Abstract
The mechanistic target of rapamycin (mTOR) is a central regulator in cell growth, activation, proliferation, and survival. Activation of the mTOR pathway underlies the pathogenesis of systemic lupus erythematosus (SLE). While mTOR activation and its therapeutic reversal were originally discovered in T cells, recent investigations have also uncovered roles in other cell subsets including B cells, macrophages, and “non-immune” organs such as the liver and the kidney. Activation of mTOR complex 1 (mTORC1) precedes the onset of SLE and associated co-morbidities, such as anti-phospholipid syndrome (APS), and may act as an early marker of disease pathogenesis. Six case reports have now been published that document the development of SLE in patients with genetic activation of mTORC1. Targeting mTORC1 over-activation with N-acetylcysteine, rapamycin, and rapalogs provides an opportunity to supplant current therapies with severe side effect profiles such as prednisone or cyclophosphamide. In the present review, we will discuss the recent explosion of findings in support for a central role for mTOR activation in SLE.
Keywords: Mitochondria, Mechanistic target of rapamycin, Systemic lupus erythematosus, Anti-phospholipid antibodies, T cells, B cells, Macrophages, Liver, Kidney
Introduction
Systemic lupus erythematosus (SLE) is a multi-system disease with underlying immune cell dysfunction and end organ damage. Recent studies have found activation of the mechanistic target of rapamycin (mTOR) both in the immune system [1, 2] and non-traditional parenchymal organs, such as the liver, which precedes the onset of disease and represents early manifestations of pathogenesis [3]. mTOR activation is a result of long-documented metabolic stress in SLE [4–7]. There is some debate on which cell type better defines SLE pathogenesis, but a common finding in T cells, B cells, macrophages, hepatocytes, and reno-vascular cells is mTOR activation [8]. Activation of the mTOR pathway is clearly not limited to SLE, but it also occurs in other autoimmune and rheumatic diseases [9]. Because of its broad role in cell dysfunction in SLE, there is increasingly strong justification for pharmacological inhibition of mTOR with rapamycin as a disease-modifying therapy (Table 1). In the present review, we will provide an update on the role of mTOR in the development of SLE and new studies that show mTOR as a viable target for effective treatment and prevention of disease flares.
Table 1.
mTOR inhibition improves clinical features of SLE/APS
Immune System
CD4 T Cells
mTOR is a sensor of the cellular energy status and can thus modify cellular activity based on available metabolites [10]. Enhanced translocation of mTOR to the outer mitochondrial membrane underlies CD4+ T cell hyperactivity in SLE [2]. In B6.Sle1.Sle2.Sle3 mice, there is increased mTOR complex 1 (mTORC1) activity, glycolysis, and mitochondrial electron transport chain (ETC.) activity [11]. In C57Bl/6 mice, inhibition of mTORC1 with rapamycin resulted in reduced glycolysis and mitochondrial ETC. activity [11]. Yin et al. normalized the metabolic phenotype of CD4+ T cells from B6.Sle1.Sle2.Sle3 by inhibiting glycolysis and the ETC. with 2-deoxyglucose (2-DG) and metformin, respectively [11]. 2-DG and metformin also resulted in reduced mTORC1 activity [11]. The suppression of mTORC1 activity was attributed to the normalization of the metabolic phenotype, but an alternative explanation may have been mTORC1 inhibition by metformin. Metformin has been shown to indirectly inhibit mTOR signaling [12]. Thus, the in vivo effect of metformin as a modifier of SLE in B6.Sle1.Sle2.Sle3, NZB/W, and chronic graft versus host disease (cGVHD) model of SLE may be due to mTOR inhibition and metabolic modulation [11].
In CD4+ T cells, the active hypomethylation of genes in the mTOR pathway by ten-eleven translocation methylcytosine dioxygenase (TET) enzymes may underlie increased expression and activation of proinflammatory cytokines such as interferon gamma (IFNγ) and interleukin 17 (IL-17). Hypomethylation of DNA in CD4+ T cells and the subsequent increased transcription are thought to be involved with SLE pathogenesis and disease activity [13, 14]. Recently, it was shown that the action of the TET enzymes in demethylation of cytosine from 5-methylcytosine to 5-hydroxymethylcytosine (5-hmC) in CD4+ cells results in enhanced IFNγ and IL-17 expression [15]. Another study found that 5-hmC was enriched in genes of the mTOR pathway of CD4+ T cells from SLE patients [16]. These data indicate that the upregulation of mTOR activity may be at both the gene and protein levels in SLE CD4+ T cells.
In SLE PMBCs, it was shown that there is depletion of E3-ubiquitin ligases casitas B-lineage lymphoma b (Cbl-B) and gene related to anergy in lymphocytes (GRAIL), especially in those with active SLE [17]. Further, messenger ribonucleic acid (mRNA) analysis of SLE T cells relative to healthy controls found reduced levels of transcription factors early growth response protein 2 (Egr2) and Egr3, both negative regulators of T cell activation that are upstream of Cbl-B [17, 18]. Banica et al. showed that SLE CD4+ T cells that were driven to expand inducible Tregs (iTregs) with rapamycin caused increased proliferation of Tregs with increased suppressive activity, especially after 21 days of iTreg expansion [17]. mTOR appears to be important in the downregulation of T cell anergy E3 ubiquitin ligases and concomitant suppression of Tregs. Rapamycin is clearly important for the normalization of T effector function and the expansion of Tregs and represents an intervention in T cell biology that can have positive therapeutic outcomes.
Th17 T Cells
There is mounting evidence that IL-17 expressing CD4+ T cells (T helper 17 cell, Th17) are responsible for depletion of Tregs and production of proinflammatory cytokines in SLE [19, 20]. The serine/threonine kinase calcium/calmodulin-dependent protein kinase IV (CaMK4) has been shown to be essential in the development of Th17 cells in SLE, but previously the mechanism of this process was unknown [21]. Recent work has found a role for CaMK4 signaling in mTOR activation in SLE. Under Th17-polarizing conditions, but not Th1 nor Th2, there is increased expression of CaMK4 [21]. In CaMK4 knockout mice, there was significantly reduced Th17 expansion under Th17-polarizing conditions and knockdown of CaMK4 produced similar results in wild-type mice [21]. In MRL/lpr mice, inhibition of CaMK4 with KN-93 resulted in the depletion of Th17 and DN T cells [21]. Administration of KN-93, and thus CaMK4 blockade, also reduced double-stranded DNA (dsDNA) antibodies, proteinuria, and mortality at 20 weeks of age [21]. Mechanistic investigation of CaMK4 in MRL/lpr mice found that it promotes cAMP-responsive element modulator alpha (CREM-α) recruitment to the IL-17 promoter in CD4+ T cells [21]. Furthermore, CaMK4 enhances AKT and mTORC1 signaling. Increased CaMK4 caused upregulation of S6K phosphorylation, and inhibition of mTOR with rapamycin blocked the development of Th17 cells [21]. Thus, CaMK4 may lie upstream of mTOR and CREM-α and independently promote Th17 expansion through either pathway.
A study of 17 children with lupus nephritis (LN) found that Stat3 activation in effector T cells correlated directly with the number of IL-17-producing cells [22]. In vitro, it was found that rapamycin blocked Stat3 activation and greatly reduced the number of IL-17 effector cells [22]. In adult SLE patients, mTOR activation promoted the expansion of IL-4-producing CD3+CD4−CD8− double negative (DN) T cells and CD4+ Th17 cells [19]. The same study found that mTOR caused the contraction of CD4+CD25+FoxP3+ Tregs [19]. Because IL-17 is likely downstream of mTOR signaling, neutralization of IL-17 in vitro promoted the expansion of Tregs independent of mTOR activity [19].
DN T Cells
N-acetylcysteine (NAC) was recently demonstrated as a potent mTOR inhibitor in SLE patients [23]. Specifically, NAC caused the depletion of phospho-S6RPhi DN T cells and expansion of CD4+CD25+FoxP3+ Tregs in SLE patients. Metabolic analysis of SLE patients found that kynurenine, a metabolite of tryptophan, stimulated mTOR activity in control PBL and SLE DN T cells [24]. In vivo, kynurenine accumulation was blocked in patients receiving NAC, a potent anti-oxidant and glutathione precursor [24].
In healthy controls, DN T cells had elevated mTOR activity, as measured by phospho-S6RP levels, relative to CD4 and CD8 T cells [25]. SLE patients had an even greater number of DN T cells with mTOR activation [25]. The mTOR activity in DN T cells indirectly correlated with fewer FoxP3+ cells in the CD3+/CD4+/CD25+ T cell population [25]. Further evaluation of these populations found that the depletion of Tregs, mTOR activation in DN T cells, and enhanced necrosis in CD3+ T cells could identify patients in active SLE flare [25]. mTOR activation in DN T cells, in combination with expansion of CD4+CD25− T cells expressing FoxP3, could predict an upcoming flare in SLE patients [25]. SLE DN T cells also produced more IL-4 than healthy controls which correlated to the production of anti-dsDNA [19, 25]. Treatment of SLE patients with rapamycin significantly rescued mTOR over-activation and reduced the number of necrotic CD4+ and DN T cells [25]. Furthermore, IL-4 production by DN T cells was significantly reduced by rapamycin treatment in SLE patients [25]. Additionally, CD4+/CD25+/FoxP3+ Tregs were expanded by rapamycin and in SLE patients [25, 26]. These data show that DN T cells and Tregs are inversely related to each other and the over-activation of the mTOR pathway underlies this imbalance in these cell subsets.
Follicular Helper T Cells
Follicular helper T (Tfh) cells are critical for germinal center (GC) formation and B cell activation. Surprisingly, mTORC1 signaling was implicated in shifting T cell differentiation away from Tfh cells, instead promoting that of Th1 cells [27]. Other studies suggest that both mTORC1 and mTORC2 are essential for Tfh cell differentiation and GC reaction under steady state and after antigen immunization and viral infection [28]. In support of Tfh development, dual activation of mTORC1 and mTORC2 drives glycolysis and lipogenesis and glucose transporter 1-mediated glucose metabolism. Such metabolic requirement matches well with accumulation of glycolytic metabolites in SLE patients’ lymphocytes [24]. Apparently, Tfh cells are far from homogenous, as they can produce not only IL-21 but also IL-17 [29•] and IL-4 [30•]. Since Tfh cells are expanded in patients with severe SLE [31], the role of mTOR pathway activation would be important to precisely define in this proinflammatory T cell subset.
B Cells
B cells have been targeted in SLE to block the production of auto-antibodies, most recently with belimumab, an inhibitor of B cell-activating factor (BAFF) [32]. BAFF has been identified as a promoter of mTOR activation [33]. In B cells, rapamycin was able to block BAFF-stimulated mTOR activation, inhibit proliferation, and induce apoptosis [33, 34].
B cell development is promoted by early B cell factor 1 (EBF1). EBF1 is targeted by microRNA (miRNA)-1246, which provides a safety switch to prevent over-activation and proliferation of B cells. In SLE patients, it was discovered that SLE B cells contain significantly reduced miRNA, thus promoting B cell stimulation [35]. Furthermore, it was found that inhibition of AKT with MK-2206 promoted the expression of miRNA-1246 [35]. Thus, mTOR activation, via AKT, promotes B cell proliferation through inhibition of miRNA-1246 with subsequent EBF1 over-expression that results in an activated B cell phenotype.
The lysosomal histidine transporter SLC15A4 was shown to be essential for the production of anti-nuclear antibodies (ANA) and type I interferon through toll-like receptor 7 (TLR7) signaling in lupus B cells in an mTOR-dependent manner [36]. In Slc15a4 knockout mice, there is reduced IgG2c in the serum [36]. In the pristine model of SLE, Slc15a4 deficiency prevented the development of anti-small nuclear ribonucleoproteins (snRNP) and anti-dsDNA antibodies after 20 weeks relative to wild-type mice [36]. When Slc15a4 knockout mice were crossed to C57BL/6lpr/lpr lupus-prone mice, there was impaired ANA production and reduced splenomegaly [36]. Stimulation of TLR7 in Slc15a4 knockout mice did not activate Irf7 transcription, which is essential for the production of type I interferon [36]. Kobayashi et al. showed that it is the histidine transport function of Slc15a4 that is essential for the immune response following TLR7 stimulation [36]. Loss of the transporter results in histidine accumulation in the lysosome.
mTOR acts as a sensor to lysosomal amino acids and is activated when translocated to the lysosome [37]. In Slc15a4-deficient B cells, there is a loss of mTOR activation as measured by phosphorylation of its downstream targets 4E-BP1 and S6K [36]. Additionally, mTOR recruitment to lysosomes was significantly reduced in Slc15a4 knockout B cells [36]. In wild-type B cells treated with rapamycin, there was a significant reduction in Irf7 protein expression [36]. Torin, a specific mTOR inhibitor, also blocked type-I IFN production, but not TNF-α [36]. Thus, mTOR is sensitive to histidine accumulation in lysosomes and is essential in the development of TLR7-driven production of ANA.
Macrophages
While mTORC1 promotes proinflammatory T cell development [38], it may reduce inflammation by shifting macrophage polarization from proinflammatory M1 to anti-inflammatory M2 phenotype [39, 40]. However, IL-4-dependent M2 polarization is also mTORC1-dependent [41]. Interestingly, M2 macrophages can also contribute to inflammation by serving as host for cytomegalovirus (CMV), which might explain the potent anti-CMV effects of mTOR inhibitors after organ transplantation [42]. Although the role of macrophage polarization in lupus nephritis has shown differences in animal models [43, 44], M2 types appear to dominate renal biopsies from patients with lupus nephritis [45]. Although further studies are clearly warranted to define the role of macrophage polarization in lupus nephritis, blockade of M1-M2 differentiation may also contribute to clinical efficacy of rapamycin in SLE.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are involved in the regulation of the immune response of B and T cells. SLE patient MSCs go through premature senescence in an mTOR-dependent manner [46]. In the presence of rapamycin, MSCs co-cultured with CD4+ T cells resulted in increased Tregs and depletion of Th17 cells in vitro [46]. Furthermore, rapamycin promoted the secretion of anti-inflammatory cytokines in these co-cultures and blocked their senescent phenotype [46]. In SLE patients, rapamycin and siRNA knockdown of mTOR resulted in the rescue of SLE patient MSCs [46]. Interestingly, SLE patient MSCs pre-treated with rapamycin and then transplanted via tail-vein injection into MRL/lpr mice resulted in some protection from lupus nephritis [46].
Liver
Liver disease, defined as a >2-fold elevation of AST or ALT, was present in 20.7 % of SLE patients independent of hepatotoxic medications [47]. Both NAC and rapamycin, but not azathioprine, cyclosporine, or cyclophosphamide, were successful in preventing liver disease [47]. Furthermore, liver disease correlated to higher SLE disease activity index (SLEDAI) scores in patients and thus is an important organ system to monitor [47].
We recently found mitochondrial dysfunction, anti-phospholipid (aPL) antibody production, and mTOR activation precedes the onset of SLE, i.e., ANA production and nephritis, in lupus-prone mice [3]. We compared oxygen consumption rates in B cells, T cells, and hepatocytes and found a 25-fold increase in oxygen consumption in hepatocytes, supporting the concept that the liver is a major source of oxidative stress which underlies SLE development [3]. In 4-week-old lupus-prone MRL/lpr mice, we identified increased oxygen consumption through complex II of the ETC., diminished state 3/state 4 ratio, and increased ΔΨm relative to mitochondrial mass indicating dysfunction prior to SLE onset [3].
The late endosomal protein Rab4A has a positive feedback loop with mTOR and promotes the depletion of the mitochondrial fission protein Drp1 and accumulation of defective mitochondria in T cells [1]. In 4-week-old MRL and MRL/lpr lupus-prone mice, we identified significant over-expression of Rab4 in the liver [3]. In MRL/lpr livers prior to SLE onset, we found 2.5-fold elevation of phospho-S6K, indicating increased mTORC1 activity. Conversely, we found reduced phospho-AKT levels in the pre-SLE livers indicating that mTORC2 is inhibited in the liver in early SLE [3]. These findings were specific to the liver, as mTOR activity was not elevated in the kidneys, thymus, or spleens of 4-week-old MRL/lpr mice.
We previously found that MRL/lpr mice responded to rapamycin which significantly reduced S6K phosphorylation in the immune system and liver [1, 3]. aPL antibodies were significantly elevated in MRL/lpr, MRL, and lpr mice at 4 weeks of age which progressed further over 10 weeks [3]. A 10-week rapamycin treatment regimen in MRL/lpr mice resulted in blockade of aPL antibody progression. We demonstrated a similar effect in NZB/W (F1) lupus mice treated with rapamycin between ages of 4 and 30 weeks [3]. Rapamycin did not rescue Rab4 over-expression in MRL/lpr livers, but did rescue Drp1 under-expression which may promote the preservation of healthy mitochondria. Additionally, rapamycin reduced the expression of NDUFS3, which acts as a promoter of oxidative stress [3, 48]. In transaldolase (TAL)-deficient mice, a model of oxidative stress in the liver, there was also increased NADH dehydrogenase iron-sulfur protein 3 (NDUFS3) expression and subsequent mTOR activation as measured by phospho-4EBP1 levels [3]. TAL knockout mice also produced elevated levels of antiphospholipid antibodies. Thus, mTOR activation and oxidative stress in the liver underlies the production of APLA and precedes the onset of SLE.
The BWF1 mouse, which produces anti-dsDNA, has glomerular inflammation, and proteinuria had increased mTOR protein levels [49]. Conversely, BWF1 mice did not have elevated levels of S6K protein [49]. mTOR activity was not measured in this study, so it cannot be determined if mTOR activity is also increased prior to the onset of SLE. Further evaluation of this model would provide more evidence for liver dysfunction as a precipitating event in SLE development.
Kidney
Rapamycin blocks the progression of lupus nephritis in MRL/lpr mice [1, 46]. Ten weeks of rapamycin treatment blocked the development of both sclerotic and crescentic glomeruli, though this is likely due to infiltration by CD4, CD8 [50], and DN T cells [51] and macrophages [43–45] not the kidney specifically [1]. As stated above, mTOR activation was not detected in the kidneys of predisease MRL/lpr mice and thus the renal epithelium is not a likely source of mTOR activation in SLE [3].
Reported Cases
Some recently reported cases give further credence to the role of mTOR in the pathogenesis of SLE. There have been two reported cases of concomitant tuberous sclerosis complex (TSC) and SLE [52, 53]. TSC1 and TSC2 negatively regulate mTORC1, and a loss of function of either results in mTOR activation which was demonstrated in the first described case of TSC/SLE [52]. mTOR activation in this patient also resulted in diminished FoxP3 levels, implicating reduced Treg activity in the development of her SLE [52].
There is a strong association between mutations of TSC, activation of the mTOR pathway, and the development of lymphangioleiomyomatosis (LAM) [54, 55]. It is then probable that activation of the mTOR pathway in SLE patients may also make them susceptible to LAM. There have been two recent case reports of LAM in SLE: on one case, an SLE patient presented with abnormal uterine bleeding [56]. Imaging studies and microscopic analysis of biopsied lymph nodes resulted in diagnoses of LAM and endometrial cancer [56]. In a second case, an SLE patient presented with thrombocytopenia and hemolytic anemia [57]. Initially, it was assumed that her symptoms were secondary to SLE, but bone marrow biopsy identified natural killer cell leukemia [57]. Imaging studies showed pulmonary LAM, angiomyolipomas in the liver, and spleen, as well as renal cysts, which qualified the patient for a diagnosis of TSC [57]. In both of these cases, a diagnosis of SLE preceded the onset of LAM by greater than a decade, thus patients with long-standing SLE should be monitored for the development of other conditions with mTOR activation. It is important to note that the activation of mTOR may be responsible for SLE/LAM overlap, but long-term immunosuppression may underlie the development of cancer in these patients due to reduced immune surveillance.
A small retrospective study of six lupus nephritis patients on rapamycin and one patient on everolimus combined with prednisone showed responsiveness with reduced proteinuria and serum creatinine [58]. One patient had to stop rapamycin due to aphthous ulcers, but in general, it appeared to be safe in this limited study.
Anti-phospholipid antibodies are a diagnostic criterion of SLE and underlie the development of the prothrombotic anti-phospholipid syndrome (APS). In a study of 72 SLE patients, which included 12 patients that also had APS, there was no significant association of mTOR activity and APS status [59]. Despite no difference in mTOR activity in T cell subsets, patients with SLE/APS had increased oxidative stress in all lymphocyte subsets and contraction of CD4+CD25+FoxP3+ Tregs relative to patients without APS [59].
Conclusion
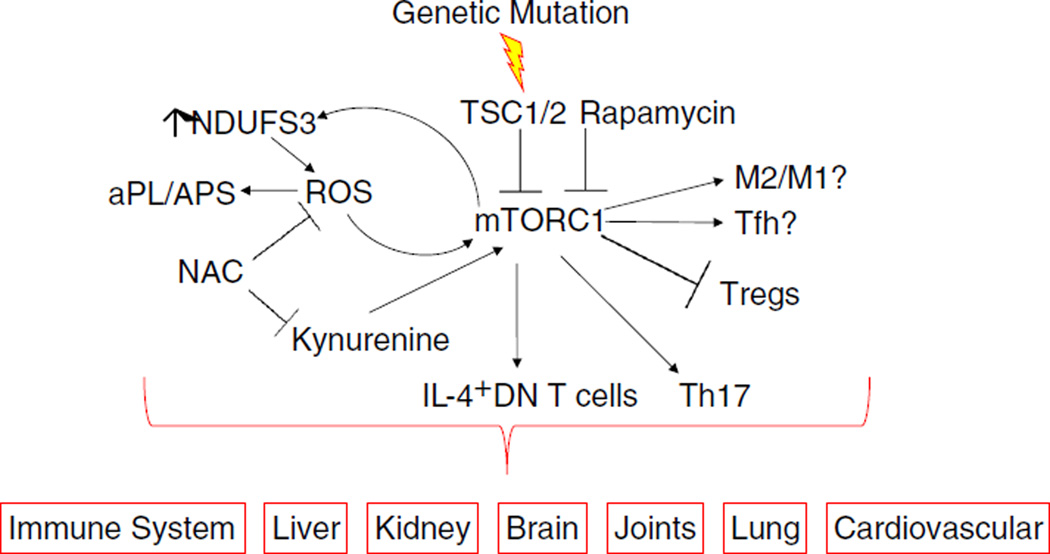
These recent discoveries about the role of mTOR show that it is activated in diverse cell populations and tissues which reflect the wide array of clinical manifestations of SLE (Fig. 1). The generalized activation of mTOR makes it a suitable target for therapeutic intervention, specifically with rapamycin, everolimus, or NAC. Recent studies in SLE have shown rapamycin and NAC to be effective in reducing disease activity with limited adverse effects. Systemic administration of rapamycin ensures inhibition of T cell, B cell, and hepatocytes that are over-activated in SLE. Rapamycin may also prevent renal failure and other prothrombotic co-morbidities in patients with APS. At present, the liver is the only organ that may be a harbinger of SLE onset via mitochondrial dysfunction, APLA production, and mTOR activation. Further studies in predisease SLE-susceptible mice may unveil other early changes in the immune system with respect to mTOR.
Fig. 1.
Schematic diagram of mTORC1 activation in the pathogenesis and biomarker-driven treatment of SLE. Additional details of biochemical checkpoints in mTOR pathway activation have been recently reviewed [9, 63]
Acknowledgments
This work was supported in part by grants AI072648, DK078922, AI122176, AI048079, and AR068052 from the National Institutes of Health, Investigator-Initiated Research Grant P0468X1-4470/WS1234172 from Pfizer, the American College of Rheumatology Research Foundation, and the Central New York Community Foundation.
Abbreviations
- 2-DG
2-Deoxyglucose
- 5-hmC
5-Hydroxmethylcytosine
- ALT
Alanine aminotransferase
- ANA
Anti-nuclear antibody
- aPL
Anti-phospholipid
- APS
Anti-phospholipid syndrome
- AST
Aspartate aminotransaminase
- BAFF
B cell-activating factor
- CaMK4
Calcium/calmodulin-dependent protein kinase IV
- Cbl-b
Casitas B-lineage lymphoma b
- cGVHD
Chronic graft versus host disease
- CREM-α
cAMP-responsive element modulator alpha
- DN T cell
CD3+CD4−CD8− double-negative T cell
- DNA
Deoxyribonucleic acid
- dsDNA
Double-stranded DNA
- EBF1
Early B cell factor 1
- Egr
Early growth response protein
- ETC.
Electron transport chain
- GRAIL
Gene related to anergy in lymphocytes
- IFNγ
Interferon gamma
- IL-17
Interleukin 17
- iTreg
inducible Treg
- LAM
Lymphangioleiomyomatosis
- LN
Lupus nephritis
- miRNA
microRNA
- mRNA
Messenger ribonucleic acid
- MSC
Mesenchymal stem cells
- mTOR
Mechanistic target of rapamycin
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- NAC
N-acetylcysteine
- NADH
Nicotinamide adenine dinucleotide
- NDUFS3
NADH dehydrogenase iron-sulfur protein 3
- PBMC
Peripheral blood mononuclear cell
- SLE
Systemic lupus erythematosus
- SLEDAI
SLE disease activity index
- snRNP
Small nuclear ribonucleoproteins
- TAL
Transaldolase
- TET
Ten-eleven translocation methylcytosine dioxygenase
- Tfh
Follicular helper T cells
- Th1
T helper 1 cell
- Th17
T helper 17 cell
- Th2
T helper 2 cell
- TLR7
Toll-like receptor 7
- TNF-α
Tumor necrosis factor alpha
- Treg
Regulatory T cell
- TSC
Tuberous sclerosis complex
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Drs. Oaks, Winans, Huang, Banki, and Perl declare no conflicts of interest relevant to this manuscript.
References
- 1.Caza TN, Fernandez DR, Talaber G, Oaks Z, Haas M, Madaio MP, et al. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis. 2014;73(10):1888–1897. doi: 10.1136/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez DR, Telarico T, Bonilla E, Li Q, Banerjee S, Middleton FA, et al. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol (Baltim, Md: 1950) 2009;182(4):2063–2073. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oaks Z, Winans T, Caza T, Fernandez D, Liu Y, Landas SK, et al. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis Rheumatol (Hoboken, N.J.) 2016 doi: 10.1002/art.39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gergely P, Jr, Grossman C, Niland B, Puskas F, Neupane H, Allam F, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46(1):175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gergely P, Jr, Niland B, Gonchoroff N, Pullmann R, Jr, Phillips PE, Perl A. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J Immunol (Baltim, Md: 1950) 2002;169(2):1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perl A, Gergely P, Jr, Puskas F, Banki K. Metabolic switches of T-cell activation and apoptosis. Antioxid Redox Signal. 2002;4(3):427–443. doi: 10.1089/15230860260196227. [DOI] [PubMed] [Google Scholar]
- 7.Tsokos GC. Systemic lupus erythematosus in 2015: cellular and metabolic requirements of effector T cells. Nat Rev Rheumatol. 2016;12(2):74–76. doi: 10.1038/nrrheum.2015.178. [DOI] [PubMed] [Google Scholar]
- 8.Canaud G, Bienaime F, Tabarin F, Bataillon G, Seilhean D, Noel LH, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 2014;371(4):303–312. doi: 10.1056/NEJMoa1312890. [DOI] [PubMed] [Google Scholar]
- 9.Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 2016;12(3):169–182. doi: 10.1038/nrrheum.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2002;99(7):4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7(274):274ra18. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Rodrigues Hoffman A, et al. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J Biol Chem. 2014;289(40):27692–27701. doi: 10.1074/jbc.M114.592576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson B, Scheinbart L, Strahler J, Gross L, Hanash S, Johnson M. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1990;33(11):1665–1673. doi: 10.1002/art.1780331109. [DOI] [PubMed] [Google Scholar]
- 14.Corvetta A, Della Bitta R, Luchetti MM, Pomponio G. 5-Methylcytosine content of DNA in blood, synovial mononuclear cells and synovial tissue from patients affected by autoimmune rheumatic diseases. J Chromatogr. 1991;566(2):481–491. doi: 10.1016/0378-4347(91)80265-e. [DOI] [PubMed] [Google Scholar]
- 15.Ichiyama K, Chen T, Wang X, Yan X, Kim BS, Tanaka S, et al. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity. 2015;42(4):613–626. doi: 10.1016/j.immuni.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M, Wang J, Liao W, Li D, Li M, Wu H, et al. Increased 5-hydroxymethylcytosine in CD4(+) T cells in systemic lupus erythematosus. J Autoimmun. 2016;69:64–73. doi: 10.1016/j.jaut.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Banica LM, Besliu AN, Pistol GC, Stavaru C, Vlad V, Predeteanu D, et al. Dysregulation of anergy-related factors involved in regulatory T cells defects in systemic lupus erythematosus patients: rapamycin and vitamin D efficacy in restoring regulatory T cells. Int J Rheum Dis. 2014 doi: 10.1111/1756-185X.12509. [DOI] [PubMed] [Google Scholar]
- 18.Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6(5):472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 19.Kato H, Perl A. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+ CD4−CD8− double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J Immunol (Baltim, Md: 1950) 2014;192(9):4134–4144. doi: 10.4049/jimmunol.1301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rother N, van der Vlag J. Disturbed T Cell signaling and altered Th17 and regulatory T cell subsets in the pathogenesis of systemic lupus erythematosus. Front Immunol. 2015;6:610. doi: 10.3389/fimmu.2015.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koga T, Hedrich CM, Mizui M, Yoshida N, Otomo K, Lieberman LA, et al. CaMK4-dependent activation of AKT/mTOR and CREM-alpha underlies autoimmunity-associated Th17 imbalance. J Clin Invest. 2014;124(5):2234–2245. doi: 10.1172/JCI73411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kshirsagar S, Riedl M, Billing H, Tonshoff B, Thangavadivel S, Steuber C, et al. Akt-dependent enhanced migratory capacity of Th17 cells from children with lupus nephritis. J Immunol (Baltim Md: 1950) 2014;193(10):4895–4903. doi: 10.4049/jimmunol.1400044. [DOI] [PubMed] [Google Scholar]
- 23.Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64(9):2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perl A, Hanczko R, Lai ZW, Oaks Z, Kelly R, Borsuk R, et al. Comprehensive metabolome analyses reveal N-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: implications for activation of the mechanistic target of rapamycin. Metabolomics: Off J Metabolomic Soc. 2015;11(5):1157–1174. doi: 10.1007/s11306-015-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai ZW, Borsuk R, Shadakshari A, Yu J, Dawood M, Garcia R, et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol (Baltim, Md: 1950) 2013;191(5):2236–2246. doi: 10.4049/jimmunol.1301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Bertucci AM, Ramsey-Goldman R, Harsha-Strong ER, Burt RK, Datta SK. Major pathogenic steps in human lupus can be effectively suppressed by nucleosomal histone peptide epitope-induced regulatory immunity. Clin immunol (Orlando, Fla) 2013;149(3):365–378. doi: 10.1016/j.clim.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, et al. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity. 2015;43(4):690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, et al. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity. 2016;45(3):540–554. doi: 10.1016/j.immuni.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Y, Li J, Wu Q, Yang P, Luo B, Xie S, et al. IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J Immunol (Baltim, Md: 1950) 2013;191(4):1614–1624. doi: 10.4049/jimmunol.1300479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17(10):1197–1205. doi: 10.1038/ni.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62(1):234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 32.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet (Lond Engl) 2011;377(9767):721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 33.Ke Z, Liang D, Zeng Q, Ren Q, Ma H, Gui L, et al. hsBAFF promotes proliferation and survival in cultured B lymphocytes via calcium signaling activation of mTOR pathway. Cytokine. 2013;62(2):310–321. doi: 10.1016/j.cyto.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Zeng Q, Zhang H, Qin J, Xu Z, Gui L, Liu B, et al. Rapamycin inhibits BAFF-stimulated cell proliferation and survival by suppressing mTOR-mediated PP2A-Erk1/2 signaling pathway in normal and neoplastic B-lymphoid cells. Cell Mol Life Sc: CMLS. 2015;72(24):4867–4884. doi: 10.1007/s00018-015-1976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo S, Liu Y, Liang G, Zhao M, Wu H, Liang Y, et al. The role of microRNA-1246 in the regulation of B cell activation and the pathogenesis of systemic lupus erythematosus. Clin Epigenetics. 2015;14(1) doi: 10.1186/s13148-015-0063-7. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi T, Shimabukuro-Demoto S, Yoshida-Sugitani R, Furuyama-Tanaka K, Karyu H, Sugiura Y, et al. The histidine transporter SLC15A4 coordinates mTOR-dependent inflammatory responses and pathogenic antibody production. Immunity. 2014;41(3):375–388. doi: 10.1016/j.immuni.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science (New York, NY) 2011;334(6056):678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercalli A, Calavita I, Dugnani E, Citro A, Cantarelli E, Nano R, et al. Rapamycin unbalances the polarization of human macrophages to M1. Immunology. 2013;140(2):179–190. doi: 10.1111/imm.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L, Yang T, Li L, Sun L, Hou Y, Hu X, et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat Commun. 2014;5:4696. doi: 10.1038/ncomms5696. [DOI] [PubMed] [Google Scholar]
- 41.Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poglitsch M, Weichhart T, Hecking M, Werzowa J, Katholnig K, Antlanger M, et al. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2012;12(6):1458–1468. doi: 10.1111/j.1600-6143.2012.04002.x. [DOI] [PubMed] [Google Scholar]
- 43.Iwata Y, Bostrom EA, Menke J, Rabacal WA, Morel L, Wada T, et al. Aberrant macrophages mediate defective kidney repair that triggers nephritis in lupus-susceptible mice. J Immunol (Baltim, Md: 1950) 2012;188(9):4568–4580. doi: 10.4049/jimmunol.1102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahu R, Bethunaickan R, Singh S, Davidson A. Structure and function of renal macrophages and dendritic cells from lupus-prone mice. Arthritis Rheumatol (Hoboken, NJ) 2014;66(6):1596–1607. doi: 10.1002/art.38410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olmes G, Buttner-Herold M, Ferrazzi F, Distel L, Amann K, Daniel C. CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res Ther. 2016;18 doi: 10.1186/s13075-016-0989-y. 90-016-0989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu Z, Tan W, Ji J, Feng G, Meng Y, Da Z, et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging. 2016;8(5):1102–1114. doi: 10.18632/aging.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Yu J, Oaks Z, Marchena-Mendez I, Francis L, Bonilla E, et al. Liver injury correlates with biomarkers of autoimmunity and disease activity and represents an organ system involvement in patients with systemic lupus erythematosus. Clin Immunol (Orlando, Fla) 2015;160(2):319–327. doi: 10.1016/j.clim.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miwa S, Jow H, Baty K, Johnson A, Czapiewski R, Saretzki G, et al. Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nat Commun. 2014;5:3837. doi: 10.1038/ncomms4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vila L, Roglans N, Baena M, Barroso E, Alegret M, Merlos M, et al. Metabolic alterations and increased liver mTOR expression precede the development of autoimmune disease in a murine model of lupus erythematosus. PLoS One. 2012;7(12):e51118. doi: 10.1371/journal.pone.0051118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winchester R, Wiesendanger M, Zhang HZ, Steshenko V, Peterson K, Geraldino-Pardilla L, et al. Immunologic characteristics of intrarenal T cells: trafficking of expanded CD8+ T cell beta-chain clonotypes in progressive lupus nephritis. Arthritis Rheum. 2012;64(5):1589–1600. doi: 10.1002/art.33488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol (Baltim, Md: 1950) 2008;181(12):8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh N, Birkenbach M, Caza T, Perl A, Cohen PL. Tuberous sclerosis and fulminant lupus in a young woman. J Clin Rheumatol: Pract Rep Rheum Musculoskelet Dis. 2013;19(3):134–137. doi: 10.1097/RHU.0b013e318289c033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carrasco Cubero C, Bejarano Moguel V, Fernandez Gil MA, Alvarez Vega JL. Coincidence of tuberous sclerosis and systemic lupus erythematosus—a case report. Reumatol Clin. 2016;12(4):219–222. doi: 10.1016/j.reuma.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Moss J, Avila NA, Barnes PM, Litzenberger RA, Bechtle J, Brooks PG, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001;164(4):669–671. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 55.Robb VA, Astrinidis A, Henske EP. Frequent [corrected] hyperphosphorylation of ribosomal protein S6 [corrected] in lymphangioleiomyomatosis-associated angiomyolipomas. Mod Pathol: Off J U S Can Acad Pathol Inc. 2006;19(6):839–846. doi: 10.1038/modpathol.3800610. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki K, Nagasaka K, Oda K, Abe H, Maeda D, Matsumoto Y, et al. A case of lymphangioleiomyomatosis associated with endometrial cancer and severe systemic lupus erythematosus. BMC Cancer. 2016;16 doi: 10.1186/s12885-016-2413-z. 390-016-2413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olde Bekkink M, Ahmed-Ousenkova YM, Netea MG, van der Velden WJ, Berden JH. Coexistence of systemic lupus erythematosus, tuberous sclerosis and aggressive natural killer-cell leukaemia: coincidence or correlated? Lupus. 2016;25(7):766–771. doi: 10.1177/0961203316636466. [DOI] [PubMed] [Google Scholar]
- 58.Yap DY, Ma MK, Tang CS, Chan TM. Proliferation signal inhibitors in the treatment of lupus nephritis: preliminary experience. Nephrol (Carlton, Vic) 2012;17(8):676–680. doi: 10.1111/j.1440-1797.2012.01646.x. [DOI] [PubMed] [Google Scholar]
- 59.Lai ZW, Marchena-Mendez I, Perl A. Oxidative stress and Treg depletion in lupus patients with anti-phospholipid syndrome. Clin Immunol (Orlando, Fla) 2015;158(2):148–152. doi: 10.1016/j.clim.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu T, Ye Y, Min SY, Zhu J, Khobahy E, Zhou J, et al. Prevention of murine lupus nephritis by targeting multiple signaling axes and oxidative stress using a synthetic triterpenoid. Arthritis Rheumatol (Hoboken, NJ) 2014;66(11):3129–3139. doi: 10.1002/art.38782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lui SL, Yung S, Tsang R, Zhang F, Chan KW, Tam S, et al. Rapamycin prevents the development of nephritis in lupus-prone NZB/W F1 mice. Lupus. 2008;17(4):305–313. doi: 10.1177/0961203307088289. [DOI] [PubMed] [Google Scholar]
- 63.Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann N Y Acad Sci. 2015;1346(1):33–44. doi: 10.1111/nyas.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]