Abstract
We explored the effects of recombinant human growth hormone (rhGH) replacement on physical and cognitive functioning in subjects with a moderate-to-severe traumatic brain injury (TBI) with abnormal growth hormone (GH) secretion. Fifteen individuals who sustained a TBI at least 12 months prior to study enrollment were identified as having abnormal GH secretion by glucagon stimulation testing (maximum GH response less than 8 ng/mL). Peak cardiorespiratory capacity, body composition, and muscle force testing were assessed at baseline and one year after rhGH replacement. Additionally, standardized neuropsychological tests that assess memory, processing speed, and cognitive flexibility, as well as self-report inventories related to depression and fatigue, were administered at baseline and 1 year after rhGH replacement. Comparison tests were performed with proper post hoc analyses. All analyses were carried out at α < 0.05. Peak O2 consumption, peak oxygen pulse (estimate of cardiac stroke volume), and peak ventilation all significantly increased (p < 0.05). Maximal isometric and isokinetic force production were not altered. Skeletal muscle fatigue did not change but the perceptual rating of fatigue was reduced by ∼25% (p = 0.06). Cognitive performance did not change significantly over time, whereas self-reported symptoms related to depression and fatigue significantly improved. The observed changes suggest that rhGH replacement has a positive impact on cardiorespiratory fitness and a positive impact on perceptual fatigue in survivors of TBI with altered GH secretion.
Keywords: : cognitive, growth hormone, physical, TBI
Introduction
Growth hormone deficiency (GHD) is known to occur in approximately 20% of all individuals who suffer from a moderate-to-severe traumatic brain injury (TBI).1,2 Physical and cognitive functions are impaired to varying degrees depending on the site and severity of the TBI. Children and adults with GHD due to factors other than TBI have been studied more thoroughly than in individuals with TBI, especially in the area of replacement therapy.3 Little is known about the effects of growth hormone (GH) replacement therapy in TBI, although recent preliminary work on cognitive functioning has been promising.4
In children and adults with GHD from causes other than TBI, reduced cardiorespiratory fitness and muscle force production have been reported.5 In response to recombinant human GH (rhGH), body composition and maximal oxygen consumption (VO2 max) improved; however, quadriceps muscle force production did not.6 Cardiac muscle has been shown to be negatively impacted by GHD.7 Cuocolo and colleagues studied cardiac function in children with GHD.8 After 6 months of therapy, they found improvements in several left ventricular variables. In addition, these investigators reported improved cardiorespiratory fitness but no change in muscle force production after rhGH therapy.
It has been shown that individuals recovering from TBI have reduced aerobic capacity, compared with an uninjured control group.9 Further, individuals with TBI who are GHD have significantly reduced cardiorespiratory fitness than patients with TBI who have normal GH levels.10
TBI-induced GHD may contribute to functional decline through direct and indirect effects on skeletal muscle. GH stimulates skeletal muscle mitochondrial function, including mitochondrial adenosine triphosphate production.11 In addition, it increases adipose tissue lipolysis and elicits a marked shift in substrate selection toward oxidation of fatty acids,11 which may reflect an increased and stable supply of energy for skeletal muscle energetic needs. Individuals with GHD have been reported to possess reduced skeletal muscle mass12,13 and strength.12–15 Increases in mass12,13,16 and strength12–17 were observed with restoration of GH to physiologic levels. Apart from a small minority of individuals included in broader studies of GHD,13 the skeletal muscle response of GHD patients with TBI to GH therapy has surprisingly only been reported in a single case study.18 In addition, changes in skeletal muscle functional or metabolic characteristics may influence the brain's perception of fatigue.19–21 Therefore, our current study is an important contribution to our understanding of the physical function response to GH replacement in patients with TBI and altered GH secretion.
Regions of the brain that are particularly vulnerable to TBI include the frontal lobe, anterior temporal lobe, corpus callosum, brain stem, and limbic structures such as the basal ganglia and hypothalamus.22 Consequently, cognitive and behavioral processes commonly disrupted by TBI include arousal, attention, speed of information processing, new learning, memory retrieval, fluency, and executive functions (including organization and planning, sequencing, multi-tasking, judgment, and abstraction).23,24
The effects of GHD and GH replacement on cognition from causes other than TBI have been relatively well studied in both childhood onset and adult onset GHD and have demonstrated positive results.25 In addition, it has been well documented in both animal models and human studies of GHD from causes other than TBI that GH replacement improves cognitive function.26 However, few studies have assessed the potential for cognitive improvement in patients with chronic TBI and rhGH replacement therapy. Kelly and coworkers failed to find significant differences in persons who were GH deficient after TBI on measures of memory and attention/concentration, compared with persons with TBI who were GH sufficient.27 The study did find greater depression and decreased quality of life for the GHD group. In another study, High and colleagues4 randomized 23 patients with TBI to either a year of rhGH replacement or placebo. A battery of neuropsychological tests and functional measures were administered before and after treatment. Significant improvements were noted on tasks that assessed fine motor dexterity and speed, speed of information processing, verbal learning and memory, and novel problem solving, suggesting that select cognitive impairments secondary to TBI and GHD are partially reversible with appropriate rhGH replacement.
Finally, the current study was undertaken to explore fatigue in patients with TBI and altered GH secretion. Complaints of fatigue are common in patients recovering from TBI.28 Central fatigue, not to be confused with apathy or depression, is typically viewed as a subjective phenomenon that can be expressed, for instance, as experiencing a lack of energy or motivation, weakness, and/or sleepiness and has been reported to greatly impact patients' lifestyles by limiting participation in therapeutic, social, and/or leisure activities.29,30 The association between depressed mood and fatigue post-TBI is not entirely clear. The consensus from prior work indicates a consistent but not necessarily causative association between subjective fatigue and psychiatric disorders in patients with TBI.
In a previous study assessing potential correlates of fatigue in patients with TBI, Ponsford and coworkers discovered that patients with symptoms related to anxiety and depression were more likely to report significant levels of fatigue; however, so were patients who experienced heightened levels of pain and cognitive dysfunction.31 Growth hormone replacement therapy may have strong potential for improving fatigue, particularly in patients with TBI, given the high prevalence of GHD in this patient population.27,32–35 Notably, no studies of the efficacy of GH replacement therapy for the reduction of fatigue in patients with TBI and altered GH secretion have been published to date. Because of the severity of physical dysfunction in a previous cohort of TBI subjects,4 we could not accurately assess their physical function in response to GH.
The current work is a follow-up study in which we selected patients with moderate-to-severe TBI and abnormal GH secretion but with the capability of performing physical function testing in response to GH replacement. In light of all the physical and cognitive impairments experienced by patients after TBI found to be GHD, our objective was to assess the effects of rhGH on physical and neuropsychological functioning in individuals who sustained a moderate-to-severe TBI.
Methods
Subjects
Fifteen individuals who sustained a TBI at least 12 months prior to enrollment and were identified as having abnormal GH secretion by glucagon stimulation testing (maximum GH response less than 8 ng/mL)36,37 were enrolled into a 1-year open-label rhGH replacement study. Exclusion criteria included: a) premorbid history of a neurological disorder (e.g., neurodegenerative disease); b) not fluent in English; c) aphasia syndrome; and/or d) inability to complete neuropsychological testing (secondary to physical limitations or low levels of cognitive functioning).
The subjects consisted of 10 males and five females with self-reported history of moderate-to-severe TBI. All subjects were community dwelling individuals and were not receiving formal rehabilitation services at the time of their participation. The racial breakdown of the sample was 13 Caucasian, one Hispanic, and one African-American. Other subject demographics are shown in Table 1. All participants experienced a closed-head TBI (9 motor vehicle accidents, 4 falls, 1 assault, and 1 gunshot wound) secondary to a motor vehicle accident or a fall and were not in litigation for purposes of remuneration for their injuries. Mean estimated pre-morbid full scale IQ, assessed using the North American Adult Reading Test38 was 107.6 (standard deviation = 8.9). A subset of these subjects (n = 9) were tested for peak muscle force production and muscle fatigue as described below. All procedures were approved by a university institutional review board.
Table 1.
Subject Characteristics (n = 15)
| Mean | SD | Median | Range | |
|---|---|---|---|---|
| Age at injury (year) | 34.3 | 15.7 | 38.6 | 10–60 |
| Age at enrollment (year) | 45.5 | 11.2 | 46.2 | 21–62 |
| Time since injury at enrollment (year) | 11.2 | 11.4 | 4.8 | 1.1–39.8* |
| Body mass index (kg/m2) | 29.4 | 4.9 | 27.2 | 22.4–40.7 |
Only one subject was 40 years post-injury. All other subjects were between 1 and 22 years post-injury.
SD, standard deviation.
Procedures: Recombinant GH administration
The GH dose was not dependent on age, sex, body mass, or body mass index. All subjects complied with the daily subcutaneous injection of rhGH daily for the 12 months of the study. At baseline, 6 months, and 12 months, subjects had blood drawn for complete blood count, basic metabolic panel, hepatic function, lipid panel, and insulin-like growth factor 1 (IGF-1). No tumor factors were measured.
The dosing scheme of rhGH used in the study was: 1) 0.2 mg/day for 2 months followed by IGF-1 check; then, 2) 0.4 mg/day for 2 months followed by IGF-1 check; then, 3) 0.6 mg/day for the remainder of the study unless IGF-1 went above 399 ng/mL. This dosing scheme is the recommended dosing in adults with GHD so as to decrease the risks of type 2 diabetes mellitus and carpal tunnel syndrome. Two subjects were on doses of less than 0.6 mg/day (0.5 mg/day) due to symptoms of carpal tunnel syndrome. In both cases, symptoms resolved on the lower dose.
Procedures: Cardiorespiratory testing
Subjects were tested using a modified39 Balke40 protocol during which electrocardiography was performed; expired gases were collected and analyzed by an automated metabolic cart. The modified Balke is a treadmill protocol and all subjects were able to ambulate independently without verbal or physical assistance. Speed was gradually increased in the first 2 min at 1% incline to a speed that an investigator determined could be maintained for 6–12 min. Incline was then increased 2% every min until the subject met two of three peak criteria (O2 plateau, heart rate >85% age-predicted maximum, respiratory exchange ratio >1.15)41 or ambulation became unsafe. This protocol has been shown to be reliable in individuals with TBI.42
Procedures: Muscle force testing
In a subset of patients (n = 9, four females, five males), maximal torque production of the knee extensors was measured during isometric and concentric isokinetic contractions using a dynamometer. In addition, muscle fatigue was measured during repetitive isokinetic contractions. Subjects were tested in a seated position with straps across the ankle and thigh of the lower extremity being tested, as well as across the hips and chest to reduce movement. Isometric efforts were conducted with a knee angle of 90°; isokinetic efforts were initiated at this knee angle, as well, with a range of motion of 75°. The right leg was tested, unless it was the leg more affected by the TBI.
The sequence of testing began with determination of maximal torque production during five consecutive maximal efforts at angular velocities of 60°/sec, then 180°/sec, with each set of maximal efforts preceded by a warm-up set of five repetitions at the same angular velocity and 2 min of rest between sets. The highest value from the five maximal efforts was taken as the maximum for each velocity. Two minutes after completion of the last 180°/sec set, subjects completed an additional warm-up practice set of 30 very low-resistance isokinetic contractions with the angular velocity set at 540°/sec (this velocity was chosen to provide minimal resistance during practice of prolonged repetitive efforts; subjects were not instructed to attempt to achieve this velocity). After a 2-min rest, maximal isometric force production was measured during three maximal efforts held for 5 sec with maximal efforts occurring approximately once each minute. The highest torque achieved during the three efforts was considered the maximal isometric strength. Similarly, maximal isokinetic strength at a velocity of 90°/sec was determined during three maximal efforts occurring approximately once every 20 sec. After a 5-min rest period, force production during fatiguing repetitive isokinetic contractions (90°/sec) was measured. Before muscle dynamometry testing and immediately after the final contraction of the muscle fatigue test, subjects were asked to rate their perceptual fatigue on a 0–10 scale (0 = no fatigue).
Procedures: Body composition
Whole–body lean mass and fat mass, leg lean mass, and whole–body percentage body fat were determined using dual energy X-ray absorptiometry.
Procedures: Neuropsychological testing
The neuropsychological test battery consisted of standardized behavioral/psychiatric inventories and cognitive measures and was administered to all participants in counterbalanced order at baseline and 12 months. The neuropsychological measures that were administered assessed learning and memory (verbal and nonverbal), verbal working memory, speed of information processing, and speeded word retrieval, and included: Digit Span from the Wechsler Adult Intelligence Scale (WAIS-III)43; California Verbal Learning Test-II44; Brief Visuospatial Memory Test-R45; Processing Speed Index from the Wechsler Adult Intelligence Scale43; Letter and Category Fluency44; Beck Depression Inventory-II (BDI-II)46; and the Fatigue Severity Scale (FSS).47
Data analysis
Oxygen pulse was calculated by dividing VO2 consumed (mL/min) by heart rate (beats/min) yielding mL/beat.48 This provides an estimate of cardiac stroke volume. Data were analyzed using SPSS v. 11.0 (SPSS, Chicago, IL.). Paired samples t-tests were performed on all pre-treatment and post-treatment measures with a Bonferroni correction for multiple comparisons. Maximal isometric and isokinetic force production before and after treatment were compared using a two-way repeated measures analysis of variance (ANOVA), with factors of time (pre-treatment vs. post-treatment) and sex (male vs. female). Torque- and power-velocity relationships were examined using regression techniques. Skeletal muscle fatigue was analyzed using a two-way repeated measure ANOVA. The exercise-induced change in perceptual fatigue before and after rhGH therapy was compared using a paired, two-tailed t-test. All analyses were performed at an alpha level of 0.05.
Results
Cardiorespiratory
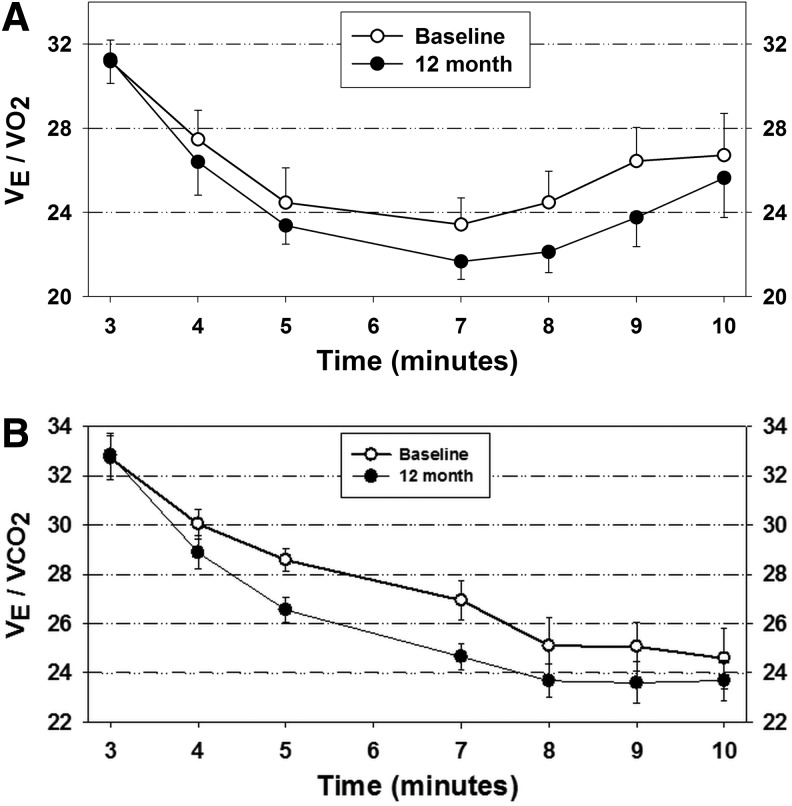
Peak cardiorespiratory responses before and after rhGH supplementation are shown in Table 2. In addition, the submaximal ventilatory equivalent for both CO2 and O2 decreased after rhGH (although not significantly), suggesting an improvement in the efficiency of breathing (Fig. 1). This could account for the fact that subjects have an overall self-reported decrease in fatigue symptoms. In addition, the subjects greater than 5 years post-injury onset tended to do better on the physiologic variables of stroke volume and minute ventilation. The less-than-5-years and greater-than-5-years subjects improved equally on VO2max. Five years was nearly the median (4.8 years) and formed two homogenous groups with respect to time since injury (1–5 years, n = 9; 18–40 years, n = 6).
Table 2.
Peak Cardiopulmonary Responses before and after rhGH (*p < 0.05, **p < 0.01).
| Variable | Baseline mean ± SD | 12 months post-rhGH mean ± SD |
|---|---|---|
| Oxygen uptake (mL/kg/min)** | 29.0 ± 7.2 | 32.1 ± 7.4 |
| Heart rate (beats/min)* | 170 ± 15 | 176 ± 13 |
| Minute ventilation (L/min)** | 90.4 ± 24.2 | 102.4 ± 23.1 |
| Respiratory exchange ratio | 1.27 ± 0.12 | 1.27 ± 0.10 |
| Oxygen pulse (mL/beat)* | 15.2 ± 4.1 | 16.4 ± 4.2 |
rhGH, recombinant human growth hormone; SD, standard deviation.
FIG. 1.
Influence of recombinant human growth hormone therapy on ventilatory equivalents (VEq) for CO2 and O2 at submaximal levels of physical work. (A) VEq O2; (B) VEq CO2. Symbols and error bars depict means and standard errors, respectively. n = 10 males, 5 females.
Body composition
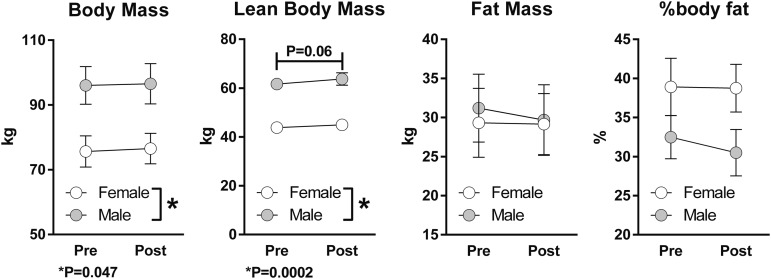
Lean body mass was, on average, higher after 1 year of rhGH therapy (p = 0.06), a marginally statistically significant effect. As expected, body mass and lean body mass were higher in male than in female subjects (Fig. 2). Had only males been considered, post-treatment lean body mass, fat mass, and % body fat would have been significantly different from pre-treatment values.
FIG. 2.
Influence of recombinant human growth hormone therapy on body composition. Symbols and error bars depict means and standard errors, respectively. n = 10 males, 5 females.
Skeletal muscle force production
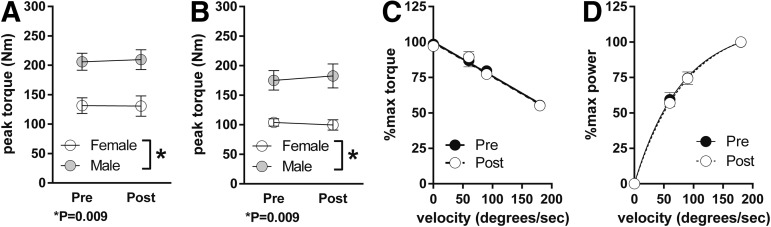
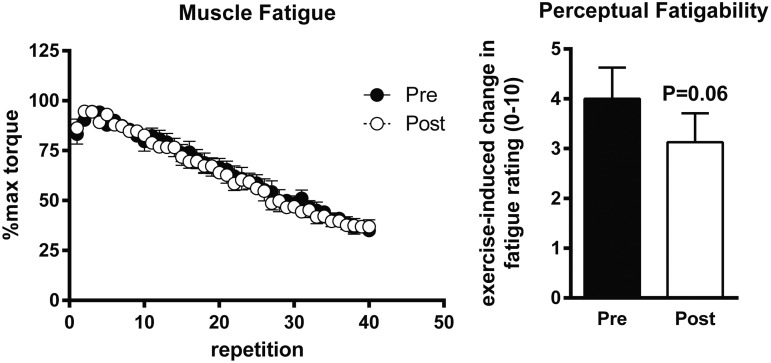
Maximal isometric (Fig. 3A) and isokinetic force (Fig. 3B, C) production were not altered by 1 year of rhGH treatment. Likewise, neither the force-velocity (Fig. 3C) nor the force-power relationship (Fig. 3D) changed in response to treatment over the range of the velocities tested. Skeletal muscle fatigue during repetitive maximal isokinetic contractions did not change in response to the year of treatment with rhGH (Fig. 4). However, the change in perceptual fatigue rating induced by the fatiguing contractions was reduced by ∼25% (Fig. 4) after 1 year of rhGH therapy (p = 0.06).
FIG. 3.
Influence of recombinant human growth hormone therapy on skeletal muscle force production. (A) Maximum torque production during isometric contraction of the knee extensor muscles. (B) Maximum torque production during maximal isokinetic contractions at 90°/sec. (C) Maximal torque versus velocity relationship. (D) Maximal power versus velocity relationship. Symbols and error bars depict means and standard errors, respectively. n = 5 males, 4 females; p values indicated refer to main effect of sex.
FIG. 4.
Influence of recombinant human growth hormone therapy on skeletal muscle fatigue and exercise-induced fatigability. Left panel: Skeletal muscle fatigue during repetitive isokinetic contractions of the knee extensors at 90°/sec. Right panel: Change in perceptual fatigue rating induced by knee extensor testing. Symbols and error bars depict means and standard errors, respectively. n = 5 males, 4 females.
Cognitive function
Within-group comparison revealed a lack of statistically significant differences for all cognitive measures. Statistically significant improvements were found for total scores of the BDI-II (p = 0.019) and FSS (p = 0.039). The BDI-II and FSS scores significantly declined over time, suggesting improved symptoms related to depression and fatigue, respectively. See Table 3 for group means and standard deviations for all cognitive variables (values shown are raw scores).
Table 3.
Cognitive and Psychiatric Variables before and after rhGH (*p < 0.05)
| Variable | Baseline mean ± SD | 12 months post-rhGH mean ± SD | p |
|---|---|---|---|
| California Verbal Learning Test-II - Total Recall | 45.7/10.8 | 51.0/9.4 | 0.159 |
| California Verbal Learning Test-II - Delayed Recall | 9.9/3.8 | 11.2/2.6 | 0.268 |
| Brief Visuospatial Memory Test-R - Total Recall | 18.7/5.7 | 22.7/5.1 | 0.053 |
| Brief Visuospatial Memory Test-R - Delayed Recall | 7.2/2.9 | 9.1/2.2 | .054 |
| Digit Span (WMS-III) | 16.0/4.5 | 17.0/3.9 | 0.520 |
| Processing Speed Index (WAIS-III) | 102.3/14.9 | 106.6/15.0 | 0.434 |
| Letter Fluency (D-KEFS) | 38.5/14.5 | 35.4/13.0 | 0.546 |
| Beck Depression Inventory (BDI-II) | 13.8/9.7 | 6.2/6.9 | 0.019* |
| Fatigue Severity Scale (FSS) | 44.3/13.5 | 34.0/12.5 | 0.039* |
rhGH, recombinant human growth hormone; SD, standard deviation; WMS-III, Wechsler Memory Scale III; WAIS-III, Wechsler Adult Intelligence Scale III; D-KEFS, Delis-Kaplan Executive Function System.
Given the large range in years in time since injury in our sample, we performed a secondary analysis. Here, the 15 subjects included in the cognitive function analysis were divided by time since injury onset. Subjects who were greater than 5 years post-injury performed significantly better (p < 0.05) on tests of verbal learning and memory, delayed recall, nonverbal/visual learning and memory, and speed of information processing.
Discussion
The results from this study suggest that one year of rhGH replacement had a positive impact on cardiorespiratory fitness, severity symptoms related to depression, and perceptual fatigue in survivors of TBI with altered GH secretion. Previous investigations49 support rhGH as a factor in improving submaximal aerobic exercise performance. The finding that chronic rhGH therapy improved cardiorespiratory fitness is in agreement with previous reports of chronic rhGH therapy in GHD adults.50,51 The reason(s) for the increase in cardiorespiratory fitness are not certain. Although no subject reported beginning a structured exercise program, it is possible that because they felt less fatigued during daily activities that they increased their physical activity levels, thereby increasing their fitness level. Anecdotally, several subjects stated they were happy to resume their pre-injury occupations and physical activity habits. This increase in occupational and functional activities post-intervention may have resulted in significant declines in symptoms related to depression.
In addition to the increase in maximal oxygen uptake, the submaximal ventilatory equivalent (VEq) for both CO2 and O2 decreased after rhGH, suggesting an improvement in the efficiency of breathing. If breathing efficiency improved, it may play a role in the subjective complaints of fatigue observed in patients recovering from TBI. Given that submaximal VEq for CO2 decreased the most, it may be that the subjects are controlling their hydrogen ion levels better and that could have a significant impact on peripheral muscle fatigue. Notably, GH has been reported to stimulate vasodilation by increasing nitric oxide production,52,53 which could have played a role in changes in cardiorespiratory fitness, including changes in VEq, particularly if GH replacement altered lung perfusion. However, there are many other biochemical processes occurring in active skeletal muscle that contribute to peripheral fatigue.54
GH replacement had no effect on any of the skeletal muscle performance variables measured in the current study. In particular, skeletal muscle fatigue was nearly identical before and after the year of treatment with rhGH (Fig. 4). Despite this, exercise-induced fatigability (Fig. 4) showed a strong tendency to be reduced. Whether this relates to changes in breathing efficiency or perfusion as discussed above, or alterations in afferent feedback or central sensory perception cannot be determined from our results. Nevertheless, the finding of reduced fatigability in response to physical activity is consistent with the significantly reduced ratings of perceptual fatigue on the FSS survey, as well as the experiences related by the majority of the patients.
While inspection of individual cases demonstrated improved cognitive scores across time following GH replacement, significant within-group findings for the entire sample were not found. These null findings were not consistent with previous work4 and are most likely attributed to a small sample size and lack of a control group in our study, as well as significant variation in time since injury onset (range, ∼1–40 years). This may suggest that individuals with greater time since injury onset represent a level of chronicity that is devoid of spontaneous recovery, unlike individuals who were 1–5 years post-injury onset. On the contrary, however, we performed a secondary analysis in which we discovered that the subset of subjects that were greater than 5 years post-injury performed significantly better on separate tests of learning and memory and processing speed than those subjects who were less than 5 years post-injury. This finding was not anticipated as the rate of spontaneous recovery in those individuals with moderate-to-severe brain injury tends to decrease over time. Thus, it is possible that a discrepancy in the rate of self-reported brain injury severity may have contributed to the null cognitive effects in this study.
Statistically significant improvement of symptoms related to depression and fatigue were noted over time. These improvements appear to be consistent with the positive physical functioning findings in this study, as well as self-report of an increase in the ability to re-engage in occupational and functional activities. While the relationship between depressed mood and fatigue following TBI is not entirely clear, there appears to be a consistent but not entirely causative relationship between the two. Of note, fatigue symptoms post-TBI can vary with improvements or declines of other factors, including pain severity, sleep quality, lifestyle changes, and/or level of independence. For instance, Schnieders and colleagues34 found that disrupted sleeping patterns, vitamin D deficiency, and symptoms related to anxiety explained approximately two-thirds of the variance in the subjective fatigue scores in patients with TBI. Similarly, Englander and coworkers55 discovered that 42–60% of the variance across two separate fatigue rating scales were explained by the patient's gender, depression severity, pain, and perceived memory or motor dysfunction. Thus, future work addressing GH replacement and levels of fatigue post-TBI must take several objective and subjective factors into consideration.
Limitations
The limitations of the current study are worth noting. As our interest was in determining the response of TBI patients to chronic GH replacement, we employed a within-subjects design that did not include a healthy control group or a group of GH-sufficient TBI patients for comparison. As such, this limited our ability to place the findings of the current study in a broader context. In addition, based on our previous findings,10 as well as consideration of subject recruitment and retention for a long-term intervention study such as this, we chose to employ an open-label design in which all subjects received therapy. To the extent that variables such as cognition, body composition, or perceptual fatigue spontaneously recover in subjects with TBI and altered GH secretion, we may have overestimated improvement that occurs with rhGH therapy. As the current study was in several respects an initial examination of the responses to long-term rhGH therapy in patients with TBI, we viewed our statistical tests from the standpoint of an exploratory analysis and did not make adjustments to reduce the false discovery rate. Finally, as the collaborative nature of the project expanded during the course of the study and additional investigators were included, muscle function testing was only available in a subset of the patients. However, the near-identical muscle strength and muscle fatigue values before and after testing mitigates this concern, as the findings suggest that an extremely large group of subjects would have to be studied to find a statistical difference related to rhGH therapy and that such differences would be of questionable practical benefit. Future studies should consider including muscle biopsies that may help explain the effect, or lack thereof, of changes in skeletal muscle function.
In addition, we readily recognize the inherent difficulty of homogeneity in doing studies on humans with TBI. Obviously, more homogeneity would be ideal, but in this population, it is difficult to accomplish. Time since injury and severity of injury should be considered important factors when designing future studies.
Acknowledgments
This work was partially funded by the generous support of the Moody Endowment and Pfizer, Inc. Recombinant growth hormone was supplied by Pfizer, Inc. This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health.
Author Disclosure Statement
BEM has equity interest in and received lecture fees from Pfizer. For the remaining authors, no competing financial interests exist.
References
- 1.Lieberman S.A., Oberoi A.L., Gilkison C.R., Masel B.E., and Urban R.J. (2001). Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J. Clin. Endocrinol. Metab. 86, 2752–2756 [DOI] [PubMed] [Google Scholar]
- 2.Agha A., Phillips J., and Thompson C.J. (2007). Hypopituitarism following traumatic brain injury (TBI). Br. J. Neurosurg. 21, 210–216 [DOI] [PubMed] [Google Scholar]
- 3.Carroll P.V., Christ E.R., Bengtsson B.A., Carlsson L., Christiansen J.S., Clemmons D., Hintz R., Ho K., Laron Z., Sizonenko P., Sonksen P.H., Tanaka T., and Thorne M. (1998). Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J. Clin. Endocrinol. Metab. 83, 382–395 [DOI] [PubMed] [Google Scholar]
- 4.High W.M.J., Briones-Galang M., Clark J.A., Gilkison C., Mossberg K.A., Zgaljardic D.J., Masel B.E., and Urban R.J. (2010). Effect of growth hormone replacement therapy on cognition after traumatic brain injury. J. Neurotrauma 27, 1565–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gullestad L., Birkeland K., Bjonerheim R., Djoseland O., Trygstad O., and Simonsen S. (1998). Exercise capacity and hormonal response in adults with childhood onset growth hormone deficiency during long-term somatropin treatment. Growth Horm. IGF Res. 8, 377–384 [DOI] [PubMed] [Google Scholar]
- 6.Whitehead H.M., Boreham C., McIlrath E.M., Sheridan B., Kennedy L., Atkinson A.B., and Hadden D.R. (1992). Growth hormone treatment of adults with growth hormone deficiency: results of a 13-month placebo controlled cross-over study. Clin. Endocrinol. 36, 45–52 [DOI] [PubMed] [Google Scholar]
- 7.Merola B., Cittadini A., Colao A., Longobardi S., Fazio S., Sabatini D., Sacca L., and Lombardi G. (1993). Cardiac structural and functional abnormalities in adult patients with growth hormone deficiency. J. Clin. Endocrinol. Metab. 77, 1658–1661 [DOI] [PubMed] [Google Scholar]
- 8.Cuocolo A., Nicolai E., Colao A., Longobardi S., Cardei S., Fazio S., Merola B., Lombardi G., Sacca L., and Salvatore M. (1996). Improved left ventricular function after growth hormone replacement in patients with hypopituitarism: assessment with radionuclide angiography. Eur. J. Nucl. Med. 23, 390–394 [DOI] [PubMed] [Google Scholar]
- 9.Mossberg K.A., Ayala D., Baker T., Heard J., and Masel B. (2007). Aerobic capacity after traumatic brain injury: comparison with a nondisabled cohort. Arch. Phys. Med. Rehabil. 88, 315–320 [DOI] [PubMed] [Google Scholar]
- 10.Mossberg K.A., Masel B.E., Gilkison C.R., and Urban R.J. (2008). Aerobic capacity and growth hormone deficiency after traumatic brain injury. J. Clin. Endocrinol. Metab. 93, 2581–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Short K.R., Moller N., Bigelow M.L., Coenen-Schimke J., and Nair K.S. (2008). Enhancement of muscle mitochondrial function by growth hormone. J. Clin. Endocrinol. Metab. 93, 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodhouse L.J., Mukherjee A., Shalet S.M., and Ezzat S. (2006). The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocr. Rev. 27, 287–317 [DOI] [PubMed] [Google Scholar]
- 13.Janssen Y.J., Doornbos J., and Roelfsema F. (1999). Changes in muscle volume, strength, and bioenergetics during recombinant human growth hormone (GH) therapy in adults with GH deficiency. J. Clin. Endocrinol. Metab. 84, 279–284 [DOI] [PubMed] [Google Scholar]
- 14.Johannsson G., Grimby G., Sunnerhagen K.S., and Bengtsson B.A. (1997). Two years of growth hormone (GH) treatment increase isometric and isokinetic muscle strength in GH-deficient adults. J. Clin. Endocrinol. Metab. 82, 2877–2884 [DOI] [PubMed] [Google Scholar]
- 15.Rutherford O.M., Beshyah S.A., Schott J., Watkins Y., and Johnston D.G. (1995). Contractile properties of the quadriceps muscle in growth hormone-deficient hypopituitary adults. Clin. Sci. (Lond.) 88, 67–71 [DOI] [PubMed] [Google Scholar]
- 16.Lafortuna C.L., Minocci A., Capodaglio P., Gondoni L.A., Sartorio A., Vismara L., Rizzo G., and Grugni G. (2014). Skeletal muscle characteristics and motor performance after 2-year growth hormone treatment in adults with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 99, 1816–1824 [DOI] [PubMed] [Google Scholar]
- 17.Gotherstrom G., Bengtsson B.A., Sunnerhagen K.S., Johannsson G., and Svensson J. (2005). The effects of five-year growth hormone replacement therapy on muscle strength in elderly hypopituitary patients. Clin. Endocrinol. (Oxf.) 62, 105–113 [DOI] [PubMed] [Google Scholar]
- 18.Bhagia V., Gilkison C., Fitts R.H., Zgaljardic D.J., High W.M., Masel B.E., Urban R.J., and Mossberg K.A. (2010). Effect of recombinant growth hormone replacement in a growth hormone deficient subject recovering from mild traumatic brain injury: a case report. Brain Inj. 24, 560–567 [DOI] [PubMed] [Google Scholar]
- 19.Amann M., Proctor L.T., Sebranek J.J., Pegelow D.F., and Dempsey J.A. (2009). Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J. Physiol. 587, 271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hampson D.B., St Clair Gibson A., Lambert M.I., and Noakes T.D. (2001). The influence of sensory cues on the perception of exertion during exercise and central regulation of exercise performance. Sports Med. 31, 935–952 [DOI] [PubMed] [Google Scholar]
- 21.Amann M., Blain G.M., Proctor L.T., Sebranek J.J., Pegelow D.F., and Dempsey J.A. (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 109, 966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson J.T. (1990). The relationship between neuropsychological function and brain damage detected by neuroimaging after closed head injury. Brain Inj. 4, 349–363 [DOI] [PubMed] [Google Scholar]
- 23.McAllister T.W. (1992). Neuropsychiatric sequelae of head injuries. Psychiatr. Clin. North Am. 15, 395–413 [PubMed] [Google Scholar]
- 24.Draper K. and Ponsford J. (2008). Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology 22, 618–625 [DOI] [PubMed] [Google Scholar]
- 25.Falleti M.G., Maruff P., Burman P., and Harris A. (2006). The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: a meta-analysis of the current literature. Psychoneuroendocrinology 31, 681–691 [DOI] [PubMed] [Google Scholar]
- 26.Nyberg F. and Hallberg M. (2013). Growth hormone and cognitive function. Nat. Rev. Endocrinol. 9, 357–365 [DOI] [PubMed] [Google Scholar]
- 27.Kelly D.F., McArthur D.L., Levin H., Swimmer S., Dusick J.R., Cohan P., Wang C., and Swerdloff R. (2006). Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J Neurotrauma 23, 928–942 [DOI] [PubMed] [Google Scholar]
- 28.Zgaljardic D.J., Durham W.J., Mossberg K.A., Foreman J., Joshipura K., Masel B.E., Urban R.J., and Sheffield-Moore M. (2014). Neuropsychological and physiological correlates of fatigue following traumatic brain injury. Brain Inj. 28, 389–397 [DOI] [PubMed] [Google Scholar]
- 29.Jha A., Weintraub A., Allshouse A., Morey C., Cusick C., Kittelson J., Harrison-Felix C., Whiteneck G., and Gerber D. (2008). A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J. Head Trauma Rehabil. 23, 52–63 [DOI] [PubMed] [Google Scholar]
- 30.Johansson B., Berglund P., and Ronnback L. (2009). Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj. 23, 1027–1040 [DOI] [PubMed] [Google Scholar]
- 31.Ponsford J.L., Ziino C., Parcell D.L., Shekleton J.A., Roper M., Redman J.R., Phipps-Nelson J., and Rajaratnam S.M. (2012). Fatigue and sleep disturbance following traumatic brain injury–their nature, causes, and potential treatments. J. Head Trauma Rehabil. 27, 224–233 [DOI] [PubMed] [Google Scholar]
- 32.Popovic V., Aimaretti G., Casanueva F.F., and Ghigo E. (2005). Hypopituitarism following traumatic brain injury. Growth Horm. IGF Res. 15, 177–184 [DOI] [PubMed] [Google Scholar]
- 33.Bavisetty S., McArthur D.L., Dusick J.R., Wang C., Cohan P., Boscardin W.J., Swerdloff R., Levin H., Chang D.J., Muizelaar J.P., and Kelly D.J. (2008). Chronic hypopituitarism after traumatic brain injury: risk assessment and relationship to outcome. Neurosurgery 62, 1080–1093 [DOI] [PubMed] [Google Scholar]
- 34.Schnieders J., Willemsen D., and de Boer H. (2012). Factors contributing to chronic fatigue after traumatic brain injury. J. Head Trauma Rehabil. 27, 404–412 [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson C.W., Pagulayan K.F., Petrie E.C., Mayer C.L., Colasurdo E.A., Shofer J.B., Hart K.L., Hoff D., Tarabochia M.A., and Peskind E.R. (2012). High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Front. Neurol. 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shalet S.M., Toogood A., Rahim A., and Brennan B.M. (1998). The diagnosis of growth hormone deficiency in children and adults. Endocr. Rev. 19, 203–223 [DOI] [PubMed] [Google Scholar]
- 37.Conceicao F.L., da Costa e Silva A., Leal Costa A.J., and Vaisman M. (2003). Glucagon stimulation test for the diagnosis of GH deficiency in adults. J. Endocrinol. Invest. 26, 1065–1070 [DOI] [PubMed] [Google Scholar]
- 38.Blair J.R. and Spreen O. (1989). Predicting premorbid IQ: a revision of the national adult reading test. Clin. Neuropsychologist 3, 129–136 [Google Scholar]
- 39.Hunter M., Tomberlin J., Kirkikis C., and Kuna S.T. (1990). Progressive exercise testing in closed head-injured subjects: comparison of exercise apparatus in assessment of a physical conditioning program. Phys. Ther. 70, 363–371 [DOI] [PubMed] [Google Scholar]
- 40.Balke B. and Ware R.W. (1959). An experimental study of “physical fitness” of Air Force personnel. U. S. Armed Forces Med. J. 10, 675–688 [PubMed] [Google Scholar]
- 41.Thompson W.R. (ed) 2010. ACSM's Guidelines for Exercise Testing and Prescription. 8th ed Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia [Google Scholar]
- 42.Mossberg K.A. and Greene B.P. (2005). Reliability of graded exercise testing after traumatic brain injury: submaximal and peak responses. Am. J. Phys. Med. Rehabil. 84, 492–500 [DOI] [PubMed] [Google Scholar]
- 43.Wechsler D. (1997). Wechsler Adult Intelligence Scale-III. Psychological Corporation: San Antonio, TX [Google Scholar]
- 44.Delis D.C., Kaplan E., Kramer J., and Ober B.A. (2000). California Verbal Learning Test-Second Edition (CVLT-II) Manual. 2nd ed Psychological Corporation: San Antonio, TX [Google Scholar]
- 45.Benedict R.H., Priore R.L., Miller C., Munschauer F., and Jacobs L. (2001). Personality disorder in multiple sclerosis correlates with cognitive impairment. J. Neuropsychiatry Clin. Neurosci. 13, 70–76 [DOI] [PubMed] [Google Scholar]
- 46.Beck A.T., Steer R.A., Ball R., and Ranieri W.F. (1996). Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. J. Pers. Assess. 67, 588–597 [DOI] [PubMed] [Google Scholar]
- 47.Krupp L.B., LaRocca N.G., Muir-Nash J., and Steinberg A.D. (1989). The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 46, 1121–1123 [DOI] [PubMed] [Google Scholar]
- 48.Astrand P.O., Rodahl K., Dahl H.A., and Stromme S.B. (2003). Textbook of Work Physiology: Physiological Basis of Exercise. 4th ed Human Kinetics: Champaign, IL [Google Scholar]
- 49.Woodhouse L.J., Asa S.L., Thomas S.G., and Ezzat S. (1999). Measures of submaximal aerobic performance evaluate and predict functional response to growth hormone (GH) treatment in GH-deficient adults. J. Clin. Endocrinol. Metab. 84, 4570–4577 [DOI] [PubMed] [Google Scholar]
- 50.Hartman M.L., Weltman A., Zagar A., Qualy R.L., Hoffman A.R., and Merriam G.R. (2008). Growth hormone replacement therapy in adults with growth hormone deficiency improves maximal oxygen consumption independently of dosing regimen or physical activity. J. Clin. Endocrinol. Metab. 93, 125–130 [DOI] [PubMed] [Google Scholar]
- 51.Nass R., Huber R.M., Klauss V., Muller O.A., Schopohl J., and Strasburger C.J. (1995). Effect of growth hormone (hGH) replacement therapy on physical work capacity and cardiac and pulmonary function in patients with hGH deficiency acquired in adulthood. J. Clin. Endocrinol. Metab. 80, 552–557 [DOI] [PubMed] [Google Scholar]
- 52.Li G., Del Rincon J.P., Jahn L.A., Wu Y., Gaylinn B., Thorner M.O., and Liu Z. (2008). Growth hormone exerts acute vascular effects independent of systemic or muscle insulin-like growth factor I. J. Clin. Endocrinol. Metab. 93, 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nystrom H.C., Klintland N., Caidahl K., Bergstrom G., and Wickman A. (2005). Short-term administration of growth hormone (GH) lowers blood pressure by activating eNOS/nitric oxide (NO)-pathway in male hypophysectomized (Hx) rats. BMC Physiol. 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robergs R.A., Ghiasvand F., and Parker D. (2004). Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 56, R502–R516 [DOI] [PubMed] [Google Scholar]
- 55.Englander J., Bushnik T., Oggins J., and Katznelson L. (2010). Fatigue after traumatic brain injury: association with neuroendocrine, sleep, depression and other factors. Brain Inj. 24, 1379–1388 [DOI] [PubMed] [Google Scholar]