Abstract
Adeno-associated virus (AAV) vectors are a commonplace tool for gene delivery ranging from cell culture to human gene therapy. One feature that makes AAV a desirable vector is its stability, in regard to both the duration of transgene expression and retention of infectivity as a viral particle. This study examined the stability of AAV serotype 1 (AAV1) vectors under different conditions. First, transducibility after storage at 4°C decreased 20% over 7 weeks. Over 10 freeze–thaw cycles, the resulting transduction efficiency became variable at 60–120% of a single thaw. Using small stainless steel slugs to mimic a biosafety cabinet or metal lab bench surface, it was found that an AAV1 vector can be reconstituted after 6 days of storage at room temperature. The stability of AAV is a desired feature, but effective decontamination procedures must be available for safety and experimental integrity. Multiple disinfectants commonly used in the laboratory for ability to inactivate an AAV1 vector were tested, and it was found that autoclaving, 0.25% peracetic acid, iodine, or 10% Clorox bleach completely prevented AAV-mediated transgene expression. These data suggest that peracetic acid should be used for inactivating AAV1 vectors on metal-based surfaces or instruments in order to avoid inadvertent transgene expression in human cells or cross-contamination of instruments.
Keywords: : adeno-associated virus, AAV, stability, decontamination, inactivation
Introduction
Adeno-associated virus (AAV)-based viral vectors are the preeminent vector for viral vector-mediated transgenesis in animal models of physiological and pathological states, as well as human-based gene therapy approaches. The prevalence of AAV as a vector stems from its low immunogenicity,1,2 high titers (∼1 × 1014 viral genomes/mL), no associated human disease,3 helper virus-free production,4 and high stability in terms of both duration of transgene expression in transduced cell and stability as a viral particle.5,6 AAV serotype 1 (AAV1) is used for neuroscience applications such as opotogenetics,7,8 pharmacogenetics,9–11 and gene silencing.12 AAV1 is also being used in human clinical trials for heart disease,13 alpha-1 antitrypsin deficiency,14 limb-girdle muscular dystrophy type 2D,15 Charcot–Marie–Tooth neuropathy,16 and Becker muscular dystrophy.17 Given the prevalence of usage and breadth of applications, establishing practical procedures in the research laboratory setting for inactivating AAV is essential for the safe use of AAV and reducing cross-contamination of instruments and equipment. The National Institutes of Health (NIH) classifies all serotypes of AAV-based vectors produced by helper-virus free methods as Risk Group 1, but depending on the transgene being expressed (e.g., oncogenes, toxic genes, etc.), the classification may be elevated. With a Risk 1 assessment, AAV vectors are often used with Biosafety Level 1 (BSL1) practices can be conducted in mixed-use laboratory space. Given the high stability of AAV described herein and by others,6,18 proper decontamination procedures such as 0.5% sodium hypochlorite, ionic detergents, or alkaline solutions (pH >9.5) must be employed to ensure safety. The recent study by Gruntman et al. examined the stability and compatibility of AAV1 in the context of human gene therapy applications. The present study focused on the issue of AAV stability and inactivation as it relates to vectors used in the basic research setting with BSL1 practices. Additionally, for laboratories that reuse equipment for multiple viruses due to cost restraints, proper AAV inactivation procedures are paramount to prevent cross-contamination. Gruntman et al. found that heating virus to 72°C and ultraviolet exposure for 10 min were the only effective means of decreasing vector activity.6 This study tested the stability and inactivation of affinity purified AAV1-based vectors under various conditions, including temperature fluctuations, dehydration, and chemical inactivators of viral particles. The data show the stability of AAV-based vectors under different conditions, and inactivation solutions are suggested for laboratories working with AAV.
Materials and Methods
Primary cortical cultures
Rat primary cortical cultures were prepared from Sprague–Dawley embryos, as described previously19 and in accordance with approved procedures by the NIH Animal Care and Usage Committee. Cells (6.0 × 104) were plated in polyethyleneimine-coated wells (96-well plate), and half-medium exchanges were performed on days in vitro (DIV) 4, 6, 8, 11, and 13. Viral transductions were performed on day 6 in culture.
AAV plasmids, packaging, purification, and titering
AAV packaging plasmids used in the study have been described and can be found on Addgene.org (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hgtb). All AAV vectors were produced by a triple transfection method4 with some modification.19 Specifically, HEK293 cells are grown in media (Dulbecco's modified Eagle's medium, high glucose [DMEM-HG; Invitrogen, Carlsbad, CA] containing 5% bovine growth serum [BGS; HyClone, Logan, UT] and 1% penicillin–streptomycin) in 20 × 150 mm dishes until 80–90% confluent. Media on each plate was changed to Iscove's modified Dulbecco's medium (IMDM; Invitrogen) containing 5% BGS (transfection media). For each 150 mm dish, 25 μg of pHelper (Stratagene, La Jolla, CA), 18 μg of AAV plasmid coding for transgene, and 7 μg of rep/cap plasmid (pAAV1; aka pXX12) coding for AAV serotype 1 (generously provided by Dr. Xiao Xiao, University of North Carolina at Chapel Hill, Chapel Hill, NC) were added to 2 mL of 0.25 M CaCl2. The solution was gently vortexed while a 2 × HEPES buffered saline (HBS) buffer (280 mM of NaCl, 50 mM of HEPES, and 1.5 mM of Na2HPO4) was slowly dripped into the solution. The DNA/CaCl2/HBS solution was dripped onto each 150 mm dish (4 mL/plate). After 15–17 h, transfection media was replaced with 25 mL/plate 293 media. Forty hours post transfection, the cells were harvested and centrifuged at 800 g for 5 min at 4°C. The pellet was resuspended in 1 mL of 50 mM of Tris–HCl pH 8.0, 150 mM of NaCl, and 2 mM of MgCl2 (resuspension buffer) per 150 mm dish. The resuspended cells were frozen and stored at −80°C until purification. AAV was affinity purified, as described previously.20 An example of affinity-purified AAV1 is shown in Supplementary Fig. S2.
AAV freeze–thaw procedure
The AAV1-CaMKII-eNpHR3.0-iRFP aliquots were placed on dry ice, and one set of aliquots was thawed for 5 min at room temperature. The aliquots were then placed back on dry ice for 5 min, and the process was repeated until all aliquots had been frozen and thawed the appropriate number of times. At DIV 6 for the primary cortical neurons (PCNs), 95 μL of media was then removed from each well of the 96-well dish, and 5 μL of AAV was added to each well. After 2 h, 100 μL of fresh PCN media was added to each well. Cells were fed with a 50% media exchange on DIVs 8, 11, and 13.
AAV 4°C testing
AAV1-CaMKII-eNpHR3.0-iRFP was thawed, diluted 1:5 and 1:10 in dilution buffer (phosphate-buffered saline containing 0.5 mM of MgCl2), and placed in a refrigerator at 4°C each week for 7 weeks. At DIV 6 for the PCNs, 95 μL of media was then removed from each well of the 96-well dish, and 5 μL of AAV1-CaMKII-eNpHR3.0-iRFP was added to each well. After 2 h, 100 μL of fresh PCN media was added to each well. Cells were fed with a 50% media exchange on DIVs 8, 11, and 13.
AAV drying test or “metal slug assay”
Seven days prior to transduction, one aliquot of AAV1-CMV-SP-GFP-Cre was thawed, and 5 μL of AAV was added to the center of three autoclaved slugs placed in a 24-well dish. The stainless-steel slugs were obtained from Arrow Metal Fabricators (Baltimore, MD). The slugs were 13 mm in diameter with an average thickness of 1.17 ± 0.03 mm. This was repeated each day for 1 week. At DIV 6 for the PCNs, 95 μL of media was then removed from each well of the 96-well dish, and 5 μL of AAV1-EF1α-DIO-mCherry was added to each well. AAV1-CMV-SP-GFP-Cre (5 μL) was dropped on the slug and immediately transferred to appropriate wells of the 96-well dish of PCNs. Dilution buffer (10 μL) was added to each of the remaining slugs and transferred to appropriate wells of the 96-well well plate. After 2 h, 100 μL of fresh PCN media was added to each well. Cells were fed with a 50% media exchange on DIVs 8, 11, and 13.
AAV autoclaving test
AAV1-CMV-GFP-Cre (5 μL) was added to the center of nine slugs placed in a 24-well dish. Dilution buffer (5 μL) was also added to the center of nine slugs and allowed to dry for 90 min. The slugs were separated into groups of three. Two sets of AAV and two sets of dilution buffer were autoclaved in two different autoclaves (121°C exposure for 30 min with 30 min drying time), while the other set of AAV and dilution buffer were kept at room temperature. The media (95 μL) was then removed from each well of the 96-well dish of PCNs (DIV 6), and 5 μL of AAV1-EF1α-DIO-mCherry was added to each well. Dilution buffer (10 μL) was added to each of the slugs and transferred to appropriate wells of the 96-well well plate. After 2 h, 100 μL fresh PCN media was added to each well. Cells were fed with a 50% media exchange on DIVs 8, 11, and 13.
AAV decontamination testing
AAV1-CMV-SP-GFP-Cre (2 μL) was added to 18 μL of 10% bleach solution, Gladiator (Cello, Havre de Grace, MD), 3.9% peracetic acid (PAA; Sigma–Aldrich, St. Louis, MO), povidone iodine solution (Thermo Fisher Scientific, Waltham, MA), cavicide (Metrex, Orange, CA), 70% isopropanol (Sigma–Aldrich), or dilution buffer. For pre-made PAA solutions are 5–6% PAA in ViorOXLS&D (PeroxyChem, Philadelphia, PA), 0.23% PAA Accel 5 RTU (Virox Technologies, Oakville, ON), and 0.05% PAA VIRASEPT (Ecolab, St Paul, MN). After 5 min and 30 min, 480 μL was added to each mixture to bring the volume up to 0.5 mL. The mixtures were placed in 20,000 MWCO dialysis cassettes (Pierce Chemical, Dallas, TX) and dialyzed in dilution buffer, changing the dialysis buffer three times, with each dialysis lasting at least 2 h. Solutions were removed from the dialysis cassettes, placed in an Amicon spin column (10 KDa; Merck Millipore, Billerica, MA), and spun at 4,000 g for 40 min at 4°C. The concentrated solution was placed in a 4°C refrigerator overnight. At DIV 6 for the PCNs, 95 μL of media was then removed from each well of the 96-well dish, and 5 μL of AAV1-EF1α-DIO-mCherry was added to each well. AAV/decon solution (10 μL) was added to each appropriate well of the 96-well plate (n = 3). After 2 h, 100 μL of fresh PCN media was added to each well. Cells were fed with a 50% media exchange on DIVs 8, 11, and 13.
Immunocytochemistry and image analysis
The media was removed from each well, and 100 μL of fresh 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA) was added to each well for 1 h at room temperature. The PFA was removed, and 200 μL of PBS was added to each well. The plates were then sealed with parafilm to prevent evaporation and aluminum foil to prevent photobleaching, and stored in a 4°C cabinet. For DAPI staining, 100 μL of PBS containing 0.1% Triton X-100 (Sigma–Aldrich) and DAPI (Invitrogen) diluted to 10 μg/mL were added to each well, and the cells were incubated for 10 min at room temperature. The cells were then washed twice in PBS, sealed with parafilm and aluminum foil, and placed in the 4°C cabinet until imaging.
For the AAV drying, decontamination, and autoclaving tests, images were taken with a Nikon Eclipse TE2000-E inverted microscope (Nikon, Melville, NY) equipped with a iXon3 (Andor, Belfast, United Kingdom) and NIS elements software (Nikon). Cells were imaged using three different filters: UV filter for DAPI nuclear staining, FITC green for GFP, and TRITC for mCherry. Exposure times were kept constant for each filter.
For the 4°C and freeze–thaw testing, plates were scanned on the Odyssey infrared scanner (Licor, Lincoln, NE) with the following settings: resolution 42 μm, quality high, focus offset 3.54 cm, and intensity 5.5. The LiCor Odyssey software v3.0 was used to calculate integrate intensities of iRFP expression for each well.
Results
4°C storage
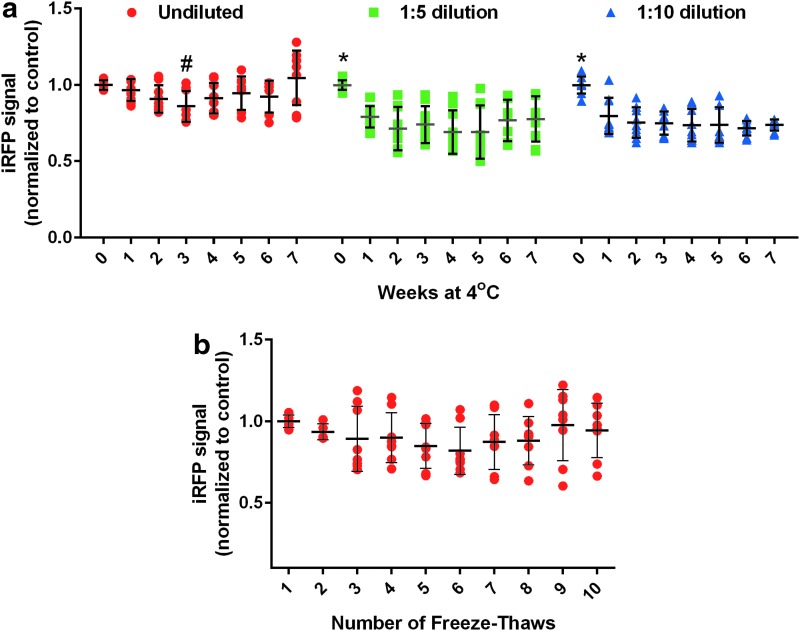
As part of routine use of viral vectors, there are practical considerations such as the storage conditions and how these conditions affect the activity or transduction efficiency. Using an AAV1 vector expressing an infrared fluorescent protein under the control of a neuronal promoter (AAV1-CaMKII-eNpHR3.0-iRFP), this study examined the transduction of primary cortical neurons after storage at 4°C for up to 7 weeks. For undiluted virus (2.1 −5.9 × 1012 vg/mL), there was up to a 20% loss in transgene expression over 7 weeks at 4°C (Fig. 1a) with a trend of improved but more variable transduction over time. Virus diluted 1:5 or 1:10 had a slightly higher loss (up to 40%) over 7 weeks, with the biggest decrease in activity during the first week.
Figure 1.
Adeno-associated virus (AAV) stability at 4°C and after freeze–thaw cycles. (a) AAV1-CaMKII-eNpHR3.0-iRFP was diluted and stored at 4°C for up to 7 weeks. Rat primary cortical neurons were transduced on DIV6 with AAV and imaged on day in vitro (DIV) 15. Graphs show each sample normalized to control with mean and standard deviation (SD). # indicates significantly different from the freshly thawed group (0 weeks at 4°C), p < 0.05; three independent experiments, three samples per experiment). * indicates significantly different from all others in group, p < 0.05). (b) AAV1-CaMKII-eNpHR3.0-iRFP was frozen and thawed up to 10 times. Rat primary cortical neurons were transduced on DIV 6 and imaged on DIV 15. Graphs show each sample normalized to control with mean and SD. No significant difference among groups (three independent experiments, two to three samples per experiment).
Freeze–thaw testing
The effect of multiple freeze–thaw cycles (10 maximal) on virus transduction was tested, using the same AAV1 vector as the 4°C stability testing described above. Compared with the first thaw of an aliquot stored at −80°C, the transduction efficiency based on transgene expression was reduced by 10% after the second freeze–thaw cycle (Fig. 1B). The efficiency of transgene expression was further decreased by another 10% on average after the third freeze–thaw cycle. Additional freeze–thaws showed increased variability in transduction ranging from 60% to 120% of the initial thaw (Fig. 1B).
Dehydration testing
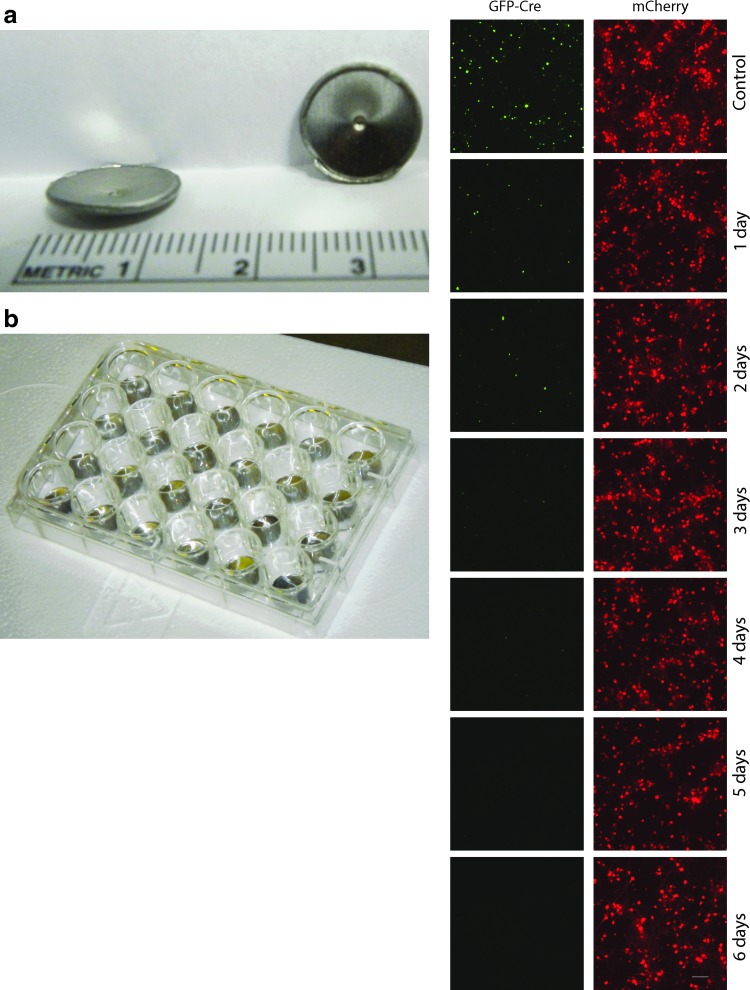
AAV viral vectors are produced in biosafety cabinets with stainless-steel surfaces. For neuroscience applications, such as optogenetic experiments in rodents, steel syringes or cannula are often used to infuse AAV vectors into the brain. The “metal slug assay” was developed to examine the stability of AAV on stainless-steel surfaces. Concave metal slugs generated as “hole punches” in stainless-steel metal sheets were obtained, and these were used to hold small volumes of concentrated virus inside 24-well plates (Fig. 2). To create a robust assay for active vector (i.e., capable of conveying transgene expression to target cells), an AAV1 vector expressing a Cre-recombinase translationally fused to green fluorescent protein (AAV-GFP-Cre) was used. In the presence of Cre-dependent reporters that use a strong promoter such as EF1a, any Cre-dependent recombination will lead to production of fluorescent reporter. AAV-GFP-Cre was applied to metal punches and allowed to dry on the slug for up to 6 days at room temperature. Reconstituted virus and a Cre-dependent AAV expressing mCherry fluorescent protein (AAV-EF1a-DIO-mCherry) were used to co-transduce rat primary cortical neurons. Although a time-dependent decrease in the amount of mCherry expression was observed, active AAV-GFP-Cre virus was detected after 6 days on the slug at room temperature (Fig. 2). Nuclear staining with DAPI demonstrated equivalent cells were present at all time points (Supplementary Fig. S3).
Figure 2.
Metal slug assay. (a) Sterile stainless-steel slugs were placed in a 24-well plate. (b) Ten microliters of AAV1-SP-GFP-Cre was placed in the center of each slug and allowed to dry at room temperature for up to 6 days. AAV was reconstituted and added to rat primary cortical neuron cultures (DIV 6), along with AAV1-EF1α-DIO-mCherry. (c) Cells were imaged on DIV 15 for GFP fluorescence (green) or mCherry fluorescence (red). Images are representative of two independent experiments with two to three samples per experiment. Scale bar = 100 μm.
Ethylene oxide and autoclave testing
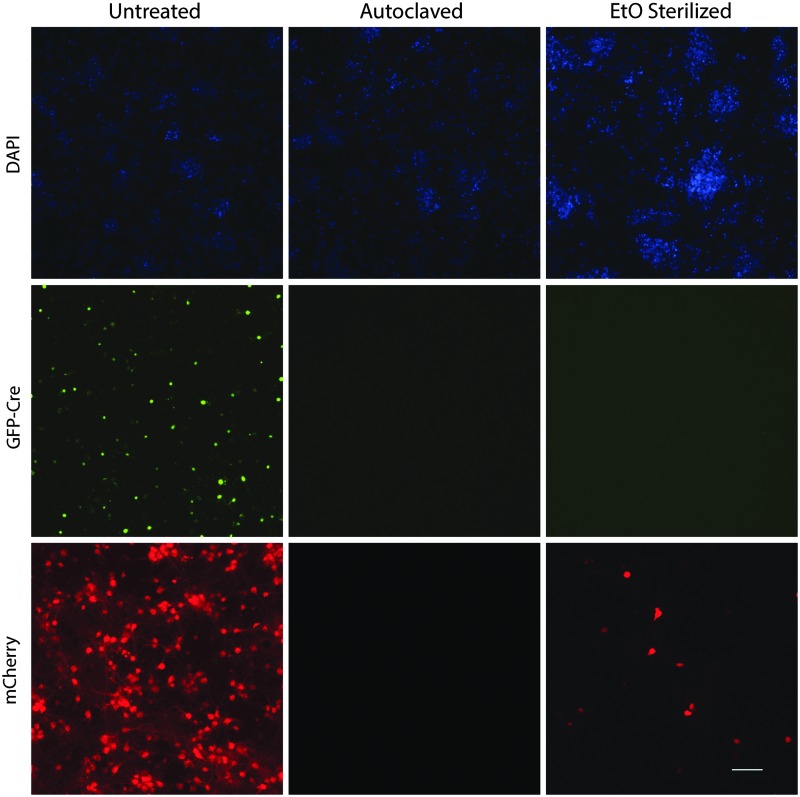
Based on the above data, the integrity of the AAV capsid is stable through temperature fluctuations and dehydration. First, ethylene oxide (EtO) was tested for its ability to inactive AAV on a metal surface. EtO is commonly used to decontaminate delicate instruments that cannot be autoclaved. EtO did not completely inactivate AAV-GFP-Cre, but steam sterilization autoclaving resulted in no detectable AAV-GFP-Cre activity (Fig. 3). Independent testing of EtO sterilizer using a Verify® disposable biological EO test pack (Steris, Mentor, OH) confirmed the EtO was functioning properly (data not shown).
Figure 3.
Autoclave and ethylene oxide (EtO) inactivation of AAV. AAV1-SP-GFP-Cre was placed in the center of stainless-steel slugs and allowed to dry. Slugs were left at room temperature and sterilized by autoclave or ETO sterilization as indicated. AAV was reconstituted and added to rat primary cortical neuron cultures (DIV 6), along with AAV1-EF1α-DIO-mCherry. Cells were imaged on DIV 15 for DAPI-stained nuclei (blue), GFP fluorescence (green), and mCherry fluorescence (red). Images are representative of two (ETO) and three (autoclave) independent experiments with three samples per experiment. Scale bar = 100 μm.
Decontamination solution testing
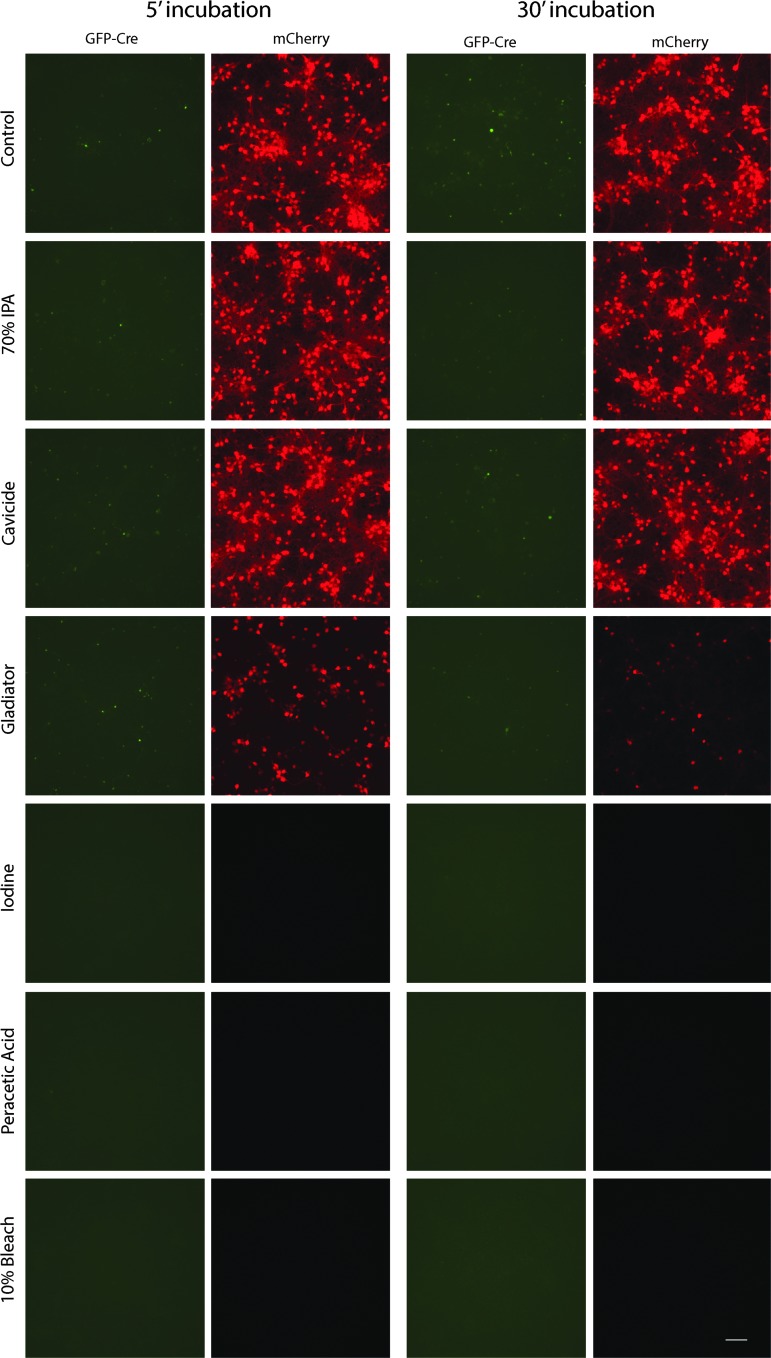
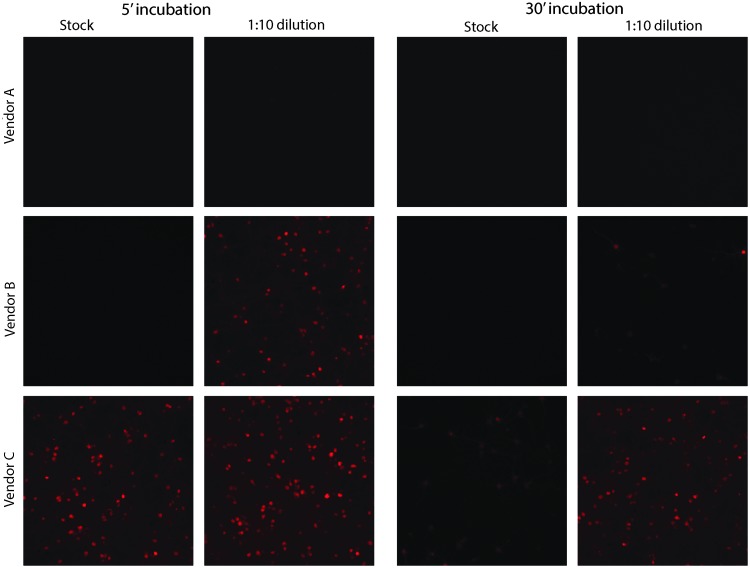
Next, the ability of a variety of commonly used laboratory disinfectants to inactivate an AAV1 vector was examined. Undiluted AAV-GFP-Cre virus was incubated in decontamination solutions for 5 or 30 min and then was diluted, dialyzed, concentrated, and used to co-transduce rat primary neurons with AAV-EF1a-DIO-mCherry (Fig. 4). Of the reagents tested, only 0.5% peracetic acid, 10% bleach, and iodine (1% available) eliminated AAV-GFP-Cre-mediated transduction (Fig. 4). The integrity of the dialysis cassettes after exposure to various decontamination solutions was tested, and any observed loss of transduction was not due to loss of virus from the cassette (Supplementary Fig. S4). Although the use of bleach and iodine was effective, they are not optimal for cleaning of stainless-steel surfaces or instruments due to their corrosive nature. PAA-based solutions are commonly used for decontamination of medical, agricultural, and food production facilities. Three ready-made, commercially available PAA-based solutions were evaluated at the working stock and 1:10 for their ability to inactivate AAV-Cre-GFP. As before, virus was incubated in various decontamination solutions for 5 or 30 min and then was diluted, dialyzed, concentrated, and used to co-transduce rat primary neurons with AAV-EF1a-DIO-mCherry (Fig. 5). Vendor A's stock solution (5–6% PAA) was 100% effective at 5 min using stock or 1:10 dilution. Vendor B's stock solution (0.23% PAA) was 100% effective at the stock solution at 5 min but required 30 min at 1:10 to inactivate AAV-GFP-Cre. Vendor C's stock solution (0.05%) required 30 min and did not completely inactivate AAV. Based on these data, decontamination solutions with 0.25% PAA for 5 min are recommended for inactivating AAV1 vectors.
Figure 4.
Inactivation of AAV using decontamination solutions. AAV1-SP-GFP-Cre was incubated at room temperature for 5 or 30 min in decontamination solutions (1:10 ratio of AAV to indicated decontamination solution). Solutions were diluted, dialyzed overnight, and concentrated. The AAV was added to rat primary cortical neuron cultures (DIV 6), along with AAV1-EF1α-DIO-mCherry. Cells were imaged on DIV 15 for GFP fluorescence (green) or mCherry fluorescence (red). Images are representative of one (iodine), four (peracetic acid [PAA]), and five (70% IPA, Gladiator, Cavicide, 10% bleach, control) independent experiments with three samples per experiment. Scale bar = 100 μm.
Figure 5.
Comparison of PAA solutions for AAV inactivation. PAA solutions were obtained from three different vendors (Vendor A = 5–6% PAA, Vendor B = 0.23% PAA, Vendor C = 0.05% PAA). AAV1-SP-GFP-Cre was incubated at room temperature for 5 or 30 min in decontamination solutions (1:10 ratio of AAV to indicated dilution of decontamination solution). Solutions were diluted, dialyzed overnight, and concentrated. The AAV was added to rat primary cortical neuron cultures (DIV 6) along with AAV1-EF1α-DIO-mCherry. Cells were imaged (three independent samples) on DIV 15 for mCherry fluorescence (red). Scale bar = 100 μm.
Discussion
AAV vectors are currently the predominant choice for viral vector-mediated transgenesis in cell culture, rodent and primate models, and human gene therapy. Here, the stability of AAV1 was tested under conditions of temperature fluctuation, dehydration, and decontamination solutions. AAV1 can be stored undiluted at 4°C for at least 7 weeks with a 15–20% reduction in transgene expression. After 10 freeze–thaw cycles, AAV1 showed up to 40% reduction in the ability to confer transgene expression and was highly variable after three freeze–thaw cycles. A novel assay was developed to assess the stability of AAV1 on metal surfaces. Using this assay, it was demonstrated that when AAV1 was air dried and left on metal surface for up to 6 days, transduction-capable virus could be recovered. Lastly, it was found that decontamination solutions should be carefully selected to ensure inactivation of AAV1-based vectors while not affecting the integrity of equipment or instruments.
The study examined how storage temperature affects the ability of AAV1 to transduce primary cortical neurons. The data suggest that for short-term storage (a few weeks), the undiluted virus should be stored at 4°C rather than be subject to multiple freeze–thaw cycles. In the present experiments, multiple freeze–thaw cycles were performed in a single setting, but the study did not test how extended storage at −80°C would affect the number of freeze–thaw cycles. The study by Gruntman et al. showed that a vector stored at 4°C for a year was slightly less stable than the same vectors stored at −80°C for 1 year. They also demonstrated that AAV thawed two to four times did not reduce expression of their transgene expressed in serum of injected animals compared to vector with one freeze–thaw cycle.6 Collectively, using different assay system, the present findings are consistent with previous published work that AAV is stable at 4°C to −80°C. The specific stability profile for a given serotype and vector backbone should be empirically determined, but the data provided herein and by Gruntman et al. can be used for comparison.
A novel assay was developed to measure AAV stability on stainless-steel metal surfaces (the “metal slug assay”). AAV1 is prepared in biosafety cabinets with metal surfaces and injected into animals with metal syringes. In the case of rodent neurosurgery, the syringes and needles are costly and may be reused. Ensuring AAV1 is inactivated is important for the safety of the user and for avoiding cross-contamination of samples. The choice of testing an AAV1 vector co-expressing Cre recombinase and GFP served two purposes. First, the detection of GFP fluorescence indicates a relatively high level of viral transduction compared to Cre activity, which can be below detection by immunofluorescence but detected by a Cre-dependent reporter protein (i.e., mCherry). Second, the enzymatic activity of Cre serves as an amplifier of AAV-mediated transgene expression by switching many copies of mCherry, even if few genomes (in theory only one) of AAV-Cre enter the cell. For AAV vectors expressing enzymes such as recombinase (Cre, FLP, etc.) or Cas9 for genome editing, dedicated syringes/needles are recommended and should be cleaned with 0.25% PAA for 5 min.
The expansive tropism of AAV vectors comes from the many available serotypes, which allows for targeting of cell types of different tissues and different species. For the current study, a commonly used serotype, AAV1, was chosen. Here, the vector genomes contain inverted terminal repeats (ITRs) from AAV serotype 2, but the rep and cap genes are from serotype 1.21 Throughout this article, “AAV1” is used, but it is also referred to as “AAV2/1” in other publications. An advantage of AAV1 is that it can be highly purified using affinity-based purification methods (Supplementary Fig. S1), thereby making the final product a solution of proteinaceous viral particles that consists of DNA genome and three viral proteins (VP1, VP2, and VP3). Because the AAV used in this study is highly pure, it is felt that its stability is not affected by contaminating cellular proteins from the viral production. The stability of AAV1 purified by a different method with higher levels of contaminating proteins was not evaluated, but the purification method should be considered when extrapolating the present results to other preparations of AAV1. The translatability of the present data from AAV1 to other serotypes is also unclear. However, the overall dodecahedral structure of the AAV nucleocapsid is similar between serotypes.22 Further studies are needed for additional serotypes of AAV vectors, and this study serves as a general guideline for evaluating stability of AAV vectors and provides a rationale for carefully reviewing safety practices when working with AAV-based vectors.
Overall, the data presented herein demonstrate that AAV1 is very stable as a virion, and appropriate decontamination procedures should be employed to promote the safe use of all AAV vectors and to reduce the possibility cross-contamination among experiments.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute on Drug Abuse. We acknowledge Dr. Ira Baum, Dr. Christopher Richie, Ms. Lowella Fortuno, Ms. Carrie Wertheim, and Dr. Rajtarun Madangopal for technical contributions and experimental suggestions. We thank Ms. Janette Lebron and Dr. Deon Harvey for assistance with editing the manuscript. We thank Arrow Metal Fabricators of Baltimore, MD, for contributing the metal slugs used for testing. A portion of this work was presented at the American Society for Gene and Cell Therapy Meeting 2014 and was published as an abstract.
Author Disclosure
No competing financial interests exist.
References
- 1.Ferreira V, Petry H, Salmon F. Immune responses to AAV-vectors, the glybera example from bench to bedside. Front Immunol 2014;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 2013;4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998;72:2224–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rayaprolu V, Kruse S, Kant R, et al. Comparative analysis of adeno-associated virus capsid stability and dynamics. J Virol 2013;87:13150–13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruntman AM, Su L, Su Q, et al. Stability and compatibility of recombinant adeno-associated virus under conditions commonly encountered in human gene therapy trials. Hum Gene Ther Methods 2015;26:71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackman SL, Beneduce BM, Drew IR, et al. Achieving high-frequency optical control of synaptic transmission. J Neurosci 2014;34:7704–7714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calu DJ, Kawa AB, Marchant NJ, et al. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J Neurosci 2013;33:214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchant NJ, Whitaker LR, Bossert JM, et al. Behavioral and physiological effects of a novel kappa-opioid receptor-based DREADD in rats. Neuropsychopharmacology 2016;41:402–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bock R, Shin JH, Kaplan AR, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci 2013;16:632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDevitt RA, Tiran-Cappello A, Shen H, et al. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep 2014;8:1857–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seif T, Chang SJ, Simms JA, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 2013;16:1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zsebo K, Yaroshinsky A, Rudy JJ, et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res 2014;114:101–108 [DOI] [PubMed] [Google Scholar]
- 14.Brantly ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A 2009;106:16363–16368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol 2009;66:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahenk Z, Galloway G, Clark KR, et al. AAV1.NT-3 gene therapy for Charcot-Marie-Tooth neuropathy. Mol Ther 2014;22:511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendell JR, Sahenk Z, Malik V, et al. A Phase 1/2a follistatin gene therapy trial for Becker muscular dystrophy. Mol Ther 2015;23:192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dismuke DJ, Tenenbaum L, Samulski RJ. Biosafety of recombinant adeno-associated virus vectors. Curr Gene Ther 2013;13:434–452 [DOI] [PubMed] [Google Scholar]
- 19.Howard DB, Powers K, Wang Y, et al. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology 2008;372:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson MJ, Wires ES, Trychta KA, et al. SERCaMP: a carboxy-terminal protein modification that enables monitoring of ER calcium homeostasis. Mol Biol Cell 2014;25:2828–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabinowitz JE, Rolling F, Li CW, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 2002;76:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther 2006;14:316–327 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.