Abstract
Several chemokines have important functions in mucosal immunity. While there are many chemokines, 4 of them (CCL25, CCL28, CXCL14, and CXCL17) are especially important in mucosal immunity because they are homeostatically expressed in mucosal tissues. Of these, only CCL25 and CCL28 have been widely recognized as mucosal chemokines. In this study, we review the physiology of these chemokines with specific emphasis on their function in mucosal immunity. CCL25 recruits certain important subsets of T cells that express CCR9 to the small intestine. These CCR9+ T cells also express the integrin α4β7 and have been shown to play important roles in the control of intestinal inflammation. CCL28 recruits CCR10+ IgA plasmablasts to the lactating mammary gland. The role of CXCL14 in mucosal immunity is less well defined, but a Cxcl14−/− mouse exhibits significant metabolic abnormalities. Finally, CXCL17 was the last chemokine to be described and signals through a new chemokine receptor (GPR35/CXCR8), which is expressed in a subset of macrophages that are recruited to mucosal tissues by this chemokine. We conclude that these 4 chemokines play very important roles in mucosal immunity and their continued functional characterization will likely identify novel therapeutic targets.
Keywords: : Mucosa, chemokines, CCL25, CCL28, CXCL14, CXCL17
Introduction
The chemokines are a superfamily of chemotactic cytokines that include 48 ligands and 19 receptors. The ligands are small (8-12KDa) secreted proteins that interact with specific receptors. Their receptors are members of the class A G-protein coupled receptor superfamily (Zlotnik and Yoshie 2012). The chemokines exhibit a large variety of functions, but are best known for their ability to chemoattract cells. Responding cells that express a given chemokine receptor migrate along a chemokine gradient until they reach a certain chemokine concentration, in the nanomolar range (Maravillas-Montero and others 2015). This leads to either a downregulation of the expression of the receptor or desensitization. Once this occurs, the migrating cells either stop migrating or may continue their migratory path in response to another chemokine gradient (Sanz and Kubes 2012; Johnson and Jackson 2014).
Chemokines have been divided into 2 kinds depending on their expression patterns. These include homeostatic or inflammatory chemokines. Interestingly, the chromosomal location of the genes encoding each group differs from the other, a phenomenon that is the result of molecular evolution. CC “inflammatory” chemokines are mainly located in a gene cluster in human chromosome 17, while CXC “inflammatory” chemokines are mainly found at the human chromosome 4 (Zlotnik and Yoshie 2012). The genes encoding inflammatory chemokines have likely been selected to provide evolutionary advantages to each species depending on their infectious experience and are likely “younger” evolutionary events that occurred after species began to diverge from each other.
In some cases, a given chemokine may exist in 1 species, but not another. A typical example is CXCL8 (IL-8), which exists in the human but not in the mouse genome (Zlotnik and Yoshie 2012). Their expression is typically induced by inflammatory or infectious events, and they are important mediators of the recruitment of immune system cells that participate in these processes. Examples include CXCL8, which recruits mainly neutrophils (Ohnuma and others 2008), and CCL5, which recruits macrophages (Soria and Ben-Baruch 2008).
In contrast, homeostatic chemokines are expressed in specific tissues in a programmed manner, without (apparent) exogenous signals that may trigger their expression. The genes encoding homeostatic chemokines are well conserved during evolution, likely reflecting their important functions in the organism, and they are typically located in isolated locations in the genome because they have been under evolutionary pressure not to diverge. Examples of homeostatic chemokines include CXCL12 in the bone marrow (Sugiyama and others 2006) and CCL19 and CCL21 in lymph nodes (Ebert and others 2005). They usually have very important functions in development. As a result, mice that do not express these chemokines usually exhibit dramatic phenotypes (including nonviability). The high conservation of their genes is the result of evolutionary pressure that rendered any of their mutants unable to either survive or reproduce.
Yet a third type of chemokine includes those that exhibit characteristics of both inflammatory and homeostatic chemokines. They can be expressed homeostatically in some tissues while their expression can also be modulated by inflammatory stimuli in other tissues. These are called “Dual” chemokines. An example is the recently described chemokine CXCL17, whose expression is homeostatic in the upper gastrointestinal (GI) system (Fig. 1; Table 1), but in the lower GI its expression is observed only when inflammation and/or tumors are present (described in detail below).
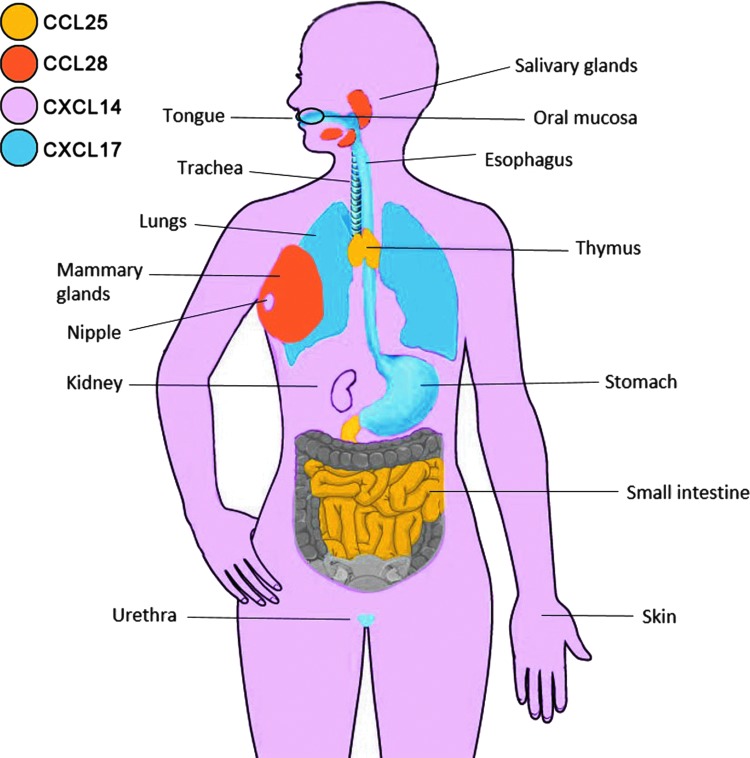
FIG. 1.
Expression of mucosal chemokines in the human body. CCL25, in yellow, is expressed in the small intestine and thymus. CCL28, in orange, is expressed in salivary and mammary glands (upon onset of lactation). CXCL14, in pink, is widely expressed, but is prominent in oral mucosa, nipple, kidneys, and skin. CXCL17, in blue, is mainly expressed in the upper gastrointestinal tract (tongue, esophagus, and stomach), in the respiratory tract (trachea and lungs), and in the urethra.
Table 1.
Expression of the Mucosal Chemokines CCL25, CCL28, CXCL14, and CXCL17 in the Normal Human Body
| Chemokine | Organs |
|---|---|
| CCL25 | Small intestine, thymus |
| CCL28 | Salivary gland, (lactating) mammary gland, trachea, bronchus, thyroid, tongue superior, colon, urethra |
| CXCL14 | Kidney, skin, mammary gland, oral mucosa, tongue, esophagus, stomach, jejunum, small intestine, colon, adipose tissue, pericardium, Aorta, vena cava, trachea, bronchus, pituitary gland, uterus, cervix, vagina, lymph node, spleen, tonsil, central nervous system |
| CXCL17 | Oral mucosa, tongue, esophagus, stomach, duodenum, urethra, trachea, bronchus, lung, pancreas, fallopian tube, cervix, vagina |
Chemokine genes are expressed in most tissues. Their mode of action (i.e., the establishment of gradients) requires that they be produced in large quantities by the cells that produce them. As a result, it is common to find chemokines among the highest expressed genes, for example, when performing gene array analyses. This characteristic also favored their identification through bioinformatics (Rossi and others 1997).
Many chemokines were originally reported as associated with particular tissues. Interestingly, some of them showed very high tissue expression specificity (Kunkel and Butcher 2002). A good example is CCL27, which is only expressed in the skin (Morales and others 1999). This specific expression predicted that CCL27 would exhibit a skin-specific function. This was confirmed when it became apparent that it chemoattracts a population of T cells (CLA+) that is specific to the skin (Homey and others 2002). The latter is the largest organ of the body, and, along with the mucosa, it represents “barrier” tissues that both protect and regulate our interactions with the outside environment.
Much interest has recently been focused in the mucosa. It has long been known that it exhibits specialized immune system functions. The first chemokine identified as mucosal was CCL28, which binds CCR10 (Pan and others 2000; Wang and others 2000). The second mucosal-associated chemokine was CCL25, which was originally identified as a thymus-expressed chemokine (TECK) (Vicari and others 1997). However, the thymus quickly involutes and in adults the main expression site for CCL25 is therefore the small intestine (Wurbel and others 2000) where it chemoattracts CCR9+ lymphocytes (Kunkel and others 2000; Papadakis and others 2000). Together CCL25 and CCL28 represent the best known mucosal chemokines to date.
Several years ago we became interested in mucosal chemokines. We analyzed the expression of all known chemokines in a comprehensive database of gene expression, body index of gene expression (BIGE) (Lee and others 2005; Roth and others 2006), which contains more than 115 tissues of the human body. We identified 4 chemokines with significant mucosal expression: CCL28, CCL25, CXCL14, and CXCL17 (Table 1; Fig. 1). As described above, CCL28 is expressed by many mucosal epithelial tissues, while CCL25 is expressed by intestinal cells.
CXCL14 exhibits relatively wide expression in the human body. It was originally called BRAK (for Breast and Kidney chemokine) (Hromas and others 1999). However, the BIGE database indicates that it is also expressed in the central nervous system, and, significantly, in many other mucosal tissues as well. Finally, CXCL17 was the last chemokine ligand to be described (Pisabarro and others 2006; Weinstein and others 2006). We have identified it as a mucosal chemokine based on its expression pattern (Burkhardt and others 2012).
We should clarify that what identifies these 4 chemokines as mucosal is their homeostatic expression patterns, that is, under normal nonpathogenic conditions, these chemokines are normally expressed at high levels in various mucosal tissues, suggesting that they have mucosal-specific functions. Under inflammatory conditions, however, many other chemokines can be expressed in mucosal sites (including many inflammatory chemokines). The difference is that the latter are not expressed in the normal (noninflamed) mucosa. In the present review we will therefore focus our attention in these 4 mucosal chemokines (CCL25, CCL28, CXCL14, and CXCL17) because they represent an area of research that has received relatively little attention, despite its potential importance in both innate and acquired mucosal immunity.
General characteristics of mucosal chemokines
As described above, chemokine genes have been subject to specific evolutionary pressures, which have influenced their location in the genome. None of the genes encoding the 4 mucosal chemokines are located in the CCL or CXCL inflammatory gene clusters. Human CCL28 and CXCL14 are located in human chromosome 5, while the genes encoding human CCL25 and CXCL17 are located in chromosome 19. However, while the genes encoding CCL25 and CXCL17 are located in the same chromosome, they are not located in the vicinity of each other, suggesting that they evolved independently. The same is true for the genes encoding CCL28/CXCL14. These observations suggest that these chemokines are closer to the homeostatic, rather than the inflammatory, categories. However, as we discuss below, some of these are more properly “Dual” chemokines depending on the tissue that expresses them.
The second important characteristic of the mucosal chemokines is that all of them have been described to have broad antimicrobial activity. The latter is not a characteristic commonly associated with chemokines, although many have been shown to exhibit antimicrobial activity against certain microbes (Yung and Murphy 2012). However, all 4 mucosal chemokines have been shown to exhibit broad antimicrobial activity (Hieshima and others 2003; Maerki and others 2009; Burkhardt and others 2012; Erickson and others 2016). Furthermore, some pathogens have been shown to have developed mechanisms to counteract the antimicrobial activity of some of these chemokines (Erickson and others 2016). Taken together, these observations strongly suggest that broad antimicrobial activity may be one of the hallmark characteristics of mucosal chemokines and raises the possibility that they may be able to influence the composition of the mucosal microbiome.
A third interesting characteristic of mucosal chemokines is their extremely specific expression pattern in mucosal tissues. An excellent example is the expression of mucosal chemokines in the tongue. CXCL14 is very highly expressed in the taste buds, but not at all in the (adjacent) lingual epithelium. Conversely, CXCL17 is strongly expressed in the lingual epithelium, but not in the taste buds (Burkhardt and others 2012). The physiological reasons for these highly specific expression patterns are currently unknown, but they strongly suggest that these chemokines have multiple (and highly specific) functions in mucosal tissues. This expression suggests that while we broadly know the expression of these mucosal chemokines in the human body, their specific expression in various mucosal tissues still remains to be adequately studied.
CCL25
CCL25 was the first mucosal chemokine discovered. Ironically, we did not first identify it as a mucosal chemokine; instead, it was described as TECK (Rossi and others 1997). We did note that it was also expressed in the small intestine. CCL25 plays a very important role in T cell development. It is required for T cell precursors from the bone marrow to enter the thymus (Williams and others 2008). Its receptor is CCR9 (Zabel and others 1999) and is expressed by intestinal homing T lymphocytes (Zabel and others 1999). Importantly, CCR9 is expressed at the DN3 stage of early T cell development, a stage when thymocytes undergo a process called “β selection” when only thymocytes that have successfully rearranged the β chain of the T cell receptor (in frame) express it along with the pre-T alpha chain. Successful “β selection” allows developing thymocytes to differentiate to the DN4 stage of thymocyte development and become CD4+CD8+ thymocytes, which will undergo positive and negative selection (Norment and others 2000).
In addition, the CCL25/CCR9 axis has been identified as critical to recruit T cell precursors from the bone marrow to home to the thymus (Wilkinson and others 1999). While CCL25 is therefore very important in T cell development, we will focus in this study on its role in mucosal immunity, specifically, in the small intestine.
CCL25 is expressed by mucosal epithelial cells in the small intestine, and it chemoattracts CCR9+ T cells that home to the small intestine (Meurens and others 2007). CCL25 expression in the intestine may exhibit a homeostatic pattern, although some reports suggest that its expression depends on the presence of microbiota (Hermsen and others 2008). What is clear is that the CCL25/CCR9 axis recruits specific subsets of CCR9+ T cells to the small intestine (Papadakis and others 2000). Importantly, these CCR9+ intestine-homing T cells also express the integrin α4β7 (Papadakis and others 2000) and have been associated with intestinal autoimmune diseases, including Crohn's disease (Saruta and others 2007; Stenstad and others 2007). Importantly, an antibody against the α4β7 integrin has been approved as a treatment for inflammatory bowel disease, presumably through its ability to target this population of intestinal-homing T cells (Jovani and Danese 2013). Interestingly, an antagonist of the CCL25/CCR9 axis was not effective against Crohn's disease (Feagan and others 2015). It is possible that the antagonist was not able to neutralize or prevent the homing of CCR9+ T cells into the Crohn's lesions in the small intestine, while the anti-α4β7 antibody may have eliminated these cells in vivo.
Chemokines and their receptors have also been shown to be major players in defining metastatic destinations during cancer (Muller and others 2001; Zlotnik and others 2011). The CCL25/CCR9 axis also provides a very clear example of the role for chemokines in cancer metastasis. In melanoma, certain tumors can express CCR9 (Letsch and others 2004). It has been observed that if melanoma tumors express CCR9, there is a high correlation with metastasis to the intestine (where CCL25 is expressed) (Amersi and others 2008). What makes this observation especially interesting is that the intestine is normally not a major metastatic destination. This observation highlights the potential of chemokines to direct tumor cell metastasis in vivo.
CXCL17
CXCL17 is a 119 amino acid protein with a 22 amino acid signal peptide. In humans, the gene encoding CXCL17 is located in chromosome 19q13.2. This was the last chemokine to be described, and therefore, we do not have much information about its function. Furthermore, its receptor was unknown and was only reported very recently (Maravillas-Montero and others 2015).
Pisabarro and others originally described it as a “dendritic cell and macrophage chemokine,” due to its ability to chemoattract these cells, a function they detected through its ability to induce migration of human peripheral blood mononuclear cells in transwell migration assays (Pisabarro and others 2006). These data have been confirmed through analysis of a Cxcl17−/− mouse, where we identified a paucity of macrophages in the lungs (Burkhardt and others 2014).
Under homeostatic conditions, CXCL17 exhibits a unique mucosal expression pattern. It is strongly expressed in the respiratory tract (trachea, bronchial, and bronchoalveolar epithelium), as well as in the upper digestive tract (lingual epithelium of the tongue, salivary glands, esophagus, and glandular stomach), and in the vagina and prostate (Burkhardt and others 2012; Lee and others 2013). Under pathogenic conditions, overexpression of CXCL17 has been observed when inflammation is present, such as in idiopathic pulmonary fibrosis, some aggressive types of gastrointestinal, breast, and lung cancer, endometrial carcinoma, and early stages of pancreatic carcinoma intraductal papillary mucinous adenoma (Burkhardt and others 2012; Matsui and others 2012; Lee and others 2013; Ohlsson and others 2016). We have observed that CXCL17 is strongly induced in the human colonic epithelial cell line T-84 when incubated with pro-inflammatory cytokines (TNFα, IFNγ) (unpublished results). Taken together, these data indicate that CXCL17 follows an inflammatory expression pattern in the small intestine/colon. Therefore, we conclude that CXCL17 is a “Dual’ chemokine that is expressed under homeostatic conditions in various tissues, but it can be upregulated under inflammatory microenvironments in some tissues (intestine) that normally do not express this chemokine.
Although Cxcl17 expression is induced under inflammatory conditions, it may also have an anti-inflammatory function. In vitro experiments evaluating the expression of pro-inflammatory markers such as IL-6, TNF-α/β, and iNOS have shown a decrease in its expression in J774 cells or primary macrophages pretreated with Cxcl17 and then stimulated with lipopolysaccharide (LPS), compared with the macrophages that received only LPS treatment (Pisabarro and others 2006).
As mentioned before, the expression of Cxcl17 is associated with bad prognosis and tumor development. This may be linked to an angiogenic role of Cxcl17. The chemokine was initially described as a vascular chemokine (VCC-1) because its expression correlates with the expression of vascular endothelial growth factor (Pisabarro and others 2006). To better understand the pro-tumoral function of Cxcl17 in angiogenesis, several groups have induced the expression of CXCL17 in cell lines and tested their tumorigenic activity in vivo. They have found bigger tumors, vasculature-rich areas, and CD31+ cell recruitment in mice injected with CXCL17-transfected cells compared with control cells transfected with the vector alone (Pisabarro and others 2006).
CXCL17 was an orphan chemokine, because its receptor was unknown. We have recently identified its receptor. The monocytic cell line THP-1 responds to CXCL17 by inducing calcium fluxes or chemotaxis in vitro, and its response increases following incubation with prostaglandin E2 (PGE2) (Maravillas-Montero and others 2015). We therefore predicted that this cell line expressed the CXCL17 receptor.
The strong macrophage chemotactic activity of CXCL17 is only mirrored by CCL2/CCR2, a well-known macrophage chemoattractant axis. We therefore first ruled out that CXCL17 signaled through CCR2 (using Calcium flux assays). Once we did this, we predicted that CXCL17 signaled through an as yet unknown receptor. We further predicted that it would be a class A GPCR. We therefore performed a bioinformatics analysis of macrophage-expressed orphan GPCRs, which we reasoned would be the candidates to be the CXCL17 receptor. This yielded a list of 10 candidate molecules. We prioritized them based on their structural features, since we predicted that the receptor should resemble other chemokine receptors. When we transfected one of these receptors (GPR35) into BAF-3 cells, we were able to render them responsive to CXCL17 as judged by calcium flux responses. Importantly, GPR35 had already been shown to be expressed by macrophages and to exhibit a mostly mucosal expression pattern (Okumura and others 2004). In addition, it exhibits many structural features of other chemokine receptors, including a DRY box. Taken together, these results indicate that GPR35 is a novel chemokine receptor that according to the chemokine receptor nomenclature we have renamed as CXCR8 (Maravillas-Montero and others 2015).
CXCL17 is also a strong macrophage chemoattractant in vivo. Importantly, genomewide association studies have linked GPR35/CXCR8 to ulcerative colitis and primary sclerosing cholangitis (Ellinghaus and others 2013). The latter observation, along with recent data documenting its ability to recruit immature myeloid cells and its inflammatory expression pattern in the intestine and colon, strongly suggests that the CXCL17/CXCR8 axis is important in human disease.
CXCL14
CXCL14, originally known as BRAK (Breast and Kidney chemokine) (Hromas and others 1999) and also as MIP-2γ or BMAC, is a 111 amino acid small protein homeostatically expressed in epithelial tissues (Meuter and Moser 2008). Together with CXCL12, it is one of the highest conserved chemokines (all the way from zebrafish to humans) (Nomiyama and others 2008). The proposed function for CXCL14 is immune surveillance, as it is able to chemoattract macrophages, immature dendritic cells, and mast cells to some epithelial tissues such as the skin (specifically to the epidermis), gastrointestinal tract, and kidney. However, it is also strongly expressed in the CNS, but not in lymphoid organs such as thymus, spleen, or lymph nodes (Meuter and Moser 2008).
CXCL14 can bind CXCR4, inhibiting the migration of cells in response to the CXCL12-CXCR4 axis. Despite its interaction, CXCL14 does not induce signaling through CXCR4 (as evaluated through calcium mobilization, DMR, and MAPK assays) (Otte and others 2014). This could be a mechanism to maintain homeostasis and avoid hyperimmune reactions in tissues highly exposed to microbes.
Like CCL28, CXCL14 exhibits broad antimicrobial activity. Specifically, CXCL14 exhibits antimicrobial activity against Gram-negative E.coli, Gram-positive Staphylococci species, Propionibacteria, Pseudomonas aeruginosa, Streptococcus species, and the yeast C. albicans. It is likely to be an important mechanism providing protection to the lung and skin and could be relevant in local immune defense of other barrier tissues, due to its strong expression in epithelial organs (Maerki and others 2009; Dai and others 2015).
A role for CXCL14 as a metabolic regulator (in female mice) was discovered by comparing the metabolic responses of wild-type and Cxcl14-/- mice. The expression of CXCL14 is upregulated in tissues such as adipose, liver, and skeletal muscle in a model of obesity and type 2 diabetes, promoting the infiltration of macrophages into those tissues. The expression of CXCL14 impacts the expression of adipokines, including adiponectin, RBP4, and the cytokine IL-6, and as a consequence the development of insulin resistance and impairment of glucose metabolism in obese mice are observed (Nara and others 2007).
In another study, behavioral differences were observed. Lower body weight and reduced birth rate were observed in Cxcl14−/− compared with wild-type mice. The decrease in body weight could be due to decrease of Npy and Agrp expression in the hypothalamus of Cxcl14−/− compared to control mice. The expression of Cxcl14 in the central nervous system and the changes in neuropeptides, in addition to the metabolic changes described before, suggest an important role for Cxcl14 in coordinating feeding behavior (Tanegashima and others 2010).
An important observation that may be related to the feeding behavior abnormalities observed in Cxcl14−/− mice is the fact that CXCL14 is expressed in the taste buds, where it is one of the highest expressed genes (Hevezi and others 2009). These observations strongly suggest that it has a function in the taste buds, either in the regulation of taste perception or, alternatively, in maintenance, cellular organization, or development of taste buds, since these structures are replaced every 2–3 weeks.
Taken together, these observations suggest that CXCL14 plays very important functions in the mucosa. This is an area that requires more study because at the present time the data available raise more questions than it answers.
CCL28
CCL28 is the best known mucosal chemokine. It binds CCR10 (originally called GPR2) (Wang and others 2000). It shares this receptor with the skin-specific chemokine CCL27 (Morales and others 1999). The latter observation indicates that CCL27 and CCL28 are derived from a common ancestral gene, and 1 specialized to the skin, while the other 1 specialized to the mucosa. CCL28 is expressed in many mucosal tissues, mostly by epithelial cells, and it attracts IgA plasmablasts (Lazarus and others 2003), which express CCR10 (Kunkel and others 2003).
CCL28 is homeostatically expressed in many mucosal tissues and particularly in the salivary glands, indicating that it is normally present in saliva and it therefore “bathes” the digestive tract. In other tissues, like the human colon, it behaves like an inflammatory chemokine since its expression is induced by pro-inflammatory cytokines like IL-1 (Ogawa and others 2004) and is upregulated in inflammatory human diseases like inflammatory bowel disease (Arijs and others 2011) or periodontitis (Ertugrul and others 2013). Therefore, like CXCL17, it is a “Dual” (homeostatic/inflammatory) chemokine. Besides its normal expression in mucosa it has been found expressed in some pathogenic conditions like the Reed–Stenberg cells of Hodgkin's lymphoma (Hanamoto and others 2004). The functional significance of the latter observation is not clear.
CCL28 is an important chemokine associated with IgA responses. In the mammary gland, it is induced upon the onset of lactation under the effect of estrogen and it chemoattracts IgA plasmablasts that will produce the IgA found in colostrum and milk (Wilson and Butcher 2004). Accordingly, Ccr10−/− mice exhibit a deficiency in IgA in the milk, but the production of IgA in the gastrointestinal tract is minimally impacted (Morteau and others 2008). However, another study found that IgA responses in the gastrointestinal tract of Ccr10−/− mice are not normal, in particular IgA memory responses to pathogen reinfection were severely impaired. These observations strongly suggest that the role of the CCL28/CCR10 axis in IgA responses involves the recruitment of IgA plasmablasts to sites of developing responses. However, if the plasmablasts can arrive there by other mechanisms the development of IgA responses can proceed normally. These data are also consistent with the conclusion that the principal receptor for CCL28 is CCR10. The functional significance of the reported ability of CCL28 to bind CCR3 (Pan and others 2000) remains unclear.
The broad antimicrobial activity of CCL28 has been the focus of several studies. The original report documenting its antimicrobial activity noted that it is particularly effective against Candida albicans (Hieshima and others 2003). In fact, its terminal 28 amino acid peptide also displayed selective candidacidal activity. Its C-terminus displays significant sequence similarity to histatin-5, a histidine-rich peptide found in human saliva (Hieshima and others 2003). A second study has identified a sequence of highly charged amino acids in the C-terminal region of CCL28 that mediates its antimicrobial activity (Liu and Wilson 2010). Taken together, these observations strongly suggest that CCL28 plays an important antimicrobial function in mucosal tissues. This conclusion is also supported by the fact that its levels in saliva drop significantly in diseases such as Sjögren's syndrome (Hernandez-Molina and others 2015), suggesting that it may be partially responsible for observed susceptibility to oral microbial infections observed in this disease.
Clearly, the CCL28/CCR10 axis is a very interesting and important player in mucosal immunity. We should mention that CCR10 expression has also been detected in T regulatory cells (Facciabene and others 2011) and Th22 cells (Duhen and others 2009). The latter represents a potentially important player in immune responses through their production of IL22. CCL28 has been shown to be induced in tumors under hypoxic conditions, and it has been suggested that it recruits Tregs that promote tumor tolerance and angiogenesis (Facciabene and others 2011). The fact that both CCL27 (skin specific) and CCL28 (mucosal specific) bind CCR10 also suggests common links between skin and mucosal immunity (which may be both innate and acquired). Further studies are required to understand the functional significance of these observations, but it is likely that the CCL28/CCR10 axis will be shown to be important in other immune responses.
Conclusions
It should be apparent from the previous sections that we still know relatively little about the function of these mucosal chemokines. Therefore, our first conclusion is that they represent a very promising relatively novel field of research. In support of this conclusion, in spite of the fact that we have covered most of the topics described in the literature that involve the physiology of these chemokines, our review is still rather small. We would like therefore to identify several obvious areas for future research.
The first is that mucosal tissues exhibit their own immune system physiology, and this is an area where mucosal chemokines are very likely to play important roles. Several lymphoid populations specific to mucosal sites have been described, like the recruitment of CCR9+ T cells by CCL25 to the intestine (Papadakis and others 2000; Saruta and others 2007). Therefore, homeostatic mucosal chemokines are excellent candidates to be involved in the recruitment of mucosal-specific lymphoid and myeloid populations some of which remain to be identified. Another emerging example is the CXCL17/CXCR8 axis, which represents a powerful macrophage-recruitment mechanism that operates in the mucosa (Burkhardt and others 2014; Maravillas-Montero and others 2015). In our experience, the CXCL17/CXCR8 axis rivals the CCL2/CCR2 axis in “macrophage-recruitment potency” in vivo. Given that CCL2/CCR2 axis is the most powerful (chemokine-mediated) macrophage-recruitment mechanism described to date (Deshmane and others 2009), the question arises as to why did evolution design a second, chemokine-mediated, macrophage-recruitment mechanism (CXCL17/CXCR8) that (appears to mainly) operate in mucosal tissues? The answer to this question likely involves the nature of the macrophages recruited by each of these chemokine axes. The macrophages recruited by the CCL2/CCR2 axis will likely be shown to mediate different functions than those recruited by the CXCL17/CXCR8 axis. This already appears to be the case for CCR2-expressing versus CX3CR1-expressing macrophages (the latter being recruited by the CX3CL1/CX3CR1 axis). Indeed, both CCR2 and CX3CR1 are now considered important macrophage subset biomarkers (Kzhyshkowska and others 2016). We predict that GPR35/CXCR8 will also become an important macrophage subset biomarker. It remains to be shown whether it specifically labels mucosal-bound macrophages. Recently, the existence of functional macrophage subsets has been postulated, with the most “popular” subsets being labeled “M1 and M2” depending on whether they express pro-inflammatory or anti-inflammatory cytokines. While this definition may be too simple, it has contributed to the acceptance of the concept of macrophage/monocyte functional subsets (Ushach and Zlotnik 2016). We therefore predict that GPR35/CXCR8 will define yet another functional macrophage subset.
Importantly, the immune system functions of different mucosal tissues are also likely to differ. The functions of the trachea/bronchus mucosa may differ from the esophagus, for example. Yet they also share many immune system functions (for example, production of IgA). The mucosal chemokines that we highlight in this article are expressed in most mucosal tissues. However, we still lack basic information about their expression is several mucosal sites. For example, we do not know whether they are expressed in the eye, but given that the eye also involves several typical mucosal tissues (conjunctiva), it is a safe prediction that some of these chemokines will be expressed in eye tissues either in a homeostatic or inflammatory manner. The same applies to other mucosal sites like the nasal epithelium.
The pattern of expression of some of these chemokines in the digestive tract is also intriguing and further suggests other functions. We have observed that the expression pattern of CXCL17 in the digestive tract mirrors that of IL-1RA (receptor antagonist) (unpublished observations). This is an interesting observation because IL-1RA is an anti-inflammatory cytokine that specifically inhibits IL-1α and IL-1β binding to their receptors. This observation suggests that CXCL17 could also have anti-inflammatory functions. In fact, a recent article has postulated that CXCL17 is an anti-inflammatory chemokine (Lee and others 2013).
The wide-spectrum antimicrobial activity of these mucosal chemokines strongly suggests that they play an important role in defining and regulating the microbiome present in many mucosal sites. Given the high importance of the microbiome in health and disease, if this prediction was to be confirmed, it would raise the importance of these chemokines as well. Fortunately, the availability of various chemokine-deficient mice should make these experiments practical. We expect to have results that should shed light on these possibilities in the near future.
Finally, we should emphasize that the present article has focused on 4 chemokines (CCL25, CCL28, CXCL14, and CXCL17) that exhibit homeostatic expression in mucosal tissues and are therefore chemokines whose function is closely tied to mucosal tissues. There are 48 human chemokine ligands, and many of these are expressed in mucosal tissues under inflammatory or pathological conditions. We have not addressed the role of these “inflammatory” chemokines in this review because that topic is very extensive. It is our hope that we have been able to call attention to these 4 mucosal chemokines and that our review will stimulate research on their role in mucosal tissue physiology.
Acknowledgments
Supported by Grant No. R01-AI093548 from NIAID. M.H. was supported by a postdoctoral fellowship from UCMEXUS. The authors thank César Gallo for his help with Figure 1.
Author Disclosure Statement
No competing financial interests exist.
References
- Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, Faries MB, Morton DL, Hoon DS. 2008. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin Cancer Res 14(3):638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arijs I, De Hertogh G, Machiels K, Van Steen K, Lemaire K, Schraenen A, Van Lommel L, Quintens R, Van Assche G, Vermeire S, Schuit F, Rutgeerts P. 2011. Mucosal gene expression of cell adhesion molecules, chemokines, and chemokine receptors in patients with inflammatory bowel disease before and after infliximab treatment. Am J Gastroenterol 106(4):748–761 [DOI] [PubMed] [Google Scholar]
- Burkhardt AM, Maravillas-Montero JL, Carnevale CD, Vilches-Cisneros N, Flores JP, Hevezi PA, Zlotnik A. 2014. CXCL17 is a major chemotactic factor for lung macrophages. J Immunol 193(3):1468–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt AM, Tai KP, Flores-Guiterrez JP, Vilches-Cisneros N, Kamdar K, Barbosa-Quintana O, Valle-Rios R, Hevezi PA, Zuniga J, Selman M, Ouellette AJ, Zlotnik A. 2012. CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity. J Immunol 188(12):6399–6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Basilico P, Cremona TP, Collins P, Moser B, Benarafa C, Wolf M. 2015. CXCL14 displays antimicrobial activity against respiratory tract bacteria and contributes to clearance of Streptococcus pneumoniae pulmonary infection. J Immunol 194(12):5980–5989 [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. 2009. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29(6):313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10(8):857–863 [DOI] [PubMed] [Google Scholar]
- Ebert LM, Schaerli P, Moser B. 2005. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol 42(7):799–809 [DOI] [PubMed] [Google Scholar]
- Ellinghaus D, Folseraas T, Holm K, Ellinghaus E, Melum E, Balschun T, Laerdahl JK, Shiryaev A, Gotthardt DN, Weismuller TJ, Schramm C, Wittig M, Bergquist A, Bjornsson E, Marschall HU, Vatn M, Teufel A, Rust C, Gieger C, Wichmann HE, Runz H, Sterneck M, Rupp C, Braun F, Weersma RK, Wijmenga C, Ponsioen CY, Mathew CG, Rutgeerts P, Vermeire S, Schrumpf E, Hov JR, Manns MP, Boberg KM, Schreiber S, Franke A, Karlsen TH. 2013. Genome-wide association analysis in primary sclerosing cholangitis and ulcerative colitis identifies risk loci at GPR35 and TCF4. Hepatology 58(3):1074–1083 [DOI] [PubMed] [Google Scholar]
- Erickson DL, Lew CS, Kartchner B, Porter NT, McDaniel SW, Jones NM, Mason S, Wu E, Wilson E. 2016. Lipopolysaccharide Biosynthesis genes of yersinia pseudotuberculosis promote resistance to antimicrobial chemokines. PLoS One 11(6):e0157092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertugrul AS, Sahin H, Dikilitas A, Alpaslan N, Bozoglan A. 2013. Comparison of CCL28, interleukin-8, interleukin-1beta and tumor necrosis factor-alpha in subjects with gingivitis, chronic periodontitis and generalized aggressive periodontitis. J Periodontal Res 48(1):44–51 [DOI] [PubMed] [Google Scholar]
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. 2011. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 475(7355):226–230 [DOI] [PubMed] [Google Scholar]
- Feagan BG, Sandborn WJ, D'Haens G, Lee SD, Allez M, Fedorak RN, Seidler U, Vermeire S, Lawrance IC, Maroney AC, Jurgensen CH, Heath A, Chang DJ. 2015. Randomised clinical trial: vercirnon, an oral CCR9 antagonist, vs. placebo as induction therapy in active Crohn's disease. Aliment Pharmacol Ther 42(10):1170–1181 [DOI] [PubMed] [Google Scholar]
- Hanamoto H, Nakayama T, Miyazato H, Takegawa S, Hieshima K, Tatsumi Y, Kanamaru A, Yoshie O. 2004. Expression of CCL28 by Reed-Sternberg cells defines a major subtype of classical Hodgkin's disease with frequent infiltration of eosinophils and/or plasma cells. Am J Pathol 164(3):997–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsen JL, Gomez FE, Maeshima Y, Sano Y, Kang W, Kudsk KA. 2008. Decreased enteral stimulation alters mucosal immune chemokines. JPEN J Parenter Enteral Nutr 32(1):36–44 [DOI] [PubMed] [Google Scholar]
- Hernandez-Molina G, Burkhardt AM, Lima G, Zlotnik A, Betanzos JL, Bahena S, Llorente L. 2015. Absence of salivary CCL28 in primary Sjogren's syndrome. Rheumatol Int 35(8):1431–1434 [DOI] [PubMed] [Google Scholar]
- Hevezi P, Moyer BD, Lu M, Gao N, White E, Echeverri F, Kalabat D, Soto H, Laita B, Li C, Yeh SA, Zoller M, Zlotnik A. 2009. Genome-wide analysis of gene expression in primate taste buds reveals links to diverse processes. PLoS One 4(7):e6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawasaki Y, Shiba F, Shiota M, Katou F, Saito T, Yoshie O. 2003. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol 170(3):1452–1461 [DOI] [PubMed] [Google Scholar]
- Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnik A. 2002. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med 8(2):157–165 [DOI] [PubMed] [Google Scholar]
- Hromas R, Broxmeyer HE, Kim C, Nakshatri H, Christopherson K, 2nd, Azam M, Hou YH. 1999. Cloning of BRAK, a novel divergent CXC chemokine preferentially expressed in normal versus malignant cells. Biochem Biophys Res Commun 255(3):703–706 [DOI] [PubMed] [Google Scholar]
- Johnson LA, Jackson DG. 2014. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis 17(2):335–345 [DOI] [PubMed] [Google Scholar]
- Jovani M, Danese S. 2013. Vedolizumab for the treatment of IBD: a selective therapeutic approach targeting pathogenic a4b7 cells. Curr Drug Targets 14(12):1433–1443 [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. 2002. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16(1):1–4 [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med 192(5):761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. 2003. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest 111(7):1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kzhyshkowska J, Gudima A, Moganti K, Gratchev A, Orekhov A. 2016. Perspectives for monocyte/macrophage-based diagnostics of chronic inflammation. Transfus Med Hemother 43(2):66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. 2003. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol 170(7):3799–3805 [DOI] [PubMed] [Google Scholar]
- Lee J, Hever A, Willhite D, Zlotnik A, Hevezi P. 2005. Effects of RNA degradation on gene expression analysis of human postmortem tissues. FASEB J 19(10):1356–1358 [DOI] [PubMed] [Google Scholar]
- Lee WY, Wang CJ, Lin TY, Hsiao CL, Luo CW. 2013. CXCL17, an orphan chemokine, acts as a novel angiogenic and anti-inflammatory factor. Am J Physiol Endocrinol Metab 304(1):E32–E40 [DOI] [PubMed] [Google Scholar]
- Letsch A, Keilholz U, Schadendorf D, Assfalg G, Asemissen AM, Thiel E, Scheibenbogen C. 2004. Functional CCR9 expression is associated with small intestinal metastasis. J Invest Dermatol 122(3):685–690 [DOI] [PubMed] [Google Scholar]
- Liu B, Wilson E. 2010. The antimicrobial activity of CCL28 is dependent on C-terminal positively-charged amino acids. Eur J Immunol 40(1):186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerki C, Meuter S, Liebi M, Muhlemann K, Frederick MJ, Yawalkar N, Moser B, Wolf M. 2009. Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J Immunol 182(1):507–514 [DOI] [PubMed] [Google Scholar]
- Maravillas-Montero JL, Burkhardt AM, Hevezi PA, Carnevale CD, Smit MJ, Zlotnik A. 2015. Cutting edge: GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. J Immunol 194(1):29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Yokoo H, Negishi Y, Endo-Takahashi Y, Chun NA, Kadouchi I, Suzuki R, Maruyama K, Aramaki Y, Semba K, Kobayashi E, Takahashi M, Murakami T. 2012. CXCL17 expression by tumor cells recruits CD11b+Gr1 high F4/80- cells and promotes tumor progression. PLoS One 7(8):e44080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurens F, Berri M, Siggers RH, Willing BP, Salmon H, Van Kessel AG, Gerdts V. 2007. Commensal bacteria and expression of two major intestinal chemokines, TECK/CCL25 and MEC/CCL28, and their receptors. PLoS One 2(7):e677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuter S, Moser B. 2008. Constitutive expression of CXCL14 in healthy human and murine epithelial tissues. Cytokine 44(2):248–255 [DOI] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A. 1999. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci U S A 96(25):14470–14475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morteau O, Gerard C, Lu B, Ghiran S, Rits M, Fujiwara Y, Law Y, Distelhorst K, Nielsen EM, Hill ED, Kwan R, Lazarus NH, Butcher EC, Wilson E. 2008. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J Immunol 181(9):6309–6315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. 2001. Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824):50–56 [DOI] [PubMed] [Google Scholar]
- Nara N, Nakayama Y, Okamoto S, Tamura H, Kiyono M, Muraoka M, Tanaka K, Taya C, Shitara H, Ishii R, Yonekawa H, Minokoshi Y, Hara T. 2007. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J Biol Chem 282(42):30794–30803 [DOI] [PubMed] [Google Scholar]
- Nomiyama H, Hieshima K, Osada N, Kato-Unoki Y, Otsuka-Ono K, Takegawa S, Izawa T, Yoshizawa A, Kikuchi Y, Tanase S, Miura R, Kusuda J, Nakao M, Yoshie O. 2008. Extensive expansion and diversification of the chemokine gene family in zebrafish: identification of a novel chemokine subfamily CX. BMC Genomics 9:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norment AM, Bogatzki LY, Gantner BN, Bevan MJ. 2000. Murine CCR9, a chemokine receptor for thymus-expressed chemokine that is up-regulated following pre-TCR signaling. J Immunol 164(2):639–648 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Iimura M, Eckmann L, Kagnoff MF. 2004. Regulated production of the chemokine CCL28 in human colon epithelium. Am J Physiol Gastrointest Liver Physiol 287(5):G1062–G1069 [DOI] [PubMed] [Google Scholar]
- Ohlsson L, Hammarstrom ML, Lindmark G, Hammarstrom S, Sitohy B. 2016. Ectopic expression of the chemokine CXCL17 in colon cancer cells. Br J Cancer 114(6):697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma K, Takahashi N, Yamochi T, Hosono O, Dang NH, Morimoto C. 2008. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front Biosci 13:2299–2310 [DOI] [PubMed] [Google Scholar]
- Okumura S, Baba H, Kumada T, Nanmoku K, Nakajima H, Nakane Y, Hioki K, Ikenaka K. 2004. Cloning of a G-protein-coupled receptor that shows an activity to transform NIH3T3 cells and is expressed in gastric cancer cells. Cancer Sci 95(2):131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte M, Kliewer A, Schutz D, Reimann C, Schulz S, Stumm R. 2014. CXCL14 is no direct modulator of CXCR4. FEBS Lett 588(24):4769–4775 [DOI] [PubMed] [Google Scholar]
- Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, Vierra MA, Genovese MC, Butcher EC, Soler D. 2000. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol 165(6):2943–2949 [DOI] [PubMed] [Google Scholar]
- Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. 2000. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol 165(9):5069–5076 [DOI] [PubMed] [Google Scholar]
- Pisabarro MT, Leung B, Kwong M, Corpuz R, Frantz GD, Chiang N, Vandlen R, Diehl LJ, Skelton N, Kim HS, Eaton D, Schmidt KN. 2006. Cutting edge: novel human dendritic cell- and monocyte-attracting chemokine-like protein identified by fold recognition methods. J Immunol 176(4):2069–2073 [DOI] [PubMed] [Google Scholar]
- Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. 1997. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol 158(3):1033–1036 [PubMed] [Google Scholar]
- Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, Zlotnik A. 2006. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics 7(2):67–80 [DOI] [PubMed] [Google Scholar]
- Sanz MJ, Kubes P. 2012. Neutrophil-active chemokines in in vivo imaging of neutrophil trafficking. Eur J Immunol 42(2):278–283 [DOI] [PubMed] [Google Scholar]
- Saruta M, Yu QT, Avanesyan A, Fleshner PR, Targan SR, Papadakis KA. 2007. Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn's disease. J Immunol 178(5):3293–3300 [DOI] [PubMed] [Google Scholar]
- Soria G, Ben-Baruch A. 2008. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett 267(2):271–285 [DOI] [PubMed] [Google Scholar]
- Stenstad H, Svensson M, Cucak H, Kotarsky K, Agace WW. 2007. Differential homing mechanisms regulate regionalized effector CD8alphabeta+ T cell accumulation within the small intestine. Proc Natl Acad Sci U S A 104(24):10122–10127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. 2006. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25(6):977–988 [DOI] [PubMed] [Google Scholar]
- Tanegashima K, Okamoto S, Nakayama Y, Taya C, Shitara H, Ishii R, Yonekawa H, Minokoshi Y, Hara T. 2010. CXCL14 deficiency in mice attenuates obesity and inhibits feeding behavior in a novel environment. PLoS One 5(4):e10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushach I, Zlotnik A. 2016. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol 100:481.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon KB, Zlotnik A. 1997. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity 7(2):291–301 [DOI] [PubMed] [Google Scholar]
- Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, Jenkins N, Copeland NG, Gilbert DJ, Nguyen N, Abrams J, Kershenovich D, Smith K, McClanahan T, Vicari AP, Zlotnik A. 2000. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J Biol Chem 275(29):22313–22323 [DOI] [PubMed] [Google Scholar]
- Weinstein EJ, Head R, Griggs DW, Sun D, Evans RJ, Swearingen ML, Westlin MM, Mazzarella R. 2006. VCC-1, a novel chemokine, promotes tumor growth. Biochem Biophys Res Commun 350(1):74–81 [DOI] [PubMed] [Google Scholar]
- Wilkinson B, Owen JJ, Jenkinson EJ. 1999. Factors regulating stem cell recruitment to the fetal thymus. J Immunol 162(7):3873–3881 [PubMed] [Google Scholar]
- Williams KM, Lucas PJ, Bare CV, Wang J, Chu YW, Tayler E, Kapoor V, Gress RE. 2008. CCL25 increases thymopoiesis after androgen withdrawal. Blood 112(8):3255–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E, Butcher EC. 2004. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med 200(6):805–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M, Jordan BR, Malissen B, Carrier A, Naquet P. 2000. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol 30(1):262–271 [DOI] [PubMed] [Google Scholar]
- Yung SC, Murphy PM. 2012. Antimicrobial chemokines. Front Immunol 3:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Roberts AI, Ebert EC, Kassam N, Qin S, Zovko M, LaRosa GJ, Yang LL, Soler D, Butcher EC, Ponath PD, Parker CM, Andrew DP. 1999. Human G protein-coupled receptor GPR-9–6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med 190(9):1241–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Burkhardt AM, Homey B. 2011. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol 11(9):597–606 [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. 2012. The chemokine superfamily revisited. Immunity 36(5):705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]