Abstract
The timing and pace of pubertal development has been associated with psychosocial functioning, with pubertal variables represented both as predictors (e.g., earlier puberty linked with poor outcomes) and as sequelae (e.g., early stress linked with earlier puberty). However, the literature has largely not tested mediational models or prospective mechanisms of associations between puberty and psychosocial variables. In a longitudinal study including 454 youth followed over four timepoints (mean ages 10–18), structural equation modeling tested a hypothesized path from childhood maltreatment to cortisol (Time 1) to pubertal stage (Time 2), and psychosocial outcomes (Times 3 and 4). There was not support for the full hypothesized pathway in either gender. However, for boys, maltreatment was associated with attenuated cortisol, and more pubertal change predicted subsequent delinquency. For girls, cortisol predicted more pubertal change which then predicted substance use. This study demonstrates links between HPA axis function, pubertal development, and risky outcomes.
Keywords: adolescent, early experience, HPA axis, stress
INTRODUCTION
Puberty is a complex developmental phenomenon with broad implications for health and well-being. Interestingly, pubertal timing and tempo have been conceptualized both as predictors and as sequelae of mental health and environmental risk. For example, the puberty-as-predictor literature has reported evidence that “off-time” and particularly early or accelerated maturation heightens risk of psychopathology (Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1997; Negriff & Susman, 2011). At the same time, the puberty-as-outcome literature has found early environmental adversity to be associated with both a faster and slower pace of pubertal development (Ellis, 2004). However, the current literature largely lacks studies that take a mechanistic approach in order to capture how risk processes both precede and follow pubertal onset, while exploring possible mediators of associations between pubertal development indices and well-being. The current study attempts to address this gap by using a longitudinal framework in which puberty is included in the same model as prior maltreatment, subsequent risk outcomes, and possible mechanisms (i.e., cortisol).
Puberty as Predictor of Mental Health and Behavioral Outcomes
“Off-time” pubertal development has been linked with a range of deleterious consequences (Negriff & Susman, 2011). Early timing, in particular, serves as a risk for depression, eating disorders, substance use, sexual activity, and delinquency (Cavanagh, 2004; Ge, Conger, & Elder, 2001; Negriff & Trickett, 2010; Stice, Presnell, & Bearman, 2001). Fewer studies have explored the effects of pubertal tempo (rate of pubertal development), but results to date suggest that a faster tempo is also linked with detrimental outcomes. One study found that earlier timing and faster tempo independently predicted internalizing and externalizing problems for girls but not for boys, whereas an interaction between timing and tempo predicted externalizing problems for boys (Marceau, Ram, Houts, Grimm, & Susman, 2011). Another study found that faster tempo predicted depressive symptoms for boys but not girls (Mendle, Harden, Brooks-Gunn, & Graber, 2010).
Puberty as Predicted by Early Adversity
A substantial literature has focused on precursors of early pubertal development. Although this literature has been mixed and has focused almost exclusively on girls, the preponderance of findings to date suggest that extremely severe stressors (starvation, war) delay pubertal maturation, while mild-to-moderate psychosocial stressors (family dysfunction, father absence) appear to accelerate development (Ellis, 2004). For example, low SES has been associated with earlier menarche (Deardorff, Abrams, Ekwaru, & Rehkopf, 2014). Much of this research is based on evolutionary life history theory, which asserts that early environment affects the onset and rate of reproductive maturity (Belsky, Steinberg, & Draper, 1991). Within this theory, child maltreatment has been discussed as an adverse experience with all the hallmarks of the negative family context and childrearing practices that may hasten pubertal onset and accelerate progress through puberty.
A number of studies have found that child maltreatment is associated with early pubertal timing (Costello, Sung, Worthman, & Angold, 2007; Foster, Hagan, & Brooks-Gunn, 2008; Romans, Martin, Gendall, & Herbison, 2003). In a study of 68,000 women, childhood sexual abuse was associated with earlier pubertal maturation and physical abuse was linked with both earlier and later maturation (Boynton-Jarrett et al., 2013). Only one study has examined maltreatment and tempo, finding that physical abuse was linked with faster tempo of puberty (Mendle, Leve, Van Ryzin, Natsuaki, & Ge, 2011).
The effects of maltreatment on puberty may be caused by stress system alternations (Trickett & Putnam, 1993). Early adversity, including maltreatment, can alter the response of the hypothalamic-pituitary-adrenal (HPA) axis, with chronic stressful experiences resulting in hyperactivation followed by downregulation of the stress system (Carpenter et al., 2007; Elzinga et al., 2008; Gordis, Granger, Susman, & Trickett, 2008). The attenuated cortisol profile shown in individuals who have experienced maltreatment may actually hasten the onset of puberty because the stress function of the HPA axis is dampened, allowing the cascade of pubertal hormones via the hypothalamic-pituitary-gonadal (HPG) axis to commence. Indeed, early life stress may contribute to inverse coupling of the HPA and HPG axes, with the gonadal hormones being suppressed by the HPA axis and vice versa (Ruttle, Shirtcliff, Armstrong, Klien, & Essex, 2013).
The first empirical investigation of HPA axis attenuation as a predictor of puberty (Saxbe, Negriff, Susman, & Trickett, 2014) used the current sample and found that girls who showed lower cortisol excretion during a laboratory stress test had an accelerated pace of pubertal development over the following year. Another investigation also using the current sample found that maltreatment history was associated with attenuated cortisol responses to a stress task (Trickett, Gordis, Peckins, & Susman, 2014). Taken together, these results suggest that maltreatment-related attenuation of the HPA axis, which has been found in numerous studies of youth (Susman, 2006), might also accelerate pubertal development. However, Saxbe et al. (2014) only tested the effect of cortisol on puberty, and Trickett et al. (2014) only tested the effect of maltreatment on cortisol, so both studies reflect a partial test of this hypothesis. Moreover, neither study included subsequent psychosocial outcomes that have been associated with puberty, such as delinquency, depression, and substance use. The present study extends the work of the previous two studies to test maltreatment, cortisol, puberty, and psychosocial outcomes in a comprehensive longitudinal model.
To our knowledge, only one paper has used a longitudinal design to explore both antecedents and sequelae of pubertal development. Mendle, Leve, Van Ryzin, & Natsuaki (2014) studied girls in foster care over several years and measured maltreatment, internalizing symptoms, and pubertal development. Childhood maltreatment was not directly linked with internalizing symptoms, but indirect effects emerged: sexual abuse predicted earlier pubertal maturation, which in turn predicted increased internalizing symptoms. This paper helps to establish the case for pubertal development as a pathway linking early risk to subsequent dysfunction.
The Current Study
Building on the literature supporting links between maltreatment and cortisol, cortisol and puberty, and puberty and outcomes, the current study includes four assessment timepoints spanning ages 9 to 22 and includes not only maltreatment measures and subsequent depression, delinquency, and substance use but also includes cortisol response as a putative link between maltreatment and early puberty. The Mendle et al. (2014) paper sampled 100 girls who were in foster care due to maltreatment, whereas our sample of 454 youth includes both maltreated and non-maltreated boys and girls. Therefore, the present study continues in the vein of Mendle et al. (2014) but assesses youth with a variety of maltreatment experiences separately by gender.
We hypothesized that maltreatment would be linked with dampened cortisol, which in turn predicts accelerated pubertal development, which then predicts riskier psychosocial functioning (higher rates of depressive symptoms, delinquency, and substance use). Structural equation modeling was used to test this hypothesis so that interrelations between all variables could be tested simultaneously. Males and females were tested in separate models due to previous findings of different patterns between males and females for some parameters in the model.
RESEARCH DESIGN AND METHODS
Participants
Data were from the first four assessments of an ongoing longitudinal study examining the effects of maltreatment on adolescent development. At Time 1 (T1), the sample was composed of 454 adolescents aged 9–13 years (241 males and 213 females). Time 2 (T2), Time 3 (T3), and Time 4 (T4) occurred on average 1, 2.7, and 7.2 years after baseline. The retention rate between T1 and T2 was 86.1% (n = 391), between T1 and T3 was 70.9% (n = 322), and between T1 and T4 was 77.5% (n = 352). Participants not seen at T2 were more likely to be in the maltreatment group (OR 4.38, p < .01), those not seen at T3 were more likely to be Latino (OR = 3.37, p < .01) and in the maltreatment group (OR = 5.36, p < .01), and those not seen at Time 4 were more likely to be in the maltreatment group (OR = 2.45, p < .01) and male (OR = 1.86, p < .01). Descriptives of the sample for all four timepoints can be found in Table 1.
Table 1.
Sample Characteristics for Time 1, 2, 3, and 4
| Group |
||||||||
|---|---|---|---|---|---|---|---|---|
| Maltreated |
Comparison |
|||||||
| Demographic Variable | Time 1 | Time 2 | Time 3 | Time 4 | Time 1 | Time 2 | Time 3 | Time 4 |
| N | 303 | 250 | 191 | 222 | 151 | 142 | 128 | 128 |
| Age (std deviation) | 10.84 (1.15) |
12.02 (1.21) |
13.85 (1.48) |
18.28 (1.41) |
11.11 (1.15) |
12.28 (1.26) |
13.57 (1.38) |
18.15 (1.56) |
| Gender (%) | ||||||||
| Male | 50 | 48 | 46 | 47 | 60 | 60 | 57 | 56 |
| Female | 50 | 52 | 54 | 53 | 40 | 40 | 43 | 44 |
| Ethnicity (%) | ||||||||
| African American | 40 | 40 | 47 | 43 | 32 | 32 | 34 | 35 |
| Latino | 35 | 36 | 29 | 34 | 47 | 45 | 43 | 42 |
| White | 12 | 11 | 8 | 10 | 10 | 11 | 11 | 10 |
| Mixed biracial | 13 | 13 | 16 | 13 | 11 | 12 | 12 | 13 |
| Living arrangement (%) | ||||||||
| With parent | 52 | 63 | 62 | 56 | 93 | 94 | 95 | 85 |
| Foster care or extended family |
48 | 37 | 38 | 24 | 7 | 6 | 5 | 3 |
| Without caregiver | n/a | n/a | n/a | 20 | n/a | n/a | n/a | 12 |
Recruitment
The maltreatment group was recruited from active cases in the Children and Family Services (CFS) of a large west coast city. The inclusion criteria were as follows: (1) a new substantiated referral to CFS in the preceding month for any type of maltreatment; (2) child age of 9–12 years; (3) child identified as Latino, African–American, or Caucasian (non-Latino); (4) child residing in one of 10 zip codes in a designated county at the time of referral to CFS. With the approval of CFS and the Institutional Review Board of the affiliated university, potential participants were contacted and asked their willingness to participate. Of the families referred by CFS, 77% agreed to participate.
The comparison group was recruited using names from school lists of children aged 9–12 years residing in the same 10 zip codes as the maltreated sample. Comparison caregivers were contacted the same way as the maltreated group. Comparison families were cross-checked through the CFS database to ensure they had no previous or ongoing experience with child welfare agencies. Approximately 50% of the comparison families contacted agreed to participate.
Procedures
All four assessment visits (T1–T4) were conducted at an urban research university. After assent and consent were obtained from the adolescent and caretaker, respectively, the adolescent was administered questionnaires and tasks during a 4-hr protocol. Both the child and caretaker were paid for their participation according to the guidelines of the National Institutes of Health standard compensation rate for healthy volunteers.
Stress Paradigm and Saliva Collection
During the scheduling phone call, caregivers were told that the child should not eat or drink anything (other than water) for 4 hr prior to their study visit. This information was also included in a confirmation letter and a reminder phone call. During their visit, children indicated medications currently being taken (including steroids, inhaled medications, or creams/lotions), and other variables that might affect cortisol concentrations.
Six saliva samples were obtained over 90 min: the first two were collected 45 min before the stressor and 10 min before the stressor (immediately after a 5-min relaxation protocol that included soft music and a still slide of a beach scene). Adolescents then engaged in the TSST-C, a version of the Trier Social Stressor Test (TSST) modified for children (Buske-Kirschbaum, Jobst, Psych, Wustmans, & Kirschbaum, 1997). During this procedure, participants were read the beginning of a story, given 5 min to develop the next part of the story, and then spent 4 min presenting that story to an interviewer and a panel of two judges who maintained neutral facial expressions throughout the task. Next, the youth performed a challenging 4-min serial subtraction task before the judges. The third saliva sample was obtained immediately after the stressor was complete, and the fourth, fifth, and sixth samples occurred 10, 20, and 30 min after the end of the stressor, respectively.
Saliva samples were collected via passive drool through a short straw into a vial. Data collection occurred primarily in the afternoon, with an average start time of 2:45 pm (SD 73 min, range 12:24–5:27 pm). Saliva samples were immediately frozen and subsequently transported on ice to Salimetrics LLC and stored frozen at −80°C until assayed for cortisol. On the day of testing, all samples were centrifuged at 3000 rpm for 15 min to remove mucins.
MEASURES
Cortisol
Raw cortisol values, in μg/dL, were truncated if out of range (>3 SD above the mean value for each time point), a common approach with extreme cortisol values (e.g., [Dettling, Gunnar, & Donzella, 1999]). Between three and nine samples were dropped at each time point; all together, 16 youth, approximately 5% of the sample, had out-of-range values at one or more time point. Cortisol values were then log-transformed to adjust for skewness. Area under the curve with respect to ground (AUCg), a measure of total cortisol excretion across the laboratory task including baseline levels, was calculated using logged values and following the trapezoidal formula supplied by Pruessner, Kirschbaum, Meinlschmidt, & Hellhammer (2003) and included all six cortisol data points.
Pubertal Development Scale (PDS)
At T1 and T2, participants reported their level of development on five physical changes associated with pubertal development (height spurt, body hair, skin changes, breast growth/deepening of voice, menarche/facial hair) on a 4-point scale ranging from 1 (has not yet started) to 4 (has completed) (Petersen, Crockett, Richards, & Boxer, 1988). The PDS scores were converted to a 5-point scale to parallel the Tanner stages (Shirtcliff, Dahl, & Pollak, 2009).
Substance Use
The adolescents responded to the computerized Adolescent Delinquency Questionnaire (ADQ; adapted from [Huizinga & Elliott, 1986]), assessing how many times the adolescent had used alcohol and marijuana (separately) in the past 12 months (0 to 5 or more times). The separate scores on alcohol use and marijuana use were used as latent variable indicators of the adolescent’s substance use at T3 and T4.
Delinquency
Participants reported on their own delinquent behaviors within the past year via 23 items from the Adolescent Delinquency Questionnaire (ADQ; adapted from [Huizinga & Elliott, 1986]). Three scales were used: status offenses (6 items, e.g., “run away from home”), person offenses (7 items, e.g., “carried a hidden weapon”), and property offenses (10 items, e.g., “damaged or destroyed someone else’s property on purpose”). Items were summed to yield a composite score for each scale at T3 and T4 and transformed using square root +1 to reduce skewness.
Depressive Symptoms
Adolescents completed the 27-item Children’s Depression Inventory (CDI; (Kovacs, 1981, 1992). They rated statements such as “I am sad all the time” and “I feel like crying every day,” on a three-point scale (range of possible scores = 0–54). The CDI has demonstrated good reliability and been shown to correlate with other measures of childhood depressive symptoms (Kovacs, 1992).
Covariates
Covariates included age at T1, Body Mass index (a known predictor of pubertal development), race (minority/White), time of the cortisol sample (an important control variable due to the diurnal slope of cortisol), and time lapse between T1 and T2 (because of variation in the interval between assessments).
DATA ANALYSIS
Structural equation models were tested using Mplus with the MLR estimator which produces maximum likelihood parameter estimates with standard errors that are robust to non-normality (Muthén & Muthén, 2006). Latent variables were constructed for delinquency (three manifest indicators) and substance use (two manifest indicators). Maltreatment status, cortisol excretion (AUCg), pubertal stage, and depressive symptoms were included as manifest variables. T2 pubertal stage was regressed on T1 pubertal stage effectively yielding a measure of pubertal change from T1 to T2. A model was stipulated in which maltreatment status predicted T1 cortisol (controlling for sample time and race) and T1 pubertal stage (controlling for race, T1 age, and T1 BMI). T1 cortisol then predicted T2 pubertal stage (controlling for T1 pubertal stage and time lapse between T1 and T2). Cortisol was modeled to have direct effects on T3 delinquency, substance use and depressive symptoms. Direct effects from T2 pubertal stage to T3 outcomes were included in the model. Autoregressive effects from T3 to T4 variables were included as well. All dependent variables were allowed to covary. Model 1 was compared to a Model 2 in which T2 puberty and cortisol had direct effects on T4 outcomes (Model 1 only included an indirect effect via T3 outcomes) and Model 3 which included direct effects from maltreatment to T2 pubertal stage (essentially circumventing the mediation effect of cortisol). We did not include initial levels of these outcomes at T1 and T2 because we were interested in levels of these variables as outcomes rather than change from earlier timepoints. Additionally we did not include T3 and T4 pubertal status because we were interested in the initial early changes of puberty, which may have more impact on short-term functioning, rather than pubertal change throughout development. Variable level and longitudinal missingness was addressed using the MLR estimator. Overall model fit was determined using the MLR chi-square test statistic (asymptotically equivalent to the Yuan-Bentler T2* test statistic), the root mean square error of approximation (RMSEA), and comparative fit index (CFI) (Brown & Cudeck, 1993). Multiple group analysis was employed to run separate models for males and females. Significant indirect effects were calculated using the IND command in Mplus.
Models were also run using a restricted sample removing those youth with more than 2 years between T1 and T2 (n = 21) and those taking a steroid medication (n = 60). Results from the restricted sample did not differ, therefore we report full sample results.
RESULTS
Model Testing
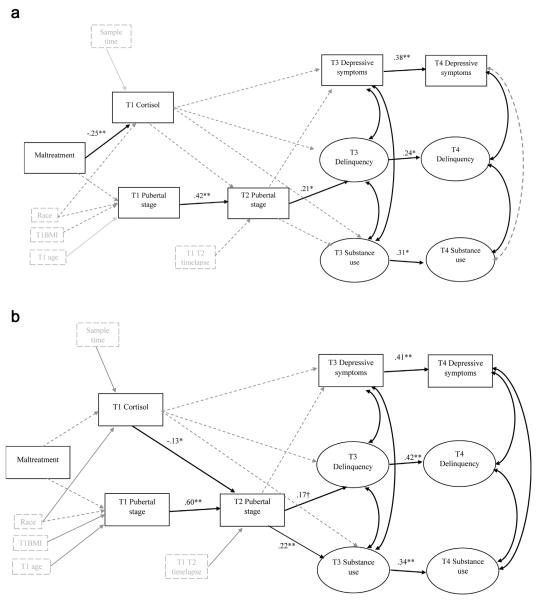
Model 1 showed adequate fit (scaled χ2 530.43 [332]; CFI = .87; RMSEA = .05; 90%CI = .04, .06), Model 2 fit was also adequate (scaled χ2 = 530.36 [320]; CFI = .87; RMSEA = .05; 90%CI = .04, .06), and Model 3 fit was similar (scaled χ2 = 502.29 [316]; CFI = .88; RMSEA = .05; 90%CI = .04, .06),) Due to the similar model fit the model which seemed most parsimonious and had more interpretable significant effects was chosen (Model 1). All parameter estimates for Model 1 can be found in Table 2.
Table 2.
Parameter Estimates for Model 1 by Sex
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| Direct effects | β | S.E. | p | β | S.E. | p |
| Maltreatment→ T1 cortisol | −.25 | .06 | ** | −.01 | .07 | ns |
| Maltreatment→ T1 pubertal stage | −.02 | .06 | ns | .02 | .06 | ns |
| T1 Cortisol → T2 pubertal stage | .03 | .06 | ns | −.13 | .06 | * |
| T2 Pubertal stage → T3 depressive symptoms | .06 | .08 | ns | .12 | .09 | ns |
| T2 Pubertal stage → T3 delinquency | .21 | .10 | * | .17 | .08 | † |
| T2 Pubertal stage → T3 substance use | .09 | .11 | ns | .22 | .08 | ** |
| T1 Cortisol → T3 delinquency | −.05 | .10 | ns | .06 | .09 | ns |
| T1 Cortisol → T3 depressive symptoms | −.11 | .08 | ns | −.03 | .07 | ns |
| T1 Cortisol → T3 substance use | −.13 | .11 | ns | .11 | .10 | ns |
| Auto-regressive effects | ||||||
| T1 Pubertal stage → T2 pubertal stage | .42 | .06 | ** | .60 | .04 | ** |
| T3 Substance use → T4 substance use | .31 | .15 | * | .34 | .13 | ** |
| T3 Delinquency → T4 delinquency | .24 | .11 | * | .42 | .11 | ** |
| T3 Depressive symptoms → T4 depressive symptoms | .38 | .07 | ** | .41 | .06 | ** |
| Covariates | ||||||
| Race → T1 pubertal stage | −.08 | .07 | ns | .11 | .07 | ns |
| T1BMI → T1 pubertal stage | .09 | .07 | ns | .10 | .05 | † |
| T1age → T1 pubertal stage | .21 | .07 | ** | .56 | .05 | ** |
| Sample time → T1 cortisol | −.24 | .06 | ** | −.23 | .07 | ** |
| Race → T1 cortisol | −.09 | .06 | ns | −.15 | .07 | * |
| T1T2 timelapse → T2 pubertal stage | .10 | .08 | ns | .16 | .07 | * |
| Correlations | r | p | r | p | ||
|
| ||||||
| Time 3 | ||||||
| Substance use ↔ delinquency | .72 | .11 | ** | .91 | .10 | ** |
| Substance use ↔ depressive symptoms | .28 | .10 | ** | .16 | .08 | † |
| Delinquency ↔ depressive symptoms | .32 | .10 | ** | .31 | .08 | ** |
| Time 4 | ||||||
| Substance use ↔ delinquency | .65 | .08 | ** | .62 | .12 | ** |
| Substance use ↔ depressive symptoms | .15 | .11 | ns | .20 | .12 | † |
| Delinquency ↔ depressive symptoms | .28 | .08 | ** | .39 | .08 | ** |
p < .05;
p < .01;
p < .08.
Note: All parameter estimates are standardized. Covariates were T1age, T1BMI, T1sample time, T1T2 timelapse, race (minority/nonminority). T1 = Time 1, T2 = Time 2, T3 = Time 3.
Males
As shown in Figure 1, there were significant direct effects from maltreatment to T1 cortisol (β = −.25, p < .01) and from T2 pubertal stage to T3 delinquency (β = .21, p < .05). No significant mediation effects were found.
FIGURE 1.
Structural equation model for (a) males and (b) females showing significant parameter estimates. Note: All parameter estimates are standardized. Covariates are indicated by grey boxes. Significant parameters between covariates and other variables are indicated by grey solid lines. Parameters included in the model but that were not significant are indicated by grey dashed lines.
Females
Significant direct effects were found from T1 cortisol to T2 pubertal stage (β = −.13, p < .05) and T2 pubertal stage to T3 substance use (β = .23, p < .01) one trend direct effect was found from T2 pubertal stage to T3 delinquency (β = .14, p < .08). One trend mediation effect emerged, T1 cortisol to T2 pubertal stage to T3 substance use (p = .09).
DISCUSSION
This study is the first to test a comprehensive longitudinal model linking childhood maltreatment to cortisol responding; cortisol responding to pubertal development; and pubertal development to psychosocial outcomes. Hypotheses were partially supported, with different associations emerging for girls versus boys.
For boys, maltreatment was associated with attenuated cortisol patterns, supporting the attenuation hypothesis (Susman, 2006) and replicating Trickett et al. (2014). Cortisol excretion and pubertal change were not significantly associated for boys (consistent with the previous paper, Saxbe et al. (2014), which found effects only for girls). This may to due, in part to gender differences in HPA responses as well as the different timing of pubertal maturation for boys than girls. In line with Marceau et al. (2011), more pubertal change from Time 1 to Time 2 predicted delinquency at the following assessment. Although Mendle et al. (2010) found that faster tempo for boys predicted less rapid decreases in depressive symptoms, we did not find links between pubertal change and depressive symptoms for boys. Our findings may have differed because we included depression only at Time 3 and 4 (mid-to-late adolescence), while Mendle et al. (2010) used an age range of 8–14. The long term effects of pubertal development on psychological outcomes are not well understood, and the present study indicates that perhaps pubertal effects on depression may be limited to short term assessments. Additionally, counter to Castellanos-Ryan et al. (2013), we did not find an association between pubertal change and substance use for boys, which may be due, in part, to the different operationalization of the puberty construct.
For girls, maltreatment did not predict attenuated cortisol. This is inconsistent with other literature showing the effects of maltreatment on cortisol response for girls (De Bellis & Putnam, 1994; MacMillan et al., 2009). However, studies of sexual abuse and the stress response have shown initial elevation of the HPA axis which transitions to attenuation in late adolescence and early adulthood (Trickett, Noll, Susman, Shenk, & Putnam, 2010). Thus it may be that the girls in the present study were still in the period of initial elevation and had not yet downregulated or were in the midst of downregulation. More longitudinal, prospective research is needed to determine whether adversity-related attenuation in cortisol occurs prior or subsequent to puberty. Consistent with Saxbe et al. (2014) and Ellis et al. (2011), there was a significant association between lower cortisol and more pubertal change from Time 1 to Time 2. Additionally, more pubertal change predicted substance use (and, at a trend level, delinquency) at the third assessment. Again, similar to boys, and in line with Mendle et al. (2010), pubertal change and depressive symptoms were not associated for girls. Only one mediation effect emerged, at a trend level, linking attenuated cortisol to subsequent more pubertal change to subsequent substance use. In other words, the association between girls’ attenuated cortisol and subsequent increases in substance use was accounted for by more pubertal change between Time 1 and 2. This mediation effect should be interpreted cautiously and warrants replication because the significance level was only a trend. No other mediation effects emerged for girls or boys.
Our study had several limitations. First, the age range may cover several developmental periods during which the outcomes may be more or less prevalent. Although we accounted for age in the model, this does not account for possible developmental period differences. Second, the pubertal measures were self-report, which have shown to be biased differently for boys and girls (Dorn, Susman, Nottelmann, Inoff-Germain, & Chrousos, 1990). Also, the pubertal change measure we used may be indexing pubertal tempo (i.e., rate of change) or those adolescents more in the midst of change (which is related to age). Pubertal tempo is more commonly measured with more than two data-points, thus we are limited in our inference of this measure. The cortisol measure (AUCg) is reflective of general HPA axis functioning rather than in response to a stress task. This has implications for the interpretation of the results. As we were interested in levels of the outcomes at Time 3 and 4, not change from Time 1, we only included psychosocial outcomes at Time 3 and 4. This is a limitation in the sense that our outcomes at Time 3 and 4 do not control for initial levels of psychosocial functioning. Similarly, we did not include puberty at Time 3 and 4 because we were interested in the effects of early pubertal change on later outcome, not on the effect of pubertal development across adolescence. Studies have shown that early changes are more deleterious than those in mod-adolescence, which informed our inclusion of puberty only at Time 1 and 2. Unreported maltreatment that occurred in the comparison group could not be accounted for, which may reduce the power to detect group differences. We were unable to examine different types of maltreatment due to limited size of maltreatment type groups. Research has shown that certain types of maltreatment (e.g., sexual abuse) may have stronger impact on the HPA functioning and pubertal development. This study does not address the different mechanisms that may operate for the various outcomes, which may have implications for which outcomes are more or less linked to pubertal tempo for males versus females.
In conclusion, the current study provides partial support for a model in which alterations in HPA axis functioning affect the rate of pubertal development across one year, which, in turn, affects substance use in mid-late adolescence. Interestingly, this pathway only emerged for girls, demonstrating that HPA activity may have a more substantial effect on the HPG for females than males. Other work has suggested that early life stress may affect the coupling of the HPA-HPG axes in girls, such that girls exposed to greater stress show a more adult-like negative coupling in early rather than late adolescence (Ruttle et al., 2013). However, our analyses suggest that males’ cortisol patterns may be more affected by childhood maltreatment, which may lead to other (non-pubertal) health and psychological risks in the future. The findings from this study add to the literature on the disparate effects of early adversity on stress responding between genders and point to HPA functioning as a key mechanism linking pubertal development with health and well-being.
REFERENCES
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Boynton-Jarrett R, Wright RJ, Putnam FW, Lividoti Hibert E, Michels KB, Forman MR, Rich-Edwards J. Childhood abuse and age at menarche. Journal of Adolescent Health. 2013;52(2):241–247. doi: 10.1016/j.jadohealth.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Sage Publications; Newbury Park: 1993. pp. 136–162. [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Weir LM, Mello AF, Mello MF, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Parent S, Vitaro F, Tremblay RE, Seguin JR. Pubertal development, personality, and substance use: A 10-year longitudinal study form childhood to adolescence. Journal of Abnormal Psychology. 2013;122(3):782–796. doi: 10.1037/a0033133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh SE. The sexual debut of girls in early adolescence: The intersection of race, pubertal timing, and friendship group charateristics. Journal of Research on Adolescence. 2004;14(3):285–312. [Google Scholar]
- Costello EJ, Sung M, Worthman C, Angold A. Pubertal maturation and the development of alcohol use and abuse. Drug and Alcohol Dependence. 2007;88S:S50–S59. doi: 10.1016/j.drugalcdep.2006.12.009. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Putnam FW. The psychology of childhood maltreatment. Child and Adolescent Psychiatric Clinics of North America. 1994;3(4):663–678. [Google Scholar]
- Deardorff J, Abrams B, Ekwaru JP, Rehkopf DH. Socioeconomic status and age at menarche: An examination of multiple indicators in an ethnically diverse cohort. Annals of Epidemiology. 2014;24(10):727–733. doi: 10.1016/j.annepidem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day childcare centers: Relations with age and temperament. Psychoneuroendocrinology. 1999;24:519–536. doi: 10.1016/s0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Nottelmann ED, Inoff-Germain G, Chrousos GP. Perceptions of puberty: adolescent, parent, and health care personnel. Developmental Psychology. 1990;26:322–329. [Google Scholar]
- Ellis BJ. Timing of Pubertal Maturation in Girls: An Integrated Life History Approach. Psychological Bulletin. 2004;130(6):920–958. doi: 10.1037/0033-2909.1130.1036.1920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce T, Deardorff J, Essex MJ. Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology. 2011;23:85–99. doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, Van Pelt J, Spinhoven P. Dimished cortisol responses to psychosocial stress assocaited with lifetime adverse events: Astudy of healthy young subjects. Psycho-neuroendocrinology. 2008;33(2):227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Foster H, Hagan J, Brooks-Gunn J. Growing up fast: Stress exposure and subjective “weathering” in emerging adulthood. Journal of Health and Social Behavior. 2008;49:162–177. doi: 10.1177/002214650804900204. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH. Pubertal transition, stressful life events and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37(3):404–417. doi: 10.1037/0012-1649.1037.1033.1404. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones and Behavior. 2008;53(1):96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(12):1768–1776. doi: 10.1097/00004583-199712000-199700026. [DOI] [PubMed] [Google Scholar]
- Huizinga D, Elliott DS. Reassessing the reliability and validity of self-report delinquency measures. Journal of Quantitative Criminology. 1986;2(4):293–327. [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica: International Journal of Child & Adolescent Psychiatry. 1981;46(5–6):305–315. [PubMed] [Google Scholar]
- Kovacs M. Child Depression Inventory Manual. Multi-Health System; Toronto: 1992. [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Neic A, Schmidt LM. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the Youth Mood Project. Biological Psychiatry. 2009;66(1):62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonliner growth models. Developmental Psychology. 2011;47(5):1374–1388. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Harden PK, Brooks-Gunn J, Graber JA. Development’s tortoise and hare: Pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Developmental Psychology. 2010;46(5):1341–1353. doi: 10.1037/a0020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Leve LD, Van Ryzin M, Natsuaki MN. Linking childhood maltreatment with girls’ internalizing symptoms: Early puberty as a tipping point. Journal of Research on Adolescence. 2014;24(4):689–702. doi: 10.1111/jora.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Leve LD, Van Ryzin M, Natsuaki MN, Ge X. Associations between early life stress, child maltreatment, and pubertal development among girls in foster care. Journal of Research on Adolescence. 2011;21(4):871–880. doi: 10.1111/j.1532-7795.2011.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuén LK, Muthén BO. Mplus user’s guide. Muthén & Muthén; Los Angeles, CA: 2006. [Google Scholar]
- Negriff S, Susman EJ. Pubertal timing, depression and externalizing problems: A framework, review, and examination of gender differences. Journal of Research on Adolescence. 2011;21(3):717–746. [Google Scholar]
- Negriff S, Trickett PK. The relationship between pubertal timing and delinquent behavior in maltreated male and female adolescents. Journal of Early Adolescence. 2010;30(4):518–542. doi: 10.1177/027243160338180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Kirschbaum C, Meinlschmidt G, Hell-hammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Romans SE, Martin JM, Gendall K, Herbison GP. Age of menarche: the role of some psychosocial factors. Psychological Medicine. 2003;33:933–939. doi: 10.1017/s0033291703007530. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klien MH, Essex MJ. Neuroendrocrine coupling across adolescence and the longitudinal influence of early life stress. Developmental Psychobiology. 2013 doi: 10.1002/dev.21138. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Negriff S, Susman EJ, Trickett PK. Attenuated HPA axis functioning predicts accelerated pubertal development in girls one year later. Development and Psychopathology. 2014 doi: 10.1017/S0954579414000790. epub ahead of print. DOI http://dx.doi.org/10.1017/S0954579414000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Development. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Developmental Psychology. 2001;37(5):608–619. doi: 10.1037/0012-1649.1037.1035.1608. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience & Biobehavioral Reviews. 2006;30(3):376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Gordis E, Peckins MK, Susman EJ. Stress reactivity in maltreated and comparison male and female young adolescents. Child Maltreatment. 2014;19(1):27–37. doi: 10.1177/1077559513520466. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology. 2010;22(1):165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett PK, Putnam FW. Impact of child sexual abuse on females: Toward a developmental, psychobiological integration. Psychological Science. 1993;4(2):81–87. [Google Scholar]