Abstract
Objective:
This study evaluates the patterns of prescription drugs use among women attending antenatal clinic at Sultan Qaboos University Hospital (SQUH) and SQUH Family and Community Medicine clinic (FAMCO), Oman.
Methods:
The study was a descriptive retrospective cross-sectional study on pregnant women who attended the antenatal clinic at SQUH and FAMCO from February to April 2014 and received a prescription containing at least one drug. Patients’ information was extracted from SQUH electronic records.
Results:
A total of 105 pregnant women were included in the study. Among the recruited pregnant women, 35 (33.3%) had at least one chronic disease. The average number of drugs prescribed per patient per prescription during the period of pregnancy was 2.33 ± 1.43. Vitamins and minerals were the most frequently prescribed class of drugs (30.60%) followed by analgesics (11.19%) and antidiabetic drugs (10.13%). According to the Food and Drug Administration risk classification, most of the prescribed drugs were from category B (30.0%) and C (27.14%). No drug was prescribed from category X. There was a significant decrease in prescribing category A drugs over the three trimesters (20.7%, 12.7%, and 9.3%, respectively) (P < 0.047).
Conclusion:
The study gives an overview of the extent of drug prescription during pregnancy and increases the awareness of health-care providers and women about the potential risks of drug use during pregnancy.
KEY WORDS: Drug use, pregnant, prescription
Drug prescribing during pregnancy is common and essential for treating a preexisting condition or a condition that develops during pregnancy.[1] It is estimated that 33–69% of pregnant women are prescribed medications, including vitamins and minerals, during their pregnancy.[2] A study from the USA found that the use of medication in pregnancy occurred in 88.8% of all pregnancies.[3] Prevalence estimates from Europe range from 26% in Serbia to 93% in France.[4] A multinational web-based study found that 81.2% of pregnant women used at least one medication.[4]
Exposing pregnant women to drugs has been of particular concern with regard to the development of congenital malformations and stillbirths. Abnormalities occur in approximately 2–3% of all newborns with an estimated 1% the result of prescription drug use.[5] It has been suggested that 10% of these abnormalities may be causally associated with prescription drug use.[6] There are only about twenty drugs or groups of drugs that are known to be teratogenic in humans.[6] However, the safety profile of these medications is not directly established in human pregnancy. This is because clinical trials of drugs exclude pregnant women due to ethical and legal reasons.[6] Therefore, there is a lack of knowledge about the harmful effects of these drugs, and drug use cannot always be avoided in pregnancy.
The Food and Drug Administration (FDA) has classified drugs used in pregnancy into five categories: (A, B, C, D, and X). Most drugs are placed in category C, which means that drugs are given only if the benefits outweigh the risks to the fetus. Drugs that fall into categories D and X showed a positive evidence of fetal risk. Several studies have indicated the existence of prescriptions of class D and X drugs in pregnant women.[7] A study on a Canadian population found that about 20% of pregnant women are exposed to FDA category C, D, and X drugs at least once during their pregnancy.[8]
Few studies come from developing countries. A study from Pakistan found that all the pregnant women attending the antenatal clinics received a prescription containing at least one drug and that <1% of the pregnant women attending tertiary care hospitals in Pakistan are prescribed teratogenic drugs.[9] Another study from India found that an average of 1.73, 2.89, and 2.49 drugs per pregnant women was used during first, second, and third trimester of pregnancy, respectively.[10] A study from Saudi Arabia found that 69% of pregnant women were prescribed at least one drug with a mean of 2.87 medications per woman.[11] A questionnaire-based study in Oman found an increase in the use of herbal supplements, whereas the use of vitamins and minerals was optimal.[8]
Due to the gap of knowledge about the safety profile of drugs in pregnancy, it is difficult to make decisions about the potential benefits/risks to the fetus. This study aimed to assess and evaluate the patterns of prescription drugs use among women attending antenatal clinic at Sultan Qaboos University Hospital (SQUH) and SQUH Family and Community Medicine clinic (FAMCO), Oman. The study might, therefore, identify the need for interventional measures to improve the rational drug prescribing in the prenatal period. Data from this study will also shed light on the prenatal care in Oman and provide a comparison with other countries. Furthermore, describing the patterns of drug exposure in pregnant women during different stages of gestation will increase the awareness of physicians and mothers which will help in employing appropriate measures to avoid the use of drugs that pose a risk to the fetus.
Methods
This was a descriptive retrospective cross-sectional study on pregnant women who attended the antenatal clinic at SQUH and FAMCO from February to April 2014 and received a prescription containing at least one drug. Both Omani and nonOmani pregnant women were included from all age groups. Patients’ information was extracted from the hospital electronic records. Ethical approval was obtained through the Medical Research and Ethics Committee at the College of Medicine and Health Sciences.
Data were analyzed using the software Statistical Package for Social Sciences version 21 (SPSS™, Chicago, IL, USA). Descriptive analysis was used for continuous data using mean ± standard deviation and for categorical data using actual numbers and percentages. Chi-square test was used to find association between different categorized data.
Results
General characteristics of pregnant women
A total of 105 pregnant women were included in the study. A total of 474 medications (encompassing 70 different drugs) were prescribed in a total of 204 prescriptions. The average age of the patients was 36.74 ± 4.17 years. Among the recruited pregnant women, 35 (33.3%) had at least one chronic disease. Among these, 35.90% were diabetic, 17.95% were hypertensive, and 12.82% had thalassemia. The characteristics of pregnant women included in this study are shown in Table 1.
Table 1.
Background characteristics and gestational history of recruited women (n=105)

Pregnancy outcomes
Out of 105 pregnancies included, 41 (39.05%) delivered during the period of the study. The characteristics of pregnancy outcomes are shown in Table 2.
Table 2.
Pregnancy outcomes (n=41)

Drug utilization pattern among patients
The average number of drugs prescribed per patients per prescription during the period of pregnancy was 2.33 ± 1.43 ranging between 1 and 7 drugs. The distribution of drugs among patients per prescription during the period of pregnancy is shown in Table 3. The average number of drugs per prescription in each trimester is as following: first trimester 2.17 ± 1.40, second trimester 2.25 ± 1.04, and third trimester 2.40 ± 1.45. There was a slight increase in the number of utilized drugs over the three trimesters, but was not statically significant (P < 0.665). The majority of drugs were administered by the oral route (n = 302, 63.71%) followed by injection (n = 146, 30.80%) and topical administration (n = 16, 3.38%).
Table 3.
The number of drugs prescribed per patient per prescription to 105 patient

Table 4 represents the top twenty drugs prescribed to all patients. Prenatal multivitamin tablet was the most commonly prescribed drug (n = 68, 14.30%) followed by folic acid (n = 28, 5.90%) and paracetamol (n = 24, 5.10%). Figure 1 shows the most frequently administered classes of drugs. Vitamins and minerals were the most commonly prescribed class of drugs (30.60%) followed by analgesics (11.19%) and antidiabetic drugs (10.13%).
Table 4.
The top twenty drugs prescribed to 105 patients (n=474)
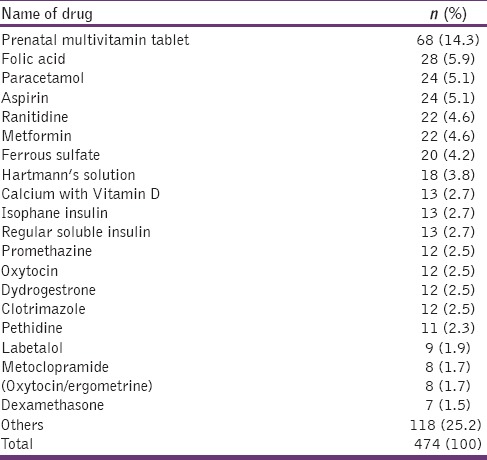
Figure 1.
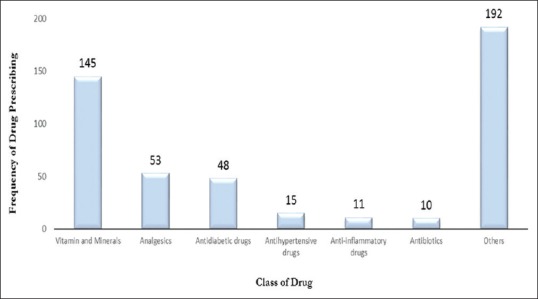
Frequency of Drug Classes Prescription (N = 474)
Most of the drugs were prescribed in the normal visits of pregnant women for follow-up of normal pregnancy (30.19%). About 18.24% of the drugs were prescribed for unspecified supervision of high-risk pregnancy followed by 15.10% for gestational diabetes mellitus, 12.58% in the day of delivery, and 3.77% for vaginal itching and discharge.
Among the total of 70 different drugs that were prescribed for 105 women during pregnancy, 21 (30.00%) were of category B;,19 (27.14%) were of category C, 8 (12.86%) were of category A, and 1 (1.43%, magnesium sulfate) was of category D. No drug from category X was prescribed.
The average number of drugs prescribed per patients per episode during the first trimester of pregnancy was 2.17 ± 1.40. The most prescribed drugs in the first trimester were folic acid (20.69%) followed by aspirin (15.52%), prenatal tablet (15.52%), dydrogestrone (10.34%), and metformin (5.17%). The average number of drugs prescribed per patients per episode during the period of the second trimester was 2.25 ± 1.04. The most prescribed drugs in the second trimester were prenatal tablet (16.56%) followed by aspirin (7.63%), folic acid (6.37%), and paracetamol (5.10%). The average number of drugs prescribed per patients per episode during the third trimester period of pregnancy was 2.40 ± 1.45. The most prescribed drugs in the third trimester were prenatal tablet (12.36%) followed by Hartmann's solution (10.42%), paracetamol (5.79%), ranitidine (5.79%), and oxytocin (5.41%). There was a significant decrease in prescribing of category A over the three trimesters (20.7%, 12.7%, and 9.3%, respectively) (P < 0.047). There was no significant pattern observed for category B (P < 0.883) and category C (P < 0.813).
Discussion
All pregnant women attended the antenatal clinic received a prescription containing at least one drug during their visits were included in the study. The average number of drugs per prescription in our study (2.33 ± 1.43) is slightly higher than the WHO recommended an average number of drugs per prescription of 2.0. A study in Saudi Arabia (n = 727) showed an average of 4.17 drugs per prescription, whereas a study in Pakistan (n = 3769) showed an average of 1.66 drugs.[6,11] A study in the USA (n = 1626) showed an average of 2.2 drugs per prescription, whereas a study in Italy showed an average of 1.8.[2,12] One reason for the slightly higher average number of drugs per prescription compared to the WHO standards is that about a third of the included women have a least one chronic disease, and this percent of women is expected in a country where the prevalence of chronic diseases is high, especially hypertension and diabetes. Despite this, keeping the mean number of drugs per prescription as low as possible is always preferable to reduce the risk to the fetus and mother also.
The average number of drugs per prescription over the three trimesters showed an increasing pattern. A study conducted in West Indies showed the same pattern where 1.32, 1.22, and 0.94 drugs per woman, were used during the first, second, and third trimesters of pregnancy, respectively.[13] This pattern is predicted as drug prescription in the first trimester is always avoided because it is a critical period where organogenesis is accruing and exposing to drugs considered one of the risk factors of developing malformations.
The majority of drugs in our study were administered by the oral route (n = 302, 63.71%) followed by injection (n = 146, 30.80%). The reason of this is because the most common of prescribed drugs were tablets that are administrated orally. Vitamins and minerals were the most commonly prescribed class of drugs (30.60%) followed by the analgesics (11.19%) and antidiabetic (10.13%). Almost a similar pattern of prescription was reported in a study in Pakistan where antianemic drugs including iron preparations and vitamin and mineral supplements (79.4%) were the most frequently prescribed drugs followed by analgesics (6.2%) and antibacterial (2.2%).[9] A number of studies conducted in developing countries have reported vitamins\minerals among the most commonly prescribed medications in pregnancy including a study in Oman, Saudi Arabia, and Ethiopia.[7,11,14] A reason that may be suspected to contribute to this finding is that pregnancy is a critical period where body demand to different vitamins, minerals, and iron supplements is increased. However, a systematic review of literatures for studies conducted in developed countries showed that anti-infective agents were widely prescribed in each study ranging from 27% in Germany to 42% in France.[2]
Out of 105 patients, 35 had chronic disease and 30.90% of them had diabetes that is why antidiabetic drugs came in the third place. Prenatal tablet was the most prescribed drug followed by folic acid, paracetamol, and aspirin [Table 4]. A study conducted among Chinese women showed that 65.2% of studied women were prescribed folic acid.[15] Folic acid is usually recommended to be given in the first trimester to prevent the bad effect of its deficiencies such as spina bifida and encephalopathy. In our study, the most prescribed drug in the first trimester was folic acid (20.69%).
Most of the 70 different drugs that were prescribed in our study were prescribed from category B and C, similar to a study from the USA that showed the same pattern where 2.4% of drugs were from category A, 50.0% category B, and 37.8% from category C.[16] The reason of our findings is that most of vitamins and minerals fall into these two categories. In the study of Ethiopia, 4% of the pregnant women were prescribed drugs from category D or X, whereas a study conducted in Taiwan found that 2446 (1.1%) were from category D or X drugs (0.6% for category D and 0.5% for category X).[14] Data from the UK showed that one in every 164 women had received the drug from category X.[17] It is estimated that 3.9–4.6% of pregnant women in the USA receive category D and X drug.[16] Overall, most of the published literatures showed low percentages of prescription of categories D and X including our study. These two categories are always avoided because they are known to be teratogenic. However, the concern is about the 20 (28.57%) drugs which are unclassified or unknown. This strongly supports the need of detailed profile for each drug to avoid unwanted bad effects either to the fetuses or mothers. There was a significant decrease in prescribing of category A over the three trimesters (first: 20.7%, second: 12.7%, and third: 9.3%) (P < 0.047). This can be explained as because most of these prescriptions are vitamins and minerals and its known that in gynecology practice, the need of vitamins and minerals decreases as the pregnancy is advancing.
The study collected the electronic data of pregnant women attended SQUH and FAMCO clinic which are a tertiary care health service. The data will therefore pick patients coming from different regions of the country. The study, however, has its limitations. Our sample size is small compared with other published studies that studied drugs use during pregnancy. However, the sample size of our study is in accordance with the WHO recommendation for practice assessment in individual facilities which requires that a minimum of 100 samples per facility should be collected for the purpose of evaluation. Due to the short duration of the study, seasonal variation may have introduced a source of bias. This study was conducted in a tertiary hospital in the capital of Oman, which limits the generalizability of the results.
Conclusion
In this study, the average number of drugs per prescription was slightly higher compared to the WHO standards. Vitamin and minerals were the most prescribed drugs followed by analgesics. Most of the prescribed drugs fell in category B and C. Only one drug fell in category D and no drug classified as category X.
The result of this type of studies gives an overview of the extent of drug prescription during pregnancy and increases the awareness of health-care providers and women about the potential risks of drug use during pregnancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Thomas SH, Yates LM. Prescribing without evidence – Pregnancy. Br J Clin Pharmacol. 2012;74:691–7. doi: 10.1111/j.1365-2125.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daw JR, Hanley GE, Greyson DL, Morgan SG. Prescription drug use during pregnancy in developed countries: A systematic review. Pharmacoepidemiol Drug Saf. 2011;20:895–902. doi: 10.1002/pds.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S National Birth Defects Prevention Study. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205:51.e1–8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupattelli A, Spigset O, Twigg MJ, Zagorodnikova K, Mårdby AC, Moretti ME, et al. Medication use in pregnancy: A cross-sectional, multinational web-based study. BMJ Open. 2014;4:e004365. doi: 10.1136/bmjopen-2013-004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Santis M, Straface G, Carducci B, Cavaliere AF, De Santis L, Lucchese A, et al. Risk of drug-induced congenital defects. Eur J Obstet Gynecol Reprod Biol. 2004;117:10–9. doi: 10.1016/j.ejogrb.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Webster WS, Freeman JA. Prescription drugs and pregnancy. Expert Opin Pharmacother. 2003;4:949–61. doi: 10.1517/14656566.4.6.949. [DOI] [PubMed] [Google Scholar]
- 7.Al-Riyami IM, Al-Busaidy IQ, Al-Zakwani IS. Medication use during pregnancy in Omani women. Int J Clin Pharm. 2011;33:634–41. doi: 10.1007/s11096-011-9517-y. [DOI] [PubMed] [Google Scholar]
- 8.Wen SW, Yang T, Krewski D, Yang Q, Nimrod C, Garner P, et al. Patterns of pregnancy exposure to prescription FDA C, D and X drugs in a Canadian population. J Perinatol. 2008;28:324–9. doi: 10.1038/jp.2008.6. [DOI] [PubMed] [Google Scholar]
- 9.Rohra DK, Das N, Azam SI, Solangi NA, Memon Z, Shaikh AM, et al. Drug-prescribing patterns during pregnancy in the tertiary care hospitals of Pakistan: A cross sectional study. BMC Pregnancy Childbirth. 2008;8:24. doi: 10.1186/1471-2393-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma R, Kapoor B, Verma U. Drug utilization pattern during pregnancy in North India. Indian J Med Sci. 2006;60:277–87. [PubMed] [Google Scholar]
- 11.Al-Humayyd MS, Babay ZH. Pattern of drug prescribing during pregnancy in Saudi women: A retrospective study. Saudi Pharm J. 2006;14:201–7. [Google Scholar]
- 12.Kulaga S, Zargarzadeh AH, Bérard A. Prescriptions filled during pregnancy for drugs with the potential of fetal harm. BJOG. 2009;116:1788–95. doi: 10.1111/j.1471-0528.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 13.Pinto Pereira LM, Nayak BS, Abdul-Lateef H, Matmungal V, Mendes K, Persad S, et al. Drug utilization patterns in pregnant women: A case study at the Mount Hope Women's Hospital in Trinidad, West Indies. West Indian Med J. 2010;59:561–6. [PubMed] [Google Scholar]
- 14.Kebede B, Gedif T, Getachew A. Assessment of drug use among pregnant women in Addis Ababa, Ethiopia. Pharmacoepidemiol Drug Saf. 2009;18:462–8. doi: 10.1002/pds.1732. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Qi X, Hao J, Huang Z, Zhang Z, Xing X, et al. Pattern of drug use during the first trimester among Chinese women: Data from a population-based cohort study. Eur J Clin Pharmacol. 2010;66:511–8. doi: 10.1007/s00228-009-0781-x. [DOI] [PubMed] [Google Scholar]
- 16.Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Hardy JR, Leaderer BP, Holford TR, Hall GC, Bracken MB. Safety of medications prescribed before and during early pregnancy in a cohort of 81,975 mothers from the UK General Practice Research Database. Pharmacoepidemiol Drug Saf. 2006;15:555–64. doi: 10.1002/pds.1269. [DOI] [PubMed] [Google Scholar]


