Abstract
Chlorophyll (Chla) and chlorophyllin (CHL) were shown previously to reduce carcinogen bioavailability, biomarker damage, and tumorigenicity in trout and rats. These findings were partially extended to humans, where CHL reduced excretion of aflatoxin B1 (AFB1)-DNA repair products in Chinese unavoidably exposed to dietary AFB1. However, neither AFB1 pharmacokinetics nor Chla effects were examined. We conducted an unblinded crossover study to establish AFB1 pharmacokinetic parameters among four human volunteers, and to explore possible effects of CHL or Chla cotreatment in three of those volunteers. For protocol 1, fasted subjects received an Institutional Review Board–approved dose of 14C-AFB1 (30 ng, 5 nCi) by capsule with 100 mL water, followed by normal eating and drinking after 2 hours. Blood and cumulative urine samples were collected over 72 hours, and 14C- AFB1 equivalents were determined by accelerator mass spectrometry. Protocols 2 and 3 were similar except capsules also contained 150 mg of purified Chla or CHL, respectively. Protocols were repeated thrice for each volunteer. The study revealed rapid human AFB1 uptake (plasma ka, 5.05 ± 1.10 h−1; Tmax, 1.0 hour) and urinary elimination (95% complete by 24 hours) kinetics. Chla and CHL treatment each significantly impeded AFB1 absorption and reduced Cmax and AUCs (plasma and urine) in one or more subjects. These initial results provide AFB1 pharmacokinetic parameters previously unavailable for humans, and suggest that Chla or CHL co-consumption may limit the bioavailability of ingested aflatoxin in humans, as they do in animal models.
Aflatoxin is a potent, naturally occurring carcinogenic mycotoxin that is associated with the growth of two types of mold: Aspergillus flavus and Aspergillus parasiticus. Food and food crops most prone to aflatoxin contamination include corn and corn products, cottonseed, peanuts and peanut products, tree nuts, and milk. Humidity, high temperatures, and other environmental conditions such as insect infestation can encourage aflatoxin growth on crops. Consequently, aflatoxins can invade the food supply at anytime during production, processing, transport, and storage. Evidence of acute aflatoxicosis in humans has been reported primarily in developing countries lacking the resources to effectively screen aflatoxin contamination from the food supply (1).
Because aflatoxins, particularly aflatoxin B1 (AFB1), are potent carcinogens in some animals, there is interest in the effects of long-term exposure to low levels of these important mycotoxins on humans. Epidemiologic studies in Asia and Africa revealed a positive association between dietary aflatoxins and liver cancer (2). High levels of aflatoxins in combination with infection with hepatitis B seem to act synergistically to increase risk of hepatocellular carcinoma (3). Intervention programs have been directed toward reducing hepatitis virus infection through immunization and screening of foods for aflatoxin levels. There is also interest in addition of dietary agents that may reduce the bioavailability of aflatoxins by binding in the intestine and limiting absorption, or by increasing metabolism and reducing systemic exposure. Intervention agents currently under investigation include apiginin clays, Oltipraz, and chlorophyll (Chla) or its derivative (4–8). Chla and its derivatives have a long, well-known history of uses for medicinal and therapeutic preparations (9). Both natural Chla and its water-soluble derivative sodium copper chlorophyllin (CHL) have been extensively studied for a variety of significant biological activities (10–14). A double-blinded, placebo-controlled intervention trial in Qidong, People's Republic of China showed that CHL intervention can reduce aflatoxin-DNA adduct excretion among individuals in a population at high risk for liver cancer (8). This study clearly showed a decrease in a biomarker of AFB1-DNA damage in individuals chronically exposed to relatively high doses of aflatoxin through their diet. The mechanisms that produce this effect in humans are undefined but may include limiting absorption in the intestine, systemic complexation with bioavailable forms of CHL, or induction of phase II enzymes leading to increased aflatoxin metabolism (7, 15, 16). Preclinical studies in rats and rainbow trout suggested that CHL acts primarily by binding certain aflatoxins, heterocyclic amines, and polycyclic aromatic hydrocarbons to reduce bioavailability, genomic damage, and tumor induction (17–19).
Microdosing pharmacokinetic studies are increasingly being used in early translational, or phase 0, human studies to evaluate the kinetics of compounds at subtherapeutic/subtoxic doses. By taking advantage of sensitive methods such as accelerator mass spectrometry (AMS) for quantifying drug concentrations, kinetic parameters can be evaluated in humans, and new approaches to medical intervention can be tested safely (20). AMS brings three distinct advantages to biochemical research: relevant chemical doses, very small sample sizes, and very low radioactive exposures due to long-lived isotopes and/or high levels of isotope dilution.
The purpose of the present study was to investigate AFB1 pharmacokinetics in human volunteers using microdosing techniques and AMS analysis, and to explore possible effects of Chla and CHL cotreatment on AFB1 pharmacokinetic parameters. The kinetics of low-dose AFB1 was investigated in three volunteers who received a 30-ng dose of 14C-labeled aflatoxin, without or with CHL or Chla cotreatment. AMS was used to measure the levels of aflatoxin equivalents in plasma and urine following oral administration. Pharmacokinetic modeling of absorption and disposition was done to evaluate the disposition of AFB1 at doses that are within the acceptable levels found in foods according to limits set by the United States Department of Agriculture (21).
Materials and Methods
Chemicals
14CAFB1 (Moraveck Biochemicals, Inc.) was a generous gift of Ronald T. Riley (R.B. Russell Research Center, Athens, GA). Radio-chemical and chemical purity was confirmed to be >97% by high performance liquid chromatography equipped with an online radioisotope detector and a photodiode array detector. The specific activity was determined to be 50 mCi/mmol by liquid scintillation counter. Chla was isolated from spinach and purified by counter current chromatography following our published method (22). CHL (Rystan) was the same lot used in the China intervention trial (7). The lactose and gelatin capsules were obtained from the College of Pharmacy at Oregon State University (OSU).
Capsule preparation
Capsules were prepared by filling the cap with lactose and adding 50 µL 14CAFB1 in ethanol (0.6 ng/µL, 0.1 nCi/µL). For the intervention, 150 mg of Chla in ethanol or 150 mg of powered CHL were added to the cup of the capsules. For 14CAFB1 and Chla, the ethanol was allowed to evaporate before the capsules were sealed. Each capsule was prepared fresh as needed. We note that the dose of aflatoxin chosen for this study is ~1/20th of the amount allowed in a 30-gram peanut butter sandwich, at the Food and Drug Administration action level of 20 ppb.
Human volunteers
Design
The protocol design for this study, and all revisions, were approved by both Institutional Review Boards at OSU and Lawrence Livermore National Laboratory (LLNL). Participants were recruited from a group with sufficient scientific knowledge to evaluate the toxicologic issues and risks of the study. Written informed consent was obtained from all enrolled volunteers. After the consent form was read and signed and any questions were answered, the volunteers provided their medical history and underwent a limited physical examination. Volunteer characteristics are provided in Table 1. Permission for the administration of 14C-radiolabeled aflatoxin was obtained from the OSU Radiation Safety Committee. Information was also obtained on any medication that the subjects received which may have affected the metabolism and disposition of 14CAFB1. All clinical procedures were conducted with the technical assistance of the Integrated Environmental Health Sciences Clinical core within the Environmental Health Sciences Center at OSU.
Table 1.
Study subject information
| Subject | Gender | Height | Weight | Age |
|---|---|---|---|---|
| 1 | Male | 6′2″ | 180 | 65 |
| 2 | Male | 6′0″ | 197 | 46 |
| 3 | Female | 5′4″ | 138 | 32 |
| 5 | Male | 6′4″ | 212 | 59 |
Compliance
Subjects 1, 2, and 5 completed the entire study in full compliance with the specified protocols. Subject 3 completed all three cycles of protocol 1 (AFB1 only) and one cycle of protocol 3. Only plasma samples were amenable to analysis for this subject, the results of which were comparable with those of other subjects and included in the AFB1-only analyses. Subject 4 was enrolled into the study but excluded before the first dose due to illness. The first AFB1-only exposure trial conducted with subject 1 was a range-finding experiment that resulted in a slight revision for all subsequent experiments and subjects. Specifically, three additional urine collections and blood draws were added and the study period was increased from 36 to 72 h to allow for a more complete pharmacokinetic analysis.
Study design
The initial design called for serial treatment of three volunteers. The three protocols used were as follows: Protocol 1—At 8 a.m., fasted subjects were given a gelatin capsule containing 14C-AFB1 (30 ng, 5 nCi), which they swallowed with 100 mL water. Normal eating and drinking was resumed at 10 a.m., beginning with a specified and provided breakfast. Blood (3 mL) was drawn by a qualified nurse at times 0, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3, 4, 8, 12, 24, 48, and 72 h. Each volunteer was given the choice between a catheter (time points 0–12 h) and multiple venipunctures. Total urine samples were collected at intervals of 0 to 2 h, 2 to 4 h, 4 to 8 h, 8 to 12 h, 12 to 24 h, and every subsequent 24 h until the end of study. This protocol was repeated thrice to obtain baseline data for each subject. Protocol 2—Nominally, a similar protocol was followed, except that the capsule also contained 150 mg of purified Chla. This protocol was repeated thrice. Protocol 3—Protocol 1 was followed, except that the capsule also contained 150 mg of CHL. This protocol was also repeated thrice. The order of protocol treatments was not totally randomized in this pilot study. Each subject received the three exposure cycles for protocol 1 (AFB1 alone) in succession to establish baseline AFB1 pharmacokinetic data in the absence of possible carryover effects from CHL or Chla intervention. Initial data indicated that 14C doses were adequate for detection, and that clearance rates in plasma and urine were sufficiently rapid that exposure intervals of 7 d or greater could be conducted without interference from baseline carryover. Volunteers then received the three CHL and three Chla interventions, which were given in differing orders within and among subjects to minimize the potential that one intervention might bias the subsequent treatment. Each subject thus received nine test treatments: AFB1 alone (repeated thrice), with Chla (repeated thrice), and with CHL (repeated thrice). Subjects were free living (i.e., not confined to the testing facility) during each 72-h study period.
AMS analysis
Plasma and urine samples were shipped to LLNL where AMS was used to measure the ratio of 14C to total carbon. Specifically, samples were thawed at room temperature, and an aliquot of each sample (typically 20 µL blood and 100 µL urine) was placed in quartz tubes and dried in a vacuum centrifuge. The carbon content of each sample was converted to CO2 by combustion, and the CO2 was quantitatively reduced to graphite in the presence of zinc and titanium hydride, condensing onto cobalt at ~500°C for 4 h (23, 24). 14C/C ratios (units of amol 14C/mg C) in the graphite samples were then quantified using the 1 MV accelerator mass spectrometer at LLNL. The 14C/C ratios in the samples were converted to 14C contents (amol 14C/mL urine or plasma) using the density and carbon contents of the plasma and urine samples (25). For plasma samples, the value used for carbon content were assumed to be 4.2% (26). As the percentage carbon is more variable for urine, each individual urine sample was analyzed at LLNL for carbon content, using an Exeter 440 CHN Analyzer. Densities of the plasma and urine samples were assumed to be 1g/cc. For each human subject dosing 14C carbon contents of “time 0,” plasma and urine samples were subtracted from the 14C carbon contents of the other samples. Excess 14C concentration was converted to aflatoxin equivalents (parent compound and all metabolites) using the specific activity of the dose (50 mCi/mmol), together with the compound molecular weight of 312.
Pharmacokinetic analysis
Pharmacokinetic parameters of AFB1 equivalents were first calculated using noncompartmental methods. Urine excretion over time, maximum plasma concentration (Cmax), and time to Cmax (Tmax) were determined by observation from the collected concentration verses time data. WinNonlin (v. 4.1.a) was used to perform noncompartmental analysis for estimation of kinetic parameters for aflatoxin with and without intervention. Area under the curve (AUC) was calculated for the intervals 0 to t, 0 to 8, 0 to 24, and 0 to inf, where t is the time of the last measurable concentration and inf is infinity, using the linear trapezoidal method. Differences between control and intervention groups were analyzed using one-way ANOVA followed by Dunnett's multiple comparison test and were considered significant for P values of <0.05. Analysis of urine data beyond the assessment of cumulative excretion for AFB1 with and without intervention was not done because of the inability to distinguish between free AFB1 and its metabolites. Additionally, to estimate absorption and disposition rate constants, a two-compartment model with first-order input and first-order elimination was fit to each of the plasma concentration–time profiles using WinNonlin (v. 4.1.a) software. Estimated parameters included A and B (coefficients, or zero-time intercepts), ka (absorption rate constant), α and β (disposition rate constants), and Tlag (absorption lag time). For plasma data, because the order could not be completely randomized, linear in order trends were examined as possible alternate explanations for observed treatment effects. In only one analysis (Cmax within subject 5) was there such evidence due to both an overall strong linear trend coupled with similar trends within the replicate runs for each treatment group.
Results
AFB1 pharmacokinetics
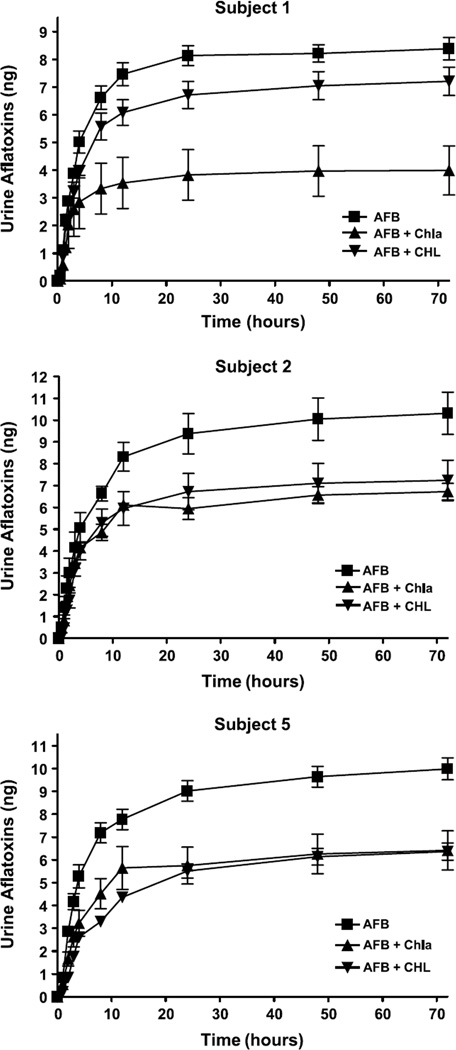
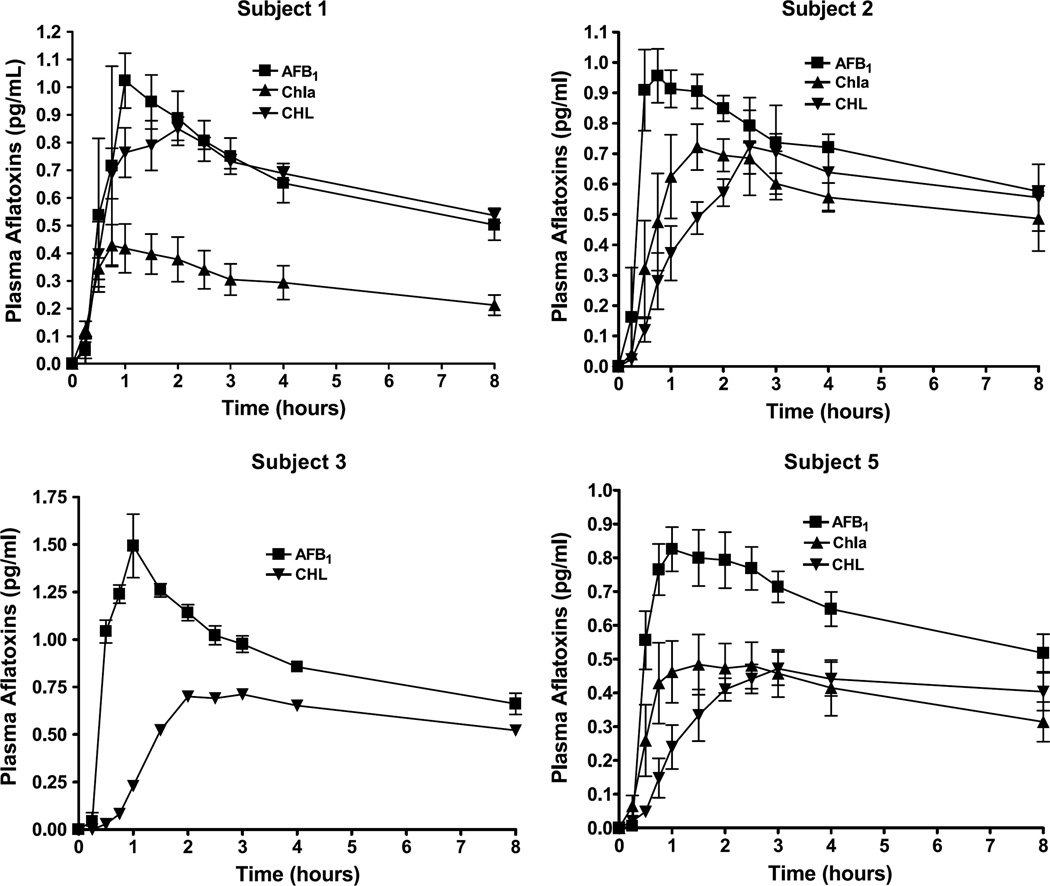
Urine excretion over the 72-hour period for AFB1 challenge with and without intervention is presented in Fig. 1 as total cumulative excretion for each subject. Excretion was rapid with >95% of the total urine AFB1 equivalents produced within the first 24 hours. As seen in Fig. 2, absorption of AFB1 equivalents into systemic circulation was also rapid with peak concentrations achieved within ~1 hour. Noncompartmental calculations are presented in Table 2 for all subjects with a breakdown of individual pharmacokinetic parameters presented in Table 3. The parameters presented for AFB1 showed that absorption of AFB1 equivalents into plasma follow a first-order process. A two-compartment model with first-order input and elimination was fitted to each plasma data set, yielding parameters that are also presented in Tables 2 and 3. These data showed that AFB1 equivalents fit a two-compartment model of absorption and elimination with a rapid distribution phase followed a slower elimination phase.
Fig. 1.
Cumulative urinary excretion of aflatoxin equivalents in subjects 1, 2, and 5. Aflatoxin amount is derived from AMS analysis based on total 14C and is presented as mean ± SD (n = 3 independent trials). Total aflatoxin excretion at 72 h was assessed by one-way ANOVA followed by Dunnett's multiple comparison test comparing AFB1 alone to interventions for each subject.
Fig. 2.
Pharmacokinetic profiles of aflatoxin equivalents from plasma samples collected at 0.25, 0.5, 0.45, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, and 8.0 h. Aflatoxin concentration is derived from AMS analysis based on total 14C from aliquots of plasma samples and is presented as mean ± SD (n = 3 independent trials).
Table 2.
Mean pharmacokinetic parameters of AFB1 equivalents in plasma
| Parameter | Unit | Aflatoxin | |
|---|---|---|---|
| Mean ± SD | CV% | ||
| Cmax | pg/mL | 0.941 ± 0.154 | 16.3 |
| Tmax | h | 1.02 ± 0.31 | 30.7 |
| AUC0-t | h*pg/mL | 25.6 ± 6.3 | 24.7 |
| AUC0-inf | h*pg/mL | 54.7 ± 18.7 | 34.3 |
| AUC_Extrap | % | 50.9 ± 8 | 15.7 |
| AUC0–8 | h*pg/mL | 5.4 ± 0.7 | 13.2 |
| AUC0–24 | h*pg/mL | 12.4 ± 1.8 | 14.7 |
| CL/F | L/h | 0.66 ± 0.39 | 59.9 |
| Vd/F | L | 62.3 ± 7.8 | 12.5 |
| ka | h−1 | 5.05 ± 1.10 | 19.5 |
| Á | h−1 | 0.24 ± 0.06 | 24.4 |
| β | h−1 | 0.01 ± 0.003 | 26.8 |
| t1/2α (harmonic mean) | h | 2.86 | — |
| t1/2β (harmonic mean) | h | 64.4 | — |
Table 3.
Individual pharmacokinetic parameters of aflatoxin equivalents
| Aflatoxin alone | With Chla | With CHL | ||
|---|---|---|---|---|
| Subject 1 | Cmax (pg/mL) | 1.02 ± 0.17 | 0.44 ± 0.14* | 0.86 ± 0.09 |
| Tmax (h) | 0.99 ± 0.02 | 0.83 ± 0.15 | 1.86 ± 0.33* | |
| t1/2α (h) | 2.06 ± 0.28 | 3.15 ± 1.50 | 2.93 ± 1.46 | |
| ka (absorption rate constant) | 4.78 ± 0.01 (2) | 3.97 ± 0.45 (2) | 2.54 ± 0.60† | |
| AUC0-t (h*pg/mL) | 22.57 ± 9.93 | 10.27 ± 3.40 | 27.20 ± 2.69 | |
| AUC0–24 | 11.94 ± 2.51 | 4.97 ± 1.61* | 12.55 ± 1.25 | |
| Subject 2 | Cmax (pg/mL) | 0.97 ± 0.15 | 0.73 ± 0.13 | 0.74 ± 0.26 |
| Tmax (h) | 0.92 ± 0.51 | 1.67 ± 0.77 | 4.16 ± 3.34 | |
| t1/2α (h) | 4.39 ± 2.83 | 8.26 (1) | 7.75 ± 2.66 (2) | |
| ka (absorption rate constant) | 7.76 ± 1.13 (2) | 3.28 (1)† | 0.97 ± 0.47* | |
| AUC0-t (h*pg/mL) | 29.00 ± 1.05 | 21.87 ± 3.71 | 23.83 ± 8.30 | |
| AUC0–24 | 13.40 ± 0.77 | 9.60 ± 3.02 | 11.15 ± 3.51 | |
| Subject 5 | Cmax (pg/mL) | 0.83 ± 0.12 | 0.50 ± 0.13† | 0.49 ± 0.07† |
| Tmax (h) | 1.16 ± 0.29 | 2.35 ± 0.79 | 2.17 ± 0.77 | |
| t1/2α (h) | 3.74 ± 0.26 | 3.67 ± 1.14 | 2.96 (1) | |
| ka (absorption rate constant) | 3.43 ± 0.40 | 2.91 ± 1.43 | 1.27 ± 1.08 | |
| AUC0-t (h*pg/mL) | 25.23 ± 5.34 | 14.10 ± 4.77† | 17.77 ± 3.80 | |
| AUC0–24 | 11.88 ± 2.07 | 6.42 ± 1.56† | 8.17 ± 1.47 |
NOTE: 1 and 2 denotes n = 1 and n = 2 respectively, because either two or one of the replicate runs could not be fit to a two compartment model. Mean and SD over (n =) 3 replicate runs except where noted in parentheses.
P < 0.01 vs Aflatoxin alone (Dunnett adjusted).
P < 0.05.
Effects of Chla and CHL intervention
Responses to intervention with Chla and CHL were qualitatively similar among the three volunteers completing the study, but with some evidence for inter-individual variability in degree of response. AFB1 equivalents excreted over 72 hours are presented in Fig. 1 and reveal a consistent trend toward reduced urinary excretion. The effects were significant for Chla intervention in all volunteers (P < 0.01 for volunteer 1 and P < 0.05 for volunteers 2 and 5). Although CHL showed a similar trend toward protection, intervention reached significance in subject 5 (P < 0.01) but not subjects 1 (P = 0.08) or 2 (P = 0.08). Subject 1 excreted an average of 29.0 ± 2.31% of the dose in the first 72 hours when given AFB1 alone, whereas intervention reduced the amount of the initial AFB1 dose to 13.3 ± 5.10% for Chla and 24.0 ± 2.95% for CHL. Similar effects were seen in subject 2 with an average of 34.4 ± 5.60% for AFB1, 22.4 ± 2.17% for Chla, and 24.1 ± 5.37% for CHL, whereas subject 5 excreted 33.3 ± 2.79% of the AFB1 dose, 21.4 ± 4.94% with Chla, and 21.2 ± 2.17% with CHL.
Changes in plasma concentrations of AFB1 equivalents following intervention in each subject mirrored those seen in urine. Apparent in Fig. 2 is a consistent trend for reduction in mean plasma concentrations during the initial absorption phase of the plasma concentration time curve. These data were fit to a two-compartment model as described in Materials and Methods, and individual pharmacokinetic parameters of aflatoxin equivalents are presented in Table 3. For some of the profiles following AFB1 alone or with CHL intervention, it was determined that a two-compartment model was overparameterized; thus, for these cases, a one-compartment model with first-order input and first-order output was fit to the data. As seen in Table 3, subject 1 showed a robust and highly significant response to Chla intervention that resulted in significant reductions in Cmax (P < 0.01) and AUC0–24 hours (P < 0.01). The response to CHL by this subject was less pronounced, with significant changes in Tmax (P < 0.01) and the absorption rate constant (P < 0.05). Subject 2 response was not significant for changes in Cmax (P = 0.27) or AUC0–24 (P = 0.33), although a similar pattern of reduced plasma AFB1 equivalents was observed. The absorption rate constant was significantly reduced with both Chla (P < 0.05) and CHL (P < 0.05) intervention in this subject. Subject 5 also responded to both Chla and CHL intervention with a significant reduction in Cmax (P < 0.05) and, for Chla, a significant change in AUC0-t and AUC0–24 (P < 0.05). Figure 2 also includes the plasma data for subject 3. Although this subject did not complete protocols 2 and 3, the one intervention trial with CHL gave a result consistent with protection by this agent.
Discussion
AFB1 pharmacokinetics in human subjects
The intestinal absorption of AFB1 at levels commonly found in the diet had not been previously measured in humans. The present study used AMS techniques, which permitted an investigation of the absorption and pharmacokinetics of AFB1 and its metabolites in four human volunteers, using doses of 14C and AFB1 that fall within an allowable range of safety. We did not attempt in this initial study to discriminate between free AFB1 and its various metabolites and conjugates, which limits interpretation relating to potential inter-individual or treatment-related differences in metabolic capability. Based on total 14C equivalents, AFB1 was rapidly absorbed into plasma in all volunteers with first-order kinetics. The ka of 5.05 ± 1.10 h−1 is similar to the in situ absorption rate constant in the rat of 5.84 ± 0.05 h−1 reported by Ramos and Hernandez (27). Following absorption, AFB1 equivalents were rapidly cleared following a first-order process with a t1/2α (harmonic mean) of 2.86 and a t1/2β (harmonic mean) of 64.4 hours. The t1/2α that we obtained is consistent with excretion patterns reported for AFB1 in Rhesus monkey by Wong and Hsieh (28) that suggest terminal excretion of AFB1 is primarily though the kidney, where 40% of the dose being eliminated as either unbound (~30%) or chloroform extractable from the urine (~10%) within the first 24 hours. Although we could not distinguish between AFB1 and its metabolites or conjugates in this study, we speculate that the α phase is likely free aflatoxin, whereas the β phase is most likely aflatoxin conjugated to albumin and other plasma proteins. The long β phase observed is not surprising considering that in the monkey, 100 hours following administration of AFB1, 13.6% of the dose remained in the liver with 5.8% remaining in the plasma, suggesting that a significant fraction of the dose has a long residence time (28). This is also consistent with reports of the linear formation of AFB1-albumin and AFB1-DNA adducts over an 80,000-fold dose range when Fischer rats were challenged with AFB1 (29). The patterns of excretion observed in our study suggest a similar phenomenon that occurs in humans as in rats with small amounts of aflatoxin found in the diet distributed throughout the body in roughly the same proportions as would be expected from larger doses.
Effects of CHL and Chla on AFB1 uptake and distribution
Many studies have characterized the molecular dosimetry of AFB1 as a measure of possible carcinogenic effects in the absence and presence of intervening agents (28, 30–32). One such agent, CHL, has been characterized extensively for its cancer chemopreventive potential in animals and humans (8, 17, 33–35). More recently, cancer chemopreventive effects of natural Chla itself have been reported in rodent and fish models, through mechanisms that involve reduced systemic carcinogen uptake (17, 18). The present study now extends these findings by establishing the kinetics of low-dose aflatoxin absorption in each of four human volunteers, and the effects of CHL and Chla intervention on specific AFB1 pharmacokinetic parameters in three of those subjects. The results show that intervention by CHL and Chla produced similar, and frequently significant, effects on AFB1 uptake and distribution among all individuals. In all individuals, Chla intervention produced a significant 40% to 60% reduction in excretion of urinary aflatoxin equivalents (Fig. 1). For CHL, the extent of protection in subject 5 was identical to that provided by Chla. A similar but non-significant trend for CHL protection was seen for subject 2 and, to a lesser extent, for subject 1. We interpret these results as direct evidence for CHL- and Chla-mediated reductions in systemic uptake of aflatoxin in humans, as in preclinical models (19, 32, 33). An alternative interpretation could be that a single cotreatment with either Chla species alters AFB1 metabolism in a direction that would reduce urinary excretion, for instance, through rapid induction of phase II detoxication pathways (16). This interpretation we believe is highly unlikely, especially since interference effects become apparent within 30 minutes of cotreatment, but is not formally ruled out by this study.
The plasma data are largely consistent with what was observed in urine, and support the notion that CHL and Chla limit AFB1 uptake from the human gastrointestinal tract. Evidence for this hypothesis would be provided by intervention-mediated reductions in ka, Cmax, and initial AUC; increases in Tmax; and absence of effect on elimination rate. As seen in Table 2, CHL and Chla intervention produced mean pK values consistent with this set of expectations for 33 of the 36 possible intervention-control comparisons. These apparent changes are, however, statistically significant in only 8 of the 36 possible comparisons with controls (AFB1-only). Interpretation of the AUC data is especially complicated by the fact that our AMS detection approach did not distinguish among the free and bound states of parent AFB1 and its metabolites. Thus, the plasma evidence for reduction in systemic AFB1 uptake by cotreatment with chlorophylls is consistent with, but less compelling than, that for urine data in this pilot study. Future design expansions might include more sampling from 0 to 1 hour, to more precisely determine ka and Tmax values, and use of separation methods to distinguish among the bound and unbound aflatoxins, which would improve sensitivity to detect intervention effects.
Alternative mechanisms of protection by chlorophylls
A number of recent reports suggest that certain phytochemicals, as well as the Chla derivative CHL, may act by triggering induction of metabolic processes that reduce AFB1 toxicity and genomic damage. However, studies with animal models for AFB1 carcinogenesis in our laboratory have provided evidence inconsistent with such a mechanism for CHL or Chla chemoprotection in the whole animal. For example, extensive dietary pretreatment of rainbow trout with a high (and otherwise chemopreventive) dosage of CHL, followed by a single aqueous treatment with AFB1, failed to reduce AFB1-DNA adduction or hepatocarcinogenesis under conditions where CHL cotreatment with dietary AFB1 was effective (36). This result showed that preloading the animal with CHL, even at a dose sufficient to turn the liver, skin, and bile green, did not provide any form of induction that could protect against AFB1. Instead, CHL and AFB1 coexposure was necessary and sufficient for protection. In a second study with this model, CHL cotreatment was shown to significantly reduce the bioavailability of AFB2, an AFB1 analogue that interacts equally as strongly with CHL in vitro but lacks the 8,9 double bond essential to be genotoxic or carcinogenic (19). In the case of this simply defined metabolic profile, CHL cotreatment produced significantly reduced AUC and Cmax, and delayed Tmax, which can be interpreted as definitive evidence supporting the reduce bioavailability hypothesis in vivo. This result shows that metabolic changes are not required to observe reduced uptake and altered pharmacokinetics of aflatoxins. A more recent study in the rat (17) revealed that cotreatment with either CHL or Chla significantly elevated fecal excretion of AFB1 equivalents, reduced hepatic AFB1-DNA adduction, and reduced preneoplastic lesions in the liver and colon in the absence of any detectable induction of hepatic phase II enzyme activities in vivo. In two companion studies, dietary CHL and Chla provided strong protection against dibenzo(a,l)pyrene uptake and tumorigenesis in the rainbow trout, without significant alterations in gene expression by microarray analysis (37).6 Indeed, the only observed molecular effect of these chlorophylls was to reverse the dose-responsive alterations in expression that were induced by the carcinogen itself, as one would expect if the primary mechanism in vivo were simple reduction in carcinogen bioavailability. In sum, these studies provide substantial evidence that chlorophylls can strongly inhibit uptake of aflatoxins and polycyclic aromatic hydrocarbons in the whole animal, in the absence of any detectable alterations in phase II activities.
Although treatments were not completely randomized in the present study, the design attempted to eliminate possible effects from prior CHL or Chla exposures. We elected to first administer three cycles of AFB1 alone (protocol 1) to each subject, which was then followed by six treatment cycles that were randomized to include three each of protocols 2 and 3. This procedure assured that prior treatments with CHL or Chla could not have modified the baseline results with AFB1 alone. Under protocols 2 and 3, CHL or Chla were given along with AFB1, not before, again to eliminate the possibility that prior treatment might provoke enzyme induction. Figure 2 shows that protective effects against AFB1 uptake were evident well within 30 minutes of CHL or Chla treatment, a time-scale that does not favor a requirement for alterations in metabolic enzyme expression. This time scale is potentially compatible with inhibition of hepatic enzyme catalysis as a protective mechanism, but data are lacking to establish if rapid inhibition of metabolic enzymes could take place under the conditions of this study, and if so, would produce the effect of reduced appearance of AFB1 equivalents in urine and plasma. Based on present data, we conclude that the changes in pharmacokinetic parameters due to Chla and CHL cotreatment reflect an ability of these blocking agents to reduce the amount of aflatoxin absorbed in the intestine, in humans as well as animal models.
Although our results are consistent with reduced uptake as the primary protective mechanism, the precise mechanisms through which this occurs are not fully established. A number of previous studies have indicated that Chla as well as CHL can form strong noncovalent complexes in vitro with AFB1, dibenzo(a,l)pyrene, heterocyclic amines, and other carcinogens with at least some partial planarity and aromaticity. This interaction, and formation of a macromolecular complex with carcinogen, may be expected to reduce the rate of carcinogen uptake from the intestine, as has been shown directly for CHL in vitro using the Caco2 monolayer transport model (34) and in vivo using the rat intestinal loop absorption model (37). We have not, however, envisioned a means to detect or quantify such complexes in the intragastric milieu in vivo, and so cannot be certain of their mandatory participation in chemoprevention. We also note some indication of inter-individual variability in response to CHL, with subject 1 appearing less responsive to protection than the other subjects. This would not be expected if reduced uptake were solely the result of intragastric CHL-AFB1 interaction. Potential explanations include variable gastric environments that might alter the strength of such interactions, or polymorphisms in certain proteins that might provide variability in the ability of CHL to mediate uptake (e.g., transport proteins) or alter phase I metabolism (e.g., CYP3A4). In addition, the rarity of plasma 0- to 72-hour AUC reduction by CHL (and Chla) seems at odds and not consistent with our former study (19) of AFB2 pharmacokinetics in the trout. However, interpretation is limited by lack of information on the identity of equivalents, including parent compound and its bound and unbound metabolites. It is noteworthy that the previous China CHL study (7) lacked any demonstration that sustained CHL intervention altered steady-state plasma aflatoxins. Additional studies will be essential to more fully define and elucidate mechanisms of protection by Chlas in human subjects.
Mechanistic implications of CHL and Chla chemistry
CHL is a semisynthetic mixture of sodium copper salts derived from Chlas a and/or b. Although the content of different CHL mixtures may vary, two compounds commonly found in commercial CHL mixtures are trisodium copper chlorin e6 and disodium copper chlorin e4. During the synthesis of CHL, the labile Chla magnesium atom is replaced with a very tightly bound copper, three carboxyl groups are revealed, and the lipophilic phytol tail is lost. As a consequence, CHL and Chla differ greatly in water solubility. Although one might expect the resulting partitioning differences to produce differing chemopreventive properties, these species in fact have exhibited comparable strengths of interaction in vitro with such carcinogens as AFB1 and dibenzo(a,l)pyrene, similar potencies in antimutagenicity assays, comparable reduction of carcinogen uptake in vivo, and comparable inhibition of tumor response in fish and rodent models (17–19). In all these studies, the various Chlas were given ample opportunity for interaction with the carcinogen during the in vitro and in vivo assays. In a general chemoprevention strategy, however, water solubility differences may prove important as determinants of target organ accessibility by Chlas in vivo. As noted, CHL is taken up readily in all species examined, and can reach at least micromolar concentrations in human serum, whereas evidence is lacking for significant uptake of Chla following oral treatment. Consequently, chemoprevention by dietary Chla may be limited by mechanisms restricted to the alimentary tract, whereas CHL may find broader chemopreventive application owing to a potential to reach many more target organs in the whole animal. As a practical matter, structural differences also render CHL considerably more stable than Chla against oxidation, and thus more readily available and affordable for large-scale experimentation and application in chemoprevention.
Acknowledgments
We thank the Integrative Health Sciences Facility Core of the Environmental Health Sciences Center at OSU for the assistance in sample collection and statistical support.
Grant support: National Cancer Institute grants CA090890 and CA65525, NIEHS grants P50 ES00210 and ES03850, and by funding from USANA Health Sciences, Inc., Salt Lake City, UT. AMS analysis was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under contract no. DE-AC52-07NA27344 and was supported by NIH's National Center for Research Resources, Biomedical Technology Program (P41 RR013461).
Footnotes
T. McQuistan, M. Simonich, C. Jubert, J. Hendricks, C. Pereira, D. Williams, R. Dashwood, and G. Bailey, unpublished observations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Simjee S. Foodborne diseases. Totowa (NJ): Humana Press; 2007. [Google Scholar]
- 2.Turner PC, Moore SE, Hall AJ, Prentice AM, Wild CP. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ Health Persp. 2003;111:217–220. doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liaw Y-F, Sollano JD. Factors influencing liver disease progression in chronic hepatitis B. Liver Int. 2006;26:23–29. [Google Scholar]
- 4.Phillips TD. Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicol Sci. 1999;52:118–126. doi: 10.1093/toxsci/52.suppl_1.118. [DOI] [PubMed] [Google Scholar]
- 5.Phillips TD, Lemke SL, Grant PG. Characterization of clay-based enterosorbents for the prevention of aflatoxicosis. Adv Exp Med Biol. 2002;504:157–171. doi: 10.1007/978-1-4615-0629-4_16. [DOI] [PubMed] [Google Scholar]
- 6.Kensler TW, He X, Otieno M, et al. Oltipraz chemoprevention trial in Qidong, People's Republic of China: modulation of serum aflatoxin albumin adduct biomarkers. Cancer Epidemiol Biomarkers Prev. 1998;7:127–134. [PubMed] [Google Scholar]
- 7.Egner PA, Wang JB, Zhu YR, et al. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc Natl Acad Sci U S A. 2001;98:14601–14606. doi: 10.1073/pnas.251536898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egner PA, Munoz A, Kensler TW. Chemoprevention with chlorophyllin in individuals exposed to dietary aflatoxin. Mutat Res. 2003;523–524:209–216. doi: 10.1016/s0027-5107(02)00337-8. [DOI] [PubMed] [Google Scholar]
- 9.Kephart JC. Chlorophyll derivatives-their chemistry, commercial preparation, and uses. Econ Botany. 1955;9:3–38. [Google Scholar]
- 10.Edwards BJ. Treatment of chronic leg ulcers with ointment containing soluble chlorophyll. Physiotherapy. 1954;40:177–179. [PubMed] [Google Scholar]
- 11.Bowers WF. Chlorophyll in wound healing and suppurative disease. Am J Surg. 1947;73:37. doi: 10.1016/0002-9610(47)90287-0. [DOI] [PubMed] [Google Scholar]
- 12.Larato DC, Pfau FR. Effects of a water-soluble chlorophyllin ointment on gingival inflammation. N Y State Dent J. 1970;36:291–293. [PubMed] [Google Scholar]
- 13.Tawashi R, Cousineau M, Sharkawi M. Effect of sodium copper chlorophyllin on the formation of calcium oxalate crystals in rat kidney. Invest Urol. 1980;18:90–92. [PubMed] [Google Scholar]
- 14.Young RW, Beregi JS., Jr Use of chlorophyllin in the care of geriatric patients. J Am Geriatr Soc. 1980;28:46–47. doi: 10.1111/j.1532-5415.1980.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 15.Egner PA, Stansbury KH, Snyder EP, Rogers ME, Hintz PA, Kensler TW. Identification and characterization of chlorin e(4) ethyl ester in sera of individuals participating in the chlorophyllin chemoprevention trial. Chem Res Toxicol. 2000;13:900–906. doi: 10.1021/tx000069k. [DOI] [PubMed] [Google Scholar]
- 16.Fahey JW, Stephenson KK, Dinkova-Kostova AT, Egner PA, Kensler TW, Talalay P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammalian phase 2 cytoprotective genes. Carcinogenesis. 2005;26:1247–1255. doi: 10.1093/carcin/bgi068. [DOI] [PubMed] [Google Scholar]
- 17.Simonich MT, Egner PA, Roebuck BD, et al. Natural chlorophyll inhibits aflatoxin B1-induced multi-organ carcinogenesis in the rat. Carcinogenesis. 2007;28:1294–1302. doi: 10.1093/carcin/bgm027. [DOI] [PubMed] [Google Scholar]
- 18.Simonich MT, McQuistan T, Jubert C, et al. Low-dose dietary chlorophyll inhibits multi-organ carcinogenesis in the rainbow trout. Food Chem Toxicol. 2008;46:1014–1024. doi: 10.1016/j.fct.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi T, Schimerlik M, Bailey G. Mechanisms of chlorophyllin anticarcinogenesis: dose-responsive inhibition of aflatoxin uptake and biodistribution following oral co-administration in rainbow trout. Toxicol Appl Pharmacol. 1999;158:132–140. doi: 10.1006/taap.1999.8695. [DOI] [PubMed] [Google Scholar]
- 20.Sandhu P, Vogel JS, Rose MJ, et al. Evaluation of microdosing strategies for studies in preclinical drug development: demonstration of linear pharmacokinetics in dogs of a nucleoside analog over a 50-fold dose range. Drug Metab Dispos. 2004;32:1254–1259. doi: 10.1124/dmd.104.000422. [DOI] [PubMed] [Google Scholar]
- 21.Dickens JW. Aflatoxin control program for peanuts. Am Oil Chem Soc. 1977;54:225A–228A. [Google Scholar]
- 22.Jubert C, Bailey G. Isolation of chlorophylls a and b from spinach by counter-current chromatography. J Chromatogr A. 2007;1140:95–100. doi: 10.1016/j.chroma.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 23.Vogel JS. Rapid production of graphite without contamination for biomedical AMS. Radiocarbon. 1992;34:344–350. [Google Scholar]
- 24.Ognibene TJ, Bench G, Vogel JS, Peaslee GF, Murov S. A high-throughput method for the conversion of CO2 obtained from biochemical samples to graphite in septa-sealed vials for quantification of 14C via accelerator mass spectrometry. Anal Chem. 2003;75:2192–2196. doi: 10.1021/ac026334j. [DOI] [PubMed] [Google Scholar]
- 25.Ognibene TJ, Bench G, Brown TA, Peaslee GF, Vogel JS. A new accelerator mass spectrometry system for 14C-quantification of biochemical samples. Int J Mass Spectrom. 2002;218:255–264. [Google Scholar]
- 26.Vogel JS. Accelerator mass spectrometry for human biochemistry: the practice and the potential. Nucl Instrum Meth B. 2000;172:884–891. [Google Scholar]
- 27.Ramos AJ, Hernandez E. In situ absorption of aflatoxins in rat small intestine. Mycopathologia. 1996;134:27–30. doi: 10.1007/BF00437049. [DOI] [PubMed] [Google Scholar]
- 28.Wong ZA, Hsieh DPH. The comparative metabolism and toxicokinetics of aflatoxin B1 in the monkey, rat, and mouse. Toxicol Appl Pharm. 1980;55:115–125. doi: 10.1016/0041-008x(80)90227-6. [DOI] [PubMed] [Google Scholar]
- 29.Cupid BC, Lightfoot TJ, Russell D, et al. The formation of AFB1-macromolecular adducts in rats and humans at dietary levels of exposure. Food Chem Toxicol. 2004;42:559–569. doi: 10.1016/j.fct.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Bailey GS, Loveland PM, Pereira C, Pierce D, Hendricks JD, Groopman JD. Quantitative carcinogenesis and dosimetry in rainbow trout for aflatoxin B1 and aflatoxicol, two aflatoxins that form the same DNA adduct. Mutat Res. 1994;313:25–38. doi: 10.1016/0165-1161(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 31.Gorelick NJ. Risk assessment for aflatoxin: I. Metabolism of aflatoxin B1 by different species. Risk Anal. 1990;10:539–559. doi: 10.1111/j.1539-6924.1990.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 32.Kensler TW, Gange SJ, Egner PA, et al. Predictive value of molecular dosimetry: individual versus group effects of oltipraz on aflatoxin-albumin adducts and risk of liver cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:603–610. [PubMed] [Google Scholar]
- 33.Dashwood R, Liew C. Chlorophyllin-enhanced excretion of urinary and fecal mutagens in rats given 2-amino-3-methylimidazo[4,5-f]quinoline. Environ Mol Mutagen. 1992;20:199–205. doi: 10.1002/em.2850200308. [DOI] [PubMed] [Google Scholar]
- 34.Mata JE, Yu Z, Gray JE, Williams DE, Rodriguez-Proteau R. Effects of chlorophyllin on transport of dibenzo(a l)pyrene, 2-amino-1-methyl-6-phenylimidazo-[4,5-b]pyridine, and aflatoxin B1 across Caco-2 cell monolayers. Toxicology. 2004;196:117–125. doi: 10.1016/j.tox.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Dashwood RH. The importance of using pure chemicals in (anti)mutagenicity studies: chlorophyllin as a case in point. Mutat Res-Fund Mol M. 1997;381:283–286. doi: 10.1016/s0027-5107(97)00221-2. [DOI] [PubMed] [Google Scholar]
- 36.Breinholt V, Arbogast D, Loveland P, et al. Chlorophyllin chemoprevention in trout initiated by aflatoxin B1 Bath treatment: an evaluation of reduced bioavailability vs. target organ protective mechanisms. Toxicol Appl Pharmacol. 1999;158:141–151. doi: 10.1006/taap.1999.8696. [DOI] [PubMed] [Google Scholar]
- 37.Dashwood RH. Protection by chlorophyllin against the covalent binding of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) to rat liver DNA. Carcinogenesis. 1992;13:113–118. doi: 10.1093/carcin/13.1.113. [DOI] [PubMed] [Google Scholar]