natural ubiquitous collector of amino (-NH2) groups, α-KG detoxifies toxicants such as ammonia by converting them to glutamic acid, as ammonia by itself is known to inhibit antioxidant enzymes by activating NMDA receptors[17] and by its participation in the nonenzymatic (NADPH-independent) oxidative decarboxylation of hydrogen peroxide decomposition.[18] Thus α-KG by stabilizing cellular redox status has the potential to ameliorate APAP-induced hepatotoxicity. Our results corroborate with earlier findings wherein exogenous α-KG was shown to inhibit hydrogen peroxide-induced oxidative stress in human erythrocytes and cultured neurons.[19,20]
In this study, APAP-induced significant LPO in rat liver, as confirmed by significantly raised MDA levels, which were suppressed after supplementation of α-KG. Besides replenishing the intracellular GSH pool, another important function of α-KG consists of the formation of carnitine[21] which leads to physiological normalization of fat metabolism offering protection against LPO. Our results are supported from previous investigations on the effect of α-KG on MDA levels, where it was observed that α-KG prevented LPO in rat livers when they were exposed to alcohol[4] or sodium valproate.[22]
The elevated levels of serum liver enzymes (ALT, AST, and ALP) are indicative of cellular leakages and loss of functional integrity of hepatocytes, which were seen in APAP hepatotoxic group. Treatment with α-KG efficiently prevented these elevations. Histopathological studies were confirmatory to the biochemical findings and showed reduction in degree of necrosis in rats supplemented with α-KG along with APAP, showing the beneficial effect of α-KG in maintaining the hepatocytes integrity and metabolic function.
Conclusion
The present study reported the hepatoprotective effects of α-KG due to its redox state stabilizing potential. Treatment with α-KG ameliorated APAP induced hepatotoxicity. Thus α-KG could be a potential therapeutic agent in treating various oxidative stress mediated diseased liver states.
Financial support and sponsorship
University Grant Commission and INMAS.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013;17:587–607, viii. doi: 10.1016/j.cld.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H, Williams CD, McGill MR, Xie Y, Ramachandran A. Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem Toxicol. 2013;55:279–89. doi: 10.1016/j.fct.2012.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sass MD. Utilization of alpha-ketoglutarate by red blood cells for glutathione synthesis. Nature. 1963;200:1209–10. doi: 10.1038/2001209a0. [DOI] [PubMed] [Google Scholar]
- 4.Velvizhi S, Nagalashmi T, Essa MM, Dakshayani KB, Subramanian P. Effects of alpha-ketoglutarate on lipid peroxidation and antioxidant status during chronic ethanol administration in Wistar rats. Pol J Pharmacol. 2002;54:231–6. [PubMed] [Google Scholar]
- 5.Mittal G, Singh T, Kumar N, Bhatnagar A, Tripathi RP, Tulsawani R, et al. Radiolabeling and dose fixation study of oral alpha-ketoglutarate as a cyanide antidote in healthy human volunteers. Clin Toxicol (Phila) 2010;48:509–15. doi: 10.3109/15563650.2010.496371. [DOI] [PubMed] [Google Scholar]
- 6.Zhu JH, McClung JP, Zhang X, Aregullin M, Chen C, Gonzalez FJ, et al. Comparative impacts of knockouts of two antioxidant enzymes on acetaminophen-induced hepatotoxicity in mice. Exp Biol Med (Maywood) 2009;234:1477–83. doi: 10.3181/0904-RM-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 8.Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37:837–41. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 9.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 10.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 11.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 12.Miner DJ, Kissinger PT. Evidence for the involvement of N-acetyl-p- quinoneimine in acetaminophen metabolism. Biochem Pharmacol. 1979;28:3285–90. doi: 10.1016/0006-2952(79)90123-0. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–7. [PubMed] [Google Scholar]
- 14.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handbook of Experimental Pharmacology. Springer, Berlin Heidelberg; 2010. pp. 369–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puntel RL, Nogueira CW, Rocha JB. Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res. 2005;30:225–35. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 16.Birck R, Zimmermann E, Wassmer S, Nowack R, van der Woude FJ. Calcium ketoglutarate versus calcium acetate for treatment of hyperphosphataemia in patients on maintenance haemodialysis: A cross-over study. Nephrol Dial Transplant. 1999;14:1475–9. doi: 10.1093/ndt/14.6.1475. [DOI] [PubMed] [Google Scholar]
- 17.Kosenko E, Kaminski Y, Lopata O, Muravyov N, Felipo V. Blocking NMDA receptors prevents the oxidative stress induced by acute ammonia intoxication. Free Radic Biol Med. 1999;26:1369–74. doi: 10.1016/s0891-5849(98)00339-6. [DOI] [PubMed] [Google Scholar]
- 18.Velvizhi S, Dakshayani KB, Subramanian P. Effects of alpha-ketoglutarate on antioxidants and lipid peroxidation products in rats treated with ammonium acetate. Nutrition. 2002;18:747–50. doi: 10.1016/s0899-9007(02)00825-0. [DOI] [PubMed] [Google Scholar]
- 19.Sokolowska M, Oleszek A, Wlodek L. Protective effect of alpha-keto acids on the oxidative hemolysis. Pol J Pharmacol. 1999;51:429–34. [PubMed] [Google Scholar]
- 20.Desagher S, Glowinski J, Prémont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 1997;17:9060–7. doi: 10.1523/JNEUROSCI.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hulse JD, Ellis SR, Henderson LM. Carnitine biosynthesis. Beta-hydroxylation of trimethyllysine by an alpha-ketoglutarate-dependent mitochondrial dioxygenase. J Biol Chem. 1978;253:1654–9. [PubMed] [Google Scholar]
- 22.Murugesan V, Subramanian P. Enhancement of circulatory antioxidants by alpha-ketoglutarate during sodium valproate treatment in Wistar rats. Pol J Pharmacol. 2003;55:31–6. [PubMed] [Google Scholar]


