Abstract
The inflammatory response regulates congestive heart failure (CHF) development. T-cell activation plays an important role in tissue inflammation. We postulate that CD28 or B7 deficiency inhibits T-cell activation and attenuates CHF development by reducing systemic, cardiac and pulmonary inflammation. We demonstrated that chronic pressure overload-induced end-stage CHF in mice is characterized by profound accumulation of activated effector T-cells (CD3+CD44high cells) in the lungs and a mild but significant increase of these cells in the heart. In knockout (KO) mice lacking either CD28 or B7, there was a dramatic reduction in the accumulation of activated effector T cells in both hearts and lungs of mice under control conditions and after transverse aortic constriction (TAC). CD28 or B7 KO significantly attenuated TAC-induced CHF development, as indicated by less increase of heart and lung weight, and less reduction of LV contractility. CD28 or B7 KO also significantly reduced TAC-induced CD45+ leukocyte, T-cell and macrophage infiltration in hearts and lungs, lowered pro-inflammatory cytokine expression (such as TNF-α and IL-1β) in lungs. Furthermore, CD28/B7 blockade by CTLA4-Ig treatment (250μg/mouse every 3 days) attenuated TAC-induced T cell activation, LV hypertrophy, and LV dysfunction. Our data indicate that CD28/B7 deficiency inhibits activated effector T-cell accumulation, reduces myocardial and pulmonary inflammation, and attenuates the development of CHF. Our findings suggest that strategies targeting T-cell activation may be useful in treating CHF.
Keywords: congestive heart failure, T-cell activation, heart, lung, inflammation, leukocytes
Introduction
The inflammatory response is believed to play an important role in the development of congestive heart failure (CHF). Increased myocardial leukocyte infiltration and elevated systemic and cardiac pro-inflammatory cytokine levels are commonly associated with CHF, 1, 2 and several of these factors, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, have been demonstrated to accelerate left ventricle (LV) hypertrophy, cardiac fibrosis and LV dysfunction.3–5 In addition to inflammation of myocardial tissue, severe CHF results in profound leukocyte infiltration in the lungs of experimental animals and CHF patients,6–9 provoking the development of type-2 pulmonary hypertension that drives the transition from LV failure to right ventricular (RV) hypertrophy and failure.
T-cell activation plays a central role in inflammation.10 T-cell activation requires at least two signals to become fully activated. The first signal occurs after the engagement of T cell receptor (TCR) by antigen-major histocompatibility complex proteins present on the surface of antigen presenting cells (APC). The second important signal is the engagement of costimulatory molecules (like CD28 or ICOS) on the T cell with one of the B7 proteins (B7.1 (CD80) and B7.2 (CD86)), which are also expressed on APC. CD28 is a potent T-cell co-stimulator. Inhibition of CD28/B7 signaling with antibodies or genetic ablation of CD28 or B7.1/B7.2 in mice attenuates T-cell activation.10, 11
Previous studies have demonstrated that CD4+ T cells accumulate in the LV of mice with CHF and contribute to the pathogenesis of CHF.12, 13 In this study, we found that end-stage CHF is associated with profound infiltration and accumulation of activated effector T cells (CD3+CD44high cells) in the heart and lungs, as well as a significant increase in the percentage of effector memory CD4+ and CD8+ T cells (TEM, CD44highCD62Llow T cells) in the lungs. To demonstrate a role for CD28 function in CHF development, we tested the hypothesis that transverse aortic constriction (TAC)-induced LV failure and lung remodeling would be attenuated in CD28 or B7-knockout (KO) mice. Our findings indicate that genetic disruption of either CD28 or B7 protein significantly attenuates TAC-induced cardiac and pulmonary inflammation, lung remodeling, as well as LV hypertrophy and dysfunction. The beneficial effect of CD28 or B7 deletion was associated with a dramatic reduction of TAC-induced T-cell activation in the heart and lungs.
Materials and methods
Detailed methods are available in the online-only Data Supplement. Primary antibodies and primers used in this study are listed in Table S1 and Table S2.
Mice and TAC Procedure
Animals and experimental design
CD28 KO (B6.129S2-Cd28tm1Mak/J, stock No. 002666) and B7 KO (B6.129S4-Cd80tm1Shr Cd86tm2Shr/J, stock No. 003610) mice and C57BL/6J wild-type (WT) mice were obtained from Jackson Laboratory. The fusion protein CTLA4-Ig (abatacept, 250 μg), which inhibits CD28/B7 interactions, was administered intraperitoneally every 3 days beginning 1 day before TAC. An isotype control antibody human IgG was used as a vehicle control. Male mice 4–5 weeks of age were subjected to a procedure using a 27G needle to create the transverse aortic constriction (TAC), a model that mimics clinical systemic hypertension and aortic stenosis, as previously described.2 Data were collected 8 weeks after TAC. Left ventricle (LV) hypertrophy and cardiac function were assessed. Experimental studies were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Data Analysis
A normality test (Shapiro-Wilk) provided by SigmaPlot was used to determine whether data were normally distributed. If data were normally distributed, the data are presented as mean ± SEM. Student’s t-test was used to test for differences between 2 groups. Two-way ANOVA followed by a Bonferroni correction post-hoc test was used to test for differences among more than 2 groups. If the physiological data were not normally distributed or the sample size in one of the experimental groups was less than 10, the data are presented as median (quartiles). Non-parametric test (Mann-Whitney or Kruskal-Wallis) followed by the Bonferroni post hoc correction was performed. All pairwise p-values are two-sided. The null hypothesis was rejected at P < 0.05.
Results
End-Stage CHF Caused a Significant Accumulation of Activated T-Cells in Lungs
We examined T-cell activation and T-cell accumulation in hearts and lungs of mice with end-stage CHF produced by TAC. As shown in Figure S1A and S1B, ratios of LV weight and lung weight to body weight were increased 2.3-fold and 2.8-fold in CHF mice, respectively. Echocardiography showed that mice with TAC had a significant decrease of LV EF (36.0% in CHF vs 77.8% in control mice) (Figure S1C), indicating the development of CHF.
Flow cytometry analysis showed that the percentage of activated effector T cells (CD3+CD44high cells that display rapid effector function in inflamed peripheral tissues) was more than doubled in the lungs of CHF mice (27.2 ± 1.7% in control vs 58.1 ± 4.6% in CHF) but not changed in the LV (11.3 ± 0.9% in control vs 11.5 ± 0.6% in CHF) (Figure S1D and S1E). However, total numbers of CD3+ T cells were significantly increased in both LV tissues and lungs of CHF mice. Specifically, CD3+ T-cell infiltration was 1.4 ×104 cells per LV and 178 ×104 cells per lung in CHF mice (Figure S1F). In addition, CD45+ leukocyte infiltration was dramatically increased in CHF mice. CD45+ cell infiltration was 1.7 ×105 cells per LV and 106 ×105 cells per lung in CHF mice (Figure S1G). These data indicate that T-cell activation and accumulation at end-stage CHF are dramatic in the lung, but relatively mild in the LV. Moreover, CHF mice also exhibited a significantly increased spleen weight and an increased percentage of TEM within the CD4+ T-cell subset in splenocytes and peripheral blood cells (Figure S2 and S3). Flow cytometry gating strategies are presented in Figure S4–S7.
CD28 Deficiency Attenuated TAC-Induced CHF Development
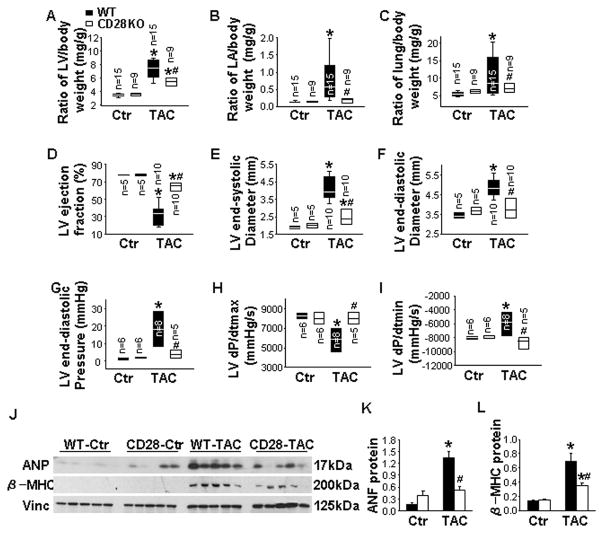
To determine whether T-cell activation affects cardiopulmonary adaptation to LV-pressure overload, WT and CD28 KO mice were examined under control conditions and 8 weeks after TAC. LV systolic pressure was similar in WT and CD28 KO mice under control conditions (96.3 ± 9.9 mmHg in WT-control vs 96.5 ± 2.6 mmHg in CD28 KO-control). TAC significantly increased LV systolic pressure after 8 weeks, which was comparable in WT and CD28 KO mice (165.2 ± 9.9 mmHg in WT-TAC vs 175.3 ± 4.7 mmHg in CD28 KO-TAC) (Figure S8A and S8B). Under control conditions, CD28 deficiency had no detectable effect on the ratio of LV, left atria (LA) or lung weight to body weight (Figure 1A–1C and Table S3). Echocardiographic measurements showed that CD28 deficiency did not affect LV function under control conditions (Figure 1D–1F). These data indicate that CD28 deficiency has no detectable effect on cardiac structure or function under control conditions.
Figure 1. CD28 deficiency attenuates transverse aortic constriction (TAC)-induced cardiac dysfunction and pulmonary congestion.
Data were collected from wild-type (WT) and CD28 knockout (CD28 KO) mice under control conditions (Ctr) or 8 weeks after TAC. A to C, The ratio of left ventricle (LV), left atria (LA) and lung weight to body weight of mice. D to F, Echocardiographic measurements of LV ejection fraction, LV end-systolic diameter and LV end-diastolic diameter from hearts. G to I, Hemodynamics of LV end-diastolic pressure, LV maximum rate of rise of pressure (dP/dtmax) and LV maximum rate of decline of pressure (dP/dtmin) from hearts. J to L, Western blot of ANP, β-MHC and vinculin (loading control) in LV lysates of mice. *P<0.05 vs control group; #P<0.05 vs TAC group of WT mice.
TAC caused severe LV hypertrophy (3.6 ± 0.2 mg/kg in WT-control vs 7.5 ± 0.3 mg/kg in WT-TAC) and markedly decreased LV ejection fraction (LVEF, 78.1 ± 2.6% in WT- control vs 32.1 ± 3.2% in WT-TAC) in WT mice (Figure 1A and 1D). CD28 deficiency significantly attenuated the TAC-induced LV hypertrophy (7.5 ± 0.3 mg/kg in WT-TAC vs 5.6 ± 1.1 mg/kg in CD28 KO-TAC), increase of LA weight (0.84 ± 0.18 mg/kg in WT-TAC vs 0.17 ± 0.04 mg/kg in CD28 KO-TAC), and increase of lung weight (10.5 ± 1.3 mg/kg in WT-TAC vs 7.1 ± 1.4 mg/kg in CD28 KO-TAC) (Figure 1A–1C and Table S3). Echocardiographic measurements showed that CD28 deficiency significantly attenuated the TAC-induced decrease of LV EF (32.1 ± 3.2% in WT-TAC vs 64.5 ± 14.3% in CD28 KO-TAC), increase of LV end-systolic diameter (LVESD, 4.1 ± 0.2 mm in WT-TAC vs 2.5 ± 0.6 mm in CD28 KO-TAC), and increase of LV end-diastolic diameter (LVEDD, 4.9 ± 0.1 mm in WT-TAC vs 3.8 ± 0.9 mm in CD28 KO-TAC) (Figure 1D–1F). CD28 deficiency also attenuated the TAC-induced increase of LV end-diastolic pressure (LVEDP, 18.4 ± 3.3 mmHg in WT-TAC vs 3.9 ± 1.1 mmHg in CD28 KO-TAC), decrease of LV maximum rate of rise of LV systolic pressure (dP/dtmax, 5997 ± 827 mmHg/s in WT-TAC vs 8038 ± 1797 mmHg/s in CD28 KO-TAC) and increase of LV minimum rate of decline of LV systolic pressure (dP/dtmin, −5943 ± 864 mmHg/s in WT-TAC vs −8930 ± 2005 mmHg/s in CD28 KO-TAC) (Figure 1G–1I). Moreover, CD28 deficiency reduced TAC-induced increases of myocardial atrial natriuretic peptide (ANP) and β-myosin heavy chain (β-MHC) protein content (Figure 1J–1L). Together, these data indicate that CD28 deficiency protects hearts against TAC-induced LV hypertrophy and CHF.
B7 Deficiency Attenuated TAC-Induced CHF Development
Since CD28 deficiency attenuated the TAC-induced LV hypertrophy and dysfunction, we utilized B7 KO mice to examine the effect of B7 deficiency on TAC-induced CHF. LV systolic pressure was comparable in WT and B7 KO mice under control conditions, as well as 8 weeks after TAC (Figure S8C and S8D). We found that B7 deficiency had no effect on the ratio of LV, LA or lung weight to body weight under control conditions (Figure 2A–2C and Table S4). Echocardiographic measurements showed that B7 deficiency did not affect LV function under control conditions (Figure 2D–2F).
Figure 2. B7 deficiency attenuates transverse aortic constriction (TAC)-induced cardiac dysfunction and pulmonary congestion.
Data were collected from wild-type (WT) and B7 knockout (B7 KO) mice under control conditions (Ctr) or 8 weeks after TAC. A to C, The ratio of left ventricle (LV), left atria (LA) and lung weight to body weight of mice. D to F, Echocardiographic measurements of LV ejection fraction, LV end-systolic diameter and LV end-diastolic diameter from hearts. G to I, Hemodynamics of LV end-diastolic pressure, LV maximum rate of rise of pressure (dP/dtmax) and LV maximum rate of decline of pressure (dP/dtmin) from hearts. J to L, Western blot of ANP, β-MHC and vinculin (loading control) in LV lysates of mice. *P<0.05 vs control group; #P<0.05 vs TAC group of WT mice.
B7 deficiency significantly attenuated the TAC-induced LV hypertrophy (6.9 ± 0.5 mg/kg in WT-TAC vs 5.1 ± 1.0 mg/kg in B7 KO-TAC), increase of LA weight (0.64 ± 0.16 mg/kg in WT-TAC vs 0.15 ± 0.03 mg/kg in B7 KO-TAC), and increase of lung weight (8.6 ± 1.1 mg/kg in WT-TAC vs 4.9 ± 1.1 mg/kg in B7 KO-TAC) (Figure 2A–2C and Table S4). Echocardiographic measurements showed that B7 deficiency significantly attenuated the TAC-induced decrease of LV EF (48.4 ± 6.2% in WT-TAC vs 67.5 ± 11.8% in B7 KO-TAC), increase of LVESD (3.4 ± 0.4 mm in WT-TAC vs 2.4 ± 0.4 mm in B7 KO-TAC), and increase of LVEDD (4.5 ± 0.5 mm in WT-TAC vs 3.8 ± 0.7 mm in B7 KO-TAC) (Figure 2D–2F). In addition, B7 deficiency also attenuated the TAC-induced increase of LVEDP (21.3 ± 6.1 mmHg in WT-TAC vs 4.7 ± 0.9 mmHg in B7 KO-TAC), decrease of LV dP/dtmax (5567 ± 1426 mmHg/s in WT-TAC vs 8730 ± 218 mmHg/s in B7 KO-TAC) and increase of LV dP/dtmin (−5579 ± 1491 mmHg/s in WT-TAC vs −8788 ± 1290 mmHg/s in B7-TAC) (Figure 2G–2I). Moreover, B7 KO significantly reduced the TAC-induced increases of myocardial ANP and β-MHC protein expression (Figure 2J–2L). These data indicate that B7 deficiency protects hearts against TAC-induced LV dysfunction and CHF.
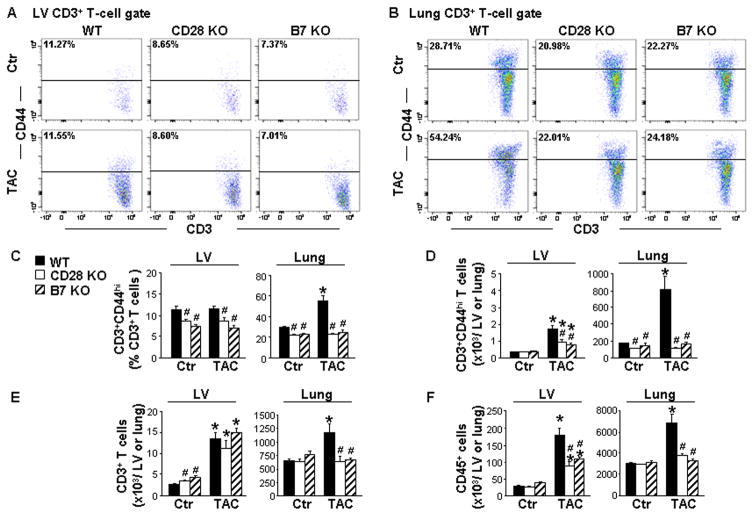
CD28 or B7 Deficiency Inhibited CD3+ T-Cell Activation and Accumulation in Hearts and Lungs of Mice after TAC
To determine the effect of CD28/B7 signaling on T-cell activation and inflammation, we assessed changes in the percentage and number of CD3+ T cells, activated effector T cells, and early activated T cells (CD3+CD69+ cells) in LV tissues and lungs using flow cytometry analysis. The gating strategies are presented in Figure S4 and S5. Under control conditions, CD28 or B7 KO significantly reduced the percentage of activated effector T cells in both LV tissues (11.3 ± 0.9% in WT-control, 8.7 ± 0.4% in CD28 KO-control, and 7.4± 0.4% in B7 KO-control) and lungs (28.7 ± 1.2% in WT-control, 21.0 ± 1.7% in CD28 KO-control, and 22.3 ± 1.0% in B7 KO-control). TAC significantly increased the percentage of activated effector T cells in lungs, but did not increase the percentage of these cells in LV tissues. Notably, CD28 or B7 deficiency significantly attenuated the TAC-induced increase of the percentage of activated effector T cells in lungs (54.2 ± 5.2% in WT-TAC, 22.0 ± 1.1% in CD28 KO-TAC, and 24.2 ± 2.1% in B7 KO-TAC) and in LV tissues (11.5 ± 0.6% in WT-TAC, 8.6 ± 0.7% in CD28 KO-TAC, and 7.0 ± 0.6% in B7 KO-TAC) after TAC (Figure 3A–3C). Moreover, CD28 or B7 deficiency considerably reduced TAC-induced accumulation of activated effector T cells in both LV tissues and lungs (Figure 3D). CD28 KO or B7 deficiency also significantly reduced TAC-induced increases of early activated T cells in LV tissues and lungs (Figure S9). In addition, CD28 or B7 deficiency significantly attenuated TAC-induced increases in total CD3+ T cells in lungs (10.6 ± 1.5 ×105 in WT-TAC, 5.9 ± 0.96 ×105 in CD28 KO-TAC, and 6.3 ± 0.35 ×105 in B7 KO-TAC), but did not significantly affect TAC-induced CD3+ T-cell accumulation in LV tissues (Figure 3E). Furthermore, CD28 or B7 deficiency had no effect on the total number of CD45+ cells in LV tissues (34.0 ± 1.9 ×103 in WT-control, 28.1 ± 0.7 ×103 in CD28 KO-control, and 37.3± 6.0 ×103 in B7 KO-control) or lungs (3041 ± 63 ×103 in WT-control, 2901 ± 77 ×103 in CD28 KO-control, and 3130± 111 ×103 in B7 KO-control) under control conditions. However, CD28 or B7 deficiency significantly attenuated TAC-induced increases of CD45+ cells in LV tissues (18.3 ± 2.1 ×104 in WT-TAC, 9.0 ± 1.13 ×104 in CD28 KO-TAC, and 10.9± 0.66 ×104 in B7 KO-TAC) and lungs (683 ± 85 ×104 in WT-TAC, 37.4 ± 2.47 ×104 in CD28 KO-TAC, and 33.20± 1.48 ×104 in B7 KO-TAC) (Figure 3F). Together, these data demonstrate that pressure overload causes T-cell activation in lungs in mice with end-stage CHF. CD28 or B7 deficiency is effective in attenuating CD3+ T-cell activation, as well as CD3+ T-cell and CD45+ cell accumulation in LV tissues and lungs in mice after TAC.
Figure 3. CD28 or B7 deficiency inhibits CD3+ T-cell activation and leukocyte accumulation in the left ventricle (LV) and lungs after transverse aortic constriction (TAC).
Flow cytometry data were collected from wild-type (WT) and CD28 or B7 knockout (KO) mice under control conditions (Ctr) or 8 weeks after TAC. A to C, Flow cytometry plots and quantitative data represent the percentage of CD3+CD44high T cells (activated effector T cells) in LV tissues and lungs. D to F, Quantitative data represent total numbers of CD3+CD44high T cells, CD3+ T cells and CD45+ cells in LV tissues and lungs. n=5–6 per group. *P<0.05 vs control group; #P<0.05 vs TAC group of WT mice.
CD28 or B7 Deficiency Attenuated TAC-Induced LV Fibrosis
Inflammation causes cardiac fibrosis, which exacerbates LV hypertrophy and CHF development. As shown by Sirius red/Fast green staining in Figure S10A and S10B, CD28 or B7 deficiency did not affect LV fibrosis under control conditions. However, CD28 or B7 deficiency significantly attenuated TAC-induced LV fibrosis. CD28 or B7 deficiency also significantly reduced TAC-induced increases in LV mRNA content of TGF-β, a pro-fibrogenic cytokine associated with fibrosis development (Figure S10C). These data demonstrate that CD28/B7 signaling contributes to pressure overload-induced LV fibrosis.
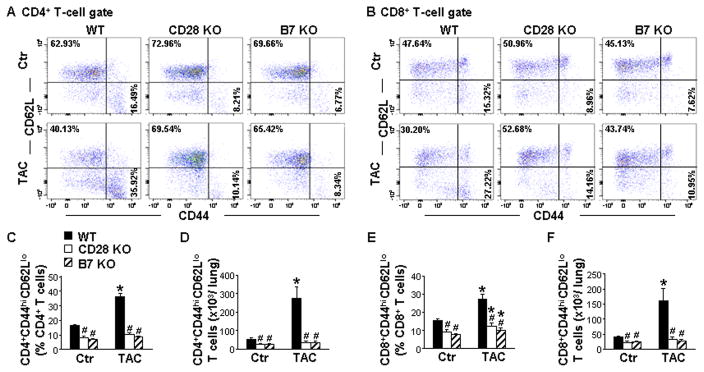
CD28 or B7 Deficiency Inhibited TAC-Induced T cell Activation and Accumulation in Both CD4+ and CD8+ Subsets in Lungs
Since CD28 or B7 deficiency significantly attenuated the TAC-induced CD3+ T-cell activation and accumulation in lungs, we further determined the effect of CD28 or B7 deficiency on the activation and accumulation of CD4+ and CD8+ T-cells. As shown in Figure 4A, 4C, 4B and 4E, under control conditions, CD28 or B7 deficiency resulted in a ~50% decrease in the percentage of TEM within the CD4+ or CD8+ T-cell subset. TAC significantly increased the percentage of CD4+ and CD8+ TEM (2.2 and 1.8-fold, respectively). CD28 or B7 deficiency totally abolished the TAC-induced increases in the percentage of CD4+ TEM, and largely reduced the increase in the percentage of CD8+ and CD3+ TEM (Figure 4A, 4C, 4B, 4E and S11A). In addition, CD28 or B7 deficiency markedly attenuated TAC-induced increases in CD4+, CD8+ and CD3+ TEM in lungs of mice (Figure 4D, 4F and S11B). Moreover, CD28 or B7 deficiency abolished the TAC-induced increase in the percentage of early activated T cells in their corresponding CD4+ and CD8+ T-cell subset and their total numbers in lungs (Figure S12). CD28 or B7 deficiency also significantly reduced the TAC-induced increases in CD4+ and CD8+ activated effector T cells (Figure S13). These data indicate that CD28 or B7 deficiency is highly effective in inhibiting pressure overload-induced T-cell activation in both CD4+ and CD8+ subsets in lungs.
Figure 4. CD28 or B7 deficiency inhibits transverse aortic constriction (TAC)-induced CD4+ and CD8+ T-cell activation and accumulation in the lung.
Flow cytometry data of lungs were collected from wild-type (WT) and CD28 or B7 knockout (KO) mice under control conditions (Ctr) or 8 weeks after TAC. A and C, Flow cytometry plots and quantitative data represent the percentage of CD44highCD62Llow T cells (effector memory T cells, TEM) within the CD4+ population of lungs. D, Total numbers of CD4+CD44highCD62Llow T cells in lungs. B and E, Flow cytometry plots and quantitative data represent the percentage of CD44highCD62Llow T cells within the CD8+ population of lungs. F, Total numbers of CD8+CD44highCD62Llow T cells in lungs. n=5–6 per group. *P<0.05 vs control group; #P<0.05 vs TAC group of WT mice.
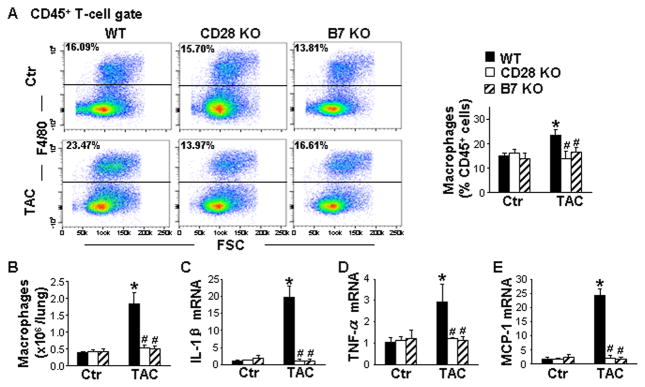
CD28 or B7 Deficiency Attenuated TAC-Induced Lung Macrophage Infiltration and Pro-Inflammatory Cytokine Expression
Because activated T cells regulate macrophage function, we further determined whether CD28 or B7 deficiency affected the number of lung macrophages expressing the macrophage marker F4/80 (Figure S5). Under control conditions, the percentage and total number of macrophages was not altered in either CD28 KO or B7 KO mice (Figure 5A and 5B). TAC significantly increased the percentage and total number of macrophages in the lung, which was notably reduced in CD28 and B7 deficient mice (Figure 5A and 5B). Furthermore, CD28 or B7 deficiency also significantly suppressed TAC-induced increases of lung mRNA levels of IL-1β, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) (Figure 5C–5E). These data demonstrate that CD28 or B7 deficiency is effective in attenuating TAC-induced lung macrophage infiltration and pro-inflammatory cytokine expression.
Figure 5. CD28 or B7 deficiency attenuates transverse aortic constriction (TAC)-induced lung macrophage infiltration and pro-inflammatory cytokine expression.
Data were collected from lungs of wild-type (WT) and CD28 or B7 knockout (KO) mice under control conditions (Ctr) or 8 weeks after TAC. A, Flow cytometry plots and quantitative data represent the percentage of macrophages (F4/80+) in the lung. B, Quantitative data of flow cytometry represent the total number of macrophages in the lung. C to E, Quantitative RT-PCR results of IL-1β, TNF-α and MCP-1 mRNA levels in lung lysates. n=5–6 per group. *P<0.05 vs control group; #P<0.05 vs TAC group of WT mice.
CTLA4-Ig Attenuated TAC-Induced T-Cell Activation and LV Dysfunction
To further determine whether inhibition of T-cell activation can attenuate CHF development, the fusion protein CTLA4-Ig, which attenuates CD28/B7 signaling, was administrated to the mice prior to TAC. TAC markedly increased LV systolic pressure after 8 weeks, which was comparable in CTLA4-Ig-treated and vehicle-treated mice (Figure S14A). CTLA4-Ig treatment significantly reduced the TAC-induced increases in the percentage of CD4+ TEM (17.1 ± 0.9% in control, 22.7 ± 1.4% in TAC-vehicle, and 14.2 ± 1.7% in TAC-CTLA4-Ig) (Figure S14B), as well as the percentage of CD4+CD69+ T cells in lungs (Figure S14C), indicating CTLA4-Ig treatment inhibited T-cell activation in our experimental setting.
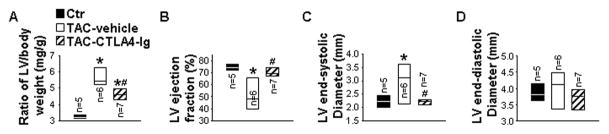
CTLA4-Ig treatment significantly attenuated the TAC-induced LV hypertrophy (3.25 ± 0.05 mg/kg in control, 5.66 ± 0.21 mg/kg in TAC-vehicle, and 4.72 ± 0.25 mg/kg in TAC+CTLA4-Ig) (Figure 6A). Moreover, echocardiographic measurements showed that CTLA4-Ig treatment also attenuated the TAC-induced decrease of LV EF (74.6 ± 1.2% in control, 52.4 ± 5.3% in TAC-vehicle, and 70.9 ± 1.5% in TAC-CTLA4-Ig), and increase of LVESD (2.2 ± 0.1 mm in control, 3.0 ± 0.3 mm in TAC-vehicle, and 2.2 ± 0.1 mm in TAC-CTLA4-Ig) (Figure 6B and 6C). LVEDD was not altered (Figure 6D). These data indicate that inhibition of CD28/B7 signaling by CTLA4-Ig treatment protects hearts against TAC-induced LV hypertrophy and dysfunction.
Figure 6. CD28/B7 blockade attenuates transverse aortic constriction (TAC)-induced cardiac hypertrophy and dysfunction.
Data were collected from mice under basal conditions (Ctr), or treated with human-Ig (vehicle) or CTLA4-Ig under TAC conditions. A to C, The ratio of left ventricle (LV), left atria (LA) and lung weight to body weight of mice. D to F, Echocardiographic measurements of LV ejection fraction, LV end-systolic diameter and LV end-diastolic diameter from hearts. *P<0.05 vs control group; #P<0.05 vs TAC group of vehicle mice.
Discussion
This study demonstrated that LV failure produced by chronic pressure overload caused an increase in total number of activated effector T cells and TEM in the LV and lungs. Using CD28/B7 KO mice or CTLA4-Ig to block CD28/B7 interactions, we also demonstrated that intact CD28/B7 signaling contributed significantly to TAC-induced LV hypertrophy and dysfunction, lung remodeling, and RV hypertrophy. CD28/B7 interaction strongly enhanced myocardial and pulmonary infiltration of activated T cells and CD45+ leukocytes, and resulted in increased fibrosis and cytokine expression in these tissues. These findings suggest that T-cell activation contributes to CHF development and that inhibition of T-cell activation may be useful in attenuating CHF development and progression by suppressing inflammatory responses.
The increased T-cell accumulation in LV and lung tissues in CHF mice appears to be a result of increased antigen-specific T-cell recruitment as well as nonspecific T-cell recruitment secondary to the pro-inflammatory environment in these tissues. The increased adhesion molecules (such as E-selectin and P-selectin) and chemokines on the resident cells, and the selectin ligands and chemokine receptors on T cells contribute to the T-cell accumulation in these tissues. Increased selectin ligands and chemokine receptors during T cell differentiation14 are known to promote T-cell recruitment into the inflamed sites15. In addition, antigen-presenting cells (such as dendritic cells and macrophages) might also enhance antigen-specific T-cell recruitment by presenting resident-cell debris or through other signaling mechanisms.16 Recent studies from Dr. Harrison and his associates have demonstrated that oxidized self- antigen(s) contributes to Ang-II induced T-cell activation and hypertension in mice.17, 18 In the context that oxidative stress is increased during CHF development, oxidized proteins in heart and lungs are likely contributors to the TAC-induced T cell activation. However, it is not clear what endogenous antigen(s) mediate T-cell activation in CHF.
CHF dramatically increased the total number and the percentage of activated effector T cells in the lungs. To our surprise, end-stage CHF caused only a mild increase in the total number of activated effector T cells in LV tissues and did not affect the percentage of these cells. The accumulation of activated effector T cells in LV tissues and lungs in mice with CHF indicate that these tissues emit TAC-induced signals that promote T-cell infiltration or promote tissue specific T-cell priming during CHF development. The profound increase of leukocytes, macrophages, T cells and activated T cells in the present study is consistent with our previous observation using the same CHF model. 13, 19 The significant increase of activated effector T cells in peripheral blood and spleen in CHF mice indicates that T-cell activation is a systemic response during CHF development. Our findings that CHF caused a significant increase in the percentage of activated effector T cells in the lungs, but unchanged percentage of these cells in the LV, suggest that T-cell activation has a greater impact on pulmonary inflammation than on LV inflammation in animals with end-stage CHF.
Since previous studies demonstrated that T cells contribute to the pathogenesis of CHF and that CD4+ T cells promote the transition from LV hypertrophy to heart failure12, 13, the dramatic increase in CD4+ TEM might promote TAC-induced CHF progression by modulating lung inflammatory responses. The reduced accumulation of TEM in LV tissues and lungs by CD28/B7 deficiency or CD28/B7 blockade after TAC is in agreement with reports that T-cell activation contributes to tissue inflammation in other disease models, such as inflammatory responses in skin diseases20, organ transplant-induced inflammation and rejection21–24 and cardiovascular diseases including hypertension and atherosclerosis25, 26. Our finding supports an important role of T cell activation in CHF development and progression, and a critical role for CD28/B7 signaling in regulating T-cell activation during CHF development.
Although CD28 or B7 deficiency completely abolished the TAC-induced increases in CD4+ TEM, we observed only a partial attenuation of the TAC-induced increases in CD8+ TEM in CD28 KO or B7 KO mice. The finding that CD28 or B7 deficiency led to a stronger inhibition of T-cell activation in CD4+ T cells than in CD8+ T cells is likely related to the constitutive expression of CD28 on CD4+ T cells, and a relatively high abundance of CD28 expression on CD4+ T cells as compared to that on CD8+ T cells in mice.27 The finding that CD28 or B7 deficiency only partially inhibited the TAC-induced tissue inflammatory responses in LV tissues and lungs suggests that additional pro-inflammatory signaling pathways independent of CD4+ and CD8+ T-cell activation may also play important roles in CHF-associated inflammatory responses and CHF development.
The present study has several limitations. First, both CD28 and B7 deficiency significantly attenuated TAC-induced LV hypertrophy and dysfunction, as well as tissue inflammation. The dramatically reduced TAC-induced lung inflammation and remodeling in CD28 and B7 deficient mice is likely to be a collective effect of the reduced cardiac, pulmonary and systemic T-cell activation in these mice. However, we are unable to determine the relative contribution of cardiac, pulmonary and systemic T-cell activation in the CHF progression and lung remodeling. In addition, as global CD28 KO and B7 KO mouse strains were used for our study, some of the observed phenotypes may be a result of chronic adaptation to the global gene deletion. Furthermore, the present study could not determine whether the cardioprotective effects in CD28 KO and B7 KO mice are because of CD28 and B7 gene deletion in leukocytes or in other cell types.
In summary, we demonstrate that CD28/B7 signaling contributes significantly to chronic pressure overload-induced cardiac and pulmonary inflammation and the development of CHF. Our study suggests that strategies that target CD28/B7 signaling may be useful in treating CHF.
Clinical Perspective
Congestive heart failure (CHF) is accompanied by increased leukocyte accumulation and increased inflammatory cytokine expression in hearts and lungs, which exacerbates the development and progression of CHF. The interaction of the CD28 co-receptor with B7 proteins regulates T-cell activation. In this article, we demonstrate that CD28 or B7 deficiency attenuates systolic overload-induced CHF development by reducing cardiac and pulmonary inflammation and remodeling. Our study provides direct evidence that strategies targeting T-cell activation may be useful in treating CHF.
Supplementary Material
Novelty and Significance.
What Is New?
CD28/B7 deficiency inhibits effector T-cell accumulation, reduces myocardial and pulmonary inflammation, and attenuates the development of congestive heart failure (CHF).
What Is Relevant?
CHF development is associated with T-cell activation-involved inflammatory responses. Thus, inhibition of T-cell activation by blocking CD28/B7 signaling may be a promising strategy in treating CHF.
Summary
CD28/B7 signaling contributes to chronic pressure overload-induced cardiac and pulmonary inflammation and the development of CHF, suggesting strategies targeting T-cell activation may be useful for CHF treatment.
Acknowledgments
Sources of funding
This study was supported by U.S. Public Health Service Grants HL021872, HL098669, HL098719, HL102597, HL089249, R01HL105406, research grants 81470512 and 81570355 from National Natural Science Foundation, and T32HL069764 from the National Institutes of Health, and Research Grant 09GRNT2260175 from the American Heart Association.
Footnotes
Disclosures
None.
References
- 1.Diwan A, Tran T, Misra A, Mann DL. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med. 2003;3:161–182. doi: 10.2174/1566524033361537. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Xu X, Fassett J, Kwak D, Liu X, Hu X, Falls TJ, Bell JC, Li H, Bitterman P, Bache RJ, Chen Y. Double-stranded RNA-dependent protein kinase deficiency protects the heart from systolic overload-induced congestive heart failure. Circulation. 2014;129:1397–1406. doi: 10.1161/CIRCULATIONAHA.113.002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Menyar AA. Cytokines and myocardial dysfunction: state of the art. J Card Fail. 2008;14:61–74. doi: 10.1016/j.cardfail.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031–1041. doi: 10.1097/00004872-199816070-00018. [DOI] [PubMed] [Google Scholar]
- 5.Weber KT, Jalil JE, Janicki JS, Pick R. Myocardial collagen remodeling in pressure overload hypertrophy. A case for interstitial heart disease. Am J Hypertens. 1989;2:931–940. doi: 10.1093/ajh/2.12.931. [DOI] [PubMed] [Google Scholar]
- 6.Pereda D, Garcia-Alvarez A, Sanchez-Quintana D, Nuno M, Fernandez-Friera L, Fernandez-Jimenez R, Garcia-Ruiz JM, Sandoval E, Aguero J, Castella M, Hajjar RJ, Fuster V, Ibanez B. Swine model of chronic postcapillary pulmonary hypertension with right ventricular remodeling: long-term characterization by cardiac catheterization, magnetic resonance, and pathology. J Cardiovasc Transl Res. 2014;7:494–506. doi: 10.1007/s12265-014-9564-6. [DOI] [PubMed] [Google Scholar]
- 7.Wagenvoort CA. Lung biopsy findings in secondary pulmonary hypertension. Heart Lung. 1986;15:429–450. [PubMed] [Google Scholar]
- 8.Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y, Weir EK. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension. 2012;59:1170–1178. doi: 10.1161/HYPERTENSIONAHA.111.186072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Hou L, Kwak D, Fassett J, Xu X, Chen A, Chen W, Blazar BR, Xu Y, Hall JL, Ge JB, Bache RJ, Chen Y. Increasing Regulatory T Cells With Interleukin-2 and Interleukin-2 Antibody Complexes Attenuates Lung Inflammation and Heart Failure Progression. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.07084. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell costimulation: a therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant. 2006;6:876–883. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 11.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 12.Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, Delage C, Calise D, Dutaur M, Parini A, Pizzinat N. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–2124. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]
- 13.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velazquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P. Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure. Circ Heart Fail. 2015;8:776–787. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowe JB. Glycosylation in the control of selectin counter-receptor structure and function. Immunol Rev. 2002;186:19–36. doi: 10.1034/j.1600-065x.2002.18603.x. [DOI] [PubMed] [Google Scholar]
- 15.Tietz W, Allemand Y, Borges E, von Laer D, Hallmann R, Vestweber D, Hamann A. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol. 1998;161:963–970. [PubMed] [Google Scholar]
- 16.Dandel M, Wallukat G, Englert A, Hetzer R. Immunoadsorption therapy for dilated cardiomyopathy and pulmonary arterial hypertension. Atheroscler Suppl. 2013;14:203–211. doi: 10.1016/j.atherosclerosissup.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Kirabo A, Fontana V, de Faria AP, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Saleh MA, Kirabo A, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126:50–67. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131:1019–1030. doi: 10.1161/CIRCULATIONAHA.114.008788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo S, Kooshesh F, Wang B, Fujisawa H, Sauder DN. Contribution of the CD28 molecule to allergic and irritant-induced skin reactions in CD28 −/− mice. J Immunol. 1996;157:4822–4829. [PubMed] [Google Scholar]
- 21.Haspot F, Seveno C, Dugast AS, Coulon F, Renaudin K, Usal C, Hill M, Anegon I, Heslan M, Josien R, Brouard S, Soulillou JP, Vanhove B. Anti-CD28 antibody-induced kidney allograft tolerance related to tryptophan degradation and TCR class II B7 regulatory cells. Am J Transplant. 2005;5:2339–2348. doi: 10.1111/j.1600-6143.2005.01018.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Krummey SM, Badell IR, Wagener M, Schneeweis LA, Stetsko DK, Suchard SJ, Nadler SG, Ford ML. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J Exp Med. 2014;211:297–311. doi: 10.1084/jem.20130902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szot GL, Zhou P, Sharpe AH, He G, Kim O, Newell KA, Bluestone JA, Thistlethwaite JR., Jr Absence of host B7 expression is sufficient for long-term murine vascularized heart allograft survival. Transplantation. 2000;69:904–909. doi: 10.1097/00007890-200003150-00040. [DOI] [PubMed] [Google Scholar]
- 24.Birsan T, Hausen B, Higgins JP, Hubble RW, Klupp J, Stalder M, Celniker A, Friedrich S, O’Hara RM, Morris RE. Treatment with humanized monoclonal antibodies against CD80 and CD86 combined with sirolimus prolongs renal allograft survival in cynomolgus monkeys. Transplantation. 2003;75:2106–2113. doi: 10.1097/01.TP.0000066806.10029.7A. [DOI] [PubMed] [Google Scholar]
- 25.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.