Abstract
Self-assembly of proteins and peptides into amyloid fibrils involves multiple distinct intermediates and late-stage fibrillar polymorphs. Understanding the conditions and mechanisms that promote the formation of one type of intermediate and polymorph over the other represents a fundamental challenge. Answers to this question are also of immediate biomedical relevance since different amyloid aggregate species have been shown to have distinct pathogenic potencies. One amyloid polymorph that has received comparatively little attention are amyloid spherulites. Here we report that self-assembly of the intrinsically disordered polymer poly(L-glutamic) acid (PLE) can generate amyloid spherulites. We characterize spherulite growth kinetics, as well as the morphological, optical and tinctorial features of this amyloid polymorph previously unreported for PLE. We find that PLE spherulites share both tinctorial and structural characteristics with their amyloid fibril counterparts. Differences in PLE's molecular weight, polydispersity or chemistry could not explain the selective propensity toward either fibril or spherulite formation. Instead, we provide evidence that PLE polymers can exist in either a collapsed globule or an extended random coil conformation. The collapsed globule consistently produces spherulites while the extended coil assembles into disordered fibril bundles. This results suggests that these 2 PLE conformers directly affect the morphology of the resulting macroscopic amyloid assembly.
Keywords: amyloid, birefringence, polyglutamic acid, polymer conformation, polymorphism, spectroscopy
Introduction
Understanding the mechanisms that regulate amyloid self-assembly from partially denatured proteins and peptides is critical, in part, because of the prominent role amyloid formation plays in multiple human diseases1-3 and its emergent association with biologically functional processes.4-6 In addition, amyloid self-assembly is an important model system for elucidating the basics of molecular self-assembly7, and the surprising range of novel biological, mechanical, optical, and even electrical functionalities amyloid fibril assemblies can display.8-14 The generic utility of amyloid self-assembly as functional biomaterials at multiple length scales is enhanced by the diversity of macroscopic assembly geometries amyloid self-assemblies can generate, including nanotubes and nanoribbons.15-19 One of the challenges in understanding amyloid self-assembly is that very same variety of distinct assembly structures observed either transiently or as distinct end-products. Small amyloid oligomers and so called protofibrils are well-known intermediates of amyloid fibril growth.20 While there is strong evidence that these intermediates might be the main pathogenic aggregate species in amyloid diseases,21,22 their roles as either precursors or competitors of fibril formation, their internal structures, and the mechanisms by which they form and later on dissolve have remained contentious.23 Similarly, late-stage fibrillar products of amyloid assembly can display a wide range of polymorphs, which appear related to their variable propensity toward bundling and forming twisted ribbons.24-26 One late-stage polymorph of amyloid assembly that, in comparison, has received little attention are large spherical aggregates.27-30
Here we report on the formation of compact spherulites using polyglutamic acid (PLE) under acidic growth conditions. Amyloid fibril grown from polyamino acids established that the propensity toward amyloid formation by polypeptides originated from their backbone and their tendency toward intermolecular hydrogen bonding.31 We initially intended to use PLE as model system to map out the effects of charge repulsion and charge screening on amyloid assembly.32 However, instead of producing the frequently observed frayed amyloid bundles, growth experiments with one specific PLE stock consistently generated highly compact spherical aggregates with rather narrow size distributions. We characterized the tinctorial, optical and spectroscopic characteristics of PLE spherulites and compared them to those of the frayed amyloid bundles generated by a different source of PLE. Using both light scattering and thioflavin T fluorescence, we determined the kinetics of PLE spherulite formation which matched the classical Johnson, Mehl, Arvrami and Kolmogorov (JMAK) theory of spherulite growth. After dismissing several possible explanations for the variant assembly behavior, we noticed that the PLE monomers existed in one of 2 distinct solution conformations: either an extended random coil or a collapsed globular conformation. These two distinct conformations of PLE polymers directly correlated with the macroscopic assembly morphology of PLE aggregates as either frayed amyloid bundles or spherulites.
Results and Discussion
Assembly of PLE into spherulites vs. fibril bundles
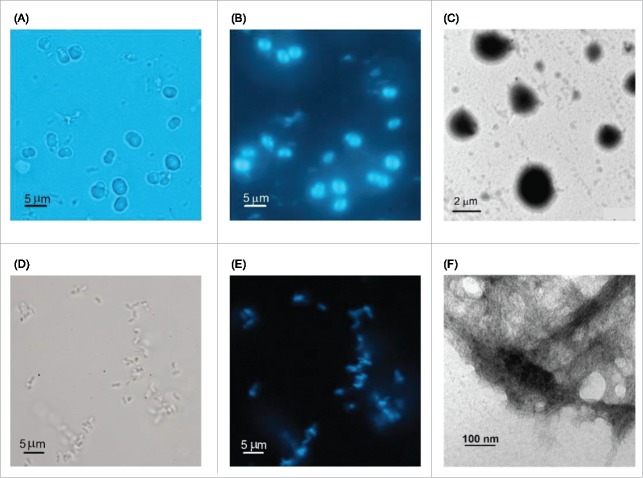
We used PLE stocks from Alamanda Polymers with average molecular weights (MW) of either 7.5, 30 or 60 kDa. Typical conditions for amyloid fibril growth of PLE were imposed by dissolving the lyophilized PLE stocks in 50 mM citrate buffer at or slightly below pH 4.0, and by incubating solutions at temperatures near 40°C.31 Solutions also contained 10 μM thioflavin T (ThT), which is a common indicator dye for monitoring amyloid fibril formation.33,34 We observed the formation of visible aggregates within 24–48 hours. However, the morphology of the resulting aggregates were strikingly different from the commonly reported frayed amyloid fibrils.35,36 Aggregates assembled from Alamanda PLE stock reproducibly yielded micron-sized spherical aggregates of rather uniform size within a given batch (Fig. 1A). Spherulites formed independent of the average molecular weight of the stock, the incubation temperature or the salt concentration used for incubation. Similar to PLE amyloid fibrils, the spherical aggregates displayed bright ThT fluorescence (Fig. 1B), suggestive of an underlying amyloid-like structural organization. TEM images (Fig. 1C) yielded the same dense spheroids seen in light microscopy and did not reveal any additional structural information or hints of intermediates to spherulite formation. To our knowledge this is the first report of spherulite growth with PLE.
Figure 1.
Morphology of Sigma vs. Alamanda PLE Aggregates. Light and transmission electron microscopy (TEM) images of aggregates grown from (A-C) Alamanda PLE vs. (D-F) Sigma PLE at 1 mg/mL, pH 3.6, 50 mM citrate buffer and 40 °C. Bright field image of (A) spherical Alamada PLE aggregates vs. (D) disorganized Sigma PLE aggregates. (B, E) Thioflavin T fluorescence images of same aggregates as in (A) and (D), respectively. (C, F) TEM images of Alamanda vs. Sigma PLE aggregates grown under the same conditions.
For comparison, we used PLE from Sigma-Aldrich with stated molecular weight ranges of 15–50 or 50–100 kDa. PLE aggregates obtained under identical growth conditions from these Sigma PLE stocks showed little organization at the micron scale, as assessed by light microscopy (Fig. 1D). Their intense ThT fluorescence, though, implied that they had the typical amyloid-like organization (Fig. 1E). Transmission electron microscopy (TEM) images confirmed that the aggregates were composed of dense bundles of fibrils at the sub-micron scale (Fig. 1F). This morphology closely resembles prior reports of PLE assemblies under these growth conditions. Overall, PLE from these 2 different sources reproducibly assembled into distinct macroscopic aggregates: either into the frequently observed bundled amyloid fibrils with little organization at the micron scale, or into uniform spherical aggregates reaching several microns in size. We therefore characterized the structural organization and growth kinetics of PLE spherulites and investigated the potential origin of the distinct assembly behavior of different PLEs.
Structural organization of PLE spherulites
Structured spherulites can be formed by a wide variety of polymers, including many synthetic polymers, liquid crystals, lipids, polysaccharides and proteins.37-40 Detailed information on the structural organization of spherulites is sparse due to the difficulties of performing high-resolution structure determinations on micron-sized aggregates.41 One characteristic feature of ordered spherulite assemblies is their optical birefringence, resulting in a “Maltese Cross” pattern when observed under crossed polarizers.28,42,43 As shown in Figure 2A, the spherulites grown with Alamanda PLE do display this characteristic birefringence pattern, indicating a high level of structural organization within the spherulites. We presumed that these ordered spherulites had an underlying amyloid-like organization, as characterized by the presence of β-sheet like intermolecular hydrogen bonds formed across adjacent polypeptide backbones. Spherulite growth in colloidal systems can involve long-lived intermediates of spherulite formation,44 but those are not common for amyloid spherulites.18 Using TEM, we could not identify any morphologically distinct precursors to PLE spherulite formation, either. Given that spherulite formation is a nucleation-limited growth process (see below), any pre-nucleation structures, if present, would be short-lived and, therefore, difficult to trap. The intense staining of PLE spherulites by the amyloid indicator-dye ThT (Fig. 1E), their well-defined birefringence and their formation under conditions that promote fibril formation, all suggest that PLE spherulites have an underlying amyloid-like organization. ThT, though, can also stain non-amyloidoic protein structures such as collagen fibrils.45 Since the alternative amyloid dye Congo Red cannot be used at these highly acid pH values we looked, instead, for a recently identified intrinsic fluorescence emission that is strongly augmented upon amyloid fibril formation.46,47 As shown in Figure 2B, PLE spherulites indeed displayed this dramatic enhancement in intrinsic fluorescence emission that is associated with amyloid formation.
Figure 2.
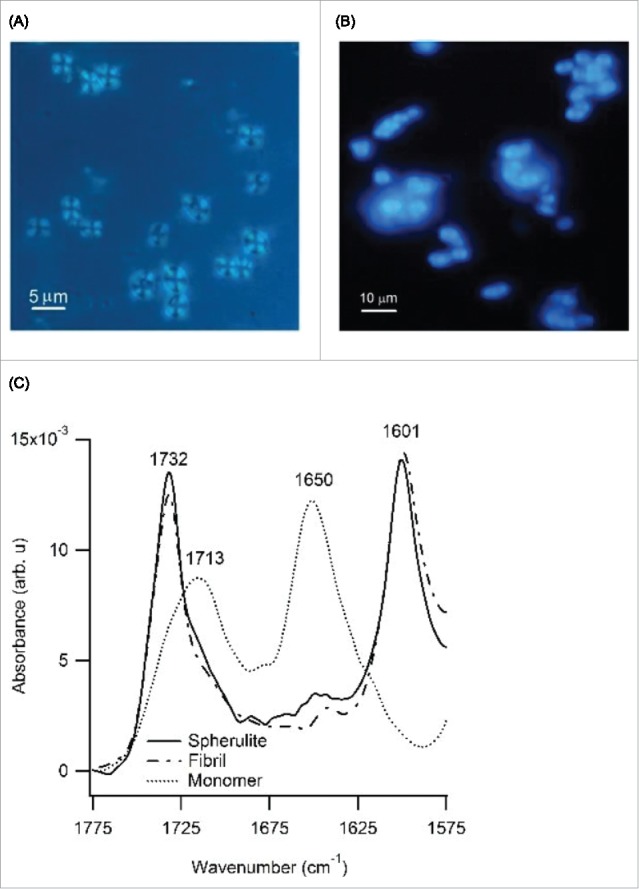
Optical Microscopy and IR Spectroscopy of PLE Spherulites (A) Same spherulites as in (Fig. 1D, E) imaged between crossed polarizers which reveals the Maltese cross pattern characteristic of structured polymeric spherulites, (B) Unstained PLE spherulites display a very intense intrinsic fluorescence emission associated with amyloid formation (ex. 380 nm, em. 455 nm) (C) Amide I band of the FTIR spectra for Alamanda PLE spherulites (solid line), Sigma PLE bundled fibrils (dot-dash line) and Alamanda PLE monomers (dotted line). Peak amplitudes of spectra were normalized to same heights for comparison.
As further confirmation of the underlying amyloid-like organization of PLE within spherulites we compared spherulite IR absorption to those of PLE fibrils using ATR-FTIR spectroscopy. Figure 2C is the superposition of the Amide I band from IR spectra for PLE spherulites (solid line), PLE amyloid fibrils (dot-dashed line) and of freshly dissolved PLE polymers (dashed line), all under the same solution conditions. For PLE monomers, the peaks near 1650 cm−1 and 1713 cm−1 are within few wavenumbers of prior observations of their α-helix conformation.48 Aggregation into spherulites and fibrils induced identical spectral changes with prominent new peaks emerging at 1601 cm−1 and 1732 cm−1. The 1732 cm−1 band is associated with COOH stretching modes. The 1601 cm−1 peak represents the β-sheet structure in the fibril state for this peptide. This peak is shifted by about 10 cm−1 toward lower wavenumbers when compared to amyloid fibrils formed by various proteins,49 which is a peculiarity of fibrils formed by pure enantiomers of PLE.50 The slight difference between our peak positions and those in ref.48 likely arises from the use of deuterium oxide as solvent in this prior work. Attempts at CD spectroscopy from PLE spherulites failed due to the rapid settling out of spherulites from solution.
The combination of the intense ThT staining, of dramatically enhanced intrinsic fluorescence, and of FTIR spectra that are indistinguishable from their fibrillar counterparts indicates that the underlying structural organization of PLE spherulites is amyloid-like, i.e. that individual monomers are cross-linked via intermolecular hydrogen bonds. The optical birefringence, in addition, speaks to the overall centrosymmetric organization of the amyloid-structures within PLE spherulites. Hence PLE can form amyloid-like spherulites similar to those observed with insulin, Alzheimer-associated Aβ, and prion proteins.27,29,37,51
Growth kinetics of PLE spherulites
Since spherulite growth of PLE has not been described previously, we characterized its kinetics using static and dynamic light scattering (for MW ≥ 30 kDa), as well as the fluorescence emission of ThT (10 μM). Figure 3A shows the superposition of the typical changes in the static light scattering and ThT fluorescence emission for a PLE solution (MW = 30 kDa, c = 2 mg/mL) at 37°C, pH 4.1 incubated for 24 hours. The semi-logarithmic display of the same data (see Fig. 3A, insert) highlights that, under these growth conditions, there is no discernable lag period. The kinetic traces obtained from either mode of monitoring are very similar. That these distinct signals should yield similar time-courses is not self-evident. ThT fluorescence is proportional to the amount of amyloid material formed while the light scattering intensity, to first order, increases with the square of the molecular weight of the aggregates.52,53 We have observed a similar proportionality associated with amyloid fibril growth by hen egg-white lysozyme.54 We argued there that the similarity of these 2 kinetics signals indicates that the underlying growth kinetics are dominated by the rate of new aggregate formation over the rate for growth/elongation of existing aggregates. PLE spherulite growth kinetics therefore replicates that feature of amyloid fibril growth, as well. Figure 3B documents the changes in the peak sizes of PLE aggregates extracted from measurements of the particle size distribution (PSD) using DLS. Initially, DLS only detects a PLE monomer peak. Around 6 h of incubation, the PSD becomes bimodal with a new aggregate peak of about 70 nm radius emerging. The formation of this new peak in DLS coincides with the sharp upturn in scattering intensity, which is added to Figure 3B as reference.
Figure 3.
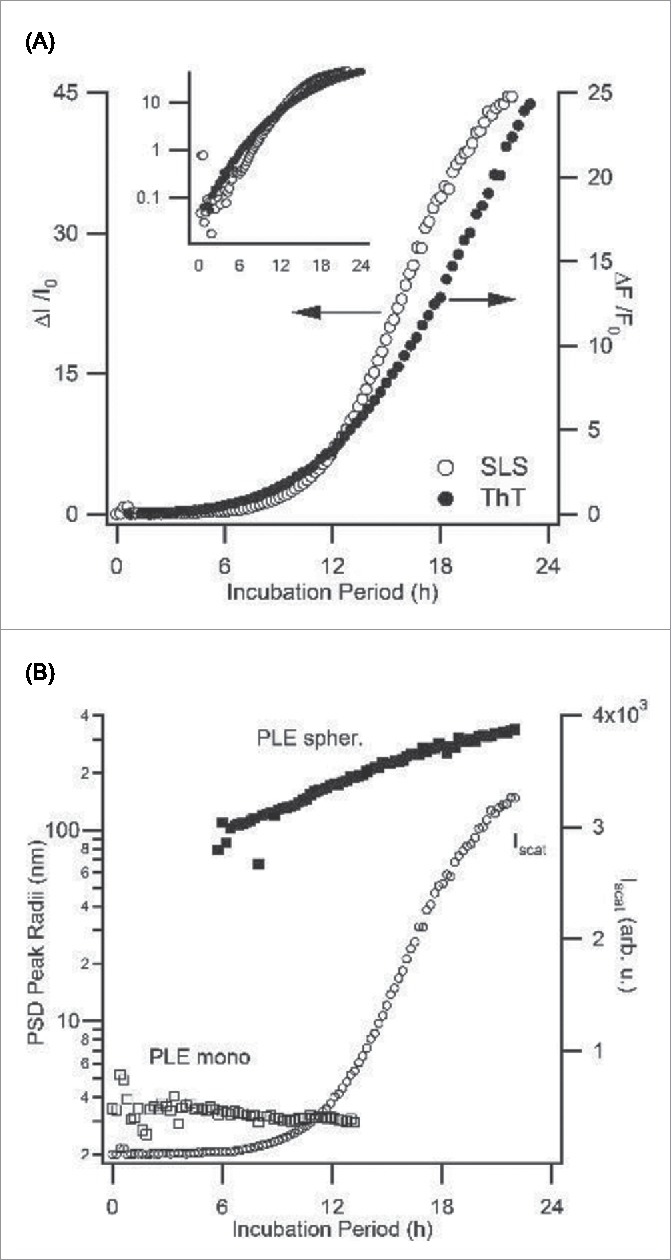
Kinetics of PLE Spherulite Assembly: Light Scattering vs. ThT Fluorescence (A) Superposition of the fractional increases in light scattering intensity ΔI/I0 vs. ThT fluorescence intensity ΔF/Fo during spherulite growth of PLE (30 kDa, 2 mg/mL) in 50 mM citrate at pH 4.1, 50 mM NaCI, incubated at T = 37 °C. The insert shows the same data on a semi-log plot which indicates the lack of a lag phase under these conditions (B) Plot of ΔI/I0 from (A) against the corresponding number of peaks in the particle size distribution (PSD), as obtained from dynamic light scattering. The appearance of a prominent new aggregate peak around 6 hours coincides with the dramatic upswing in scattering intensity.
The ThT and light scattering data in Figure 3 indicate that both of these measurements provide comparable information about the kinetics of PLE spherulite growth. Using ThT fluorescence, however, allowed us to explore the kinetics of PLE assembly over a wider range of polymer concentrations and/or molecular weights.55 Subsequent experiments therefore focused on ThT kinetics. Figure 4A displays the variation in PLE spherulite assembly for 7.5 kDa PLE at pH 3.6 as function of PLE concentration. At the highest concentration (1 mg/mL) PLE displays the same instantaneous onset of aggregate growth seen with 30 kDa PLE at 2 mg/mL and pH 4.1 (Fig. 3A). The semi-log display of ThT fluorescence highlights that spherulite growth does start to develop a well-defined lag period as PLE concentrations decrease. The variability in this lag period shows a surprisingly small sample-to-sample variability (Fig. 4B). At high driving forces for aggregation, we observed that PLE assemblies at the level of light microscopy became increasingly ramified and no clear birefringence was detectable. However, inspection with TEM confirmed that this increasing “disorder” at the micron scale arose from a rapid decrease in the size of the underlying spherulites down to just a few tens of nanometers (Fig. 4C), instead of a transition in the growth habit to fibrillar morphologies. Hence Alamanda PLE yielded spherulites over the entire range of parameters mapped out in these experiments, and with sizes ranging from just a few tens of nanometers up to several microns.
Figure 4.
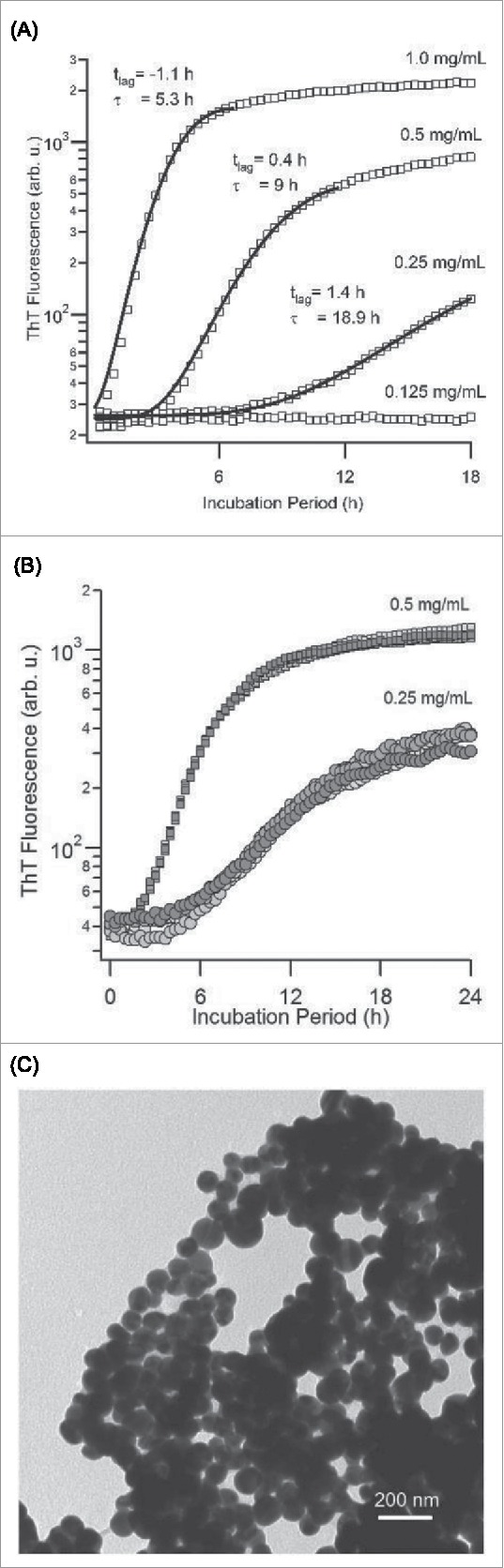
Concentration-dependence of PLE Spherulite Assembly (A) Increase in ThT fluorescence emission at 485 nm (445 nm ex.) during spherulite assembly of Alamanda PLE (7.5 kDa) at the indicated PLE concentrations (40 °C, pH 3.6, 50 mM NaCI). Solid lines and tlag/C parameters represent the results of fits through the data using the JMAK spherulite growth model (B) Kinetics traces similar to (A) for four identical samples of PLE at 0.5 mg/mL and 0.25 mg/mL, respectively, highlighting the high degree of reproducibility of spherulite kinetics (C) TEM image of nanoscale Alamanda PLE spherulites obtained at high driving force (PLE concentrations ≥ 2 mg/mL).
Analytical predictions of the growth rates of nucleated spherulite formation go back to the JMAK models of Kolmogorov and Johnson, Mehl and Avrami.56,57 The JMAK model presumes a uniform rate of nucleus formation and growth which does not account for monomer depletion over time. The time course for a kinetics signal X(t) following this 3-dimensional growth is then given by
Here A, B represent the indicator-dependent signal baseline and saturation values. The exponential term, in turn, contains the time constants tlag and τ related to the lag period and the growth rate of the spherulites, respectively.58 Figure 4A shows fits through the ThT fluorescence responses during PLE spherulites growth using the above prediction. The fitting parameters are as indicated. The overall agreement with the data is surprisingly good. Deviations are noticeable, though, when extending the fit into the upper plateau phase of the data. This is not surprising since depletion effects, which are not included in the JMAK model, will become important at high spherulite concentrations. In addition, the plateau phase in the experimental data displays a slight upward drift which might arise from heterogeneities in local spherulite concentrations and sizes that develop as solute concentrations near the solubility limit for PLE spherulites.
Dependence of spherulite assembly on pH and temperature
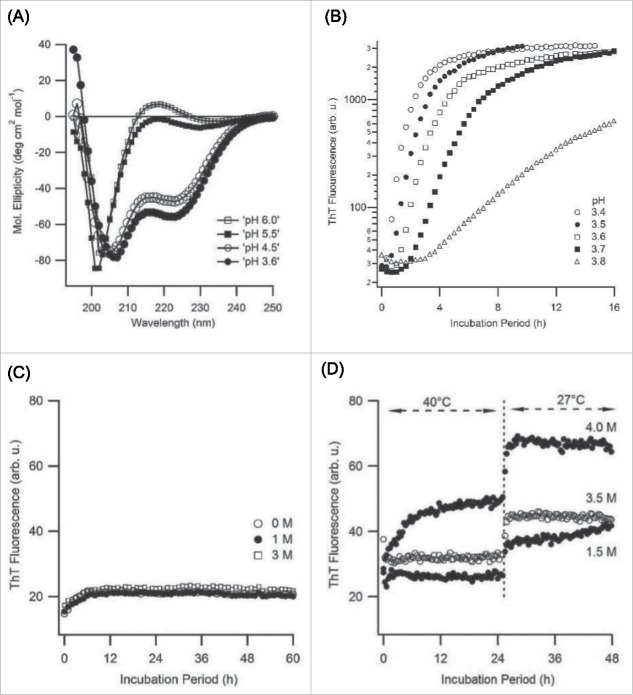
PLE has been previously employed for investigating how its pH-induced helix-to-coil transition alters amyloid fibril assembly.31 Given the distinctly different aggregation behavior of Alamanda PLE we initially confirmed that the helix-to-coil transition of its monomers was preserved. Figure 5A displays CD spectra of Alamanda PLE samples between pH 3.6 and pH 6.0, which show the typical transition from a dominant α-helical (minima at 208 and 222 nm) to a random coil (single minimum near 200 nm) conformation. This closely matches with the reported pH-induced helix-coil transition of PLE monomers.59 By choosing pH values on either side of the transition, we next tested whether Alamanda PLE would form spherulites in either conformation. Figure 5B documents the deceleration of PLE spherulite growth rates as the solution pH approached the edge for the α-helix conformation (∼ pH 4.5). However, the highly cooperative helix-coil transition of PLE monomers with pH (Fig. 5A) also coincides with a rapid increase in PLE net charge. Hence, it is not immediately clear whether the decline in spherulite growth rates is dominated by charge effects or by a preference for the α-helix conformation of PLE. We tried to separate these effects by screening out salt effects using solutions of very high ionic strength. PLE when incubated at pH 6.0 and with salt concentrations ranging up to several molar of NaCl (Fig. 5C) showed no discernible ThT response at any salt concentration. No spherulites were visible in optical microscopy even after 2 weeks of incubation. As control, we incubated PLE at pH 4.5, at which point it is almost exclusively in the α-helix conformation (Fig. 5A). ThT only registered weak responses over 2 d of incubation (Fig. 5D). However, after one week visible spherulites had formed at all salt concentrations. The absence of any spherulite formation at pH 6 even in high ionic strength solutions and the ability to induce fibril formation at pH 4.5 is consistent with the idea that the α-helix conformation of PLE monomers actually promotes ordered spherulite assembly. One issue confounding this interpretation is the known shift of the helix-coil transition to more acidic values upon increases in ionic strength.59 Since alkali and halogen ions interfere with UV-CD spectroscopy to quantify this shift, we did not attempt to untangle these mutual dependences on solution pH and ionic strength.
Figure 5.
pH Dependence of Spherulite Growth (A) Far UV circular dichroism (CD) spectra of Alamanda PLE (7.5 kDa, 1 mg/mL, 37 °C) as function of solution pH. Alamanda PLE undergoes the typical cooperative transition from an alpha-helix to a random-coil conformation between pH 4.5 and 5.5.67,58 (B) Slow down in the kinetics of PLE spherulite growth as pH approaches the edge for the alpha-helix conformation (C, D) ThT fluorescence responses from 1 mg/mL solutions of 7.5 kDa PLE incubated at high salt concentrations either (C) in the random coil state (pH 6.0) or (D) near the edge of the alpha helical conformation (pH 4.5).
During our experiments we noticed, though, that PLE spherulite growth rates were reduced with increasing solution temperatures. This effect is documented in Figure 6A. Using CD spectroscopy we found that elevated temperatures did disrupt the α-helical conformation of PLE and push its monomers toward a random coil conformation (Fig. 6B). This destabilization of the α-helical conformation, if anything, seemed to suppress rather than promote spherulite growth. Furthermore, we did not find that spherulite formation by PLE required a transition of the monomers to a β-sheet conformation. This observation provides additional support for the suggestion in our pH data that the α-helical conformation of PLE actually favors amyloid spherulite formation. Our observations are distinct from poly-l-lysine (PLK) fibril growth at basic pH.31 There, elevated temperatures (65°C) were required to destabilize the α-helix of poly-l-lysine in order to induce amyloid formation.
Figure 6.
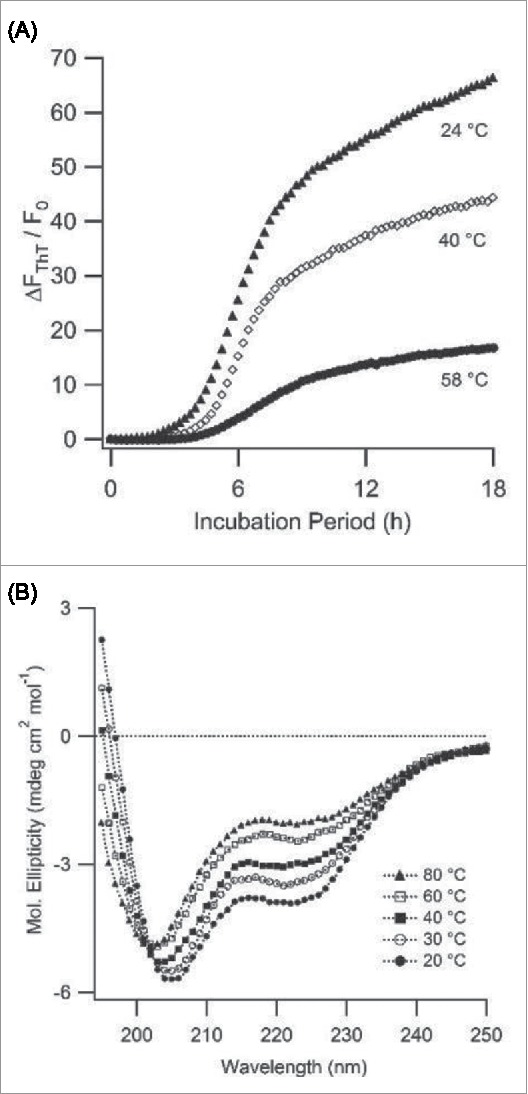
Temperature Effects on Monomer Structure and Spherulite Growth (A) Self-assembly of PLE (0.5 mg/mL, pH 3.6, 50 mM NaCI) into spherulites monitored at three different temperatures using ThT (10 [μM) as indicator dye. (B) CD spectra of PLE (0.2 mg/mL, pH = 3.6) vs. temperature. CD spectra indicate a gradual transition from α-helix to random coil conformation as temperature increases.
Compact globule vs. random coil conformation of PLE as origin of distinct assembly behavior
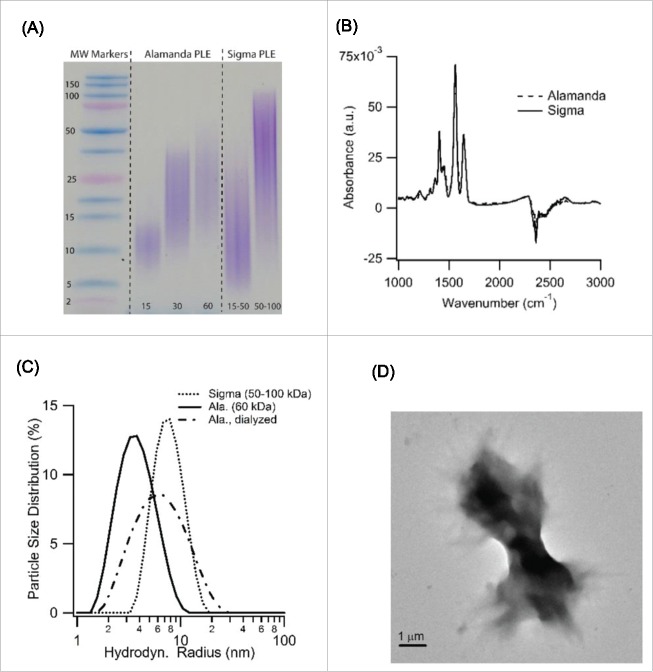
The above data provide details about the structure and growth kinetics of PLE spherulites, as well as their dependence on solution parameters and monomer characteristics. However, they don't indicate why the 2 different stocks of PLE we used produce amyloid aggregates of such distinctly different morphologies. We originally presumed that the observed differences related to the stated differences in molecular weight polydispersity of Alamanda PLE vs. those by other manufacturers. When subjecting the PLE stocks from Alamanda and Sigma to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) we found that the actual differences in polydispersity were modest, at best, and independent of their average molecular weights (Fig. 7A). These modest differences in polydispersity seemed insufficient to explain the dramatic differences in amyloid self-assembly. Similarly, the CD data of PLE monomers in Figure 5A indicate that there were no discernible differences in the secondary structure content between Alamanda PLE monomers and that reported for various other sources of PLE used in earlier studies. FTIR spectra of Alamanda vs. Sigma PLE were similarly indistinguishable from each other (Fig. 7B), suggesting that the 2 stock materials did not differ noticeably in their chemical composition.
Figure 7.
Globule vs. Random Coil Conformation of PLE underlying Transition in Growth Behavior (A) SDS-PAGE analysis of Alamanda vs Sigma PLE stocks of various average molecular weights. Overall polydispersity of Alamanda PLE, while reduced, was not significantly different from Sigma PLE. (B) FTIR spectra of Sigma vs. Alamanda PLE at pH 7 in deuterium oxide reveal no obvious chemical differences between either PLE stock. (C) In situ particle size distribution of Alamanda (60 kDa, solid line) vs. Sigma PLE (50–100 kDa, dotted line) dissolved at pH 7 and 400 mM NaCI. The hydrodynamic radius Rh of Alamanda PLE is more than a factor of two smaller than that of Sigma PLE. Extensive dialysis of Alamanda PLE nearly doubles its Rh (dashed-dotted line) (D) TEM images of Alamanda PLE aggregates obtained from dialyzed PLE monomers incubated at pH 3.6.
The hydrodynamic radii of monomers for these 2 PLE variants under identical solution conditions, however, were dramatically different. While the hydrodynamic radius of 60 kDa Alamanda PLE was slightly below 4 nm, the corresponding radius of 75–150 kDa PLE from Sigma was near 10 nm (Fig. 7C). This 2.fold5- difference in radius is inconsistent with the scaling of the hydrodynamic radius with the square root of the polymers molecular weight, as expected for polymers in a random coil conformation. This is the presumed conformation of PLE monomers for the measurement conditions used for Figure 7C. The large difference in solution radius persisted independent of the pH used to measure monomer radii (e.g. see monomer peak in Fig. 3B). We therefore hypothesize that Alamanda PLE was trapped in a highly compacted globule conformation while Sigma PLE assumed an extended random coil conformation. Such a coil-to-globule transition of PLE, mediated by specific ion absorption onto PLE monomers, has been recently proposed to explain changes in PLE solution viscosity upon addition of alcohol.60 We subjected the hypothesis of an ion-induced monomer collapse as the underlying cause for PLE spherulite growth to 2 tests. First, we attempted to reverse the specific ion adsorption onto PLE using extensive dialysis. This should recover the expanded random-coil conformation of PLE and increase its hydrodynamic radius. As shown in Figure 7C, this was indeed observed. Furthermore, the extended conformation of Alamanda PLE monomers should then “revert” to form disordered amyloid fibril bundles, typically observed with PLE in the more extended solution conformation. As shown by the TEM images in Figure 7D, incubation of dialyzed Alamanda PLE did recover the bundled fibril morphology observed with Sigma PLE under the same growth conditions.
Conclusion
Formation of ordered spherulites is an aggregate morphology commonly observed for both synthetic39,61 and natural polymers.38 Formation of spherulites has also been reported for a variety of proteins during in vitro experiments.18,19 Amyloid spherulites have been detected in vivo for the Alzheimer peptide Aβ27 and in mice expressing mutations of the human prion protein.30 Our experiments indicate that the polyamino acid PLE can also assemble into highly ordered spherulite structures. The intense ThT fluorescence (Fig. 1E), intrinsic fluorescence response, and the peaks in the FTIR spectra of spherulites (Fig. 2B) imply that the underlying organization of these spherulites involves the same type of intermolecular hydrogen bonds as those for PLE amyloid fibrils.48,50 For the most part, spherulite assembly by PLE displays growth kinetics and dependencies on PLE charge and charge screening that are consistent with those for PLE fibril growth performed by other labs.31,35 However, we find that the α-helical conformation of PLE, which has been reported to inhibit fibril growth, appears to promote spherulite formation (Fig. 6B).
The investigation into the cause for the observed spherulite formation by Alamanda PLE suggests that PLE monomers can exist in either a collapsed globular state or an extended random-coil conformation. Such a globule-coil transition is commonly associated with hydrophobic polymers such as poly(N-isopropylacrylamide)62-64 or the counterion induced collapse of deoxy-ribonucleic acids.65 Our data (Fig. 7C) imply that PLE undergoes a collapse similar to DNA upon counterion adsorption. This collapsed state, in turn, promotes the formation of spherulite assemblies instead of disordered fibrils. Our observation is consistent with the generic consensus that the formation of folded polymer states is a prerequisite of ordered spherulite formation in synthetic polymers.58 The existence of the collapsed state and its effects on PLE self-assembly suggest that changes in PLE conformation provide yet another dimension in the parameter space that modifies and controls amyloid assembly.
Materials and Methods
Chemicals
Sodium salts of poly-L-glutamic acid (PLE) were purchased either from Alamanda Polymers (Huntsville, AL) or from Sigma-Aldrich. Nominal molecular weights of PLE polymers were either 7.5, 30 or 60 kDa (Alamanda) or 15–50 and 50–100 kDa (Sigma-Aldrich). Stated polydispersity indices of Alamanda stocks were 1.01, as determined by gel phase chromatography. Ultrapure grade Thioflavin T (ThT) dye was obtained from Anaspec Inc. (Fremont, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA) and were reagent grade or better. Solutions were prepared using 18 MΩ water from a reverse osmosis unit (Barnstead E-pure, Dubuque, IA).
Amyloid growth with poly-L-glutamic acid (PLE) stocks
Solutions of PLE were prepared by dissolving the lyophilized PLE salts in 50 mM citrate buffer with pH values between 3.4 and 6.0. This buffer concentration was sufficient to maintain pH even after addition of PLE. Solution pH was measured using an AR15 pH meter (Fisher Scientific). NaCl concentrations were adjusted by addition of dry ingredient or use of concentrated (2M) stock solutions. Prior to measurements, solutions were routinely filtered through 220 nm PVDF syringe filters (Fisher Scientific, Pittsburgh, PA) and, for light scattering measurements, additionally through 50 nm PES filters (Tisch Scientific, North Bend, OH). For dialysis, 0.5 mL of an 8 mg/mL solution of Alamanda PLE was dialyzed against 15 mL of water with at least 5 exchanges of the water over 3 d.
Optical microscopy
For optical microscopy, spherulites were resuspended in solution by gently inverting cuvettes multiple times. Small aliquots were deposited on a microscope cover slip and placed on the stage of an inverted research microscope (IX70, Olympus USA, Center Valley, PA). Images were acquired using a 40 × 0.75 NA water immersion objective (UFluor2, Olympus) and recorded with either a DSLR camera (EOS Rebel T3, Canon) or an electron-multiplying CCD camera (Ixon DV-885K, Andor Technology Ltd., Belfast UK). ThT fluorescence was excited using a 455 nm light emitting diode (M455L3, Thorlabs, Newton, NJ) and a filter cube with a 445/20 nm bandpass, a 458 nm dichroic, and a 482/35 nm emission filter (Brightline series, Semrock, Rochester, NY). Intrinsic fluorescence associated with amyloid formation was excited using a 385 nm light emitting diode (M385L2, Thorlabs, Newton, NJ) and imaged using a standard DAPI filter cube. For observations of optical activity, a polarizer was placed above the condenser and the T2 IX-AN analyzer was inserted into the optical path below the microscope objective.
Transmission electron microscopy
Typically 1mg/mL of PLE solution was diluted 10-fold in buffer and then deposited onto carbon-coated grids. After drying for 3–5 minutes samples were negatively stained with 0.5% aqueous uranyl acetate solution. Samples were imaged with a FEI Morgagni TEM at 60kV using an Olympus MegaView III camera.
Static and dynamic light scattering (SLS and DLS)
The scattering intensities, mean hydrodynamic radii and size distributions of PLE monomers in solution were assessed using a Zetasizer Nano S (Malvern Instruments, UK). Prior to scattering measurements, solutions were filtered through 50 nm pore size syringe filters and placed into 1 cm path length glass cuvettes. SLS and DLS was used to monitor the growth kinetics of 30 kDa PLE at 2 mg/mL. Each data point for those measurements represents the average of 18 measurements of the autocorrelation function of scattered light with a typical acquisition time of 10 seconds. Measurements were taken at 15–30 minute time intervals. Changes in the mean hydrodynamic radius of the particles in solution were extracted from the autocorrelation functions using standard inversion algorithms.
Thioflavin-T fluorescence measurements
ThT stock solutions at 1 mM were prepared by dissolving the dye in deionoized water and passing it through a 220 nm syringe filter. Actual ThT concentrations were determined from its optical absorbance using α412 nm = 32,000 M−1 cm-1. 66 For measurements of PLE spherulite assembly, 10 µM of ThT was added to solutions and samples were incubated in either a single-cuvette spectrofluorometer (Fluoromax-4, Horiba Scientific, Edison, NJ) or a fluorescence plate reader (Spectramax M5, Molecular Devices, Sunnyvale, CA). Dye fluorescence was excited at 440 nm and emission collected at 485 nm at regular time intervals.
Fourier-transform infrared (FTIR) spectroscopy
FTIR spectra from monomeric or aggregated PLE samples were acquired using a Vertex 70 spectrometer (Bruker Optics, Billerica, MA) with a BioATRCell II attenuated total reflectance accessory. For native PLE samples, a 4 mg/mL solution of 7.5 kDa PLE was prepared in pH 3.6, 50 mM citrate buffer. For measurements from spherulites, 1 mg/mL of 7.5 kDa PLE was incubated at pH 3.6 in 50mM citrate at 37°C. The presence of spherulites was confirmed using microscopy. Samples were centrifuged at 18,000g for 15 hours (accuSpin 1R, Fisher Scientific). The supernatant was removed and the spherulites were resuspended in fresh buffer. For comparison of the FTIR spectra of Alamanda vs. Sigma monomers (Fig. 7B), 23 mg/mL of the lyophilized stocks were directly dissolved in D2O. Typically, FTIR spectra were acquired using a resolution of 4 cm−1 and atmospheric compensation. Buffer spectra were subtracted from all reported spectra.
Circular dichroism (CD) spectroscopy
CD measurements were performed on an AVIV Model 215 (Aviv Biomedical, Lakewood, NJ) or a Jasco J-815 (Jasco Inc.., Easton, MD) CD spectrometer. Spectra of monomeric 7.5 kDa PLE at 1 mg/mL (Aviv) or 0.2 mg/mL (Jasco) were collected at 37°C for multiple pH values. Buffer concentration was reduced to 10 mM citrate to minimize background absorption. Wavelength scans were acquired between 190 and 260 nm in 1 nm increments. Three scans with 10 seconds acquisition times each were averaged. Averaged buffer spectra were subtracted from the sample spectra. Temperature scans were performed between 20°C and 80°C in 10°C increments using 0.2 mg/mL of 7.5 kDa PLE at pH 3.6 in 10 mM citrate buffer alone. Three scans at a rate of 20 nm/minute were averaged for each buffer and sample. In all cases, buffer spectra were subtracted from the corresponding sample spectra.
Gel chromatography
Sodium dodecyl sulfate PAGE (SDS PAGE) was used to determine the polydispersity of PLE stocks from Alamanda Polymers and from Sigma Aldrich for multiple average molecular weights. We used 10–20% gradient Tris-tricine gels (Criterion, Bio-Rad) and a sodium dodecyl sulfate (SDS) running buffer without glycine. An aliquot of 3.6 μg/μL of PLE solution was mixed 1:2 with Laemmli sample buffer (Bio-Rad) with reducing agent (β-mercapto-ethanol). Thirty μL of the 1.2 μg/μL sample solutions was loaded onto precast gels and run at constant voltage (110 V). The gel was rinsed with DI water, then fixed with a 10% acetic acid, 40% methanol solution for 30 minutes, and subsequently stained with 1.5M methylene blue solution and destained with DI water.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful for access to the CD spectrophotometer provided by Dr. Uversky at USF Health and for use of the TEM in the Department of Cell, Micro- and Molecular Biology at USF.
Funding
This work was supported, in part, by NIH Grant GM097723 (M.M.).
References
- 1.Koo EHJ, Lansbury PT, Kelly JW. Amyloid diseases: abnormal protein aggregation in neurodegeneration Proc Natl Acad Sci USA 1999; 96:9989-90; PMID:10468546; http://dx.doi.org/ 10.1073/pnas.96.18.9989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease Annu Rev Biochem 2006; 75:333-66; PMID:16756495; http://dx.doi.org/ 10.1146/annurev.biochem.75.101304.123901 [DOI] [PubMed] [Google Scholar]
- 3.Uversky VN, Fink AL. Conformational constraints for amyloid fibrillation: the importance of being unfolded, Biochim Biophys Acta 2004; 1698:131-53; PMID:15134647; http://dx.doi.org/ 10.1016/j.bbapap.2003.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid – from bacteria to humans. Trends Biochem Sci 2007; 32:217-24; PMID:17412596; http://dx.doi.org/ 10.1016/j.tibs.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 5.Otzen D, Functional amyloid: turning swords into plowshares Prion 2010; 4:256-64; PMID:20935497; http://dx.doi.org/ 10.4161/pri.4.4.13676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KPR, Simon R, Schubert D, et al.. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 2009; 325:328-32; PMID:19541956; http://dx.doi.org/ 10.1126/science.1173155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazit E. Mechanisms of amyloid fibril self-assembly and inhibition. FEBS J 2005; 272:5971-8; PMID:16302962; http://dx.doi.org/ 10.1111/j.1742-4658.2005.05022.x [DOI] [PubMed] [Google Scholar]
- 8.Smith JF, Knowles TPJ, Dobson CM, MacPhee CE, Welland ME, Characterization of the nanoscale properties of individual amyloid fibrils. Proc Natl Acad Sci U S A 2006; 103:15806-11; PMID:17038504; http://dx.doi.org/ 10.1073/pnas.0604035103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick AWP, Park ST, Zewail AH, Exceptional rigidity and biomechanics of amyloid revealed by 4D electron microscopy. Proc Natl Acad Sci U S A 2013; 110:10976-81; PMID:23784773; http://dx.doi.org/ 10.1073/pnas.1309690110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrigan AM, Müller C, Krebs MRH. The formation of nematic liquid crystal phases by hen lysozyme amyloid fibrils. J Am Chem Soc 2006; 128:14740-1; PMID:17105248; http://dx.doi.org/ 10.1021/ja064455l [DOI] [PubMed] [Google Scholar]
- 11.Cherny I, Gazit E. Amyloids: not only pathological agents but also ordered nanomaterials. Angew Chem Int Ed 2008; 47:4062-9; PMID:18412209; http://dx.doi.org/ 10.1002/anie.200703133 [DOI] [PubMed] [Google Scholar]
- 12.Scheibel T, Parthasarathy R, Sawicki G, Lin X-M, Jaeger H, Lindquist SL. Conducting nanowires built by controlled self-assembly of amyloid fibers and selective metal deposition. Proc Natl Acad Sci U S A 2003; 100:4527-32; PMID:12672964; http://dx.doi.org/ 10.1073/pnas.0431081100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrau S, Zhang F, Herland A, Mammo W, Andersson MR, Inganas O. Integration of amyloid nanowires in organic solar cells. Appl Phys Lett 2008; 93:023307; http://dx.doi.org/ 10.1063/1.2949073 [DOI] [Google Scholar]
- 14.Malisauskas M, Meskys R, Morozova-Roche LA, Ultrathin silver nanowires produced by amyloid biotemplating. Biotechnol Prog 2008; 24:1166-70; PMID:19194928; http://dx.doi.org/ 10.1002/btpr.49 [DOI] [PubMed] [Google Scholar]
- 15.Lu K, Jacob J, Thiyagarajan P, Conticello VP, Lynn DG. Exploiting amyloid fibril lamination for nanotube self-assembly, J Am Chem Soc 2003; 125:6391-3; PMID:12785778; http://dx.doi.org/ 10.1021/ja0341642 [DOI] [PubMed] [Google Scholar]
- 16.Adler-Abramovich L, Aronov D, Beker P, Yevnin M, Stempler S, Buzhansky L, Rosenman G, Gazit E. Self-assembled arrays of peptide nanotubes by vapour deposition. Nat Nano 2009; 4:849-54; PMID:19893524; http://dx.doi.org/ 10.1038/nnano.2009.298 [DOI] [PubMed] [Google Scholar]
- 17.Lashuel HA, LaBrenz SR, Woo L, Serpell LC, Kelly JW. Protofilaments, filaments ribbons, and fibrils from peptidomimetic self-assembly: implications for amyloid fibril formation and materials science. J Am Chem Soc 2000; 122:5262-77; PMID:22339465; http://dx.doi.org/ 10.1021/ja9937831 [DOI] [PubMed] [Google Scholar]
- 18.Krebs MRH, MacPhee CE, Miller AF, Dunlop IE, Dobson CM, Donald AM. The formation of spherulites by amyloid fibrils of bovine insulin. Proc Natl Acad Sci USA 2004; 101:14420-4; PMID:15381766; http://dx.doi.org/ 10.1073/pnas.0405933101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bromley EHC, Krebs MRH, Donald AM, Aggregation across the length-scales in b-lactoglobulin. Faraday Discuss 2005; 128:13-27; PMID:15658764; http://dx.doi.org/ 10.1039/b403014a [DOI] [PubMed] [Google Scholar]
- 20.Kodali R, Wetzel R. Polymorphism in the intermediates and products of amyloid assembly. Curr Opin Struct Biol 2007; 17:48-57; PMID:17251001; http://dx.doi.org/ 10.1016/j.sbi.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 21.Dahlgren KN, Manelli AM, Stine WB, Baker J, Lorinda K, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-b peptides differentially affect neuronal viability, J Biol Chem 2002; 277:36046-53; PMID:12058030; http://dx.doi.org/ 10.1074/jbc.M201750200 [DOI] [PubMed] [Google Scholar]
- 22.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenisis, Science 2003; 300:486-9; PMID:12702875; http://dx.doi.org/ 10.1126/science.1079469 [DOI] [PubMed] [Google Scholar]
- 23.Uversky VN. Mysterious oligomerization of the amyloidogenic proteins. FEBS J 2010; 277:2940-53; PMID:20546306; http://dx.doi.org/ 10.1111/j.1742-4658.2010.07721.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fändrich M, Meinhardt J, Grigorieff N. Structural polymorphism of Alzheimer Ab and other amyloid fibrils. Prion 2009; 3:89-93; PMID:19597329; http://dx.doi.org/ 10.4161/pri.3.2.8859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fodera V, van de Weert M, Vestergaard B. Large-scale polymorphism and auto-catalytic effect in insulin fibrillogenesis. Soft Matter 2010; 6:4413-9; http://dx.doi.org/ 10.1039/c0sm00169d [DOI] [Google Scholar]
- 26.Adamcik J, Mezzenga R. Study of amyloid fibrils via atomic force microscopy. Curr Opin Colloid Interface Sci 2012; 17:369-76; http://dx.doi.org/ 10.1016/j.cocis.2012.08.001 [DOI] [Google Scholar]
- 27.Exley C, House E, Collingwood JF, Davidson MR, Cannon D, Donald AM. Spherulites of amyloid-b42 in vitro and in alzheimer's disease. J Alzheimers Dis 2010; 20:1159-65; PMID:20413877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers SS, Krebs MRH, Bromley EHC, Linden EVD, Donald AM. Optical Microscopy of Growing Insulin Amyloid Spherulites on Surfaces in Vitro. Biophys J 2006; 90:1043-54; PMID:16272436; http://dx.doi.org/ 10.1529/biophysj.105.072660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ban T, Morigaki K, Yagi H, Kawasaki T, Kobayashi A, Yuba S, Naiki H, Goto Y. Real-time and single fibril observation of the formation of amyloid β spherulitic structures. J Biol Chem 2006; 281:33677-83; PMID:16959773; http://dx.doi.org/ 10.1074/jbc.M606072200 [DOI] [PubMed] [Google Scholar]
- 30.Sigurdson CJ, Joshi-Barr S, Bett C, Winson O, Manco G, Schwarz P, Ruelicke T, Nilsson KPR, Margalith I, Raeber A, et al. Spongiform encephalopathy in transgenic mice expressing a point mutation in the b2-a2 loop of the prion protein. J Neurosci 2011; 31:13840-7; PMID:21957246; http://dx.doi.org/ 10.1523/JNEUROSCI.3504-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fändrich M, Dobson CM. The behaviour of polyamino acids reveals an inverse side chain effect in amyloid structure formation. EMBO J 2002; 21:5682-90; PMID:12411486; http://dx.doi.org/ 10.1093/emboj/cdf573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill SE, Miti T, Richmond T, Muschol M. Spatial extent of charge repulsion regulates assembly pathways for lysozyme amyloid fibrils. PLoS ONE 2011; 6:e18171.1-12; PMID:21483680; http://dx.doi.org/ 10.1371/journal.pone.0018171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeVine H. Thioflavine T interaction with amyloid b-sheet structures. Amyloid 1995; 2:1-6; http://dx.doi.org/ 10.3109/13506129509031881 [DOI] [Google Scholar]
- 34.Ban T, Hamada D, Hasegawa K, Naiki H, Goto Y. Direct observation of amyloid fibril growth monitored by thioflavin T fluorescence. J Biol Chem 2003; 278:16462-5; PMID:12646572; http://dx.doi.org/ 10.1074/jbc.C300049200 [DOI] [PubMed] [Google Scholar]
- 35.Colacu M, Park J, Blanch H. The kinetics of aggregation of poly-glutamic acid based polypeptides. Biophys Chem 2008; 136:74-86; PMID:18538463; http://dx.doi.org/ 10.1016/j.bpc.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulara A, Lakhani A, Wojcik S, Nieznanska H, Keiderling TA, Dzwolak W. Spiral superstructures of amyloid-like fibrils of polyglutamic acid: an Infrared absorption and vibrational circular dichroism study. J Phys Chem B 2011; 115:11010-6; PMID:21842891; http://dx.doi.org/ 10.1021/jp206271e [DOI] [PubMed] [Google Scholar]
- 37.Krebs MRH, Bromley EHC, Rogers SS, Donald AM. The mechanism of amyloid spherulite formation by bovine insulin. Biophys J 88:2013-21; PMID:15596515; http://dx.doi.org/ 10.1529/biophysj.104.051896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamley IW. Liquid crystal phase formation by biopolymers. Soft Matter 2010; 6:1863-71; http://dx.doi.org/ 10.1039/b923942a [DOI] [Google Scholar]
- 39.Norton DR, Keller A. The spherulitic and lamellar morphology of melt-crystallized isotactic polyPropylene. Polymer 1985; 26:704-16; http://dx.doi.org/ 10.1016/0032-3861(85)90108-9 [DOI] [Google Scholar]
- 40.Ring SG, Miles MJ, Morris VJ, Turner R, Colonna P. Spherulitic crystallization of short chain amylose. Int J Biol Macromol 1987; 9:158-60; http://dx.doi.org/ 10.1016/0141-8130(87)90044-4 [DOI] [Google Scholar]
- 41.Yagi N, Ohta N, Iida T, Inoue K. A microbeam X-ray diffraction study of insulin spherulites. J Mol Biol 2006; 362:327-33; PMID:16919294; http://dx.doi.org/ 10.1016/j.jmb.2006.07.041 [DOI] [PubMed] [Google Scholar]
- 42.Smith MI, Sharp JS, Roberts CJ. Giant amyloid spherulites reveal their true colours. Soft Matter 2012; 8:3751-5; http://dx.doi.org/ 10.1039/c2sm25147g [DOI] [Google Scholar]
- 43.Keller A. The spherulitic structure of crystalline polymers. Part I Invest Polarizing Microscope 1955; 17:291-308 [Google Scholar]
- 44.Heijna MCR, Theelen MJ, van Enckevort WJP, Vlieg E. Spherulitic growth of hen egg-white lysozyme grystals. J Physical Chem B 2007; 111:1567-73; http://dx.doi.org/ 10.1021/jp0643294 [DOI] [PubMed] [Google Scholar]
- 45.Morimoto K, Kawabata K, Kunii S, Hamano K, Saito T, Tonomura BI. Characterization of Type I collagen fibril formation using thioflavin T fluorescent dye. J Biochem 2009; 145:677-84; PMID:19204013; http://dx.doi.org/21456595 10.1093/jb/mvp025 [DOI] [PubMed] [Google Scholar]
- 46.Sharpe S, Simonetti K, Yau J, Walsh P. Solid-state NMR characterization of autofluorescent fibrils formed by the elastin-derived peptide GVGVAGVG. Biomacromolecules 2011; 12:1546-55; PMID:21456595; http://dx.doi.org/ 10.1021/bm101486s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan FTS, Pinotsi D, Kaminski Schierle GS, Kaminski CF. Structure-pecific intrinsic fluorescence of protein amyloids used to study their kinetics of aggregation in bio-nanoimaging Protein Misfolding and Aggregation, Vladimir NU, and Lyubchenko YL Editors. Academic Press: Waltham MA: 2013; 147-55 [Google Scholar]
- 48.Fulara A, Dzwolak W. Bifurcated hydrogen bonds stabilize fibrils of poly(l-glutamic) acid. J Phys Chem B 2010; 114:8278-83; PMID:20509699; http://dx.doi.org/ 10.1021/jp102440n [DOI] [PubMed] [Google Scholar]
- 49.Zandomeneghi G, Krebs MRH, McCammon MG, Fändrich M. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci 2004; 13:3314-21; PMID:15537750; http://dx.doi.org/ 10.1110/ps.041024904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fulara A, Lakhani A, Wójcik S, Nieznańska H, Keiderling TA, Dzwolak W. Spiral superstructures of amyloid-like fibrils of polyglutamic acid: an infrared absorption and vibrational circular dichroism study. J Phys Chem B 2011; 115:11010-6; PMID:21842891; http://dx.doi.org/ 10.1021/jp206271e [DOI] [PubMed] [Google Scholar]
- 51.Sigurdson CJ, Manco G, Schwarz P, Liberski P, Hoover EA, Hornemann S, Polymenidou M, Miller MW, Glatzel M, Aguzzi A. Strain fidelity of chronic wasting disease upon murine adaptation. J Virol 2006; 80:12303-11; PMID:17020952; http://dx.doi.org/ 10.1128/JVI.01120-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cummins HZ, Pike R. Photon correlation and light beating spectroscopy in Nato Advanced Studies Institute. 1973; New York: Plenum Press [Google Scholar]
- 53.Berne BJ, Pecora R. Dynamic Light Scattering: with Applications to Chemistry, Biology and Physics. 1976; New York: Wiley [Google Scholar]
- 54.Foley J, Hill SE, Miti T, Mulaj M, Ciesla M, Robeel R, Persichilli C, Raynes R, Westerheide S, Muschol M. Structural fingerprints and their evolution during oligomeric vs. oligomer-free amyloid fibril growth J Chem Phys 2013; 139:121901/1-12; PMID:24089713; http://dx.doi.org/ 10.1063/1.4811343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miti T, Mulaj M, Schmit JD, Muschol M. Stable metastable and kinetically trapped amyloid aggregate phases. Biomacromolecules 2015; 16:326-35; PMID:25469942; http://dx.doi.org/ 10.1021/bm501521r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cahn JW. The time cone method for nucleation and growth kinetics on a finite domain. Mater Res Soc Symp P 1996; 398:425-38http://dx.doi.org/ 10.1557/PROC-398-425 [DOI] [Google Scholar]
- 57.Cahn JW. Johnson-mehl-avrami kinetics on a finite growing domain with time and position dependent nucleation and growth rates. Trans Indian Inst Met 1997; 50:573-80 [Google Scholar]
- 58.Gránásy L, Pusztai T, Tegze G, Warren JA, Douglas JF. Growth and form of spherulites. Phys Rev E 2005; 72:011605; PMID:16089977; http://dx.doi.org/ 10.1103/PhysRevE.72.011605 [DOI] [PubMed] [Google Scholar]
- 59.Nilsson S, Zhang W, Helix-coil transition of a titrating polyelectrolyte analyzed within the Poisson-Boltzmann cell model: effects of pH and salt concentration. Macromolecules 1990; 23:5234-9; http://dx.doi.org/ 10.1021/ma00227a010 [DOI] [Google Scholar]
- 60.Hasuike M, Kuroki S, Satoh M. Double conformational transition of alkali metal poly(l-glutamate)s in aqueous ethanol: counterion mixing effect revisited. Biophys Chem 2012; 165–166:48-55; PMID:22464848; http://dx.doi.org/ 10.1016/j.bpc.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 61.Toda A, Keller A. Growth of polyethylene single crystals from the melt: morphology. Colloid Polym Sci 1993; 271:328-42; http://dx.doi.org/ 10.1007/BF00657415 [DOI] [Google Scholar]
- 62.Hirotsu S, Hirokawa Y, Tanaka T. Volume-phase transitions of ionized N-isopropylacrylamide gels J Chem Phys 1987; 87:1392-5; http://dx.doi.org/ 10.1063/1.453267 [DOI] [Google Scholar]
- 63.Schild HG. Poly (N-Isopropylacrylamide): experiments theory and application. Prog Polym Sci 1992; 17:163-249; http://dx.doi.org/ 10.1016/0079-6700(92)90023-R [DOI] [Google Scholar]
- 64.Parmar AS, Hill S, Vidyasagar A, Bello C, Toomey R, Muschol M. Frequency and temperature dependence of poly(N-isopropylacrylamide) gel rheology. J Appl Polym Sci 2012; 127:1527-37 [Google Scholar]
- 65.Wilson RW, Bloomfield VA. Counterion-induced condensation of deoxyribonucleic acid. Light-Scattering Study Biochem 1979; 18:2192-6; PMID:444448 [DOI] [PubMed] [Google Scholar]
- 66.Kuznetsova IM, Sulatskaya AI, Uversky VN, Turoverov KK. Analyzing thioflavin T binding to amyloid fibrils by an equilibrium microdialysis-based technique. PLoS ONE 2012; 7:e30724; PMID:22383971; http://dx.doi.org/ 10.1371/journal.pone.0030724 [DOI] [PMC free article] [PubMed] [Google Scholar]