Abstract
Forkhead box Class O is one of 19 subfamilies of the Forkhead box family, comprising 4 human transcription factors: FOXO1, FOXO3a, FOXO4, and FOXO6, which are involved in many crucial cellular processes. FOXO3a is a tumor suppressor involved in multiple physiological and pathological processes, and plays essential roles in metabolism, cell cycle arrest, DNA repair, and apoptosis. In its role as a transcription factor, the FOXO3a binds a consensus Forkhead response element DNA sequence, and recruits transcriptional coactivators to activate gene transcription. FOXO3a has additional functions, such as regulating p53-mediated apoptosis and activating kinase ATM. With the exception of the structured DNA-binding forkhead domain, most of the FOXO3a sequence comprises intrinsically disordered regions (IDRs), including 3 regions (CR1-3) that are conserved within the FOXO subfamily. Numerous studies have demonstrated that these IDRs directly mediate many of the diverse functions of FOXO3a. These regions contain post-translational modification and protein-protein interaction sites that integrate upstream signals to maintain homeostasis. Thus, the FOXO3a IDRs are emerging as key mediators of diverse regulatory processes, and represent an important target for the future development of therapeutics for FOXO3a-related diseases.
Keywords: apoptosis, cancer, FOXO3a, intrinsically disordered protein (IDP), protein-protein interaction, post-translational modification, transcriptional regulation
Introduction
The Forkhead box (Fox) family of transcription factors contains more than 100 members, which can be divided into 19 subclasses based on phylogenetic analysis (from FOXA to FOXS).1,2 The Fox genes originated in unicellular organisms, and during evolution, the family expanded through multiple duplication events. Fox genes are present exclusively in animals and fungi, but not in plants.3 Fox transcription factors can directly activate or repress gene transcription, or function through interacting with other transcription factors to affect gene regulation. Fox family proteins have acquired distinct as well as cooperative functions involving regulation of many key biological processes.4 For instance, FOXA transcription factors function in organ development;5 FOXC1 and FOXC2 play roles in cardiovascular development;6 FOXP2 exhibits a unique role in humans where it is required for language acquisition,7 while FOXP3 is a key regulator of the immune system response and is also involved in cancer.8
The class O subfamily (FOXO) proteins have been identified in many species, including C. elegans, D. rerio, D. melanogaster, G. gallus, mouse, and human.9 There are 4 mammalian FOXO proteins: FOXO1 (FKHR), FOXO3a (FKHRL1), FOXO4 (AFX), and FOXO6. The first 3 members are regulated by the PI3K/Akt pathway,10,11 and are ubiquitously expressed, although the expression level of each protein varies significantly in different organs or tissues.12 This review focuses on FOXO3a, which is highly expressed in liver and brain, and was identified through cDNA library screening and localized to chromosomal region 6q21.13,14 Chromosomal rearrangements occurring in some leukemias result in the production of a chimeric protein in which FOXO3a is fused to MLL.13 Although there is a degree of functional redundancy within the FOXO subfamily, FOXO3a exhibits some unique roles.15 FOXO3a knockout mice are viable, but females show age-dependent infertility.16 Recent biodemographic studies have established a strong relationship between FOXO3a genotype and human longevity in diverse human populations, including Hawaiian men of Japanese ancestry,17 German,18 Italian,19 Han Chinese,20 and Ashkenazi Jewish peoples,21 while no such correlation has been revealed for FOXO1, FOXO4, or FOXO6.22
FOXO3a has been established as a bona fide tumor suppressor, and inhibition of FOXO3a by abnormally activated Akt has been implicated in leukemia, liver, breast, and prostate cancers.23-25 Deletion of somatic FOXO genes in mice resulted in cancer-prone phenotypes, with increased incidence of thymic lymphomas and hemangiomas.16 FOXO3a regulates the expression of genes involved in apoptosis, cell cycle arrest, cell differentiation and stress resistance,26 including Bim, PUMA, cycling G2, p27, and Manganese-Superoxide Dismutase.27-31 Several oncogenic pathways negatively modulate FOXO3A activity, including the IKKβ, ERK and PI3K/AKT signaling cascades.29,32-36 Conversely, in some colon cancer cells, FOXO3a plays a role in the development of cisplatin drug resistance.37 Further, in chronic myelogenous leukemia (CML), activated FOXO3a protects leukemia initiation cells against Imatinib treatment, possibly through its function in stress resistance.38 Moreover, FOXO3a has diverse roles in response to a variety of stimuli and dysregulation of FOXO3a is related to the development of several types of disease.39
In addition to in vitro and in vivo functional studies, structural information obtained from biochemical and structural characterization is critical to developing a more comprehensive understanding of the functional versatility of FOXO3a.40 FOXO3a is particularly rich in intrinsically disordered regions (IDRs), which lack well-defined 3D conformations, but may transiently sample multiple conformations. As much as 75% of the sequence is predicted to comprise IDRs, thus FOXO3a is considered to be an intrinsically disordered protein (IDP).41-44 Because IDRs do not form a rigid structure, IDPs are remarkably versatile and many can form distinct interactions with other proteins in response to different cellular signals. The IDRs of FOXO3a execute many important functions, such as signal integration and mediating protein-protein interactions. In this review, we discuss the current understanding of the diverse functions of the FOXO3a IDRs, and their roles in diseases, as well as biochemical and biophysical studies of the structurally ordered and disordered domains of FOXO3a that are elucidating the structure-function relationship of this multi-functional protein.
Domain Architecture of FOXO3a
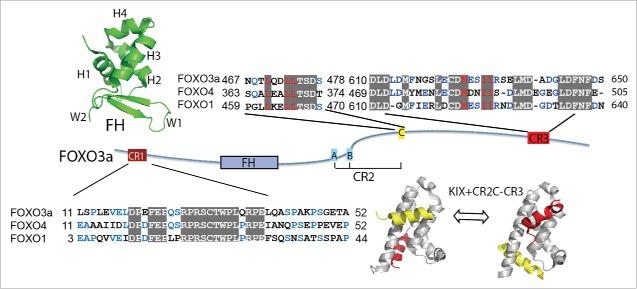
All Fox proteins have a conserved forkhead (FH) DNA-binding domain of about 100 residues and one or more transactivation domains (TADs), while other parts vary in length and sequence among different subfamilies (Fig. 1). Human FOXO3a is a 673-amino acid protein with a N-terminal FH domain that comprises a winged-helix fold. Based on primary sequence analysis,45 the rest of FOXO3a is predicted to be highly disordered, although these IDRs contain 3 regions (CR1-CR3) conserved among the class O subfamily (Fig. 2) that have been reported to comprise the TADs.4648 The overall conformation of FOXO3a thus resembles a rigid “head” followed by a flexible “tail.”
Figure 1.
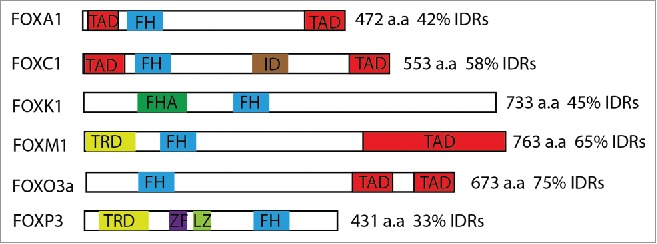
Schematic diagram of the structural organization and IDR content of representative Fox proteins. The number of residues and the percentage of IDRs are listed on the right. TAD: transcactivation domain; FH: forkhead domain; ID: inhibitory domain; FHA: forkhead-associated domain; TRD: transcriptional repressor domain; ZF: zinc finger domain; LZ: leucine zipper domain. Human Fox proteins have high ratio of IDRs.
Figure 2.
Domain architecture and sequence alignment of FOXO transcription factors. The domain architecture of FOXO3a contains a well-folded Forkhead (FH) DNA-binding domain, and 3 conserved regions, CR1-CR3. Sequence alignments of CR1, CR2C and CR3 are shown with conserved residues highlighted in gray boxes. The solution structure of FH (PDB: 2K86) is shown above and the secondary structure elements are labeled. The structures of 2 states of CR2C and CR3 bound to the KIX domain of CBP (PDB: 2LQI and 2LQH) are shown below, with CR2C colored yellow and CR3 colored red.
The forkhead DNA-binding domain
The FH DNA-binding domain is the key feature of the Fox family proteins. The crystal structure of FH domain exhibits a “winged-helix” motif, with 3 α helices and 2 large disordered loops that resemble the wings of a butterfly.49 The FH domain of FOXO proteins has a unique “GDSNS” 5-residue insertion relative to members of the other Fox subfamilies, which makes FOXO the most divergent Fox subfamily.40 All FOXO FH domains recognize a consensus DNA sequence motif, 5′-(G/C/A) (T/C/A) AAA(T/C) A-3′, named Forkhead response element (FRE).50 Much effort has focused on determining structures of FH domain, and many solution and X-ray structures of the FH domains of FOXO proteins alone or in complex with DNA have been determined.41,51-54 The FH domain of FOXO3a possesses 3 stacking major α-helices (H1–H3), an antiparallel β-sheet comprising 3 β-strands, and 2 large unstructured wings (W1, W2). The 5-residue insertion partially overlaps a short fourth helix H4, which is poorly defined in the solution structure, with boundaries that vary among different conformers (Fig. 2).41 The FOXO3a FH domain binds the double-stranded FRE DNA motif through extensive contacts involving the perpendicular insertion of helix H3 into the major groove, as well as interactions with the C-terminal region, which forms a coil structure. In addition, Wing 1 interacts with the phosphate backbone of the DNA.52 Biochemical characterization of longer FH constructs showed that the N-terminal flexible region contributes to DNA binding, although this region was not present in the constructs used in structural studies.55 The overall structure of the FH domain does not exhibit substantial changes upon DNA-binding.
The intrinsically disordered regions
IDRs are particularly abundant in proteins involved in gene transcription and translation, as well as signal transduction.42 Mammalian Fox transcription factors are generally large, with a high content of IDRs (over 30%), and most, if not all, TADs of Fox transcription factors are predicted to reside in IDRs (Fig. 1). The transcription factor FOXO3A contains extensive IDRs, however the elucidation of structural information about these regions has lagged behind that of the more extensively studied FH domain. Newly developed paradigms in biochemical methods have enabled the characterization of IDRs, and the IDRs of FOXO3a have come to light with increasing recognition of their functional importance. Within the IDRs, the conserved regions CR1-CR3 are unstructured in solution, but have the propensity to form helical structures, as evidenced by circular dichroism (CD) spectra.41,48 CR1 is a Proline-rich region, and CR2 contains 3 short separated segments (CR2A, B, and C). The CR2C and CR3 regions contain ΦxxΦΦ motifs, and were identified to be the TADs (Fig. 2).46-48 We previously showed that within the FOXO3a molecule, the FH and CR3 domains engage in a low affinity intramolecular interaction. Furthermore, we used NMR titrations to map the CR3-binding site on FH to a region comprised of the N-terminal loop, H1 and H3, which overlaps with the DNA binding site.41 Thus the FOXO3a molecule exists in a “closed” conformation, in which both the DBD and TAD are partially autoinhibited. However, competition assays revealed that FRE DNA disrupts this intramolecular interaction, displacing CR3 from FH, thus making CR3 accessible to the transcriptional coactivator and facilitating transcriptional activation.56 We speculate this intramolecular interaction sequesters both CR3 and FH from non-specific interactions. The precise sequence of the IDRs that link the conserved motifs is not well conserved in FOXO proteins, but the presence of long linkers is conserved in all mammalian FOXOs through evolution. We found that the length of the linker between CR2C and CR3 influenced transcriptional activation.48
Post-Translational Modifications
FOXO3a plays pivotal roles in cell fate determination, and it has been implicated in opposing outcomes. FOXO3a receives signals from a variety of cellular stimuli, including growth factors, metabolic stress, and oxidative stress, and transduces these signals to responses that modulate protein-protein interactions and the spatial and temporal expression of genes, resulting in either apoptosis or protective cell growth arrest.57,58 These processes are precisely regulated by reversible post-translational modifications (PTMs), including phosphorylation, acetylation, and ubiquitination. These reversible PTMs can have additive or competitive effects on the stability, subcellular localization, DNA-binding, and/or transactivation functions of FOXO3a (Table 1).59
Table 1.
Summary of known PTMs of FOXO3a
| Modification enzyme | Modification site(s) | Type of modification | Effect |
|---|---|---|---|
| Akt/PKB | T32, S253, and S315 | Phosphorylation | Translocation |
| AMPK | T179, S399, S413, S555, S588, and S626 | Phosphorylation | Transcription |
| CK1 | S317 and S320 | Phosphorylation | Translocation |
| DYRK | S324 | Phosphorylation | Translocation |
| ERK | S294, S344, and S425 | Phosphorylation | Degradation |
| Erythropoietin | T32 | Phosphorylation | Transcription |
| IKKβ | S644 | Phosphorylation | Degradation |
| MST1 | S207, S213, S299, and S230 | Phosphorylation | Translocation |
| SCF | T32 | Phosphorylation | Transcription |
| SGK | T32 and S319 | Phosphorylation | Translocation |
| CBP/p300 | K242, K245, and K259 | Acetylation | Transcription |
| PCAF | K290, K271, and K569 | Acetylation | Transcription |
| Sirt1 | K242, K245, and K262 | Deacetylation | Transcription |
| MDM2 | Not known | Ubiquitination | Degradation |
| Set9 | K271 | Methylation | Transcription |
| O-GlcNAc | Not known | O-GlcNAc | Transcription |
The IDRs of FOXO3a are enriched in sites of PTMs. Compared with structured domains, the flexibility and conformational plasticity of IDRs confer accessibility to PTM enzymes, thus many PTMs occur preferentially in IDRs.60-62 The long FOXO3a IDRs (∼500 a.a.) incorporate a large number of PTM sites, and each molecule can be modified simultaneously at multiple sites. Intricate integration of these multiple PTMs confers high-fidelity FOXO3a responses. The combination of various PTMs is critical to the function of FOXOs, thus this phenomenon has been described as the “FOXO code” akin to the “histone code” that integrates multiple PTMs of histone proteins to regulate chromatin structure.15
Phosphorylation
FOXO3a is predominantly regulated by Akt phosphorylation, which affects its subcellular localization. Activation of the PI3K pathway is triggered by many stimuli, including insulin, insulin like growth factor, epidermal growth factor and erythropoietin, which lead to activation of the downstream protein kinase Akt,9 which in turn inactivates FOXO3a by phosphorylating 3 conserved sites in the IDRs, T32, S253, and S315. Phosphorylated T32 and S253 form binding sites for 14-3-3 proteins, which facilitate nuclear exclusion of FOXO3a, thereby blocking its role in transactivation.34 Furthermore, phosphorylation of S253 and 14-3-3 binding in close proximity to the FOXO3a FH domain disrupt DNA-binding, further down-regulating its transcriptional activity.63,64 Serum and glucocorticoid-regulated kinase (SGK) is also activated downstream of PI3K, and directly phosphorylates FOXO3a at sites overlapping those recognized by Akt. Both Akt and SGK phosphorylate T32, but SGK exhibits a preference for S315 whereas Akt phosphorylates S253 more efficiently.65 Phosphorylation of S315 ‘primes’ FOXO3a for further phosphorylation by Casein kinase 1 (CK1), which is dependent on Akt/SGK-phosphorylation and has the effect of accelerating cytoplasmic sequestration.66 Dual Specificity Tyrosine Phosphorylated and Regulated Kinase 1A (DYRK1A) has been shown to phosphorylate FOXO3a and contribute to the cytoplasmic translocation as well.67 Phosphorylation-induced cytoplasmic translocation involves the binding of RanGTPase and CRM1 to a nuclear export sequence (NES) found in the IDRs of FOXO3a.68 Alternatively, phosphorylation of certain sites can induce nuclear translocation of FOXO3a. Phosphorylation of FOXO4 by JNK results in nuclear localization,69 and although these phosphorylation sites are not conserved in FOXO3a, JNK regulates FOXO3a in a phosphorylation-independent manner by shuttling it in and out of the nucleus.70,71 Recent studies have revealed that phosphorylation of FOXO3a by mammalian sterile 20-like kinase 1 (MST1) disrupts the binding of 14-3-3, allowing FOXO3a to translocate back into the nucleus.72 In addition to regulating subcellular localization, phosphorylation of FOXO3a IDRs can also affect other functions. For instance, AMPK phosphorylation of FOXO3a enhances its transcriptional activity without altering subcellular localization or DNA binding.73 Erk and Ikκβ phosphorylation of FOXO3a trigger its degradation.74,75 The phosphorylation of FOXO3a is reversible, and PP2A has been identified as a protein phosphatase that dephosphorylates FOXO3a.76 Many anti-cancer drugs (e.g. cisplatin and melatonin) induce FOXO3a dephosphorylation.77,78
Acetylation
The transcriptional function of FOXO3a is also modulated by acetylation. The transcriptional coactivator CBP and its closely related paralog p300 (CBP/p300) possess histone acetyltransferase (HAT) activity that acetylates histone tails resulting in chromatin remodeling, and can modify non-histone proteins as well, including FOXOs and other transcription factors.79-81 Acetylation of FOXO3a by CBP/p300 reduces DNA binding, thus resulting in negative feedback,77,82 while phosphorylation of T32 by erythropoietin or stem cell factor (SCF) abolishes acetylation.80 CBP/p300 acetylation sites are within or close to the FH domain (Table 1). Structural studies of FOXO3a and FOXO1 indicated that acetylation of these conserved Lys residues decreases the affinity of FH for DNA, thus reducing transcriptional activity.52,53,83 However, other studies suggest that acetylation of FOXO3a can enhance transcription of a specific group of genes.84,85 Therefore, the dual role of CBP/p300 as both transcriptional coactivator and PTM enzyme is essential in regulating FOXO3a transcription. Similar to phosphorylation, FOXO3a acetylation is reversible: Histone deacetylases (HDACs), Sirt1 and Sir2, catalyze the removal of acetyl groups from sites modified by CBP/p300.86,87 Sirt1 binds FOXO3a through the LXXLL region of CR2C,88 and the effects of deacetylation are gene and cell-type specific, either enhancing or reducing FOXO-dependent transcription.68,84,85 In general, acetylation of FOXO3a is presumed to promote cell survival,89 while deacetylation by Sirt1 promotes autophagy.90 Thus the interplay among FOXO3a, CBP/p300, and Sirt1, mediated by the FOXO3a IDRs, fine-tunes the function of FOXO3a.
Ubquitination
Degradation of FOXO3a is directly regulated by ubiquitination. It has been reported that IKKβ and the RAS-ERK pathways promote MDM2-mediated poly-ubiquitination of FOXO3a, which leads to proteasome-dependent FOXO3a degradation.32,74 MDM2 interacts with FOXO3a in vitro and in vivo, and while the FH domain is required for MDM2 binding, the ubiquitination sites have not been identified.91 Skp2-mediated FOXO3a ubiquitination and proteasomal degradation is promoted by deacetylation of FOXO3 by Sirt1 or Sirt2.92 Mono-ubiquitination, but not poly-ubquitination, is reversible, and the deubiquitination is catalyzed by Herpesvirus-associated ubiquitin-specific protease (HAUSP), which is important for stress responses.93
Other modifications
FOXO3a is also subject to other PTMs, including methylation94 and glycosylation, i.e., O-linked β-N-acetylglucosamine (O-GlcNAc) modification.95 Methylation of Lys 271 by Set9 decreases the stability of FoxO3a, but moderately increases its transcriptional activity, which represents another fine-tuning mechanism. FOXO1 has been shown to be modified by addition of O-GlcNAc, which is proposed to lead to increased expression of gluconeogenic genes as well as those involved in detoxification of reactive oxygen species. FOXO1 can undergo poly(ADP-ribosyl)ation,96 but it is not clear whether this applies to FOXO3a. Many FOXO3a PTMs interact with each other, and can function in combination or compete with each other, thus deciphering the FOXO code is essential for better understanding the functional mechanisms of FOXO3a.
Protein-Protein Interactions
FOXO3a engages in a variety of physical interactions with other proteins under physiological and pathological conditions. As a transcription factor, FOXO3a interacts with coactivators and general transcription factors to up- or down-regulate gene transcription. The FOXO3a FH domain can interact with the DNA-binding domains of other transcription factors, such as Smad and RUNX3 that bind to adjacent sites on DNA, thus enabling FOXO3a to function synergistically with these other transcription factors, dramatically enhancing the response to stimuli (Fig. 3A).97,98 Additionally, FOXO3a performs many transcriptional independent functions through cross-talk with other proteins.99 The IDRs of FOXO3a, particularly the conserved regions CR1-CR3, mediate these functionally important interactions. IDRs lack stable 3-dimensional structures, but rapidly sample a wide range of conformations.100 IDRs often fold into ordered states upon interacting with partners, and their ability to adopt different conformations enables interactions with multiple proteins.101 The coupled folding and binding of IDRs can be described by 2 mechanisms: induced fit and conformational selection.102-105 In addition to mediating promiscuous interactions, IDRs can confer high-specificity, low-affinity, rapid reversibility, and multi-valency to protein-protein interactions.106-108 Thus the presence of IDRs in FOXO3a greatly enhances its capability of binding to other proteins, and several FOXO3a-mediated interactions are discussed below.
Figure 3.
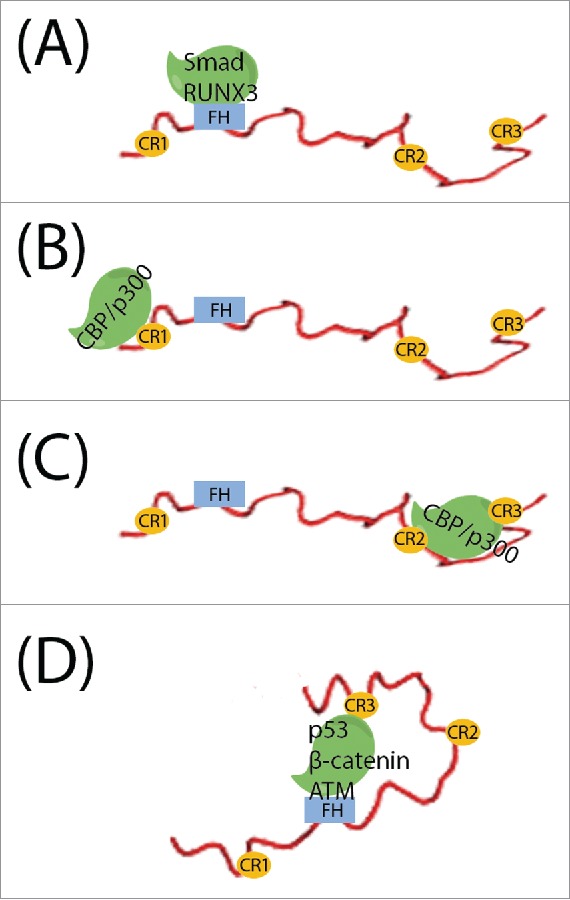
Modes of FOXO3a-mediated protein-protein interaction. (A) Only the FH domain interacts with the binding partner. (B) One IDR interacts with the binding partner. (C) Multiple regions of IDRs are involved in binding. (D) The FH domain and IDRs together mediate the interaction.
FOXO3a-CBP/p300 interaction
CBP/p300 contain several conserved domains, namely the TAZ1, TAZ2, KIX, NCBD, Bromo, and HAT domains.109,110 CBP/p300 serves as a transcriptional coactivator for many important transcription factors, including p53,111 E2A,112 and FOXO3a,48,110 and also acts as a histone acetyltransferase to remodel chromatin thereby rendering genes accessible for transcription. The interaction between CBP/p300 and FOXO3a is promiscuous and multi-valent, involving several domains and regions on both proteins. The binding between FOXO3a CR1 and the C-terminus of CBP/p300 mediates acetylation of FOXO3a by CBP/p300, whereas phosphorylation of T32, within the CR1 region, abolishes the binding and acetylation (Fig. 3B).80 However, this association does not lead to transcriptional activation, which is mediated by the C-terminal IDRs of FOXO3a. Our recent studies found that in addition to the previously known transactivation domain CR3, another IDR, CR2C also contributes to the transactivation,48 FOXO3a CR2C and CR3 both contain “ϕXXϕϕ” motifs that interact simultaneously with the KIX domain of CBP/p300 at 2 binding sites designated the “MLL-binding” and “c-Myb-binding” sites (Fig. 3C). An engineered CR2C-CR3 fusion peptide in complex with KIX exists in equilibrium between 2 equally populated conformational states. We determined the solution structures of these 2 states: In one state CR2C bound to the MLL site and CR3 bound to the c-Myb site, whereas in the other, CR2C and CR3 bound the c-Myb and MLL sites, respectively (Fig. 2). These are the first structures revealing the bound state of FOXO3a IDRs. Further, we observed that CR2C and CR3 also interact with the TAZ1/2 domains, and that AMPK phosphorylation of S626 in CR3 can enhance these interactions. Luciferase assays demonstrated that all of these interactions contribute to productive gene transactivation, indicating that a synergistic effect can be generated through multi-valent binding. The theoretical Kd value for the interaction between full-length FOXO3a and CBP/p300 proteins should be the product of the Kd values for each individual interaction between domains. This phenomenon is critical to the formation of a promoter-anchored transcriptional complex by promoting productive FOXO3a-dependent coactivator recruitment, and is equally important for rapid disassembly of the complex.
FOXO3a-p53 interaction
The tumor suppresser p53 induces apoptosis, cell senescence, or transient cell-cycle arrest, and is regarded as the guardian of the genome.113 Mutations in p53 occur in >50% of human cancers, and the mutational status of p53 is prognostic in many malignancies.114 FOXO3a has been shown to promote the translocation of p53 to the cytoplasm, thereby inducing the mitochondria-associated apoptotic pathway.115 We demonstrated a direct interaction between p53 and FOXO3a,41 mediated by the DNA-binding domain (DBD) of p53 and the FH domain and the CR3 region of FOXO3a. Interestingly the FH-CR3 linker also seems to contribute to the full binding activity (Fig. 3D). By NMR titration, we showed that FH or CR3 alone can bind to p53, exhibiting low millimolar or low micromolar Kd values, respectively. Our data further revealed that within the FOXO3a molecule, the FH and CR3 domains engage in an intramolecular interaction, which can be disrupted by the binding of p53. These results shed light on a complex interplay between the FOXO3a FH and CR3 domains and p53, which contributed to a better understanding of FOXO3a's function in critical cellular processes.
FOXO3a-nuclear hormone receptor interaction
The nuclear hormone receptors (NHRs) are ligand-inducible transcription factors that regulate cell growth, differentiation, metabolism, and reproduction.116 FOXO3a interacts with many NHRs and regulates their function in different manners. The recognition of the LXXLL motif of co-factors, including coactivators, co-repressors, and other transcription factors, is essential for the function of NHRs.117 The CR2C region of FOXO3a contains one such LXXLL motif, which may be involved in interactions with many NHRs.99 The association of FOXO3a with NHRs alters the transcriptional activity of both proteins. For instance, Cornforth et al. observed that FOXO3a interacts with androgen receptor in prostate cancer LNCaP cells, which increases transcription of the anti-apoptotic gene FLIP through the combined effects of both androgens and FOXO3a.118 In contrast, previous studies in prostate cancer cells showed that activated androgen receptor blocked FOXO's ability to bind DNA, thus reducing its transcriptional activity.119 Recently, Estrogen receptors α and β were shown to interact with FOXO3a and increase FOXO3a-dependent transcription.120,121 These interactions between NHRs and FOXO3a link steroid hormone signaling and insulin signaling in oncogenesis.
FOXO3a-β-catenin interaction
Wnt signaling activates β-Catenin and triggers its nuclear localization, where activated β-Catenin functions as the transcriptional coactivator of the T cell factor (TCF) transcription factors.122 FOXO3a sequesters β-Catenin from TCF binding, and thereby serves as a transcriptional repressor of TCF, abolishing its transactivation function in a manner that is independent of DNA-binding.123,124 It has been demonstrated that the FH domain and the C-terminal IDRs of FOXO3a and the first 8 Armadillo domains of β-Catenin are sufficient for full binding (Fig. 3D), while the FH domain is not necessary for transcription inhibition.123 This suggested that both the structured FH domain and the IDRs of FOXO3a contact β-Catenin, but it is the IDRs that execute the major inhibitory function. FOXO3a-β-catenin interaction confers resistance to PI3K and AKT inhibitors and promotes metastasis in colon cancer.125
FOXO3a-ATM interaction
Ataxia telangiectasia mutated (ATM) is a Serine/Threonine protein kinase that normally resides in an inactive state, but becomes activated in response to DNA double-strand breaks, and phosphorylates downstream effectors, including p53 and Chk1/2.126 FOXO3a not only activates ATM expression,127 but during the DNA repair process FOXO3a directly binds the FAT domain of ATM.128 Similar to FOXO3a-p53 interaction, both FH and CR3 make direct interactions with ATM (Fig. 3D). This interaction promotes ATM auto-phosphorylation at S1981, which is required for activation of its kinase activity. Knockdown of FOXO3a resulted in a failure to activate ATM and DNA repair mechanisms in response to DNA damage.
FOXO3a in Cancer Therapy
Alterations of FOXO3a function is implicated in the progression of several types of cancers, fibrosis and other diseases.129 Importantly, FOXO transcription factors may serve as putative biomarkers and therapeutic targets for specific cancers. Several cancer chemotherapy drugs activate FOXO3a, and indirect activation of FOXO3a as a therapeutic strategy has been reviewed extensively in a special issue of Current Drug Targets.130 On the other hand, although FOXO3a usually exhibits properties of a tumor suppressor, it also serves a potential protective role by maintaining cellular homeostasis. FOXO3a can protect cancer stem cells from anti-cancer drug-induced apoptosis. For example, FOXO3a interaction with β-catenin and cross-talk with TGF-β induce FOXO3a-dependent drug resistance.38,125 Thus direct inhibition of FOXO3a in combination with other therapies may be beneficial to certain patients. However, the DNA-binding FH domain, which is the only structured region of FOXO3a, has proven very difficult to target with small molecule inhibitors.131 Interactions between transcription factors and DNA are considered challenging targets because binding is usually very tight (Kd in low nanomolar range) and involves very large interface. However, targeting the IDRs of transcription factors is proving to be a viable alternative.132,133 Considering that IDRs comprise 75% of the FOXO3a sequence, it seems likely that targeting these IDRs may be the key to successful discovery of FOXO3a inhibitors. Biochemical and structural investigations of the IDRs of FOXO3a have shed light on the structure-function relationships of these regions, paving the way for structure-based drug discovery.
Summary and Perspective
The high abundance of IDRs in FOXO proteins (Fig. 1) is preserved in different species through evolution, suggesting these regions are functionally important. This structural property seems to be evolutionarily conserved in all other members of the Fox protein subfamilies as well. PTM sites located in these IDRs, provide high-level and fine-tuned functional regulation. Recent studies have focused on the IDRs of FOXO3a, with particular attention to the PTM sites, most of which are conserved in all FOXO proteins. This review summarizes the most significant current observations and findings about these IDRs. Many studies have also elucidated the functions of PTMs in other Fox proteins, such as FOXA1 and FOXM1.58 Additionally, although conservation of sequence has not been preserved during evolution, some functions of the IDRs of Fox proteins have been preserved. For example, both TADs of FOXA1 and FOXO1 can interaction with Histones and open up chromatin,134 which suggests that IDRs of other Fox transcription factors may execute this function in the same manner. Thus, it is a common property of Fox transcription factors that they receive cellular signals and subsequently respond to execute functions through their IDRs.
Atomic resolution structures of FOXO3a IDRs are currently limited to short peptide regions of TADs bound to interacting partners. More efforts are required to continue to elucidate structures of these complexes in order to reveal the mechanisms of the unfolded-to-folded transitions of these IDRs upon target binding, and to explore the structural diversity sampled by each FOXO3a IDR when bound to different target proteins. Furthermore, the linker or spacer regions, which exhibit little conservation of primary sequence, have been largely overlooked. Akin to the TADs found in FOXO3a, multiple TADs of other Fox transcription factors are sparsely distributed within a long stretches of the IDRs. Our studies of the FOXO3a-CBP/p300 interaction indicated that long linker regions enhance binding by increasing the capture radius.48 This structural advantage may also apply to other Fox members that have similar, sparsely-distributed TAD architecture within IDR-rich regions. The detailed functional mechanisms of these IDRs, especially within the high-protein concentration environment of the living cell, are not well understood. Computational modeling and bioinformatics studies may shed light on these mechanisms, and experimental validations will be essential.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank our collaborators Drs. T. Mak and K. Yamamoto (Campbell Family Cancer Research Institute) for technical support and intellectual input to our FOXO3a studies.
Funding
The FOXO3a project was supported by a Canadian Institutes of Health Research (CIHR) grant to M.I, and the NMR facility at UHN is supported by the Canada Foundation for Innovation. M.I. holds the Canada Research Chair in Cancer Structural Biology.
References
- 1.Tuteja G, Kaestner KH. SnapShot: forkhead transcription factors I. Cell 2007; 130:1160; PMID:17889656; http://dx.doi.org/ 10.1016/j.cell.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Tuteja G, Kaestner KH. Forkhead transcription factors II. Cell 2007; 131:192; PMID:17923097; http://dx.doi.org/ 10.1016/j.cell.2007.09.016 [DOI] [PubMed] [Google Scholar]
- 3.Baldauf SL. A search for the origins of animals and fungi: comparing and combining molecular data. Am Nat 1999; 154:S178-88; PMID:10527926; http://dx.doi.org/ 10.1086/303292 [DOI] [PubMed] [Google Scholar]
- 4.Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet 2009; 10:233-40; PMID:19274050; http://dx.doi.org/ 10.1038/nrg2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mango SE. The molecular basis of organ formation: insights from the c-elegans foregut. Annu Rev Cell Dev Bi 2009; 25:597-628; http://dx.doi.org/ 10.1146/annurev.cellbio.24.110707.175411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kume T. The cooperative roles of Foxc1 and Foxc2 in cardiovascular development. Adv Exp Med Biol 2009; 665:63-77; PMID:20429416 [DOI] [PubMed] [Google Scholar]
- 7.Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 2001; 413:519-23; PMID:11586359; http://dx.doi.org/ 10.1038/35097076 [DOI] [PubMed] [Google Scholar]
- 8.Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, Maclsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature 2007; 445:931-5; PMID:17237765; http://dx.doi.org/ 10.1038/nature05478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol 2006; 41:709-17; PMID:16806782; http://dx.doi.org/ 10.1016/j.exger.2006.05.015 [DOI] [PubMed] [Google Scholar]
- 10.Burgering BMT, Coffer PJ. Protein-kinase-B (C-Akt) in phosphatidylinositol-3-oh inase signal-transduction. Nature 1995; 376: 599-602; PMID:7637810; http://dx.doi.org/ 10.1038/376599a0 [DOI] [PubMed] [Google Scholar]
- 11.Kloet DEA, Burgering BMT. The PKB/FOXO switch in aging and cancer. Bba-Mol Cell Res 2011; 1813:1926-37 [DOI] [PubMed] [Google Scholar]
- 12.van der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Antioxid Redox Sign 2011; 14:579-92; http://dx.doi.org/ 10.1089/ars.2010.3419 [DOI] [PubMed] [Google Scholar]
- 13.Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics 1998; 47:187-99; PMID:9479491; http://dx.doi.org/ 10.1006/geno.1997.5122 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhou YM, Graves DT. FOXO transcription factors: their clinical significance and regulation. Biomed Res Int 2014; 2014:925350; PMID:24864265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calnan DR, Brunet A. The FoxO code. Oncogene 2008; 27:2276-88; PMID:18391970; http://dx.doi.org/ 10.1038/onc.2008.21 [DOI] [PubMed] [Google Scholar]
- 16.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, et al.. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 2007; 128:309-23; PMID:17254969; http://dx.doi.org/ 10.1016/j.cell.2006.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilicox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A 2008; 105:13987-92; PMID:18765803; http://dx.doi.org/ 10.1073/pnas.0801030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flachsbart F, Caliebeb A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A 2009; 106:2700-5; PMID:19196970; http://dx.doi.org/ 10.1073/pnas.0809594106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anselmi CV, Malovini A, Roncarati R, Novelli V, Villa F, Condorelli G, Bellazzi R, Puca AA. Association of the FOXO3A locus with extreme longevity in a southern italian centenarian study. Rejuv Res 2009; 12:95-103; http://dx.doi.org/ 10.1089/rej.2008.0827 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Wang WJ, Cao HQ, Lu JH, Wu C, Hu FY, Guo J, Zhao L, Yang F, Zhang YX, et al.. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet 2009; 18:4897-904; PMID:19793722; http://dx.doi.org/ 10.1093/hmg/ddp459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawlikowska L, Hu DL, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, et al.. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell 2009; 8:460-72; PMID:19489743; http://dx.doi.org/ 10.1111/j.1474-9726.2009.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleindorp R, Flachsbart F, Puca AA, Malovini A, Schreiber S, Nebel A. Candidate gene study of FOXO1, FOXO4, FOXO6 reveals no association with human longevity in Germans. Aging Cell 2011; 10:622-8; PMID:21388494; http://dx.doi.org/ 10.1111/j.1474-9726.2011.00698.x [DOI] [PubMed] [Google Scholar]
- 23.Dansen TB, Burgering BMT. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol 2008; 18:421-9; PMID:18715783; http://dx.doi.org/ 10.1016/j.tcb.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 24.Gomes AR, Zhao F, Lam EWF. Role and regulation of the forkhead transcription factors FOXO3a and FOXM1 in carcinogenesis and drug resistance. Chin J Cancer 2013; 32:365-70; PMID:23706767; http://dx.doi.org/ 10.5732/cjc.012.10277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbajo-Pescador S, Mauriz JL, Garcia-Palomo A, Gonzalez-Gallego J. FoxO proteins: regulation and molecular targets in liver cancer. Curr Med Chem 2014; 21:1231-46; PMID:24372208; http://dx.doi.org/ 10.2174/0929867321666131228205703 [DOI] [PubMed] [Google Scholar]
- 26.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene 2008; 27:2312-9; PMID:18391973; http://dx.doi.org/ 10.1038/onc.2008.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med 2006; 203:1657-63; PMID:16801400; http://dx.doi.org/ 10.1084/jem.20060353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunters A, de Mattos SF, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EWF. FoxO3a transcriptional regulation of bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem 2003; 278:49795-805; PMID:14527951; http://dx.doi.org/ 10.1074/jbc.M309523200 [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Gac L, Marques M, Garcia Z, Campanero MR, Carrera AC. Control of cyclin G2 mRNA expression by forkhead transcription factors: Novel mechanism for cell cycle control by phosphoinositide 3-kinase and forkhead. Mol Cell Biol 2004; 24:2181-9; PMID:14966295; http://dx.doi.org/ 10.1128/MCB.24.5.2181-2189.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stahl M, Dijkers PF, Kops GJPL, Lens SMA, Coffer PJ, Burgering BMT, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27(Kip1) and bim in response to IL-2. J Immunol 2002; 168:5024-31; PMID:11994454; http://dx.doi.org/ 10.4049/jimmunol.168.10.5024 [DOI] [PubMed] [Google Scholar]
- 31.Li MY, Chiu JF, Mossman BT, Fukagawa NK. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem 2006; 281:40429-39; PMID:17079231; http://dx.doi.org/ 10.1074/jbc.M606596200 [DOI] [PubMed] [Google Scholar]
- 32.Hu MCT, Lee DF, Xia WY, Golfman LS, Fu OY, Yang JY, Zou YY, Bao SL, Hanada N, Saso H, et al.. I kappa B kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 2004; 117:225-37; PMID:15084260; http://dx.doi.org/ 10.1016/S0092-8674(04)00302-2 [DOI] [PubMed] [Google Scholar]
- 33.Yang JY, Zong CS, Xia WY, Yamaguchi H, Ding QQ, Xie XM, Lang JY, Lai CC, Chang CJ, Huang WC, et al.. ERK promotes tumorigenesis by inhibiting FOXO3a via MCM2-mediated degradation (vol 10, pg 138, 2008). Nat Cell Biol 2008; 10:370; http://dx.doi.org/ 10.1038/ncb0308-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 1999; 96:857-68; PMID:10102273; http://dx.doi.org/ 10.1016/S0092-8674(00)80595-4 [DOI] [PubMed] [Google Scholar]
- 35.Medema RH, Kops GJPL, Bos JL, Burgering BMT. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27(kip1). Nature 2000; 404:782-7; PMID:10783894; http://dx.doi.org/ 10.1038/35008115 [DOI] [PubMed] [Google Scholar]
- 36.Kops GJPL, Dansen TB, Polderman PE, Saarloos I, Wirtz KWA, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BMT. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002; 419:316-21; PMID:12239572; http://dx.doi.org/ 10.1038/nature01036 [DOI] [PubMed] [Google Scholar]
- 37.de Mattos SF, Villalonga P, Clardy J, Lam EWF. FOXO3a mediates the cytotoxic effects of cisplatin in colon cancer cells. Mol Cancer Ther 2008; 7:3237-46; PMID:18852127; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, Nakao S, Motoyama N, Hirao A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 2010; 463:676-U111; PMID:20130650; http://dx.doi.org/ 10.1038/nature08734 [DOI] [PubMed] [Google Scholar]
- 39.Nho RS, Hergert P. FoxO3a and disease progression. World J Biol Chem 2014; 5:346-54; PMID:25225602; http://dx.doi.org/ 10.4331/wjbc.v5.i3.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obsil T, Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 2008; 27:2263-75; PMID:18391969; http://dx.doi.org/ 10.1038/onc.2008.20 [DOI] [PubMed] [Google Scholar]
- 41.Wang F, Marshall CB, Yamamoto K, Li GY, Plevin MJ, You H, Mak TW, Ikura M. Biochemical and structural characterization of an intramolecular interaction in FOXO3a and its binding with p53. J Mol Biol 2008; 384:590-603; PMID:18824006; http://dx.doi.org/ 10.1016/j.jmb.2008.09.025 [DOI] [PubMed] [Google Scholar]
- 42.Tompa P. Intrinsically disordered proteins: a 10-year recap. Trends Biochem Sci 2012; 37:509-16; PMID:22989858; http://dx.doi.org/ 10.1016/j.tibs.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 43.Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol 2014; 16:18-29; http://dx.doi.org/ 10.1038/nrm3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oldfield CJ, Dunker AK. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu Rev Biochem 2014; 83:553-84; PMID:24606139; http://dx.doi.org/ 10.1146/annurev-biochem-072711-164947 [DOI] [PubMed] [Google Scholar]
- 45.Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 2004; 337:635-45; PMID:15019783; http://dx.doi.org/ 10.1016/j.jmb.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 46.So CW, Cleary ML. MLL-AFX requires the transcriptional effector domains of AFX to transform myeloid progenitors and transdominantly interfere with forkhead protein function. Mol Cell Biol 2002; 22:6542-52; PMID:12192052; http://dx.doi.org/ 10.1128/MCB.22.18.6542-6552.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.So CW, Cleary ML. Common mechanism for oncogenic activation of MLL by forkhead family proteins. Blood 2003; 101:633-9; PMID:12393557; http://dx.doi.org/ 10.1182/blood-2002-06-1785 [DOI] [PubMed] [Google Scholar]
- 48.Wang F, Marshall CB, Yamamoto K, Li GY, Gasmi-Seabrook GM, Okada H, Mak TW, Ikura M. Structures of KIX domain of CBP in complex with two FOXO3a transactivation domains reveal promiscuity and plasticity in coactivator recruitment. Proc Natl Acad Sci U S A 2012; 109:6078-83; PMID:22474372; http://dx.doi.org/ 10.1073/pnas.1119073109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 1993; 364:412-20; PMID:8332212; http://dx.doi.org/ 10.1038/364412a0 [DOI] [PubMed] [Google Scholar]
- 50.Pierrou S, Hellqvist M, Samuelsson L, Enerback S, Carlsson P. Cloning and characterization of seven human forkhead proteins: binding site specificity and DNA bending. EMBO J 1994; 13:5002-12; PMID:7957066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weigelt J, Climent I, Dahlman-Wright K., Wikstrom M. Solution structure of the DNA binding domain of the human forkhead transcription factor AFX (FOXO4). Biochemistry 2001; 40:5861-9; PMID:11352721; http://dx.doi.org/ 10.1021/bi001663w [DOI] [PubMed] [Google Scholar]
- 52.Tsai KL, Sun YJ, Huang CY, Yang JY, Hung MC, Hsiao CD. Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of post-translational modification. Nucleic Acids Res 2007; 35:6984-4; PMID:17940099; http://dx.doi.org/ 10.1093/nar/gkm703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brent MM, Anand R, Marmorstein R. Structural basis for DNA recognition by FoxO1 and its regulation by posttranslational modification. Structure 2008; 16:1407-6; PMID:18786403; http://dx.doi.org/ 10.1016/j.str.2008.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boura E, Rezabkova L, Brynda J, Obsilova V, Obsil T. Structure of the human FOXO4-DBD-DNA complex at 1.9 A resolution reveals new details of FOXO binding to the DNA. Acta Crystallogr D Biol Crystallogr 2010; 66:1351-7; PMID:21123876; http://dx.doi.org/ 10.1107/S0907444910042228 [DOI] [PubMed] [Google Scholar]
- 55.Boura E, Silhan J, Herman P, Vecer J, Sulc M, Teisinger J, Obsilova V, Obsil T. Both the N-terminal loop and wing W2 of the forkhead domain of transcription factor FoxO4 are important for DNA binding. J Biol Chem 2007; 282:8265-75; PMID:17244620; http://dx.doi.org/ 10.1074/jbc.M605682200 [DOI] [PubMed] [Google Scholar]
- 56.Wang F, Marshall CB, Li GY, Yamamoto K, Mak TW, Ikura M. Synergistic interplay between promoter recognition and CBP/p300 coactivator recruitment by FOXO3a. ACS Chem Biol 2009; 4:1017-27; PMID:19821614; http://dx.doi.org/ 10.1021/cb900190u [DOI] [PubMed] [Google Scholar]
- 57.Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 2013; 14:83-97; PMID:23325358 [DOI] [PubMed] [Google Scholar]
- 58.Lam EW, Brosens JJ, Gomes AR, Koo CY. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer 2013; 13:482-95; PMID:23792361 [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y, Wang YC, Zhu WG. Applications of post-translational modifications of FoxO family proteins in biological functions. J Mol Cell Biol 2011; 3:276-82; PMID:21669942; http://dx.doi.org/ 10.1093/jmcb/mjr013 [DOI] [PubMed] [Google Scholar]
- 60.Mittag T, Kay LE, Forman-Kay JD. Protein dynamics and conformational disorder in molecular recognition. J Mol Recognit 2010; 23:105-16; PMID:19585546 [DOI] [PubMed] [Google Scholar]
- 61.Forman-Kay JD. Dynamic complexes of intrinsically disordered proteins and their regulation by post-translational modifications. Faseb J 2013; 27 [Google Scholar]
- 62.Gao J, Xu D. Correlation between posttranslational modification and intrinsic disorder in protein. Pacific Symposium on Biocomputing. Pac Symp Biocomput 2012; 1:94-103; PMID:22174266 [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Gan L, Pan H, Guo S, He X, Olson ST, Mesecar A, Adam S, Unterman TG. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem 2002; 277:45276-84; PMID:12228231; http://dx.doi.org/ 10.1074/jbc.M208063200 [DOI] [PubMed] [Google Scholar]
- 64.Obsil T, Ghirlando R, Anderson DE, Hickman AB, Dyda F. Two 14-3-3 binding motifs are required for stable association of forkhead transcription factor FOXO4 with 14-3-3 proteins and inhibition of DNA binding. Biochemistry 2003; 42:15264-72; PMID:14690436; http://dx.doi.org/ 10.1021/bi0352724 [DOI] [PubMed] [Google Scholar]
- 65.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 2001; 21:952-65; PMID:11154281; http://dx.doi.org/ 10.1128/MCB.21.3.952-965.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rena G, Woods YL, Prescott AR, Peggie M, Unterman TG, Williams MR, Cohen P. Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. Embo J 2002; 21:2263-71; PMID:11980723; http://dx.doi.org/ 10.1093/emboj/21.9.2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woods YL, Rena G, Morrice N, Barthel A, Becker W, Guo S, Unterman TG, Cohen P. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem J 2001; 355:597-607; PMID:11311120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 2004; 380:297-309; PMID:15005655; http://dx.doi.org/ 10.1042/BJ20040167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Essers MAG, de Vries-Smits LMM, Barker N, Polderman PE, Burgering BMT, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005; 308:1181-4; PMID:15905404; http://dx.doi.org/ 10.1126/science.1109083 [DOI] [PubMed] [Google Scholar]
- 70.Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Derijard B. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol 2010; 30:470-80; PMID:19917721; http://dx.doi.org/ 10.1128/MCB.00666-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang XW, Chen WR, Xing D. A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J Cell Physiol 2012; 227:1168-78; PMID:21604264; http://dx.doi.org/ 10.1002/jcp.22839 [DOI] [PubMed] [Google Scholar]
- 72.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 2006; 125:987-1001; PMID:16751106; http://dx.doi.org/ 10.1016/j.cell.2006.03.046 [DOI] [PubMed] [Google Scholar]
- 73.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 2007; 282:30107-19; PMID:17711846; http://dx.doi.org/ 10.1074/jbc.M705325200 [DOI] [PubMed] [Google Scholar]
- 74.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, et al.. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol 2008; 10:138-48; PMID:18204439; http://dx.doi.org/ 10.1038/ncb1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu MCT, Lee DF, Xia WY, Golfman LS, Fu OY, Yang JY, Zou YY, Bao SL, Hanada N, Saso H, Kobayashi R, Hung MC. I kappa B kinase promotes tumorigenesis through inhibition of forkhead FOXO3a (vol 117, pg 225, 2004). Cell 2007; 129:1427-8; http://dx.doi.org/ 10.1016/j.cell.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 76.Singh A, Ye M, Bucur O, Zhu SD, Santos MT, Rabinovitz I, Wei WY, Gao DM, Hahn WC, Khosravi-Far R. Protein Phosphatase 2A Reactivates FOXO3a through a Dynamic Interplay with 14-3-3 and AKT. Mol Biol Cell 2010; 21:1140-52; PMID:20110348; http://dx.doi.org/ 10.1091/mbc.E09-09-0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shiota M, Yokomizo A, Kashiwagi E, Tada Y, Inokuchi J, Tatsugami K, Kuroiwa K, Uchiumi T, Seki N, Naito S. Foxo3a expression and acetylation regulate cancer cell growth and sensitivity to cisplatin. Cancer Sci 2010; 101:1177-85; PMID:20210796; http://dx.doi.org/ 10.1111/j.1349-7006.2010.01503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carbajo-Pescador S, Steinmetz C, Kashyap A, Lorenz S, Mauriz JL, Heise M, Galle PR, Gonzalez-Gallego J, Strand S. Melatonin induces transcriptional regulation of Bim by FoxO3a in HepG2 cells. Br J Cancer 2013; 108:442-9; PMID:23257900; http://dx.doi.org/ 10.1038/bjc.2012.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 1997; 90:595-606; PMID:9288740; http://dx.doi.org/ 10.1016/S0092-8674(00)80521-8 [DOI] [PubMed] [Google Scholar]
- 80.Mahmud DLMGA, Deb DK, Platanias LC, Uddin S, Wickrema A. Phosphorylation of forkhead transcription factors by erythropoietin and stem cell factor prevents acetylation and their interaction with coactivator p300 in erythroid progenitor cells. Oncogene 2002; 21:1556-62; PMID:11896584; http://dx.doi.org/ 10.1038/sj.onc.1205230 [DOI] [PubMed] [Google Scholar]
- 81.Fukuoka M, Daitoku H, Hatta M, Matsuzaki H, Umemura S, Fukamizu A. Negative regulation of forkhead transcription factor AFX (Foxo4) by CBP-induced acetylation. Int J Mol Med 2003; 12:503-8; PMID:12964026 [PubMed] [Google Scholar]
- 82.van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci 2005; 30:81-6; PMID:15691653; http://dx.doi.org/ 10.1016/j.tibs.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 83.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A 2005; 102:11278-83; PMID:16076959; http://dx.doi.org/ 10.1073/pnas.0502738102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al.. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004; 303:2011-5; PMID:14976264; http://dx.doi.org/ 10.1126/science.1094637 [DOI] [PubMed] [Google Scholar]
- 85.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell 2004; 116:551-63; PMID:14980222; http://dx.doi.org/ 10.1016/S0092-8674(04)00126-6 [DOI] [PubMed] [Google Scholar]
- 86.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A 2004; 101:10042-7; PMID:15220471; http://dx.doi.org/ 10.1073/pnas.0400593101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salminen A, Kaarniranta K, Kauppinen A. Crosstalk between Oxidative Stress and SIRT1: impact on the aging process. Int J Mol Sci 2013; 14:3834-59; PMID:23434668; http://dx.doi.org/ 10.3390/ijms14023834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakae J, Cao Y, Daitoku H, Fukamizu A, Ogawa W, Yano Y, Hayashi Y. The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity. J Clin Invest 2006; 116:2473-83; PMID:16917544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 2004; 305:361; PMID:15192154; http://dx.doi.org/ 10.1126/science.1098219 [DOI] [PubMed] [Google Scholar]
- 90.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circul Res 2010; 107:1470-82; PMID:20947830; http://dx.doi.org/ 10.1161/CIRCRESAHA.110.227371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu W, Ma Q, Chen L, Li P, Zhang M, Ramamoorthy S, Nawaz Z, Shimojima T, Wang H, Yang Y, et al.. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem 2009; 284:13987-4000; PMID:19321440; http://dx.doi.org/ 10.1074/jbc.M901758200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang F, Chan CH, Chen K, Guan X, Lin HK, Tong Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene 2012; 31:1546-57; PMID:21841822; http://dx.doi.org/ 10.1038/onc.2011.347 [DOI] [PubMed] [Google Scholar]
- 93.van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice MM, Burgering BM. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol 2006; 8:1064-73; PMID:16964248; http://dx.doi.org/ 10.1038/ncb1469 [DOI] [PubMed] [Google Scholar]
- 94.Calnan DR, Webb AE, White JL, Stowe TR, Goswami T, Shi X, Espejo A, Bedford MT, Gozani O, Gygi SP, Brunet A. Methylation by Set9 modulates FoxO3 stability and transcriptional activity. Aging 2012; 4:462-79; PMID:22820736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem 2008; 283:16283-92; PMID:18420577; http://dx.doi.org/ 10.1074/jbc.M802240200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sakamaki J, Daitoku H, Yoshimochi K, Miwa M, Fukamizu A. Regulation of FOXO1-mediated transcription and cell proliferation by PARP-1. Biochem Biophys Res Commun 2009; 382:497-502; PMID:19281796; http://dx.doi.org/ 10.1016/j.bbrc.2009.03.022 [DOI] [PubMed] [Google Scholar]
- 97.Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 2004; 117:211-23; PMID:15084259; http://dx.doi.org/ 10.1016/S0092-8674(04)00298-3 [DOI] [PubMed] [Google Scholar]
- 98.Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem 2006; 281:5267-76; PMID:16373335; http://dx.doi.org/ 10.1074/jbc.M512151200 [DOI] [PubMed] [Google Scholar]
- 99.van der Vos KE, Coffer PJ. FOXO-binding partners: it takes two to tango. Oncogene 2008; 27:2289-99; PMID:18391971; http://dx.doi.org/ 10.1038/onc.2008.22 [DOI] [PubMed] [Google Scholar]
- 100.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Bio 2005; 6:197-208; http://dx.doi.org/ 10.1038/nrm1589 [DOI] [PubMed] [Google Scholar]
- 101.Hsu WL, Oldfield C, Meng J, Huang F, Xue B, Uversky VN, Romero P, Dunker AK. Intrinsic protein disorder and protein-protein interactions. Pacific Symposium on Biocomputing. Pac Symp Biocomput 2012; 1:116-27; PMID:22174268 [PubMed] [Google Scholar]
- 102.Boehr DD, Nussinov R, Wright PE. The role of dynamic conformational ensembles in biomolecular recognition (vol 5, pg 789, 2009). Nat Chem Biol 2009; 5:954-954; http://dx.doi.org/ 10.1038/nchembio1209-954d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vogt AD, Di Cera E. Conformational selection or induced fit? A critical appraisal of the kinetic mechanism. Biochemistry 2012; 51:5894-902; PMID:22775458; http://dx.doi.org/ 10.1021/bi3006913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gianni S, Dogan J, Jemth P. Distinguishing induced fit from conformational selection. Biophys Chem 2014; 189:33-9; PMID:24747333; http://dx.doi.org/ 10.1016/j.bpc.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 105.Rogers JM, Oleinikovas V, Shammas SL, Wong CT, De Sancho D, Baker CM, Clarke J. Interplay between partner and ligand facilitates the folding and binding of an intrinsically disordered protein. Proc Nat Acad Sci U S A 2014; 111:15420-5; PMID:25313042; http://dx.doi.org/ 10.1073/pnas.1409122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uversky VN, Oldfield CJ, Dunker AK. Showing your ID: intrinsic disorder as an ID for recognition, regulation and cell signaling. J Mol Recognit 2005; 18:343-84; PMID:16094605; http://dx.doi.org/ 10.1002/jmr.747 [DOI] [PubMed] [Google Scholar]
- 107.Liu JG, Perumal NB, Oldfield CJ, Su EW, Uversky VN, Dunker AK. Intrinsic disorder in transcription factors. Biochemistry 2006; 45:6873-88; PMID:16734424; http://dx.doi.org/ 10.1021/bi0602718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hilser VJ, Thompson EB. Intrinsic disorder as a mechanism to optimize allosteric coupling in proteins. Proc Natl Acad Sci U S A 2007; 104:8311-5; PMID:17494761; http://dx.doi.org/ 10.1073/pnas.0700329104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature 1996; 384:641-3; PMID:8967953; http://dx.doi.org/ 10.1038/384641a0 [DOI] [PubMed] [Google Scholar]
- 110.Wang F, Marshall CB, Ikura M. Transcriptional/epigenetic regulator CBP/p300 in tumorigenesis: structural and functional versatility in target recognition. Cell Mol Life Sci 2013; 70:3989-4008; PMID:23307074; http://dx.doi.org/ 10.1007/s00018-012-1254-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 1997; 89:1175-84; PMID:9215639; http://dx.doi.org/ 10.1016/S0092-8674(00)80304-9 [DOI] [PubMed] [Google Scholar]
- 112.Denis CM, Chitayat S, Plevin MJ, Wang F, Thompson P, Liu S, Spencer HL, Ikura M, LeBrun DP, Smith SP. Structural basis of CBP/p300 recruitment in leukemia induction by E2A-PBX1. Blood 2012; 120:3968-77; PMID:22972988; http://dx.doi.org/ 10.1182/blood-2012-02-411397 [DOI] [PubMed] [Google Scholar]
- 113.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell 2009; 137:413-31; PMID:19410540; http://dx.doi.org/ 10.1016/j.cell.2009.04.037 [DOI] [PubMed] [Google Scholar]
- 114.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 2012; 26:1268-86; PMID:22713868; http://dx.doi.org/ 10.1101/gad.190678.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci U S A 2006; 103:9051-6; PMID:16757565; http://dx.doi.org/ 10.1073/pnas.0600889103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev 2001; 81:1269-304; PMID:11427696 [DOI] [PubMed] [Google Scholar]
- 117.Plevin MJ, Mills MM, Ikura M. The LxxLL motif: a multifunctional binding sequence in transcriptional regulation. Trends Biochem Sci 2005; 30:66-9; PMID:15691650; http://dx.doi.org/ 10.1016/j.tibs.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 118.Cornforth AN, Davis JS, Khanifar E, Nastiuk KL, Krolewski JJ. FOXO3a mediates the androgen-dependent regulation of FLIP and contributes to TRAIL-induced apoptosis of LNCaP cells. Oncogene 2008; 27:4422-33; PMID:18391984; http://dx.doi.org/ 10.1038/onc.2008.80 [DOI] [PubMed] [Google Scholar]
- 119.Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol 2003; 23:104-18; PMID:12482965; http://dx.doi.org/ 10.1128/MCB.23.1.104-118.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sisci D, Maris P, Cesario MG, Anselmo W, Coroniti R, Trombino GE, Romeo F, Ferraro A, Lanzino M, Aquila S, Maggiolini M, Mauro L, Morelli C, Ando S. The estrogen receptor alpha is the key regulator of the bifunctional role of FoxO3a transcription factor in breast cancer motility and invasiveness. Cell cycle 2013; 12:3405-20; PMID:24047697; http://dx.doi.org/ 10.4161/cc.26421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dey P, Strom A, Gustafsson JA. Estrogen receptor beta upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene 2014; 33:4213-25; PMID:24077289; http://dx.doi.org/ 10.1038/onc.2013.384 [DOI] [PubMed] [Google Scholar]
- 122.Hoogeboom D, Burgering BM. Should I stay or should I go: beta-catenin decides under stress. Biochim Biophys Acta 2009; 1796:63-74; PMID:19268509 [DOI] [PubMed] [Google Scholar]
- 123.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005; 308:1181-4; PMID:15905404; http://dx.doi.org/ 10.1126/science.1109083 [DOI] [PubMed] [Google Scholar]
- 124.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem 2008; 283:9224-30; PMID:18250171; http://dx.doi.org/ 10.1074/jbc.M706638200 [DOI] [PubMed] [Google Scholar]
- 125.Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I, Arques O, Landolfi S, Fernandez Y, Herance JR, Gispert JD, Mendizabal L, et al.. beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med 2012; 18:892-901; PMID:22610277; http://dx.doi.org/ 10.1038/nm.2772 [DOI] [PubMed] [Google Scholar]
- 126.Derheimer FA, Kastan MB. Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS Lett 2010; 584:3675-81; PMID:20580718; http://dx.doi.org/ 10.1016/j.febslet.2010.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, Sarkar A, Grisotto M, Taneja R, Ghaffari S. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem 2008; 283:25692-705; PMID:18424439; http://dx.doi.org/ 10.1074/jbc.M800517200 [DOI] [PubMed] [Google Scholar]
- 128.Tsai WB, Chung YM, Takahashi Y, Xu Z, Hu MC. Functional interaction between FOXO3a and ATM regulates DNA damage response. Nat Cell Biol 2008; 10:460-7; PMID:18344987; http://dx.doi.org/ 10.1038/ncb1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tikhanovich I, Cox J, Weinman SA. Forkhead box class O transcription factors in liver function and disease. J Gastroenterol Hepatol 2013; 28 Suppl 1:125-31; PMID:23855308; http://dx.doi.org/ 10.1111/jgh.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singh A, Plati J, Khosravi-Far R. Harnessing the tumor suppressor function of FOXO as an alternative therapeutic approach in cancer. Curr Drug Targets 2011; 12:1311-21; PMID:21443464; http://dx.doi.org/ 10.2174/138945011796150271 [DOI] [PubMed] [Google Scholar]
- 131.Yan CH, Higgins PJ. Drugging the undruggable: Transcription therapy for cancer. Bba-Rev Cancer 2013; 1835:76-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Uversky VN. Targeting intrinsically disordered proteins in neurodegenerative and protein dysfunction diseases: another illustration of the D(2) concept. Expert Rev Proteomics 2010; 7:543-64; PMID:20653509; http://dx.doi.org/ 10.1586/epr.10.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y, Cao H, Liu Z. Binding cavities and druggability of intrinsically disordered proteins. Protein Sci 2015; 24(5):688-705; PMID:25611056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hatta M, Cirillo LA. Chromatin opening and stable perturbation of core histone: DNA contacts by FoxO1. J Biol Chem 2007; 282:35583-3; PMID:17923482; http://dx.doi.org/ 10.1074/jbc.M704735200 [DOI] [PubMed] [Google Scholar]