Abstract
Background and aims
Malignant transformation of pheochromocytomas/paragangliomas (PCC/PGL) is a rare occurrence, and predictive factors for the same are not well understood. This study aims to identify the predictors of malignancy in patients with PCC/PGL.
Materials and methods
We performed a retrospective analysis of 142 patients with either PCC or PGL registered at our institute between 2000 and 2015. Records were evaluated for clinical parameters like age, gender, familial/syndromic presentation, symptomatic presentation, biochemistry, size, number and location of tumours and presence of metastases and mode of its diagnosis.
Results
Twenty patients were found to have metastases; 13 had metastases at diagnosis and seven during follow-up. Metastases were detected by radiology (CT-neck to pelvis) in 11/20 patients (5/13 synchronous and 6/7 metachronous), 131I-metaiodobenzylguanidine in five (2/12 synchronous and 3/6 metachronous) patients and 18F-flurodeoxyglucose PET/CT in 15 (12/12 synchronous and 3/3 metachronous) patients. Malignant tumours were significantly larger than benign tumours (8.3 ± 4.1 cm, range: 3–22 cm vs 5.7 ± 2.3 cm, range: 2–14 cm, P = 0.0001) and less frequently metanephrine secreting. On linear regression analysis, tumour size and lack of metanephrine secretion were the independent predictors of malignancy.
Conclusions
Patients with primary tumour size >5.7 cm and lack of metanephrine secretory status should be evaluated for possible malignancy not only at diagnosis but also in the postoperative period. As compared to CT and 131I-MIBG scan, 18F-flurodeoxyglucose PET/CT analyses are better (sensitivity: 100%) for the diagnosis of metastases in our study.
Keywords: malignant pheochromocytoma, malignant paraganglioma, clinical predictors, 18F-flurodeoxyglucose PET/CT
Introduction
Malignant pheochromocytoma and paraganglioma (PCC/PGL) comprise approximately 10–20% of all pheochromocytomas and paragangliomas (1). According to the World Health Organization classification, the only accepted criterion for diagnosis of malignant PCC/PGL is the presence of metastases in the non-chromaffin areas (2).
Metastases in patients with PCC/PGL are commonly diagnosed by anatomical imaging and/or functional imaging (3, 4). The latter includes 123I-metaiodobenzyl guanidine (123I-MIBG), 18F-flurodeoxyglucose positron emission tomography (18F-FDG PET/CT), 18F-flurodopamine PET (18F-FDA PET/CT), 18F-flurodopa PET (18F-FDOPA PET/CT) and 68Ga-DOTATATE PET/CT (3, 4). As compared to anatomical imaging and 123I-MIBG, 18F-FDG PET/CT has been proven to be more sensitive in diagnosing metastases (5, 6, 7, 8). However, 68Ga-DOTATATE PET/CT has proven to be a superior modality as compared to 18F-FDG PET/CT in the evaluation of SDHx-associated malignant PCC/PGL (3).
Majority of the malignant PCCs/PGLs are initially diagnosed as benign tumours but subsequently present with widespread metastasis (9). Small series and case reports have tried to analyse the clinical predictors for malignancy and have suggested size (greater than 5 cm), location (sympathetic PGL) of the primary tumour, tumour necrosis, non-secretory status and dopamine secretion as clinical predictors of metastatic disease in PCC/PGL (10, 11, 12, 13, 14). Multiple population studies have suggested SDHB germline mutations as an important genetic predictor for metastasis, aggressiveness and poor outcome (15). Histopathologic features like PASS score and Ki67 have also been suggested to predict malignancy (14, 16). However, none of the clinical, biochemical, radiological and histopathological predictors for malignancy has been largely proven successful; probably due to the rarity of the disease and paucity of data. Hence, we analysed data from our PCC/PGL cohort to identify the potential clinical predictors for malignancy in PCC/PGL.
Materials and methods
This retrospective study was conducted at a tertiary health care centre, which caters to Central and Western India. A written informed consent was obtained from all participants, and the study was approved by the institutional ethics committee. The study included 142 patients (M: 66, F: 76) with PCC/PGL registered at our centre between 2000 and 2015. Of these, 10 patients (n = 2 malignant) were referred for genetic analysis from other centres where they were managed, whereas the rest were managed at our institute. Data were obtained by retrospective record analysis of the participants. Metastasis was defined as the presence of tumour cells at sites that normally lack chromaffin tissue, and the metastatic lesions were proven either by histopathology or the presence of persistently elevated catecholamine biochemistry together with concurring functional imaging findings and previous history of PCC/PGL.
Preoperatively, 24-h urinary concentrations of vanillylmandelic acid (VMA) was initially performed till the year 2008 (n = 49) followed by plasma free normetanephrine and metanephrine since 2009 (n = 101). Tumours were localised by computed tomography (CT) and/or magnetic resonance imaging (MRI). In addition, 131I-MIBG scans were performed in majority of the patients (n = 110, 92 benign tumours and 18 malignant tumours), whereas 18F-FDG PET scanning was performed in 75 (60 benign tumours; 15 malignant tumours) patients. Two experienced laparoscopic surgeons operated on majority of the patients. Selection of surgical approach was according to the discretion of the operating surgeon. The cases with evidence of metastases at diagnosis (n = 13) underwent open surgical approach (n = 9 and n = 4 deferred surgery), whereas the remaining cases who developed metastases on follow-up (n = 7) had undergone laparoscopic excision (n = 5) and open excision (n = 2; one with a paraganglioma and one with PCC with size of 22 cm) of the primary tumour.
Genetic testing (n = 139) for five susceptibility genes (VHL, RET, SDHB, SDHC and SDHD) was performed using polymerase chain reaction and Sanger sequencing for identifying mutations and multiplex ligation-dependent probe amplification (MLPA) for large deletions. Postoperative follow-up included annual blood pressure and biochemical monitoring. Postoperatively, mapping with imaging was done if biochemistry was elevated or if tumour was preoperatively non-secretory.
Statistical analysis was performed using SPSS software, version 20.0 (SPSS). Chi-square test and the Mann–Whitney U test were used for the comparison of characteristics between benign and malignant PCC/PGL. Cut-off values for tumour size were calculated by receiver-operating characteristic (ROC). A P value of <0.05 was considered significant. Linear regression analysis was done to identify the predictors of malignancy.
Results
Patient demographics
The study group comprised 142 patients (males: 66; females: 76). Of these, 96 had PCC, 28 had sPGLs, seven had multifocal disease (PCC + sPGL) and 11 had HNPGL. The mean age at diagnosis of the overall study population was 35 years (range, 6–75 years). Median duration of follow-up of the study cohort was 5.2 years (range: 6 months to 15 years).
Twenty patients (10 males and 10 females) were diagnosed with malignant PCC/PGL. Thirteen patients (65%) showed evidence of metastases at diagnosis and seven (35%) patients developed metachronous metastases after a median follow-up period of 17.5 months (range: 12–48 months). Synchronous metastases were common in bones, lungs and liver, whereas in patients with metachronous metastases, apart from bones, lungs and liver, five patients had local bed recurrence.
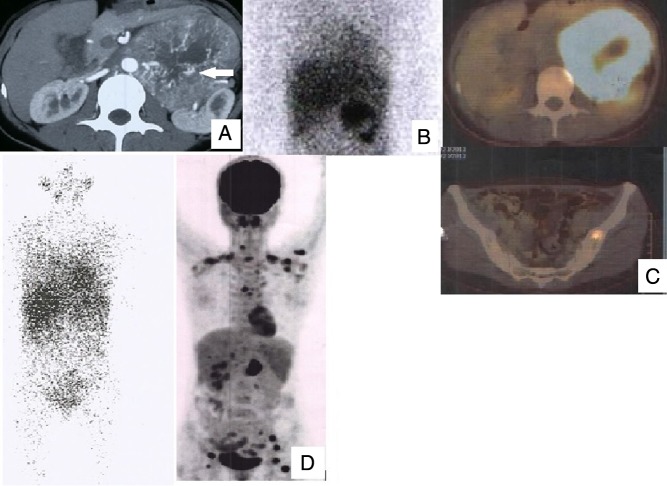
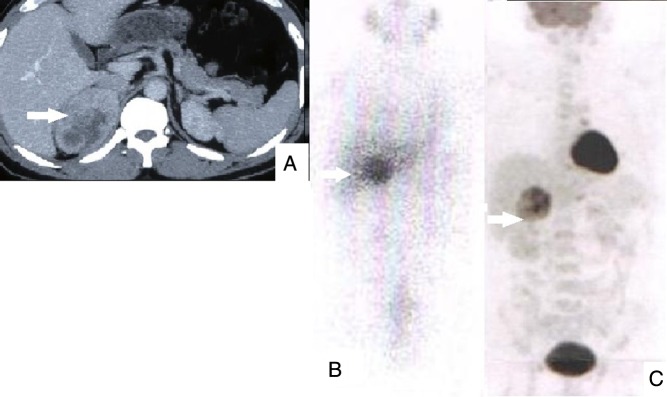
Radiology (CT-neck to pelvis) detected metastases in 55% (11/20) patients. It had sensitivity of 38.46% (5/13) for synchronous metastases (dorsal vertebrae: 3, lungs: 2) and 85.7% (6/7) for metachronous metastases (liver: 2, dorsal vertebra: 1 and lung nodules: 3). 131I-MIBG detected metastases in 27.78% (5/18). 131I-MIBG had sensitivity of 16.67% (2/12) for synchronous metastases (skull: 1, humerus and sacrum: 1) and 50% (3/6) for metachronous metastases (lung: 1, liver: 1 and humerus: 1). 18F-FDG PET/CT detected metastases in 100% (15/15). 18F-FDG PET/CT had 100% sensitivity for both synchronous as well as metachronous metastases. It performed well for both skeletal as well as soft tissue metastases. Management, follow-up and outcome of patients with malignant PCC/PGL are summarised in Table 1. Better detection of metastases with 18F-FDG PET/CT than CT and 131I-MIBG is depicted in Fig. 1, whereas Fig. 2 depicts the performance of 18F-FDG PET/CT compared to CT and 131I-MIBG in a patient with benign unilateral PCC.
Table 1.
Description of clinical characteristics, mode of diagnosis, management and outcome of individual patients with malignant PCC/PGL.
| Detection of metastases on imaging | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex/age (years) | Location | Primary tumour size (cm) | Secretory status | Site of metastasis | Time to metastasis (months) | CT scan | IMIBG | 18F FDG PET/CT | Genetics | Management |
| M/39 | Unilateral; PCC | 10 | Normetanephrine | Lung nodules; vertebral metastases | 36 | Lung nodules; vertebral metastases | Lung nodules | NA | Negative | Advised MIBG therapy but lost to follow-up |
| M/14 | Unilateral; PCC | 3 | Normetanephrine | Liver; lymph nodes | 48 | Nil | Liver nodule | Multiple liver metastases | Negative | 4 cycles of CVD chemotherapy and 3 cycles of MIBG therapy (1 curie); static disease |
| F/28 | Abdominal; sPGL | 14 | Non-secretory | Multiple skeletal metastases | At diagnosis | Nil | Metastases in the sacrum and right humerus | Multiple vertebral metastases, right clavicle, right humerus, sacrum and ribs | Negative | Deferred surgery; no progression; no symptoms |
| M/33 | Unilateral; HNPGL | 8.1 | Non-secretory | Multiple skeletal metastases; lung nodules; liver; posterior chest wall | At diagnosis | Nil | Nil | Multiple skeletal metastases lungs posterior chest wall; liver | Negative | EBRT for the primary tumour; CVD chemotherapy 2 cycles; lost to follow-up |
| F/14 | Abdominal; sPGL | 6.4 | Normetanephrine | Lung nodule; multiple skeletal metastases; lymph nodes | 48 | Lung nodule | Single skeletal metastasis: humerus | Lung nodule; axillary lymph nodes; multiple skeletal sites | Negative | Metastatectomy of lung nodule; 3 cycles of PRRT; static disease; no progression |
| F/50 | Thoracic sPGL + abdominal sPGL | 9.6 | Normetanephrine | Lung nodules | At diagnosis | Lung nodules | Nil | Multiple lung nodules | Negative | EBRT to primary tumour; 2 cycles of MIBG therapy; static disease; no progression |
| F/45 | Unilateral; PCC | 5.8 | Normetanephrine | Lungs | At diagnosis | Lung nodules | Nil | Multiple lung nodules | Negative | Deferred treatment; lost to follow-up |
| F/43 | Bilateral PCC + abdominal sPGL | 7.2 | Metanephrine | Liver; metastases | 36 | Liver nodules | nil | Liver; multiple peritoneal metastases | RET c.1901G>A | Succumbed before treatment for metastases |
| M/30 | Unilateral; PCC | 8.7 | Normetanephrine | Lung nodules; peritoneal deposits | 24 | Lung nodules | Nil | Multiple lung nodules; multiple peritoneal metastases | Negative | 4 cycles of MIBG therapy; no progression |
| F/47 | Unilateral; PCC | 10 | Non-secretory | Lungs; multiple skeletal metastases | At diagnosis | Nil | Single skull metastasis | Lung nodules; skull and pelvic metastases | Negative | Planned MIBG therapy |
| F/35 | Abdominal; sPGL | 9.4 | Normetanephrine | Liver; bones | At diagnosis | Nil | Nil | Liver nodule; multiple skeletal metastases | Negative | Surgical excision of local bed recurrence; succumbed before treatment for metastases |
| M/45 | Unilateral; PCC | 5.8 | Normetanephrine | Vertebral metastases | 12 | Vertebral metastasis | Nil | NA | Negative | Planned EBRT for vertebral metastases; lost to follow-up |
| M/55 | Unilateral; PCC | 22 | Non-secretory | Malignant pleural effusion; liver | 24 | Malignant pleural effusion; multiple liver nodules | NA | NA | Negative | Succumbed before therapy of the metastases |
| M/72 | Unilateral; PCC | 9 | Normetanephrine | Multiple lung nodules | At diagnosis | Nil | Nil | Multiple lung nodules | Negative | Refused treatment; lost to follow-up |
| F/49 | Unilateral; PCC | 4 | NA | Multiple vertebral metastases | At diagnosis | Vertebral metastasis | Nil | NA | NA | MIBG therapy one cycle; planned further cycles of MIBG; pain relief |
| F/40 | Bilateral; HNPGL | 4.7 | Non-secretory | Multiple vertebral | At diagnosis | Nil | Nil | Two vertebral metastases | Negative | EBRT given to both primary tumour as well as vertebral metastases; no new lesions; no increase in size of primary |
| M/40 | Abdominal; sPGL | 6.1 | Normetanephrine | Multiple skeletal metastases | At diagnosis | Vertebral metastasis | Nil | Multiple skeletal metastases | NA | Bisphosphonate therapy for skeletal metastases; planned EBRT |
| M/27 | Abdominal; sPGL | 5.8 | Normetanephrine | Vertebral metastases | At diagnosis | Vertebral metastasis | Nil | NA | SDHB c.251A>C | Lost to follow-up |
| M/36 | Abdominal; sPGL | 7.5 | NA | Liver; bones | At diagnosis | Nil | Nil | Liver; vertebral and long bones | SDHB c286 + 1G>A | No treatment for metastases; yet to follow-up |
| F/43 | Unilateral; PCC | 10.1 | Normetaneprine | Abdominal lymph nodes | At diagnosis | Nil | NA | Regional lymph nodes | NA | Operated with clearance of regional lymph nodes; no new lesions |
CVD, cyclophosphamide, vincristine and dacarbazine; EBRT, external beam radiotherapy; MIBG, metaiodobenzyl guanidine; PRRT, peptide receptor based radiotherapy.
Figure 1.
Case 11: 35/F, abdominal sPGL. (A) CT scan showing sPGL; (B) MIBG positive only in the primary; (C) positive FDG PET in primary and skeletal metastasis; (D) on follow-up, MIBG did not detect any lesions, but FDG detected multiple metastases.
Figure 2.
Benign right PCC. (A) CT showing primary tumour; (B) MIBG showing uptake in primary; (C) FDG PET showing uptake only in primary.
The baseline characteristics of benign and malignant PCC/PGL are summarised in Table 2. In the benign group, 31.2% (38/122) had a symptomatic presentation compared to 70% (14/20) in the malignant group (P = 0.002). Familial/syndromic association (10% (2/20) vs 7.4% (9/122), P = 0.65), normetanephrine-secreting tumours (12/18 (66.67%) vs 42/83 (50.6%), P = 0.29) and non-secretory tumours (5/18 (22.2%) vs 15/83 (18.07%), P = 0.34) were not significantly different between the malignant and benign groups, whereas metanephrine-secreting tumours (1/18 (5.55%) vs 26/83 (31.3%), P = 0.037) were significantly less in the malignant group.
Table 2.
Comparison of clinical characteristics and germline mutations between patients with benign and malignant PCC/PGL.
| Benign (n = 122) | Malignant (n = 20) | P Value | |
|---|---|---|---|
| Age (years) | 34.9 ± 14.4 | 39.3 ± 13.4 | 0.65 |
| Sex (M:F) | 56: 66 | 10:10 | 0.8 |
| Symptomatic presentation | 38/122 (31.2%) | 14/20 (70%) | 0.002 |
| Primary tumour size (cm) | 5.7 ± 2.3 | 8.3 ± 4.1 | 0.0001 |
| Range | 2 to 14 cm | 3 to 22 cm | |
| Adrenal PCC | 86/122 (70.5%) | 10/20 (50%) | 0.07 |
| sPGL | 21/122(17.2%) | 7/20(35%) | 0.07 |
| HNPGL | 9/122 (7.4%) | 2/20 (10%) | 0.65 |
| Multifocal tumours | 6/122 (4.9%) | 1 /20 (5.0%) | 1 |
| Biochemistry | |||
| Metanephrine secreting | 26/83 (31.3%) | 1/18 (5.55%) | 0.037 |
| Normetanephrine secreting | 42/83 (50.6%) | 12/18 (66.67%) | 0.29 |
| Non-secretory | 15/83 (18.07%) | 5/18 (22.2%) | 0.25 |
| SDHB germline mutations | 6/122 (4.9%) | 2/17 (11.8%) | 0.081 |
Location of the primary tumour and malignancy: no statistical significance was found when proportion of malignant tumours was compared across different locations of primary tumour: PCC (10/96), sPGL (7/28), HNPGL (2/11), multifocal (1/7); P = 0.29.
Comparison of malignancy in PCC (10/96) and sPGL (7/28): P = 0.048.
Ten (10.4%) of the 96 patients with PCCs had metastases; five of these had metachronous metastases. Of the patients with sPGLs, seven patients (25%) had metastases, of whom one had metachronous metastases. Two patients (18.2%) with HNPGL had metastases, both at the diagnosis of primary tumour. One of the seven patients with multifocal disease had metastases on follow-up. Proportion of malignancy was significantly higher in patients with sPGL than those with PCC (10.4% vs 25%, P = 0.0048). However, the frequency of PCC, sPGL, HNPGL or multifocal tumours did not differ between malignant and benign groups (Table 2). Primary tumours in malignant group were significantly larger (8.3 ± 4.1 cm, range: 3–22 cm) than benign tumours (5.7 ± 2.3 cm, range: 2–14 cm, P = 0.0001). On receiver-operative characteristic curve analysis, cut-off of 5.7 cm had the best accuracy to identify malignant tumours (sensitivity: 80%; specificity: 59%).
We have recently reported the prevalence of germline mutations in 150 PCC/PGL patients from eight different centres from India (17). One hundred and twenty patients from our institute included in that study are also part of the present cohort. In the metastatic group, 17 patients underwent genetic testing with three patients having positive germline mutations ((17.6%); SDHB: 2, RET: 1). In the benign group, 122 patients underwent genetic testing with genetic yield of 34.4% (42/122; VHL: 23, RET: 9, SDHB: 6, SDHD: 2, SDHC: 0 and NF1: 2). Prevalence of metastases was numerically higher in patients with germline mutations in SDHB (2/8, 25%) as compared to other mutation groups and mutation-negative group (14/94, 14.9%).
On linear regression analysis, including age, sex, tumour size, metanephrine secretion and presence of at least one extra-adrenal tumour as variables, only tumour size (P = 0.003) and lack of metanephrine elevation (P = 0.013) were the independent predictors of malignancy. When biochemistry was excluded from the analysis, tumour size (P < 0.001) and presence of at least one extra-adrenal tumour were identified as independent predictors of malignancy (P = 0.013).
Discussion
This is the first study that describes the prevalence, diagnostic sensitivities of commonly used anatomical and functional imaging methods and predictors of malignancy in Indian patients with malignant PCC/PGL. The prevalence of malignancy among our 142 patients with PCC/PGL was 14.08%, which is similar to that of previous reports from the rest of the world (18, 19, 20). Majority of the malignant PCCs/PGLs usually present as apparently benign tumours without metastases at initial presentation (9). In contrast, almost 2/3rd of our malignant PCC/PGL had metastases at initial presentation. Higher prevalence of metastases at initial presentation could be due to late presentation. Another reason for this phenomenon could be frequent testing at diagnosis of all our PCC/PGL patients with 18F-FDG PET/CT. On the other hand, shorter duration of follow-up and substantial rate of incomplete follow-up might have contributed for the low prevalence of metachronous metastases. The most frequent sites for metastases are lymph nodes, the skeleton, liver and lungs, which were also observed in our study (15).
18F-FDG PET/CT had the best (100%) sensitivity for both skeletal and soft tissue metastases when compared to anatomical imaging (CT-neck to pelvis) (55%) and MIBG (27.78%). Superiority of 18F-FDG PET/CT over anatomical imaging and MIBG has been demonstrated in most of the other studies (5, 6, 7, 8). Sensitivities of anatomical imaging and 18F-FDG PET/CT in our study are similar to the previous reports. Poor performance of MIBG in our study is striking and is probably due to the use of 131I isotope that could not be coupled with SPECT. Additional reason for the poor sensitivity (16.67%) to diagnose synchronous metastases may be the uptake of most of the tracer by the large primary tumour. 18F-FDA PET/CT, 18F-FDG PET/CT and 68Ga-DOTATATE PET/CT are the recommended first-line functional imaging techniques for the diagnosis of metastases in PCC/PGL tumours; however, only the latter two are presently available in India (3, 4). Due to the relatively limited availability of 68Ga-DOTATATE PET/CT in India, 18F-FDG PET/CT hence becomes the first choice to detect metastases in Indian PCC/PGL patients (21).
Many authors have attempted to find the predictors of malignancy in PCC/PGL, but no scoring system has been proven reliable (22). Age at diagnosis, gender or familial/syndromic presentation did not correlate with the presence of metastases. However, malignant tumours were commonly symptomatic as compared to their benign counterparts as reported from Korea, in which persistence or increase of symptoms was observed with progression of metastases (23).
In our study, metastatic PCC/PGL had significantly larger primary tumours than the benign ones. Tumour size was the strongest predictor of malignancy. Most of the previous studies have reported similar observations (11, 13, 24, 25, 26). In contrast, one study reported no significant difference in the primary tumour size between malignant and benign tumours (27). There is no clear cut-off of primary tumour size that distinguishes benign from malignant lesions. In our study, a cut-off of 5.7 cm provided the best accuracy (sensitivity: 80%; specificity: 59%). Among the 20% patients who had tumour size <5.7 cm and evidence of metastases, three had PCC, one had HNPGL, whereas none had sPGL. Smallest tumour diameter in our study was 3 cm, which is similar to previous reports (14). These findings suggest that even smaller tumours cannot be exempted from regular follow-up and careful vigilance.
Metanephrine-secreting tumours were rarely malignant, and this secretory status was an independent negative predictor of malignancy in our cohort. Less frequent secretion of metanephrine in malignant PCC/PGL may be due to the downregulation of phenylalanine-N-methyl transferase (PNMT) in dedifferentiated tumours and lack of metanephrine secretion by extradrenal tumours (14, 28). In contrast, one study has reported high metanephrine secretion as a predictor of malignancy (26). Isolated or co-secretion of dopamine has been reported to be a marker of malignancy, but our patients were not routinely evaluated for dopamine secretory status (12, 29, 30).
In our study, malignancy was more frequent among sPGL (25%) than PCC (10.4%) (P = 0.048). Similar observation has been noted in most of the previous studies (12, 26). The presence of at least one extra-adrenal tumour was an independent predictor of malignancy when biochemistry was excluded from the analysis. It suggests that when fractionated metanephrines are not available, extra-adrenal location can be used to predict the malignancy risk. Higher prevalence of malignancy in sPGL may be due to relative immaturity of sympathetic ganglia as compared to the adrenal medulla or due to the plausible role of adrenal cortex in the prevention of malignant transformation in PCC (10).
The absence of underlying germline mutations in five major susceptibility genes in almost 90% of malignant PCC/PGL suggests the role for different susceptibility genes in the malignant group. There is strong literature evidence to support the relationship between mutations in the SDHB gene and the presence of malignant tumours (15, 18, 19, 20). Although SDHB mutations were the most common among our malignant patients, it was not an independent predictor of malignancy.
Multiple studies have tried to analyse histological predictors of malignancy like necrosis, PASS score and Ki67 without any significant success (14, 16, 31). However, in our study, detailed histopathological data on these parameters were not sufficient to include in the analysis.
The European Society of Endocrinology (ESE) recommends the preoperative evaluation for malignancy with 18F-FDG PET/CT in patients with paraganglioma, elevated serum 3-methoxytyramine levels in plasma or urine and germline SDHB mutations (32). We suggest that patients with apparently benign tumours at initial presentation with tumour size >5.7 cm and isolated normetanephrine secretion or non-secretory status also be considered for preoperative evaluation with 18F-FDG PET/CT. In the postoperative period, evaluation for malignancy should be considered if there is elevation of catecholamines or their metabolites; in non-secretory tumours with primary tumour size > 5.7 cm, routine evaluation with 18F-FDG PET/CT may be undertaken at regular intervals after surgery. The ESE guidelines suggest life-long follow-up for large tumours and PGL (33). We recommend the same; however, in Indian set up where regular follow-up is most often not ensured, a minimum follow-up for at least initial two years should be ensured during which the chances of metastases are high. The study is limited by the retrospective design, lack of histopathology data, relatively small number of patients and shorter postoperative follow-up.
Conclusion
Primary tumour size >5.7 cm and/or lack of metanephrine secretory status independently predict malignancy. Patients with either of these features should be evaluated for possible malignancy not only at initial presentation but also during follow-up, especially in the early postoperative period. 18F-flurodeoxyglucose PET/CT has the best sensitivity, whereas 131I-metaiodobenzylguanidine has the least sensitivity for diagnosis of metastases.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This project has been funded by the Scientific and Engineering Research Board (SERB), Department of Science and Technology, Government of India (Grant# SB/SO/HS-41/2013).
References
- 1.Baudin E, Habra MA, Deschamps F, Cote G, Dumont F, Cabanillas M, Arfi-Roufe J, Berdelou A, Moon B, Al Ghuzlan A, et al. Therapy of endocrine disease: treatment of malignant pheochromocytoma and paraganglioma. European Journal of Endocrinology 2014. 3 R111–R122. ( 10.1530/EJE-14-0113) [DOI] [PubMed] [Google Scholar]
- 2.DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and Genetics of Tumours of Endocrine Organs. World Health Organization Classification of Tumours. Lyon, France: IARC Press, 2004. [Google Scholar]
- 3.Castinetti F, Kroiss A, Kumar R, Pacak K, Taieb D. 15 years of paraganglioma: imaging and imaging-based treatment of pheochromocytoma and paraganglioma. Endocrine-Related Cancer 2015. 4 T135–T145. ( 10.1530/ERC-15-0175) [DOI] [PubMed] [Google Scholar]
- 4.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF, Jr, Endocrine Society Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2014. 99 1915–1942. ( 10.1210/jc.2014-1498) [DOI] [PubMed] [Google Scholar]
- 5.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. Journal of Clinical Endocrinology and Metabolism 2009. 12 4757–4767. ( 10.1210/jc.2009-1248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rufini V, Treglia G, Castaldi P, Perotti G, Giordano A. Comparison of metaiodobenzylguanidine scintigraphy with positron emission tomography in the diagnostic work-up of pheochromocytoma and paraganglioma: a systematic review. Quarterly Journal of Nuclear Medicine and Molecular Imaging 2013. 2 122–133. [PubMed] [Google Scholar]
- 7.Cantalamessa A, Caobelli F, Paghera B, Caobelli A, Vavassori F. Role of (18)F-FDG PET/CT, (123)I-MIBG SPECT, and CT in restaging patients affected by malignant pheochromocytoma. Nuclear Medicine and Molecular Imaging 2011. 2 125–131. ( 10.1007/s13139-011-0083-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cistaro A, Niccoli Asabella A, Coppolino P, Quartuccio N, Altini C, Cucinotta M, Alongi P, Balma M, Sanfilippo S, Buschiazzo A, et al. Diagnostic and prognostic value of 18F-FDG PET/CT in comparison with morphological imaging in primary adrenal gland malignancies – a multicenter experience. Hellenic Journal of Nuclear Medicine 2015. 2 97–102. [DOI] [PubMed] [Google Scholar]
- 9.Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocrine-Related Cancer 2007. 14 569–585. ( 10.1677/ERC-07-0074) [DOI] [PubMed] [Google Scholar]
- 10.Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, Busaidy N, Cote GJ, Perrier N, Phan A, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. Journal of Clinical Endocrinology and Metabolism 2011. 96 717–725. ( 10.1210/jc.2010-1946) [DOI] [PubMed] [Google Scholar]
- 11.Szalat A, Fraenkel M, Doviner V, Salmon A, Gross DJ. Malignant pheochromocytoma: predictive factors of malignancy and clinical course in 16 patients at a single tertiary medical center. Endocrine 2011. 2 160–166. ( 10.1007/s12020-010-9422-5) [DOI] [PubMed] [Google Scholar]
- 12.John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: can malignant potential be predicted? Urology 1999. 4 679–683. ( 10.1016/S0090-4295(98)00612-8) [DOI] [PubMed] [Google Scholar]
- 13.Schovanek J, Martucci V, Wesley R, Fojo T, Del Rivero J, Huynh T, Adams K, Kebebew E, Frysak Z, Stratakis CA, et al. The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC Cancer 2014. 14 523 ( 10.1186/1471-2407-14-523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strong VE, Kennedy T, Al-Ahmadie H, Tang L, Coleman J, Fong Y, Brennan M, Ghossein RA. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery 2008. 6 759–768. ( 10.1016/j.surg.2008.02.007) [DOI] [PubMed] [Google Scholar]
- 15.Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, Bertagna X, Schlumberger M, Jeunemaitre X, Gimenez-Roqueplo AP, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. Journal of Clinical Endocrinology and Metabolism 2007. 92 3822–3828. ( 10.1210/jc.2007-0709) [DOI] [PubMed] [Google Scholar]
- 16.Liu TH, Chen YJ, Wu SF, Gao J, Jiang WJ, Lu ZH, Guan J, Wei SZ, Luo YF, Cao JL, et al. Distinction between benign and malignant pheochromocytomas. Zhonghua Bing Li Xue Za Zhi 2004. 3 198–202. [PubMed] [Google Scholar]
- 17.Pandit R, Khadilkar K, Sarathi V, Kasaliwal R, Goroshi M, Khare S, Nair S, Raghavan V, Dalvi A, Hira P, et al. Germline mutations and genotype – phenotype correlation in Asian Indian patients with pheochromocytoma and paraganglioma. European Journal of Endocrinology 2016. 175 311–323. ( 10.1530/EJE-16-0126) [DOI] [PubMed] [Google Scholar]
- 18.Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-de Paillerets B, Chabre O, Chamontin B, Delemer B, Giraud S, Murat A, et al. Genetic testing in pheochromocytoma or functional paraganglioma. Journal of Clinical Oncology 2005. 23 8812–8818. ( 10.1200/JCO.2005.03.1484) [DOI] [PubMed] [Google Scholar]
- 19.Cascón A, Pita G, Burnichon N, Landa I, López-Jiménez E, Montero-Conde C, Leskelä S, Leandro-García LJ, Letón R, Rodríguez-Antona C, et al. Genetics of pheochromocytoma and paraganglioma in Spanish patients. Journal of Clinical Endocrinology and Metabolism 2009. 94 1701–1705. ( 10.1210/jc.2008-2756) [DOI] [PubMed] [Google Scholar]
- 20.Mannelli M, Castellano M, Schiavi F, Filetti S, Giacchè M, Mori L, Pignataro V, Bernini G, Giachè V, Bacca A, et al. Italian Pheochromocytoma/Paraganglioma Network. Clinically guided genetic screening in a large cohort of Italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. Journal of Clinical Endocrinology and Metabolism 2009. 94 1541–1547. ( 10.1210/jc.2008-2419) [DOI] [PubMed] [Google Scholar]
- 21.Janssen I, Blanchet EM, Adams K, Chen CC, Millo CM, Herscovitch P, Taieb D, Kebebew E, Lehnert H, Fojo AT, et al. Superiority of [68Ga]-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clinical Cancer Research 2015. 17 3888–3895. ( 10.1158/1078-0432.CCR-14-2751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tischler AS. Pheochromocytoma: time to stamp out ‘malignancy’? Endocrine Pathology 2008. 19 207–208. ( 10.1007/s12022-008-9047-x) [DOI] [PubMed] [Google Scholar]
- 23.Park J, Song C, Park M, Yoo S, Park SJ, Hong S, Hong B, Kim CS, Ahn H. Predictive characteristics of malignant pheochromocytoma. Korean Journal of Urology 2011. 4 241–246. ( 10.4111/kju.2011.52.4.241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura N, Watanabe T, Noshiro T, Shizawa S, Miura Y. Histological grading of adrenal and extra-adrenal pheochromocytomas and relationship to prognosis: a clinicopathological analysis of 116 adrenal pheochromocytomas and 30 extra-adrenal sympathetic paragangliomas including 38 malignant tumors. Endocrine Pathology 2005. 16 23–32. ( 10.1385/EP:16:1:023) [DOI] [PubMed] [Google Scholar]
- 25.Shen WT, Sturgeon C, Clark OH, Duh QY, Kebebew E. Should pheochromocytoma size influence surgical approach? A comparison of 90 malignant and 60 benign pheochromocytomas. Surgery 2004. 136 1129–1137. ( 10.1016/j.surg.2004.05.058) [DOI] [PubMed] [Google Scholar]
- 26.Feng F, Zhu Y, Wang X, Wu Y, Zhou W, Jin X, Zhang R, Sun F, Kasoma Z, Shen Z. Predictive factors for malignant pheochromocytoma: analysis of 136 patients. Journal of Urology 2011. 5 1583–1590. ( 10.1016/j.juro.2010.12.050) [DOI] [PubMed] [Google Scholar]
- 27.Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. American Journal of Surgical Pathology 2002. 26 551–566. ( 10.1097/00000478-200205000-00002) [DOI] [PubMed] [Google Scholar]
- 28.Lee SE, Oh E, Lee B, Kim YJ, Oh DY, Jung K, Choi JS, Kim J, Kim SJ, Yang JW, et al. Phenylethanolamine N-methyltransferase down regulation is associated with malignant pheochromocytoma/paraganglioma. Oncotarget 2016. 17 24141–24153. ( 10.18632/oncotarget.8234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenhofer G, Goldstein DS, Sullivan P, Csako G, Brouwers FM, Lai EW, Adams KT, Pacak K. Biochemical and clinical manifestations of dopamine producing paragangliomas: utility of plasmamethoxytyramine. Journal of Clinical Endocrinology and Metabolism 2005. 4 2068–2075. ( 10.1210/jc.2004-2025) [DOI] [PubMed] [Google Scholar]
- 30.Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, Mannelli M, Linehan WM, Adams K, Timmers HJ, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. European Journal of Cancer 2012. 11 1739–1749. ( 10.1016/j.ejca.2011.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, Giordano TJ, Greene LA, Goldstein DS, Lehnert H, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocrine-Related Cancer 2004. 11 423–436. ( 10.1677/erc.1.00829) [DOI] [PubMed] [Google Scholar]
- 32.Plouin PF, Amar L, Dekkers OM, Fassnacht M, Gimenez-Roqueplo AP, Lenders JW, Lussey-Lepoutre C, Steichen O, Guideline Working Group European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. European Journal of Endocrinology 2016. 5 G1–G10. ( 10.1530/eje-16-0033) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a