Abstract
Introduction
Many infectious diseases are sensitive to climatic changes; specifically, flooding. This systematic literature review aimed to strengthen the quality and completeness of evidence on infectious diseases following flooding, relevant to Europe.
Methods
A systematic literature review from 2004–2012 was performed. Focused searches of the following databases were conducted: Medline, Scopus, PubMed, Cochrane Library, and Evidence Aid. Personal communications with key informants were also reviewed.
Results
Thirty-eight studies met the inclusion criteria. Evidence suggested that water-borne, rodent-borne, and vector-borne diseases have been associated with flooding in Europe, although at a lower incidence than developing countries.
Conclusion
Disease surveillance and early warning systems, coupled with effective prevention and response capabilities, can reduce current and future vulnerability to infectious diseases following flooding.
Keywords: flooding, infectious diseases, vector-borne, rodent-borne, water-borne, climate change, natural disaster
Background
Flooding has been of significant concern since the beginning of human civilization and has led to extensive morbidity and mortality. Floods are the most common natural disaster worldwide and specifically in Europe; hence, a crucial area of research.1 Between 1997 and 2006, the number of global flood events doubled.2 European vulnerability to flooding has been highlighted by recent flooding events; most notably, the Central European floods in 2002 and in 2010, the 2010 flooding in Southern France, and the 2007 flooding in several areas in the United Kingdom.
Defining what constitutes a flood can be quite complex as floods can take many forms; therefore, no universal definition exists. Generally and in the context of this review, a flood is defined as the overflow of areas that are not normally submerged with water or a stream that has broken its normal confines or has accumulated due to lack of drainage.3 Overall, different flood characteristics affect the severity of the flood event; specifically, regularity, speed of onset, velocity of flow, and depth of water. Quantifying the level of flooding has proven to be difficult; however, the Emergency Events Database (EM-DAT) provides information about flood events and the impact of floods. For a flood to be classified as a disaster or flood event by EM-DAT one of the criteria must be fulfilled: either ten or more people killed; 100 or more people affected; declaration of a state emergency; and/or call for international assistance. EM-DAT defines a flood as a significant rise in water level in a stream, lake, reservoir, or coastal region and includes general river floods, flash floods, and storm surges or coastal flooding.
Flood disasters hit some European regions very frequently, and in some circumstances every year. In Europe from 2003–2012, 19 flash floods and 162 general floods were reported by EM-DAT. In terms of the number of people affected, 7 out of the 20 most important floods ever recorded in Europe occurred during the 2000–2010 decade.4 A study concluded a rising number in flood disasters from 1950–2005 in the European Union (EU).5 According to Frei et al.6 there has been a significant trend toward increased intense winter rainfall events in Europe. Other studies do not find a rising incidence of flooding. For example Mudelsee et al.7 examined river flood patterns in Central Europe, and despite the occurrence of two flood events exceeding the 100-y flood level in 1997 and 2002, found no increased trend in extreme flood frequency over recent decades. Analyzing the more frequent, small-magnitude flood events as well as high-magnitude floods can make it easier to detect shifting trends in flood frequency.6 Flood trend analysis is essential to understand future flood risk and vulnerability.
Both climatic and non-climatic impacts, such as land-use dynamics, are expected to influence future flooding in Europe. Although considerable limitations remain in the ability to make robust projections of changes in flood size and frequency due to climate change, common projections appear to be emerging. According to the latest Intergovernmental Panel on Climate Change’s (IPCC) SREX Report8 there is a 66–100% probability that the intensity of heavy precipitation and the proportion of total rainfall will increase particularly in northern mid-latitudes and high latitudes of Europe. The highest total precipitation increases are projected to occur during the winter months. Although the IPCC states a general decrease in mean precipitation in the southern European region, rainfall may become more irregular and intense. However there remains low confidence in projections of changes in riverine floods. Climate change is likely to increase the frequency of storm surges and coastal flooding due to rise in sea levels, and threaten an additional 1.6 million people per year in Europe by the 2080s.9 Overall, changes in the climate that may affect the transmission of infectious diseases include temperature, humidity, altered rainfall, and sea-level rise.
Flooding can have a range of health impacts but this review focused solely on infectious diseases. The diseases most likely to be affected by flooding are those that require a vehicle for transfer from host to host (water-borne) or a host/vector as part of its life cycle (vector-borne).10 Flood-affected areas serve as ideal breeding grounds for pathogens and may alter vector breeding grounds and zoonotic reservoirs.11,12 Where infectious disease transmission is endemic, it can present a major public health concern following flooding.13
The risk of infectious diseases following flooding is exacerbated by the fact many factors work together to increase incidence.14 The significance of the association between precipitation and disease is potentially amplified when considering the effects of global climate change and land use changes. Flooding can alter the equilibrium of the environment and may affect the incidence and geographic range of climate-sensitive infectious diseases. A better understanding of the associations and underlying mechanisms of infectious disease outbreaks following flooding will help support evidence-based flood policies and mitigation strategies.
This systematic literature review aimed to identify and examine the relationship between infectious disease incidence and flooding in order to gain a better understanding of:
What evidence-based public health interventions are used to minimize infectious disease incidence following flooding.
Knowledge gaps and issues for further research.
Literature Review Methodology
The search strategy used was adapted from two studies (Table 1).15,16 All papers with the specified search terms in their titles, abstracts, or keywords were searched for.
Table 1. Search strategy.
| EXPOSURE (COMBINED WITH OR) dam, embankment*, flood*, hurricane*, inundation, monsoon*, overflow*, seawater intrusion, storm surge*, storm water*, tropical storm*, typhoon*, waterlogging |
| (AND) |
| OUTCOME (COMBINED WITH OR) amoebiasis, bacillary dysentery, burul*, campylo*, chikungunya, cholera, communicable disease*, contamination, crypto*, dengue, dengue virus, dermatitis, diarrhea*, diarrhea*, disease*, disease vector*, disease outbreak*, epidemic*, enteric fever, Escherichia coli, gastrointestinal, giardia*, hanta virus infections, health, health effect*, health impact*, hemorrhagic fever, hepatitis A, hepatitis E, illness, infectio*, infectious disease*, Japanese encephalitis, legionellosis, leptospirosis, lyme disease, lymphatic filariasis, malaria, morbidity, mosquito*, norovirus, naeg*, outbreak*, onchocerciasis, physical health, plague, pollut*, public health, q fever, risk factor*, rodent*, rodentborne, rodent-borne, rodent related, rodent-related, salmonellosis, sars virus, severe acute respiratory syndrome, shigellosis, schistosomiasis, tick*, tick-borne encephalitis, tularaemia, tularemia, typhoid, water, waterborne, water-borne, water related, water-related, west nile fever, vector*, vectorborne, vector-borne, vector related, vector-related, yellow fever, yersini* |
Focused searches of the following databases were conducted: Medline, Scopus, and PubMed. The Cochrane Database of Systematic Reviews was searched for further existing epidemiological reviews, as well as Evidence Aid. Further relevant articles were identified manually from cited references from each selected full-text paper. Data from gray literature were not systematically searched, but sources and advice from key experts were discussed in the accompanying text.
Inclusion criteria
Papers published from 1 January 2004 to 30 September 2012. Ahern et al.15 included studies associated with infectious disease incidence following flooding up to 2004.
Epidemiological studies.
Studies conducted in any country, because Europe experiences a wide range of climate and geographical variation.
Papers in all languages with English abstracts.
All papers where an explicit link is studied between flooding as an exposure and an infectious disease as an outcome.
Exclusion criteria
Papers concerned primarily with mental health effects, flood-related injuries, population displacement, economic costs, and disruption of food supplies.
Papers on unrelated subject areas; such as, biochemistry, molecular biology, and genetics.
Personal communications between key informants were conducted in conjunction with the literature review. The context of the questions included the current state of knowledge of the association between flooding and infectious diseases and potential solutions to mitigate the risks.
Risk of bias and quality assessment
Because flooding is a natural disaster and cannot be induced experimentally, the research evidence was unlikely to be the considered ‘gold standard’ of a systematic literature review or a randomized controlled trial. Most of the data was observational, and because of insufficient numbers of similar studies, and variation in outcome reporting no studies were excluded on the basis of study quality. A formal assessment of bias was not possible for each individual study.
Results
The initial search generated 7,861 relevant articles. After reviewing the abstracts, 106 full-text articles were examined in more detail for eligibility. Of these 106 articles, 38 peer-reviewed articles were found to fit the inclusion criteria. Increased infectious disease transmission and outbreaks following global flood events have been documented (Table 2). The study design and main results of all papers found meeting the inclusion criteria are listed in detail in Appendices A-D. Some articles and gray literature not meeting the specific inclusion criteria were incorporated into the conceptual framework to give a better contextual outline.
Table 2. Summary of studies assessing infectious disease transmission following flood events.
| COUNTRY | YEAR(S) STUDIED | INFECTIOUS DISEASE(S) | REF. |
|---|---|---|---|
| Australia | 1998–2001, 2011 | Leptospirosis, Ross River virus | (46,57) |
| Austria | 2010 | Leptospirosis | (43) |
| Bangladesh | 1983–2007 | Cholera, rotavirus, acute respiratory infection | (23–24,72–73,75–76) |
| Canada | 1975–2001 | Diarrhea | (22,26) |
| China | 1979–2000 | Schistosomiasis | (58) |
| Czech Republic | 1997,2002 | Leptospirosis, Tahyna virus | (41,54) |
| England | 2000 | Diarrhea | (28) |
| France | 2009 | Leptospirosis | (38) |
| Germany | 2005,2007 | Norovirus, leptospirosis | (29,34) |
| Guyana | 2005 | Leptospirosis | (44) |
| Italy | 1993–2010 | Hepatitis A, salmonellosis, diarrhea, leptospirosis, leishmaniasis, legionellosis | (18,36) |
| India | 2001–2006 | Leptospirosis | (33,45) |
| Indonesia | 2001–2003 | Paratyphoid fever | (27) |
| Mexico | 2007,2010 | Leptospirosis, dengue fever | (37,55) |
| Pakistan | 2010 | Diarrhea, skin and soft tissue infection, conjunctivitis, respiratory tract infection, suspected malaria | (69) |
| The Philippines | 2009 | Leptospirosis | (47) |
| Sudan | 2007 | Rift Valley fever | (56) |
| Taiwan | 1994–2009 | Leptospirosis, melioidosis, enteroviruses, dengue fever, bacillary dysentery, Japanese encephalitis | (21,40,48,74) |
| Thailand | 2012 | Melioidosis | (70) |
| United States | 2001,2004 | Diarrhea, leptospirosis | (25,32,35) |
| Vietnam | 2008 | Conjunctivitis, dermatitis | (71) |
Water-borne
Water-borne outbreaks are an acute aftermath of flood disasters, mainly as a result of contaminated drinking water supply. Intense precipitation can mobilize pathogens in the environment and transport them into the aquatic environment, increasing the microbiological agents on surface water.17-20 Chen et al.21 found extreme torrential rain (> 350mm) was a significant risk factor for enteroviruses (RR = 1.96; 95% CI 1.474–23.760) and bacillary dysentery (RR = 7.703; 95% CI 5.008–11.849). Globally, water-borne epidemics have shown an increasing trend from 1980–2006 which coincides with the increasing number of flood events.2 According to a global systematic literature review performed by Cann et al.17 the most common water-borne pathogens to be identified following flooding were vibrio spp The most common water-borne pathogens associated with heavy rainfall were campylobacter, followed by vibrio spp
Appendices A, B list published studies which have reported post-flood increases in cholera, cryptosporidiosis, non-specific diarrhea, rotavirus, and typhoid and paratyphoid.22-31 Several studies have implicated excess rainfall in water-borne disease outbreaks because of the transportation of bacteria, parasites, and viruses into water systems.22 Marcheggiani et al.18 showed a potential association between flood events and a range of water-borne infectious diseases in Italy; including, legionellosis, salmonellosis, hepatitis A, and infectious diarrhea. Reacher et al.28 performed a historical cohort study following a severe flood in 2000 in Lewes, England. The risk of gastroenteritis was significantly associated with depth of flooding in people whose households were flooded (RR = 1.7; 95% CI 0.9–3.0; p for trend by flood depth = 0.04). Additionally, an outbreak of norovirus in American tourists was linked to direct exposure to floodwater contaminated with raw sewage in Germany.29
Earlier research has shown an association between water-borne diseases and flooding in high-income countries. From 1948–1994, more than half of the water-borne disease outbreaks in the United States were preceded by heavy rainfall (p = 0.002).30 Research from Finland found that 13 water-borne disease outbreaks from 1998–1999 were associated with un-disinfected groundwater contaminated by floodwaters and surface runoff.32 Surveys in high-income countries where individuals reported their own symptoms have indicated an increase in water-borne diseases following flooding.28,30-32
Rodent-borne
Rodent-borne diseases are climate sensitive and may increase during heavy rainfall and flooding because of altered patterns of human- pathogen- rodent contact.15 Flooding and heavy rainfall have been associated with numerous outbreaks of leptospirosis from a wide-range of countries around the world.15,21,33-48 Areas at the highest risk for leptospirosis outbreaks are those where multiple risk factors are likely to coexist; such as, increased flooding risk, rising temperatures, overcrowding, poor sanitation, poor health care, poverty, and an abundance of rats and other animal reservoirs.39 Rodent-borne pathogens can be indirectly affected by ecological determinants of food sources which have an effect on the size of rodent populations. For example, lack of garbage management and collection following flooding where rubbish is left on the streets contributes to an increased rodent population.38 Appendices A, C summarize the key studies assessing the relationship between flooding and rodent-borne diseases.
Outbreaks of leptospirosis were observed in the Czech Republic following floods in 1997 and 2002.41,42 The rates of serologically confirmed cases of leptospirosis were three times higher than usual at 0.9 cases/100,000 inhabitants (average incidence rate is 0.3 cases/100,000 inhabitants). The first leptospirosis outbreak in Austria in July 2010, involved four athletes who swam in recreational waters during a triathlon.43 Heavy rains had preceded the triathlon (22mm). This outbreak demonstrates a risk of contracting leptospirosis in recreational waters, especially after heavy rainfall.
In Marseilles, France the incidence of leptospirosis identified in the laboratory increased significantly between January 2001 and July 2011 (p < 0.0001).38 Between 1991 and 2003, the rate of leptospirosis incidence in southern France was very low, 0.09 cases/100,000 inhabitants. In 2008, this incidence increased to 0.25 cases/100,000 inhabitants. The first three autochthonous cases identified in Marseilles (October 2009) were preceded by heavy rainfall. The study showed the first autochthonous case was identified after a period of flooding preceded by heavy rainfall over several days (34.6mm/day; 79.2mm/day; 137mm/day with an episode of 63mm/3hr). Similarly, the other two autochthonous cases occurred during a period of high rainfall (13.6–23.8mm).
Pellizzer et al.36 performed a sero-epidemiological study to evaluate the risk of leptospirosis in a population in Northeast Italy exposed to a severe flood event. This area is endemic for leptospirosis and exhibits and average of 4 cases/100,000 inhabitants. Seven out of 44 subjects exposed to floodwaters exhibited anti-Leptospira specific IgM antibodies and five were confirmed positive by micro-agglutination test. Re-testing a few months later found significant antibody titers greater than 100 against serovar Copenhangeni in three cases (6.8% seroconversion rate). Overall, the rate for seroconversion for leptospirosis appeared to be low, and while flooding appeared to be the sole risk factor, confirmation was not possible due to a lack of a control group.
Ahern et al.15 reviewed earlier studies addressing flood-associated outbreaks of leptospirosis from a wide-range of countries: Argentina, Brazil, Cuba, India, Korea, Mexico, Nicaragua, Portugal, and Puerto Rico. In 1997 in the Krasnodar Territory in Russia, a severe outbreak of leptospirosis took place in connection with a high flood.49 Sanders et al.50 stated that flooding after heavy rain favors leptospires. It prevents animal urine from being absorbed into the soil or evaporating; therefore leptospires may pass directly into the surface water or persist in mud. The evidence of this review, supported by several other reviews, suggests the association between leptospirosis and flooding is fairly robust even in high-income countries.
Vector-borne
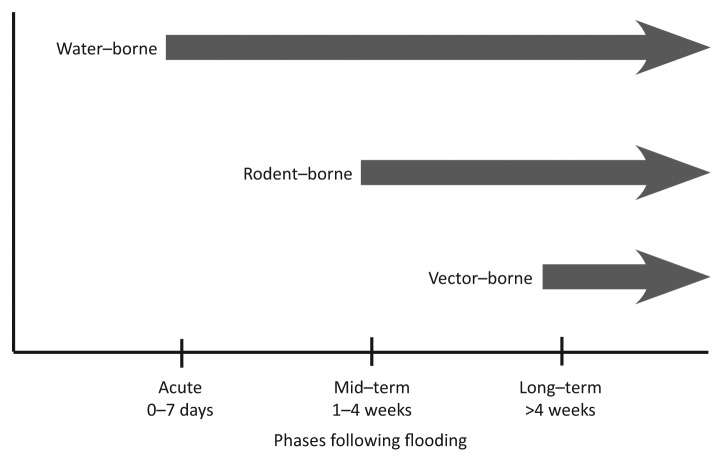
Precipitation changes are known to effect the reproduction, development, behavior, and population dynamics of arthropod vectors, their pathogens, and non-human vertebrate reservoirs.10 Mosquito-borne infections tend to increase with warming and certain changes in rainfall patterns. Vector-borne diseases are unlikely to be a problem during the onset phase of the flood, as many vector breeding habitats are expected to be overwhelmed by the flood waters.51 While flooding may initially wash out vector populations, they return when the waters recede. Receding flood water can provide ideal breeding habitats. Therefore, vector-borne diseases are likely to have mid-term to long-term impacts on health following flooding (Fig. 1). Vector-borne virus outbreaks are strictly determined by the presence of the pathogen and particular competent disease vectors.52 The current and future establishment of exotic mosquito species in Europe is a cause for serious concern, as the newly introduced species may already be disease vectors or could potentially become vectors.
Figure 1. The occurrence of infectious disease outbreaks following flooding in relation to time.
West Nile virus (WNV) emerged in Europe after heavy rains and flooding, with outbreaks in Romania in 1996–1997, the Czech Republic in 1997, and Italy in 1998.53 The 2002 flood in the Czech Republic resulted in mass mosquito breeding with a biting frequency peaking at 70 bites per person per minute.54 Specimens from 497 flood-affected residents were examined serologically for mosquito-borne viruses. Paired serum samples showed one Tahyna virus infection among 150 residents.
Jiménez-Sastré et al.55 sampled dwellings in Tabasco, Mexico, post-flood for dengue fever cases and found the geographical distribution of dengue fever cases was associated with the proximity of two permanent bodies of water. Chen et al.21 found heavy precipitation was a significant risk factor for dengue fever (RR = 1.96; 95%; CI 1.53–2.52). Additionally, more non-European studies have also examined relationships between flooding and shistosomiasis, Japanese encephalitis, Ross River virus, and Rift Valley fever.21,56-58
While evidence of vector-borne diseases associated with flooding from 2004–2012 in Europe was sparse (Appendices A, D) older studies have shown associations. In Romania, flooded basements were a significant risk factor for WNV in apartment dwellers (p = 0.01).59 In 1997, heavy rains in Moravia, Czech Republic resulted in flooding, and mosquito populations in the area amplified immediately.60 WNV activity was reported in the area. Hubálek and Halouzka61 stated environmental factors such as flooding can facilitate the re-emergence of WNV.
Summary of Possible Public Health Interventions
Public health interventions include those made before, during, and after flooding to reduce vulnerability to infectious diseases. Interventions need to take place at a variety of levels: individual, household, community, regional, national, and international.51 The public health measures cited in the literature to reduce the risk of infectious diseases as a result of flooding focus on: risk assessments, enhanced surveillance systems, and specific prevention and control measures depending upon the type of infectious disease risk.62,63
A rapid disease risk assessment should be conducted by a representative multi-agency group within the first week of the flood including: data on the flooded region and displaced persons, the main disease threats for the enhanced surveillance system, baseline data collection, and identification of priority interventions.62,63 During a flood event, hand-held devices that allow workers to enter and analyze data in the field can assist the rapid risk assessment.64
Existing disease surveillance systems can be enhanced to target specific diseases or syndromes and to support timely response actions to reduce disease impact and risk of transmission.62,63 Public health teams need to establish adequate disease surveillance systems which take into account the inherent disruption of the public health infrastructure that may occur during flooding. An enhanced surveillance system should be adaptable and context-specific, monitor key epidemiological data and compare with baseline data, monitor vulnerable groups, identify any emerging outbreaks, and result in timely public health action. In high-income countries, risk assessments and surveillance systems need to be very refined to detect small differences from baseline incidence data.51
Prevention of infectious diseases following flooding involves maintenance of health services, provision of shelter, clean water supplies, proper sanitation, regular and adequate food supply, and in some cases mass vaccination campaigns and control of disease vectors.62,63 Water and sanitation are vital elements in the transmission of water-borne diseases; hence, providing clean drinking water is a priority in the initial days following flooding. Clasen et al.65 found that household interventions were more effective in preventing diarrhea than interventions at the water-source. Interventions at the household level reviewed included: chlorination, filtration, solar disinfection, and combined flocculation and disinfection. Ejemot-Nwadiaro et al.66 found hand-washing interventions can reduce diarrhea episodes by one-third.
Rodent control is another prevention measure that needs to be considered during flooding. The local rodent species and their behaviors should be identified, water and food storage containers should be rodent-proofed, and solid waste should be properly stored, collected, and disposed.62,63 According to Bhardwaj et al.33 prompt and vigilant fever surveillance activities in pre-flooding preparedness plans, rodent control programs, and improvement of environmental sanitary conditions may help greatly reduce leptospirosis incidence.
Vector control can reduce disease transmission by rendering the environment unfavorable for the survival, development, and reproduction of the vector.62,63 Establishing surveillance for the introduction of new vector species could contribute substantially to vector-control. An expert should identify vectors responsible for local disease transmission, the factors that influence transmission, location of breeding ground, and which measures of control should be implemented. Local destruction of breeding sites after flooding has receded is extremely effective, so individuals should remove unused vessels and stagnant water when possible. Water storage containers need to be covered to protect from disease vectors, such as egg-laying female mosquitoes. Individuals can protect themselves against mosquito bites by using repellents during biting hours, mosquito nets, and screens in doors and windows.
Individual and community awareness and participation is essential for successfully reducing the risk of infectious diseases following flooding. Understanding the social and cultural influences on response behavior in the time of a flood emergency is crucial to inform the design and targeting of warnings and health education messages.1
Discussion
Some studies showed the frequency of infectious diseases can increase in the weeks to months after flooding, and Figure 1 illustrates when infectious disease outbreaks following flood events are likely to occur. However, there remains scientific uncertainty about the strength of association between infectious disease incidence and flooding. Floods can cause population displacement and changes in population density, raise concern about waste management and the availability of clean water, as well as affect the availability and access to healthcare services. All of these are risk factors for an infectious disease outbreak. Kouadio et al.13 and Watson et al.67 suggested that unless there is a substantial population displacement, there is minimal risk of infectious disease transmission and outbreaks following flooding. Overall, the risk of infectious disease following flooding is context-specific, differs between countries, and is dependent upon a number of synergistic factors. Outbreaks of leptospirosis and diarrheal diseases following flooding have been documented in Europe18,28,29,34,36,38,42 but the evidence of increased incidence of vector-borne diseases following flooding is lacking because the time lag before onset can be several months.68 Past studies have indicated possible associations between vector-borne diseases and flooding in Europe.36,59-61 European residents may be exposed to these risks while traveling. Foreign relief workers can potentially introduce infectious diseases into an area affected by flooding and these workers may be susceptible to endemic diseases that are more prevalent because of the flood.
Surveillance in flood-affected areas is fundamental to understanding the impact of flooding on infectious disease incidence. Surveillance and early warning systems may reduce current and future vulnerability. A comprehensive risk assessment could help determine priority diseases for inclusion in the enhanced surveillance system and prioritize prevention and control measures. In addition to surveillance and early warning systems to detect epidemic-prone diseases, assuring access to clean water, proper sanitation, adequate shelter, and primary healthcare services is essential.
Despite a considerable amount of research on the relationship between infectious diseases and flooding, globally and in Europe, the body of information still remains fragmentary. Many studies attempted to collect data retrospectively, had methodological shortcomings, lacked longitudinal data/baseline health data, control groups for comparison, and measures of clear disease outcomes. The studies included in this review were mainly observational studies with widely varying quality levels and study designs. Because it is unethical to conduct experimental studies on this topic, rigorous observational studies must be continue to be undertaken. Observational studies can present particular challenges because of the unpredictability of the timing and location of floods. Reporting and recall bias was very likely in many studies. Additionally, many studies relied on data from disease surveillance systems. Obtaining relevant disease surveillance data pre-, mid-, and post- flooding is frequently challenging. Population displacement can distort the rates of comparison for infectious disease incidence. The quality and robustness of disease surveillance systems can vary from country to country, and a country with a weak disease surveillance system will probably lack pre-flood baseline data. Flood damage to pre-existing public health infrastructure can exacerbate weaknesses in a disease surveillance system. Furthermore, it is difficult to attribute an increase in infectious disease incidence solely to a flood event, and therefore this issue may be under-investigated and under-reported. Finally, this systematic review is not entirely exhaustive, and there may be many other reports in gray literature, but the quality is likely to be lower than the peer-reviewed published reports identified.
Conclusions and Further Rsesearch
It is important for health officials and the public to understand that exacerbation of disease risk factors contribute to infectious disease outbreaks following flooding. Population and individual vulnerability and resilience factors can worsen or mitigate infectious diseases following flooding. The community needs to be aware of actions that can facilitate or prevent infectious disease. To mitigate infectious disease risk following flooding, those involved in flood planning, response, and recovery should be aware of the results of this systematic literature review. If climate change causes more floods, then the future health burden of infectious diseases from floods could increase. In Europe, maintenance and continuous adaptation and improvement of public health measures is important to sustain the low risk of infectious disease outbreaks following floods. Presently, there are clear research needs to improve the understanding of the association between infectious diseases and flooding:
More robust epidemiological studies on infectious diseases covering the pre-, mid-, and post-flood periods.
Further research assessing the effectiveness of public health interventions minimizing risk from infectious diseases following flooding.
Investigation of infectious disease incidence following smaller flood events.
Analysis of the differences between summer and winter flooding on infectious disease incidence.
Analysis of the differences between flash and riverine flooding on infectious disease incidence.
Appendix A. Studies assessing the relationship between infectious diseases and flooding - Multiple diseases.
| Authors | Location and Year of Flood | Design | Main Results |
|---|---|---|---|
| Ahmed et al.69 | Pakistan, 2010 | Cross-sectional study- 7,814 flood affected individuals interviewed to determine frequency of infectious diseases. | Gastrointestinal (30%), skin and soft tissue infection (33%), conjunctivitis (7%), ear, nose and throat infection (5%), respiratory tract infection (21%), suspected malaria (4%). No comparative data before flooding. |
| Bich et al.71 | Vietnam, 2008 | Cross-sectional study- rural and urban districts interviewed within 1 mo after flood about social, economic, and health impacts. In each district, a flooded commune and a less affected commune (control commune) were selected. | No statistically significant differences in proportion of dengue cases in flood affected and less affected communes. Higher proportions of pink eye and dermatitis in severely flood affected communes. In flood affected communes, 10/10 urban cases (p < 0.05) and 64/69 rural cases (p < 0.05) contracted pink eye after flood. In flood affected communes, 30/34 urban cases and 221/229 (p < 0.05) rural cases contracted dermatitis after flood. |
| Chen et al.21 | Taiwan, 1994–2008 | Routine data- analysis of a database integrating daily precipitation and temperature and an infectious disease case registry. | Heavy precipitation (130–200mm) a significant risk factor for enteroviruses (RR = 2.45; 95% CI 1.59–3.78) and dengue fever (RR = 1.96; 95% CI 1.53–2.52). Extreme torrential rain (> 350mm) a significant risk factor for enteroviruses (RR = 5.981; 95% CI 1.474–23.760) and bacillary dysentery (RR = 7.703; 95% CI 5.008–11.849). Associations between precipitation levels and enterovirus infections, Japanese encephalitis (p < 0.001), and stronger linear relationships between precipitation and bacillary dysentery, dengue fever, leptospirosis (p < 0.001). |
| Marcheggiani et al.18 | Italy, 1993–2010 | Routine data- national statistics collected by Italian Ministry of Health. | Association between hepatitis A, salmonellosis, infectious diarrhea, leptospirosis, cutaneous and visceral leishmaniasis, legionellosis and flood events from 1993–2010 seemed to exist. |
| Milojevic et al.75 | Bangladesh, 2001–2007 | Controlled interrupted time series- diarrheal incidence of a cohort of 211,000 residents classified as flooded or non-flooded in 2004. | After fully controlling pre-flood rate differences and seasonality, no clear evidence of excesses mortality or diarrhea risk during/after flooding. No evidence of excess risk from acute respiratory illnesses during flood but moderate increase in risk 6 mo after flood (RR = 1.25; 95% CI 1.06–1.47). |
| Su et al.40 | Taiwan, 2009 | Routine data- to clarify association between leptospirosis and melioidosis epidemics and flooding. | Positive correlation for leptospirosis (r = 0.54; p < 0.05) and for melioidosis (r = 0.52; p < 0.05) with cumulative rainfall. Increase in melioidosis cases significantly associated with > 500mm/day (p < 0.05). Number of leptospirosis cases positively correlated with 24-h cumulative rainfall (r = 0.71; p = 0.14). |
Appendix B. Studies assessing the relationship between infectious diseases and flooding - Water-borne.
| Authors, Year | Location and Year of Flood | Study Design | Main Results |
|---|---|---|---|
| Apisarnthanarak et al.70 | Thailand, 2012 | Case report- 5 melioidosis patients located through active case surveillance. | 5 cases reported excess flooding of homes and 0 had traditional risk factors for melioidosis. All cases survived. |
| Auld et al.22 | Canada, 2000 | Outbreak investigation- E. coli O157:H7 and Campylobacter outbreak. | Outbreak occurred several days after heavy rainfall (5-d accumulation 130–140mm). Heavy rainfall hypothesized as a causative factor of the outbreak. |
| Carrel et al.72 | Bangladesh, 1983–2003 | Longitudinal study- 21-y data cluster analysis of health surveillance and Geographic Information System to investigate temporal and spatial distribution of cholera following flood protection interventions. | 8,500 confirmed cholera cases. Two clusters of lower than expected cases, 3 clusters of higher than expected cases found (p < 0.001). Following flood protection interventions, overall decrease in cholera incidence, differences in the geography of high vs. low spatial clusters of cholera, and shifts in location of unusually high spatio-temporal cholera clusters. |
| Harris et al.73 | Bangladesh, 1998, 2004, 2007 | Routine data- comparison of pathogens in flood-associated diarrheal epidemics in 1998, 2004, and 2007. | In 2007, V. cholerae O1 (33%), rotavirus (12%), and enterotoxigenic E. coli (ETEC) (12%) were most prevalent. Significantly higher percentage of labile toxin-producing ETEC isolated in 2007 flood than in previous floods (p < 0.001). More severe dehydration seen in 2007 compared with 2004 and 1998 (p < 0.001). Findings showed alterations in clinical features and phenotypic changes of major bacterial pathogens. |
| Hashizume et al.23 | Bangladesh, 1998 | Routine data- number of observed cases of cholera and non-cholera diarrhea per week during flood and post-flood periods compared with expected numbers. | During flooding, cholera cases 5.9 times higher (95% CI 5–7) and non-cholera cases 1.8 times higher (95% CI 1.6–1.9) than expected. Post-flood period, cholera cases 2.1 times higher (95% CI 1.9–2.4) and non-cholera cases 1.2 times higher (95% CI 1.1–1.3). |
| Ko et al.74 | Taiwan, 2009 | Routine data- melioidosis outbreak. | 40 melioidosis cases identified following flooding. Onset within 4 d. |
| Qadri et al.76 | Bangladesh, 2004 | Routine data- diarrheal stools collected from patients during flooding. | Of 350 stool specimens tested, 78 positive for V. cholerae O1 (22.2%), 11 for shigella spp (3.4%), 5 for salmonella spp (1.7%). |
| Reacher et al.28 | England, 2000 | Historical cohort study- post-flooding survey interview. | Flooding associated with significant increase in risk of gastroenteritis with depth of flooding (RR = 1.7; 95% CI 0.9–3.0 p = 0.09, p for trend by flood depth = 0.04). |
| Schwartz et al.24 | Bangladesh, 1988, 1998, 2004 | Routine data- diarrheal patients during the 1988, 1998, and 2004 floods compared with non-flood periods. | During flood-related epidemics, V. cholerae most common cause of diarrhea, followed by rotavirus. Patients with V. cholerae (OR = 1.63; 95% CI 1.23–2.14; p = 0.001) and those without microbiologically identifiable V. cholerae (OR = 2.75; 95% CI 2.11–3.59; p < 0.001) more likely to have severe dehydration during floods than during non-flood periods. Median of 8.5 d (range 3–13 d) from time rivers reached flood stage until beginning of epidemics, and median of 17.5 d (range 8–36 d) for epidemics to end after rivers fell below flood stage. |
| Setzer et al.25 | United States, 2004 | Routine data- investigation of 6 target pathogens (Cryptosporisium, Giardia lamblia, Toxoplasma gondii, Helicobacter pylori, Mycobacterium avium, adenoviruses) in outpatient visits. | Statistically significant increase in outpatient visits for T. gondii (p < 0.05) and adenoviruses (p < 0.01) in severely flooded areas. Small magnitude of both effects, indicating on average < 1 extra outpatient visit each month in each severely affected county for these pathogens. Significant increase in outpatients visits for ill identified intestinal infections in both severely and moderately flooded counties (p < 0.01). |
| Schmid et al.29 | Germany, 2005 | Outbreak investigation- norovirus. | Gastrointestinal outbreak in 26 American tourists linked to direct exposure to floodwater contaminated with raw sewage. 6/10 firefighters with floodwater contact also fell ill with vomiting/diarrhea. |
| Thomas et al.26 | Canada, 1975–2001 | Longitudinal study- association between extreme rainfall and spring snowmelt and waterborne disease outbreaks. | For rainfall events greater than 93rd percentile, the relative odds of water-borne outbreak increased by 2.283 (95% CI 1.216, 4.285). |
| Vollaard et al.27 | Indonesia, 2001–2003 | Case-control study- 93 (69 typhoid and 24 paratyphoid) enteric fever cases compared with 289 non-enteric fever patient controls and 378 randomly selected community controls. | House flooding a signi□cant risk factor for paratyphoid fever; when paratyphoid group was compared with community control (OR = 4.52; 95% CI 1.90–10.73); when compared with fever controls (OR = 3.25; 95% CI 1.31–8.02). |
| Wade et al.32 | United States, 2001 | Cross-sectional study- 1,110 individuals provided flood survey health data. | House/yard flooding signi□cantly associated with gastrointestinal illness (Incidence rate ratio = 2.36; 95% CI 1.37–4.07). |
Appendix C. Studies assessing the relationship between infectious diseases and flooding - Rodent-borne.
| Authors, Year | Location and Year of Flood | Study Design | Main Results |
|---|---|---|---|
| Amilasan et al.47 | The Philippines, 2009 | Hospital-based investigation- investigating risk factors for leptospirosis mortality following flooding. Prospective surveillance and retrospective data collection. | Outbreak of 471 leptospirosis cases, 51 cases died. Patients predominately young and male. Delayed initiation of treatment, older age, jaundice, anuria, hemoptysis increased risk for death. |
| Bhardwaj et al.33 | India, 2006 | Case-control study- identifying risk factors for leptospirosis during flooding. 62 confirmed cases and 253 age and sex matched fever and healthy controls given a questionnaire. | 4 factors identified by multivariate analysis: contact of injured part with floodwater (OR 6.69; 95% CI 3.05–14.64), walking barefoot (OR 4.95; 95% CI 2.22–11.06), constant presence of rats (OR 4.95; 95% CI 1.53–16.05), spending > 4 d cleaning (OR 2.64; 85% CI 1.18–5.89). |
| Chiu et al.48 | Taiwan, 2004–2008 | Routine data- analyze characteristics of patients with laboratory-diagnosed leptospirosis and correlate onset of symptoms with exposure to floodwater. | 6 patients identified with history of contact with contaminated soil/water. 5/6 patients (83%) suffered from leptospirosis after typhoon. |
| Dechet et al.44 | Guyana, 2005 | Routine data- laboratory testing on suspected leptospirosis hospitalizations and deaths. Confirmed outbreak of leptospirosis after severe flooding. | Of 236 suspected cases admitted, 105 (44%) tested with Dip-S-Tick IgM ELISA; 52 (50%) positive, 41 (39%) negative, and 12 (11%) indeterminate. 34 deaths attributed (11 confirmed, 10 probable, 13 suspected) to leptospirosis. Of 201 patients interviewed, 89% reported floodwater contact. |
| Desai et al.34 | Germany, 2007 | Retrospective cohort study- leptospirosis in strawberry harvesters. Local rodents examined for leptospirosis. | 13 confirmed patients. Risk of disease increased with each day an individual worked in the rain with hand wounds (OR = 1.1; 95% CI, 1.04–1.14) and accidental rodent contact (OR = 4.8; 95% CI 1.5–15.9). |
| Gaynor et al.35 | United States, 2004 | Outbreak investigation- leptospirosis. | 271 persons responded to Internet survey, 90 (33%) reported febrile illness within 30 d of floodwater contact. One additional acute leptospirosis case identified. Patient 2 epidemiologically linked to Patient 1. |
| Maskey et al.45 | India, 2001–2005 | Longitudinal study- prevalence of leptospirosis. | 8 fold rise in leptospirosis in 2005 observed after heavy rainfall and water logging. 432 laboratory confirmed cases. |
| Pellizzer et al.36 | Italy, 2002 | Sero-epidemiogical study- evaluated leptospirosis risk in flood-exposed population. | 7/44 patients exposed to floodwaters exhibited anti-Leptospira specific IgM antibodies and 5 confirmed positive. Re-testing months later found significant antibody titers > 100 against serovar Copenhangeni in 3 cases (6.8% seroconversion rate). Flooding appeared to be sole risk factor, verification not possible due to lack of control group. |
| Radl et al.43 | Austria, 2010 | Outbreak investigation- leptospirosis. | 1st documented outbreak of leptospirosis in Austria. Four serologically confirmed cases, all triathlon athletes. Triathlon preceded by heavy rainfall (22mm). Cases contracted leptospirosis while swimming in recreational body of water. |
| Renato et al.37 | Mexico, 2007 | Outbreak investigation- leptospirosis. | 165 hospital cases showed febrile illness: 30 (18.2%) leptospirosis. 12/30 cases of leptospirosis confirmed serologically, all with moderate to severe floodwater contact. 4/12 positive cases died. |
| Smith et al.46 | Australia, 2011 | Routine data- leptospirosis surveillance. | 9 cases confirmed, all with floodwater exposure. 1st reported outbreak in central Queensland. |
| Socolovschi et al.38 | France, 2009 | Longitudinal-study- leptospirosis cases compared with weather conditions and garbage management strikes. | 3 autochthonous cases identified in Marseilles (October 2009) preceded by heavy rainfall. 1st autochthonous case identified after period of flooding preceded by heavy rainfall over several days (34.6 mm/day; 79.2 mm/day; 137 mm/day with an episode of 63 mm/3hr). Two autochthonous cases occurred during period of high rainfall (13.6–23.8 mm). |
| Zitek and Benes41 | Czech Republic, 1997,2002 | Routine data- leptospirosis surveillance. | Rates of reported and serologically confirmed cases of leptospirosis 3 times higher with specific morbidity (0.9 cases/100,000 inhabitants). 94 confirmed cases in 1997 and 92 confirmed cases in 2002. Two-thirds from inundation areas, half directly associated with floodwater. |
Appendix D. Studies assessing the relationship between infectious diseases and flooding - Vector-borne.
| Authors, Year | Location and Year of Flood | Study Design | Main Results |
|---|---|---|---|
| Hassan et al.56 | Sudan, 2007 | Outbreak investigation- Rift Valley fever. | 747 confirmed human cases including 230 deaths. Outbreak followed heavy rainfall with severe flooding. |
| Hubalek et al.54 | Czech Republic, 2002 | Routine data- specimens from residents in flooded area examined serologically for mosquito-borne viruses. | Antibodies detected after flood for Tahyna, Sindbis, and Batai viruses, with only activity found for Tahyna virus among 150 residents. |
| Jiménez-Sastré et al.55 | Mexico, 2010 | Cross-sectional study- convenience sampling of dengue fever in flooded colonies. | 3 cases with positive serology of IgG (0.6%) and 5 cases of positive IgM (0.9%). Geographical distribution associated with proximity to 2 permanent water bodies. |
| Tong et al.57 | Australia, 1998–2001 | Routine data- assessment of variability in environmental and vector factors on Ross River virus transmission. | Increases in high tide (RR = 1.65; 95%CI 1.2–2.26), rainfall (RR = 1.45; 95%CI 1.21–1.73), and mosquito density (RR = 1.17; 95%CI 1.09–1.27) significantly associated with rise of monthly Ross River virus. |
| Wu et al.58 | China, 1979–2000 | Longitudinal study- review of retrospective data to determine intermediate host snail dispersal patterns and acute and chronic infections of schistosomiasis after floods. | Average number of acute schistosomiasis cases recorded in flood years 2.8 times higher than in years with little to no flooding. Re-emerging and new snail infested areas in flood years on average 2.6 and 2.7 times larger than in years with normal water levels. Flooding of marshlands identified as main driver for vector dispersal. |
Glossary
Abbreviations:
- CI
confidence interval
- EM-DAT
Emergency Events Database
- EU
European Union
- IPCC
Intergovernmental Panel on Climate Change
- OR
odds ratio
- p
P-value
- RR
relative risk
- r
correlation coefficient
- SREX
Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation
- WNV
West Nile virus
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was performed within Public Health England’s department of Extreme Events and Health Protection, funded partly by the EU project “Public Health Adaptation Strategies to Extreme Weather Events—PHASE” (contract number EAHC 20101103). We would like to acknowledge Carla Stanke, Katie Carmichael, Angie Bone, Jolyon Medlock, and Jane Jones (Public Health England); Bettina Menne, Franziska Matthies, and Gerard Rockenschaub (World Health Organization Regional Office for Europe); Jan Semenza (European Centre for Disease and Control); Philippe Pirard (French Institute for Public Health Surveillance); Sari Kovats (London School of Hygiene and Tropical Medicine); Norman Parkinson (King’s College London); and Brittany Scheckelhoff.
References
- 1.Few R. Flood hazards, vulnerability, and risk reduction. In: [Few R and Matthies F, eds] Flood hazards and health: Responding to present and future risks. Tyndall Centre for Climate Change Research. 2006. Earthscan. [Google Scholar]
- 2.Adikari Y, Yoshitani J. Global trends in water-related disasters: An insight for policy-makers. The United Nations world water assessment program. International center for water hazard and risk management. 2009. Available from: http://unesdoc.unesco.org/images/0018/001817/181793e.pdf [Accessed on 6/6/12].
- 3.Keim ME. . Building human resilience: the role of public health preparedness and response as an adaptation to climate change. Am J Prev Med 2008; 35:508 - 16; http://dx.doi.org/ 10.1016/j.amepre.2008.08.022; PMID: 18929977 [DOI] [PubMed] [Google Scholar]
- 4.Jakubicka T, Vos F, Phalkey R, Marx M. Health impacts of floods in Europe: data gaps and needs from a spatial perspective. A MICRODIS REPORT. CRED. 2010. Available from: http://www.preventionweb.net/files/19820_healthimpactsoffloodsineurope1.pdf [Accessed on 7/6/12].
- 5.Barredo JI. . Major flood disasters in Europe: 1950–2005. Nat Hazards 2007; 42:125 - 48; http://dx.doi.org/ 10.1007/s11069-006-9065-2 [DOI] [Google Scholar]
- 6.Frei C, Schöll R, Fukutome S, Schmidli J, Vidale PL. . Future change of precipitation extremes in Europe: Intercomparison of scenarios from regional climate models. J Geophys Res 2006; 111; D06105 http://dx.doi.org/ 10.1029/2005JD005965; PMID: 20411040 20411040 [DOI] [Google Scholar]
- 7.Mudelsee M, Borngen M, Tetzlaff G, Grunewald U. . No upward trends in the occurrence of extreme floods in central Europe. Nature 2003; 425:841 - 3; http://dx.doi.org/ 10.1038/nature01928; PMID: 14574413 [DOI] [PubMed] [Google Scholar]
- 8.IPCC. Summary for policymakers: Managing the risks of extreme events and disasters to advance climate change adaptation [Field CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KL, Mastrandrea MD, Mach KJ, Plattner GK, Allen SK, Tignor M, and Midgley PM]. Cambridge University Press, Cambridge, UK, The Edinburgh Building, Shaftesbury Road, Cambridge CB2 8RU ENGLAND. June 2012. 582pp. [Google Scholar]
- 9.WHO. Protecting health in Europe from climate change. [Menne B, Apfel F, Kovats S, Racioppi F, eds]. Copenhagen. 2008. [Google Scholar]
- 10.Patz JA, Olson SH, Uejio CK, Gibbs HK. . Disease emergence from global climate and land use change. Med Clin North Am 2008; 92:1473 - 91, xii; http://dx.doi.org/ 10.1016/j.mcna.2008.07.007; PMID: 19061763 [DOI] [PubMed] [Google Scholar]
- 11.Baqir M, Sobani ZA, Bhamani A, Bham NS, Abid S, Farook J, et al. . Infectious diseases in the aftermath of monsoon flooding in Pakistan. Asian Pac J Trop Biomed 2012; 2:76 - 9; http://dx.doi.org/ 10.1016/S2221-1691(11)60194-9; PMID: 23569839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iver LC, Ryan ET. . Infectious diseases of severe weather-related and flood-related natural disasters. Curr Opin Infect Dis 2007; 19:408 - 14; http://dx.doi.org/ 10.1097/01.qco.0000244044.85393.9e [DOI] [PubMed] [Google Scholar]
- 13.Kouadio IK, Aljunid S, Kamigaki T, Hammad K, Oshitani H. . Infectious diseases following natural disasters: prevention and control measures. Expert Rev Anti Infect Ther 2012; 10:95 - 104; http://dx.doi.org/ 10.1586/eri.11.155; PMID: 22149618 [DOI] [PubMed] [Google Scholar]
- 14.Ligon BL. . Infectious diseases that pose specific challenges after natural disasters: a review. Semin Pediatr Infect Dis 2006; 17:36 - 45; http://dx.doi.org/ 10.1053/j.spid.2006.01.002; PMID: 16522504 [DOI] [PubMed] [Google Scholar]
- 15.Ahern M, Kovats RS, Wilkinson P, Few R, Matthies F. . Global health impacts of floods: epidemiologic evidence. Epidemiol Rev 2005; 27:36 - 46; http://dx.doi.org/ 10.1093/epirev/mxi004; PMID: 15958425 [DOI] [PubMed] [Google Scholar]
- 16.Semenza JC, Menne B. . Climate change and infectious diseases in Europe. Lancet Infect Dis 2009; 9:365 - 75; http://dx.doi.org/ 10.1016/S1473-3099(09)70104-5; PMID: 19467476 [DOI] [PubMed] [Google Scholar]
- 17.Cann KF, Thomas DR, Salmon RL, Wyn-Jones AP, Kay D. . Extreme water-related weather events and waterborne disease. Epidemiol Infect 2013; 141:671 - 86; http://dx.doi.org/ 10.1017/S0950268812001653; PMID: 22877498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcheggiani S, Puccinelli C, Della Bella V, Carere M, Blasi MF, Pacini N, et al. . Risks of water-borne disease outbreaks after extreme events. Toxicol Environ Chem 2010; 92:593 - 9; http://dx.doi.org/ 10.1080/02772240903252140 [DOI] [Google Scholar]
- 19.Martinez-Urtaza J, Saco M, de Novoa J, Perez-Piñeiro P, Peiteado J, Lozano-Leon A, et al. . Influence of environmental factors and human activity on the presence of Salmonella serovars in a marine environment. Appl Environ Microbiol 2004; 70:2089 - 97; http://dx.doi.org/ 10.1128/AEM.70.4.2089-2097.2004; PMID: 15066800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Public Health Wales, Communicable Disease Surveillance Centre. Systematic review of waterborne disease outbreaks following extreme water events. 2011 [WWW] Available from: http://www.viroclime.org/downloads/viroclime_survey_wp7.pdf [Accessed on 20/7/12].
- 21.Chen MJ, Lin CY, Wu YT, Wu PC, Lung SC, Su HJ. . Effects of extreme precipitation to the distribution of infectious diseases in Taiwan, 1994-2008. PLoS One 2012; 7:e34651; http://dx.doi.org/ 10.1371/journal.pone.0034651; PMID: 22737206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auld H, MacIver D, Klaassen J. . Heavy rainfall and waterborne disease outbreaks: the Walkerton example. J Toxicol Environ Health A 2004; 67:1879 - 87; http://dx.doi.org/ 10.1080/15287390490493475; PMID: 15371222 [DOI] [PubMed] [Google Scholar]
- 23.Hashizume M, Wagatsuma Y, Faruque ASG, Hayashi T, Hunter PR, Armstrong B, et al. . Factors determining vulnerability to diarrhoea during and after severe floods in Bangladesh. J Water Health 2008; 6:323 - 32; http://dx.doi.org/ 10.2166/wh.2008.062; PMID: 19108552 [DOI] [PubMed] [Google Scholar]
- 24.Schwartz BS, Harris JB, Khan AI, Larocque RC, Sack DA, Malek MA, et al. . Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am J Trop Med Hyg 2006; 74:1067 - 73; PMID: 16760521 [PMC free article] [PubMed] [Google Scholar]
- 25.Setzer C, Domino ME. . Medicaid outpatient utilization for waterborne pathogenic illness following Hurricane Floyd. Public Health Rep 2004; 119:472 - 8; http://dx.doi.org/ 10.1016/j.phr.2004.07.004; PMID: 15313110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas KM, Charron DF, Waltner-Toews D, Schuster C, Maarouf AR, Holt JD. . A role of high impact weather events in waterborne disease outbreaks in Canada, 1975 - 2001. Int J Environ Health Res 2006; 16:167 - 80; http://dx.doi.org/ 10.1080/09603120600641326; PMID: 16611562 [DOI] [PubMed] [Google Scholar]
- 27.Vollaard AM, Ali S, van Asten HA, Widjaja S, Visser LG, Surjadi C, et al. . Risk factors for typhoid and paratyphoid fever in Jakarta, Indonesia. JAMA 2004; 291:2607 - 15; http://dx.doi.org/ 10.1001/jama.291.21.2607; PMID: 15173152 [DOI] [PubMed] [Google Scholar]
- 28.Reacher M, McKenzie K, Lane C, Nichols T, Kedge I, Iverson A, et al. . Health impacts of flooding in Lewes: A comparison of reported gastrointestinal and other illness and mental health in flooded and non-flooded households. CDPH 2004; 7:56 - 63 [PubMed] [Google Scholar]
- 29.Schmid D, Lederer I, Much P, Pichler AM, Allerberger F. Outbreak of norovirus infection associated with contaminated flood water, Salzburg 2005. Eurosurveillance. 2005; 10(24). [DOI] [PubMed] [Google Scholar]
- 30.Patz JA. Extreme precipitation linked to waterborne disease outbreaks. ScienceDaily. Johns Hopkins University Bloomberg School of Public Health. 2001. Available at http://www.sciencedaily.com/releases/2001/08/010801081736.htm [Accessed on 7 February 2012].
- 31.Wade TJ, Sandhu SK, Levy D, Lee S, LeChevallier MW, Katz L, et al. . Did a severe flood in the Midwest cause an increase in the incidence of gastrointestinal symptoms?. Am J Epidemiol 2004; 159:398 - 405; http://dx.doi.org/ 10.1093/aje/kwh050; PMID: 14769644 [DOI] [PubMed] [Google Scholar]
- 32.Miettinen IT, Zacheus O, von Bonsdorff CH, Vartiainen T. . Waterborne epidemics in Finland in 1998-1999. Water Sci Technol 2001; 43:67 - 71; PMID: 11464771 [PubMed] [Google Scholar]
- 33.Bhardwaj P, Kosambiya JK, Desai VK. . A case control study to explore the risk factors for acquisition of leptospirosis in Surat city, after flood. Indian J Med Sci 2008; 62:431 - 8; http://dx.doi.org/ 10.4103/0019-5359.48454; PMID: 19265232 [DOI] [PubMed] [Google Scholar]
- 34.Desai S, van Treeck U, Lierz M, Espelage W, Zota L, Sarbu A, et al. . Resurgence of field fever in a temperate country: an epidemic of leptospirosis among seasonal strawberry harvesters in Germany in 2007. Clin Infect Dis 2009; 48:691 - 7; http://dx.doi.org/ 10.1086/597036; PMID: 19193108 [DOI] [PubMed] [Google Scholar]
- 35.Gaynor K, Katz AR, Park SY, Nakata M, Clark TA, Effler PV. . Leptospirosis on Oahu: an outbreak associated with flooding of a university campus. Am J Trop Med Hyg 2007; 76:882 - 5; PMID: 17488909 [PubMed] [Google Scholar]
- 36.Pellizzer P, Todescato A, Benedetti P, Colussi P, Conz P, Cinco M. . Leptospirosis following a flood in the Veneto area, North-east Italy. Ann Ig 2006; 18:453 - 6; PMID: 17089960 [PubMed] [Google Scholar]
- 37.Renato ZCI, Beatriz B, Angel B, Williams M, Miguel D. . [Later leptospirosis after flood in Tabasco, Mexico, 2007] Enf Inf Micrbiol. 2011; 31:33 - 7 [Google Scholar]
- 38.Socolovschi C, Angelakis E, Renvoisé A, Fournier PE, Marié JL, Davoust B, et al. . Strikes, flooding, rats, and leptospirosis in Marseille, France. Int J Infect Dis 2011; 15:e710 - 5; http://dx.doi.org/ 10.1016/j.ijid.2011.05.017; PMID: 21767971 [DOI] [PubMed] [Google Scholar]
- 39.Lau CL, Smythe LD, Craig SB, Weinstein P. . Climate change, flooding, urbanisation and leptospirosis: fuelling the fire?. Trans R Soc Trop Med Hyg 2010; 104:631 - 8; http://dx.doi.org/ 10.1016/j.trstmh.2010.07.002; PMID: 20813388 [DOI] [PubMed] [Google Scholar]
- 40.Su HP, Chan TC, Chang CC. . Typhoon-related leptospirosis and melioidosis, Taiwan, 2009. Emerg Infect Dis 2011; 17:1322 - 4; http://dx.doi.org/ 10.3201/eid1707.101050; PMID: 21762606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zitek K, Benes C. . [Longitudinal epidemiology of leptospirosis in the Czech Republic (1963-2003)]. Epidemiol Mikrobiol Imunol 2005; 54:21 - 6; PMID: 15807384 [PubMed] [Google Scholar]
- 42.Kriz B, Benes C, Castkova J, et al. Monitoring of the epidemiological situation in flooded areas of the Czech Republic in year 1997. In: [Davidova P, Rupes V, eds] Proceedings of the Conferecne DDD ’98. 1998. Prodebrady, the Czech Republic. National Institute of Public Health, Prague.
- 43.Radl C, Müller M, Revilla-Fernandez S, Karner-Zuser S, de Martin A, Schauer U, et al. . Outbreak of leptospirosis among triathlon participants in Langau, Austria, 2010. Wien Klin Wochenschr 2011; 123:751 - 5; http://dx.doi.org/ 10.1007/s00508-011-0100-2; PMID: 22105111 [DOI] [PubMed] [Google Scholar]
- 44.Dechet AM, Parsons M, Rambaran M, Mohamed-Rambaran P, Florendo-Cumbermack A, Persaud S, et al. . Leptospirosis outbreak following severe flooding: a rapid assessment and mass prophylaxis campaign; Guyana, January-February 2005. PLoS One 2012; 7:e39672; http://dx.doi.org/ 10.1371/journal.pone.0039672; PMID: 22808049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maskey M, Shastri JS, Saraswathi K, Surpam R, Vaidya N. . Leptospirosis in Mumbai: post-deluge outbreak 2005. Indian J Med Microbiol 2006; 24:337 - 8; http://dx.doi.org/ 10.4103/0255-0857.29413; PMID: 17185871 [DOI] [PubMed] [Google Scholar]
- 46.Smith JKG, Young MM, Wilson KL, Craig SB. Leptospirosis following a major flood in Central Queensland, Australia. Epidemiol Infect FirstView. 2012;1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amilasan AS, Ujiie M, Suzuki M, Salva E, Belo MC, Koizumi N, et al. . Outbreak of leptospirosis after flood, the Philippines, 2009. [Accessed on 5/7/12] Emerg Infect Dis 2012; 18:91 - 4; http://wwwnc.cdc.gov/eid/article/18/1/10-1892_article.htm http://dx.doi.org/ 10.3201/eid1801.101892; PMID: 22257492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiu CH, Wang YC, Yang YS, Chang FY. . Leptospirosis after typhoon in Taiwan. J Med Sci 2009; 29:131 - 4 [Google Scholar]
- 49.Kalashnikova IA, Mkrtchan MO, Shevyreva TV. . [Anti-epidemic provision for the population in emergency situations in the Krasnodar Territory]. Zh Mikrobiol Epidemiol Immunobiol 2003; 6:16 - 8; PMID: 14716969 [PubMed] [Google Scholar]
- 50.Sanders EJ, Rigau-Pérez JG, Smits HL, Deseda CC, Vorndam VA, Aye T, et al. . Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1996 [correction of 1966]. Am J Trop Med Hyg 1999; 61:399 - 404; PMID: 10497979 [DOI] [PubMed] [Google Scholar]
- 51.Ahern M, Kovats RS. The health impacts of floods. In: [Few R and Matthies F] Flood hazards and health: Responding to present and future risks. Tyndall Centre for Climate Change Research. Earthscan. 2006. [Google Scholar]
- 52.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. . A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector Borne Zoonotic Dis 2012; 12:1 - 13; http://dx.doi.org/ 10.1089/vbz.2011.0814; PMID: 21995261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO. Flooding and communicable disease fact sheet. 2006. Available at: http://www.who.int/hac/techguidance/ems/flood_cds/en/ [Accessed on 7/6/12].
- 54.Hubálek Z, Zeman P, Halouzka J, Juricová Z, Stovicková E, Bálková H, et al. . Mosquitoborne viruses, Czech Republic, 2002. Emerg Infect Dis 2005; 11:116 - 8; http://wwwnc.cdc.gov/eid/article/11/1/04-0444_article.htm http://dx.doi.org/ 10.3201/eid1101.040444; PMID: 15705333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiménez-Sastré A, Boldo-León X, Priego-Álvarez H, Quevedo-Tejero E, Zavala-González MA. . [Geographic distribution of dengue fever cases in flooded zones from Villahermosa, Tabasco, in 2010]. Rev Chilena Infectol 2012; 29:32 - 6; PMID: 22552508 [DOI] [PubMed] [Google Scholar]
- 56.Hassan OA, Ahlm C, Sang R, Evander M. . The 2007 Rift Valley fever outbreak in Sudan. PLoS Negl Trop Dis 2011; 5:e1229; http://dx.doi.org/ 10.1371/journal.pntd.0001229; PMID: 21980543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong S, Hu W, Nicholls N, Dale P, MacKenzie JS, Patz J, et al. . Climatic, high tide, and vector variables and the transmission of Ross Rover virus. Intern Med 2005; 35:677 - 80; http://dx.doi.org/ 10.1111/j.1445-5994.2005.00935.x [DOI] [PubMed] [Google Scholar]
- 58.Wu XH, Zhang SQ, Xu XJ, Huang YX, Steinmann P, Utzinger J, et al. . Effect of floods on the transmission of schistosomiasis in the Yangtze River valley, People’s Republic of China. Parasitol Int 2008; 57:271 - 6; http://dx.doi.org/ 10.1016/j.parint.2008.04.004; PMID: 18499513 [DOI] [PubMed] [Google Scholar]
- 59.Han LL, Popovici F, Alexander JP Jr., Laurentia V, Tengelsen LA, Cernescu C, et al. . Risk factors for West Nile virus infection and meningoencephalitis, Romania, 1996. J Infect Dis 1999; 179:230 - 3; http://dx.doi.org/ 10.1086/314566; PMID: 9841844 [DOI] [PubMed] [Google Scholar]
- 60.Hubalek Z, Halzouka J, Juricova Z. . West Nile fever in Czechland. Emerg Infect Dis 1999; 5:643 - 50; http://dx.doi.org/ 10.3201/eid0504.990430; PMID: 10511520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hubálek Z, Halouzka J. . West Nile fever--a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis 1999; 5:643 - 50; http://dx.doi.org/ 10.3201/eid0505.990506; PMID: 10511520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johns Hopkins and Red Cross Red Crescent. Public health guide in emergencies. 2008. Available from: http://www.jhsph.edu/research/centers-and-institutes/center-for-refugee-and-disaster-response/publications_tools/publications/_CRDR_ICRC_Public_Health_Guide_Book/Forward.pdf [Accessed on 8/8/12].
- 63.WHO. Communicable disease control in emergencies: A field manual. Connolly, M. (eds). 2005. Available from: http://www.who.int/infectious-disease-news/IDdocs/whocds200527/ISBN_9241546166.pdf [Accessed on 8/8/12].
- 64.Foltz S, Braur B. Communication, data sharing, and collaboration, at the disaster site. Comput in Civil Eng. Conference Proceeding Paper. 2005; 1-8.
- 65.Clasen TF, Roberts IG, Rabie T, Schmidt WP, Cairncross S. Interventions to improve water quality for preventing diarrhea. In: Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. 2006. [DOI] [PubMed] [Google Scholar]
- 66.Ejemot-Nwadiaro RI, Ehiri JE, Meremikwu MM, Critchley JA. Hand washing for preventing diarrhea. In: Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. 1996. [DOI] [PubMed] [Google Scholar]
- 67.Watson JT, Gayer M, Connolly MA. . Epidemics after natural disasters. Emerg Infect Dis 2007; 13:1 - 5; http://dx.doi.org/ 10.3201/eid1301.060779; PMID: 17370508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waring SC, Brown BJ. . The threat of communicable diseases following natural disasters: a public health response. Disaster Manag Response 2005; 3:41 - 7; http://dx.doi.org/ 10.1016/j.dmr.2005.02.003; PMID: 15829908 [DOI] [PubMed] [Google Scholar]
- 69.Ahmed Z, Khan AA, Nisar N. . Frequency of infectious diseases among flood affected people at district Rajanpur, Pakistan. Pak J Med Sci 2011; 27:866 - 9 [Google Scholar]
- 70.Apisarnthanarak A, Khawcharoenporn T, Mundy LM. . Flood-associated melioidosis in a non-endemic region of Thailand. Int J Infect Dis 2012; 16:e409 - 10; http://dx.doi.org/ 10.1016/j.ijid.2012.01.013; PMID: 22421023 [DOI] [PubMed] [Google Scholar]
- 71.Bich TH, Quang LN, Ha TT, Hanh TT, Guha-Sapir D. . Impacts of flood on health: epidemiologic evidence from Hanoi, Vietnam. Glob Health Action 2011; 4:6356; http://dx.doi.org/ 10.3402/gha.v4i0.6356; PMID: 21866222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carrel M, Emch M, Streatfield PK, Yunus M. . Spatio-temporal clustering of cholera: the impact of flood control in Matlab, Bangladesh, 1983-2003. Health Place 2009; 15:741 - 52; http://dx.doi.org/ 10.1016/j.healthplace.2008.12.008; PMID: 19217821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris AM, Chowdhury F, Begum YA, Khan AI, Faruque ASG, Svennerholm AM, et al. . Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am J Trop Med Hyg 2008; 79:708 - 14; PMID: 18981509 [PMC free article] [PubMed] [Google Scholar]
- 74.Ko WC, Cheung BMH, Tang HJ, Shih HI, Lau YJ, Wang LR, et al. . Melioidosis outbreak after typhoon, southern Taiwan. Emerg Infect Dis 2007; 13:896 - 8; http://dx.doi.org/ 10.3201/eid1306.060646; PMID: 17553230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milojevic A, Armstrong B, Hashizume M, McAllister K, Faruque A, Yunus M, et al. . Health effects of flooding in rural Bangladesh. Epidemiology 2012; 23:107 - 15; http://dx.doi.org/ 10.1097/EDE.0b013e31823ac606; PMID: 22082995 [DOI] [PubMed] [Google Scholar]
- 76.Qadri F, Khan AI, Faruque AS, Begum YA, Chowdhury F, Nair GB, et al. . Enterotoxigenic Escherichia coli and Vibrio cholerae diarrhea, Bangladesh, 2004. Emerg Infect Dis 2005; 11:1104 - 7; http://dx.doi.org/ 10.3201/eid1107.041266; PMID: 16022790 [DOI] [PMC free article] [PubMed] [Google Scholar]