Abstract
Despite advances in understanding autoimmune diabetes in animal models, there has been little progress in altering the natural course of the human disease, which involves progression to insulin deficiency. Studies with immunosuppressive agents have shown short-term effectiveness, but they have not induced tolerance, and continuous treatment is needed. We studied the effects of hOKT3γ1(Ala-Ala), a humanized Fc mutated anti-CD3 monoclonal antibody, on the progression of type 1 diabetes in patients with recent-onset disease in a randomized controlled trial. In general, the drug was well tolerated. A single course of treatment, within the first 6 weeks after diagnosis, preserved C-peptide responses to a mixed meal for 1 year after diagnosis (97 ± 9.6% of response at study entry in drug-treated patients vs. 53 ± 7.6% in control subjects, P < 0.01), with significant improvement in C-peptide responses to a mixed meal even 2 years after treatment (P < 0.02). The improved C-peptide responses were accompanied by reduced HbA1c and insulin requirements. Clinical responses to drug treatment were predicted by an increase in the relative number of CD8+ T-cells in the peripheral blood after the lymphocyte count recovered 2 weeks after the last dose of drug. We conclude that treatment with the anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improved C-peptide responses and clinical parameters in type 1 diabetes for at least 2 years in the absence of continued immunosuppressive medications.
Keywords: AUC, area under the response curve; mAb, monoclonal antibody; MMTT, mixed-meal tolerance test
Considerable progress has been made in understanding the targets and pathogenesis of the autoimmune response that leads to type 1 diabetes and preventing the disease in animal models, but there has been little progress in developing new treatments for patients. Previously tested agents did not have lasting effects on the disease once the drug was discontinued and therefore required continuous treatment to prevent progression of β-cell loss (1–5). For example, treatment with cyclosporin A enhanced the rates of non–insulin- and partial insulin-requiring remissions, but the clinical remission was lost once the agent was discontinued (6). The risk-to-benefit ratio was felt to be unfavorable when the toxicities and risks of long-term immune suppression including infections or the renal toxicity of cyclosporin were considered (7). Other agents including azathioprine with prednisone or anti-lymphocyte antibody with prednisone raised similar concerns (8,9). To develop a clinically useful treatment, agents that have lasting effects or induce tolerance are needed so that continuous immunosuppressive medication is not required.
Preclinical studies by Chatenoud et al. (10,11) in the NOD mouse and our studies in multidose streptozotocin-induced diabetes showed that treatment with anti-CD3 monoclonal antibody (mAb) could prevent and even induce tolerance to autoimmune responses (12). Treatment of hyperglycemic NOD mice for 5 days at the time of diagnosis induced remission in 80% of the mice, and the disease did not recur after the antibody treatment was discontinued. Mice that had been successfully treated with the anti-CD3 mAb resisted adoptive transfer of diabetes. Moreover, when NOD mice that had been treated with anti-CD3 mAb after the onset of hyperglycemia but that had persisting hyperglycemia received syngeneic islet grafts, the grafts were not destroyed. These studies indicate that anti-CD3 mAb can induce tolerance toward the pancreatic β-cell and thereby to type 1 diabetes in the NOD mouse. More recent studies suggest that the prolonged duration of the therapeutic effect may involve the induction of regulatory T-cells that can control autoimmune responses through a transforming growth factor-β–dependent mechanism (13).
In humans, an anti-CD3 mAb, OKT3, has been used for prevention of transplant rejection, but the side effects of the murine mAb rendered it unsuitable for treatment of otherwise healthy patients (14–16). Primarily to eliminate the toxicities of OKT3, Bluestone and colleagues (17,18) developed a humanized anti-CD3 mAb, hOKT3γ1(Ala-Ala), which retains the binding region of OKT3, but amino acids 234 and 235 of the human IgG1 Fc have been changed to alanines. Based on the preclinical studies that showed efficacy in murine models of diabetes even at the time of presentation with hyperglycemia and the acceptable adverse event profile that was seen in studies of renal and renal/pancreas allograft recipients and patients with refractory psoriatic arthritis (19,20), we initiated a phase I/II randomized controlled study to study the safety and efficacy of a single course of treatment with hOKT3γ1(Ala-Ala) on the loss of insulin production over 2 years in patients with new onset type 1 diabetes. We previously reported that the mAb prevented the loss of insulin production over the 1st year after diagnosis of type 1 diabetes in the initial one-half of the study entrants (21). In this study, we provide our findings from the complete study participants who were followed for 2 years. We have carried out additional studies to determine the relationships between changes in T-cells that were observed in vivo with the activation properties of the mAb in vitro. Our studies show that a single course of treatment with hOKT3γ1(Ala-Ala) can prevent the loss of insulin production for 1 year after treatment at diagnosis, and the clinical effects persist for at least 2 years. Clinical responses are not related to changes in conventional autoantibodies but are associated with an increase in the number of CD8+ T-cells in vivo. These findings, together with our previous studies in patients and in the NOD mouse, suggest that treatment with anti-CD3 mAb modifies the progression of the autoimmune process.
RESEARCH DESIGN AND METHODS
Forty-two individuals with type 1 diabetes between the ages of 7.5 and 30 years and within 6 weeks of hospital discharge or diagnosis were recruited for participation. The diagnosis of diabetes was established by American Diabetes Association criteria and the diagnosis of type 1a diabetes was verified by the presence of anti-GAD65, anti-ICA512, and/or anti-insulin autoantibodies. The demographic information on the study subjects is shown in Table 1. The study was approved by the Institutional Review Boards at Columbia Presbyterian Medical Center, National Institute of Diabetes and Digestive and Kidney Diseases, University of Utah, and the University of California at San Francisco.
TABLE 1.
Demographics of the patients
| Drug treated | Control | |
|---|---|---|
| n | 21 | 21 |
| Sex (male/female) | 14/7 | 12/9 |
| Age (years) | 13.9 ± 1.17 | 14.9 ± 1.31 |
| History of DKA | 20 | 21 |
| HbA1c at entry (%) | 8.98 ± 0.38* | 7.97 ± 0.24 |
| Fasting C-peptide | 0.207 ± 0.026 | 0.210 ± 0.015 |
| GAD (%) | 81 | 74 |
| ICA512 (%) | 71 | 74 |
| IAA (%) | 62 | 74 |
Data are means ± SE unless noted otherwise from the time of study entry.
P < 0.05.
The study design was a phase I/II randomized, controlled, open-label study to test safety and clinical efficacy of the anti-CD3 mAb. The control group underwent the same metabolic and immunologic studies as in the drug-treated group, but the patients did not receive anti-CD3 mAb and were not hospitalized. Because this was the first use of the anti-CD3 mAb in children and in patients with type 1 diabetes, the initial four drug-treated subjects were required to be >13 years old. During the period that these four subjects were enrolled, three subjects, also >13 years old, were enrolled in the control group. After these seven subjects were enrolled, the age was extended to 8–30 years old, and all subjects were randomly assigned to the drug treatment or control groups. After randomization, blood samples were drawn for immunologic studies and HbA1c, and a 4-h mixed-meal tolerance test (MMTT) was performed. For this study, the evening long- or intermediate-acting insulin and the morning insulin were withheld. After drawing samples for baseline C-peptide and glucose, the patients drank a liquid meal (6 ml/kg; Boost; Mead Johnson, Evansville, IN) over 5 min. The C-peptide levels were measured in samples drawn after 15, 30, 60, 90, 120, 150, 180, 210, and 240 min.
The drug-treated patients were hospitalized for 3–14 days. Most of the patients were treated at the Irving Center for Clinical Research at Columbia University Medical Center. Two patients were treated at the National Institutes of Health, and one patient received drug treatment in the Pediatric Clinical Research Center at University of California at San Francisco. The anti-CD3 mAb was produced by Dr. Jeffrey Bluestone. It was administered intravenously over 15 min in the following dosing: In the first 12 subjects it was day 1, 1.42 μg/kg; day 2, 5.67 μg/kg; day 3, 11.3 μg/kg; day 4, 22.6 μg/kg; and days 5–14, 45.4 μg/kg. Because of the development of anti-idiotypic antibodies after the completion of drug treatment, the dosing was modified for patients 13–21 to day 1, 460 μg/m2; day 2, 919 μg/m2; and days 3–12: 1,818 μg/m2. This dosing change was a 23% increase. All patients were observed for 6 h after each daily infusion, but some elected to remain as outpatients during the treatment period. Adverse events were scored using the National Cancer Institute common toxicity criteria.
Cell-bound mAb was measured by flow cytometry during drug administration by competition with OKT3-fluorescein isothiocyanate, and modulation of CD3 was detected by comparing the binding of OKT3d-fluorescein isothiocyanate, which recognizes an epitope on CD3 different from OKT3, in the drug-treated patient to a control subject. The maximal coating and modulation of CD4+ cells were 58.6 ± 4.1% and 45.9 ± 2.7% and of CD8+ cells were 64.3 ± 3.4% and 50 ± 3.4%. In addition to measuring the coating and modulation of CD3, samples for complete blood counts and blood chemistry analyses were drawn throughout the 14-day drug infusion period and at 2- to 3-month intervals afterward in the drug-treated patients. Every 6 months, serum and blood samples were collected from control and drug-treated patients for immunologic studies and HbA1c, and a repeat MMTT was performed.
Clinical management of diabetes.
During the 2-year study period, all patients were treated with insulin and were advised not to discontinue its use. The clinical management of the type 1 diabetes was done by the patients’ personal physicians. In addition to diet, insulin treatment generally included at least three injections of mixtures of rapid and intermediate or slow-acting insulins; about one-half of the subjects in each group used continuous subcutaneous insulin infusion.
Assay measurements.
C-peptide levels were measured at the Diabetes Research and Training Center at the University of Chicago by radioimmunoassay (22). The intra- and interassay coefficients of variation in this assay were 7 and 15%, respectively. The C-peptide response to the mixed meal was expressed as the total area under the response curve (AUC). HbA1c levels were measured by latex immunoagglutination inhibition methodology (DCA 2000; Bayer, Elkhart, IN) except in three patients followed at the National Institutes of Health where HbA1c was measured by affinity chromatography (Isolab).
Autoantibodies against GAD65, IA2/ICA512, and insulin were measured using previously described immunoprecipitation methods (23). Briefly, human GAD651–585 (complete protein) and human IA2/ICA512603–979 (full cytoplasmic portion) were labeled with [35S]methionine, during in vitro transcription and translation (TnT; Promega, Madison WI). High-performance liquid chromatography–purified human [125I]monoiodo-TyrA14 insulin was purchased (Amersham). Each serum sample was assayed in triplicate. The inter- and intra-assay coefficients of variation were all less than 16, 13, and 11% for the GADab, IA2/ICA512ab, and insulin autoantibody assays, respectively; and intra-assay coefficients of variation were less than 7% for all assays. Using the mean of triplicates on each index serum and each unknown serum, an antibody “index” was calculated as: index = (unknown serum − index negative)/(index positive − index negative). All sample indexes above the 95th percentile of normal school-aged children were confirmed by retesting before final designation.
Anti-idiotype antibodies were detected by enzyme-linked immunosorbent assay by measuring binding of human IgM, IgG, or IgA to plate-bound OKT3. The specificity of the binding was confirmed by demonstrating displacement of binding with soluble drug added to the enzyme-linked immunosorbent assay plates.
Data analysis.
An increase or decrease in the response was designated if the change in the response was greater or less than 7.5% (one-half of the interassay coefficient of variation of the C-peptide assay) of the response at study entry. A χ2 analysis was performed to test the effect of drug treatment on responses to MMTT. The effect of drug treatment on the AUC of the C-peptide response to a MMTT, HbA1c levels, and insulin dosage was evaluated by a mixed-effects ANCOVA model, using the baseline value as a covariate. The changes in CD4-to-CD8 T-cell ratios were evaluated with a similar approach. A Student’s t test was used to compare variables between drug-treated and control subjects. The statistical analyses were done using StatView (SAS, Cary, NC) and STATA (College Station, TX). A P < 0.05 was considered to be of statistical significance.
RESULTS
Recruitment and treatment of patients with new-onset type 1 diabetes with hOKT3γ1(Ala-Ala).
Forty-two patients with new-onset type 1 diabetes were randomly assigned to a drug treatment or control group. The demographic information of the two groups is shown in Table 1. One of the subjects in the control group was lost to follow-up after 6 months, and one of the subjects in the control group became pregnant after month 18 of follow-up. All patients in the drug-treated group completed the 24-month study. The groups were similar in terms of age, sex, duration of diabetes, frequency of diabetic ketoacidosis, and fasting C-peptide levels. However, the HbA1c levels were significantly higher in the drug-treated group at the time of study entry (P < 0.05).
Patients randomly assigned to drug treatment received either a 12-day (n = 9) or 14-day (n = 12) course of hOKT3γ1(Ala-Ala), which included 2 or 4 days of incremental dose escalation to 10 days of a “full dose” of drug. The most frequent adverse events included fever (36.4%) (Tmax = 39.9°C), headache (72.7%), myalgia (22.7%), or arthralgia (13.6%), all of which generally occurred with the first full dose of the drug on the 3rd or 5th day of drug administration. Other less frequent adverse events included nausea, diarrhea, or vomiting; rigors; or fatigue. These symptoms were generally mild and controlled with nonsteroidal anti-inflammatory drugs, acetaminophen, and/or antihistamine and resolved after the initial full doses of the drug. These cytokine release syndrome-like symptoms and signs are consistent with the finding of cytokine release after drug administration that has been reported previously (24). In 90.9% of drug-treated subjects, a rash developed, generally after the 5th dose of drug. The rash had an urticarial appearance and often involved the hands, but the distribution and appearance were variable between individual patients. The rash lasted for up to 3 weeks after the final dose of drug and was occasionally pruritic. A single patient developed grade 3 thrombocytopenia that led to withholding of drug. The platelet count recovered to baseline levels within 5 days.
The drug treatment caused a decrease in the number of circulating lymphocytes (Fig. 1). The nadir in lymphocyte count generally occurred after the first full dose of drug, but the number of circulating cells increased thereafter despite continued administration of the drug. By 2 weeks after the final dose of drug, the average absolute lymphocyte count had reached 80% of the baseline levels in 81% of the drug-treated individuals. The patient who developed grade 3 thrombocytopenia did not receive the full course of drug treatment, but her data are included in the intention-to-treat statistical analyses. Anti-idiotype antibodies were detected in 50% of the subjects at 6 months and in 43% of the subjects at 12 months. The anti-idiotype antibodies were not detectable at dilutions of serum greater than 1:1,000.
FIG. 1.
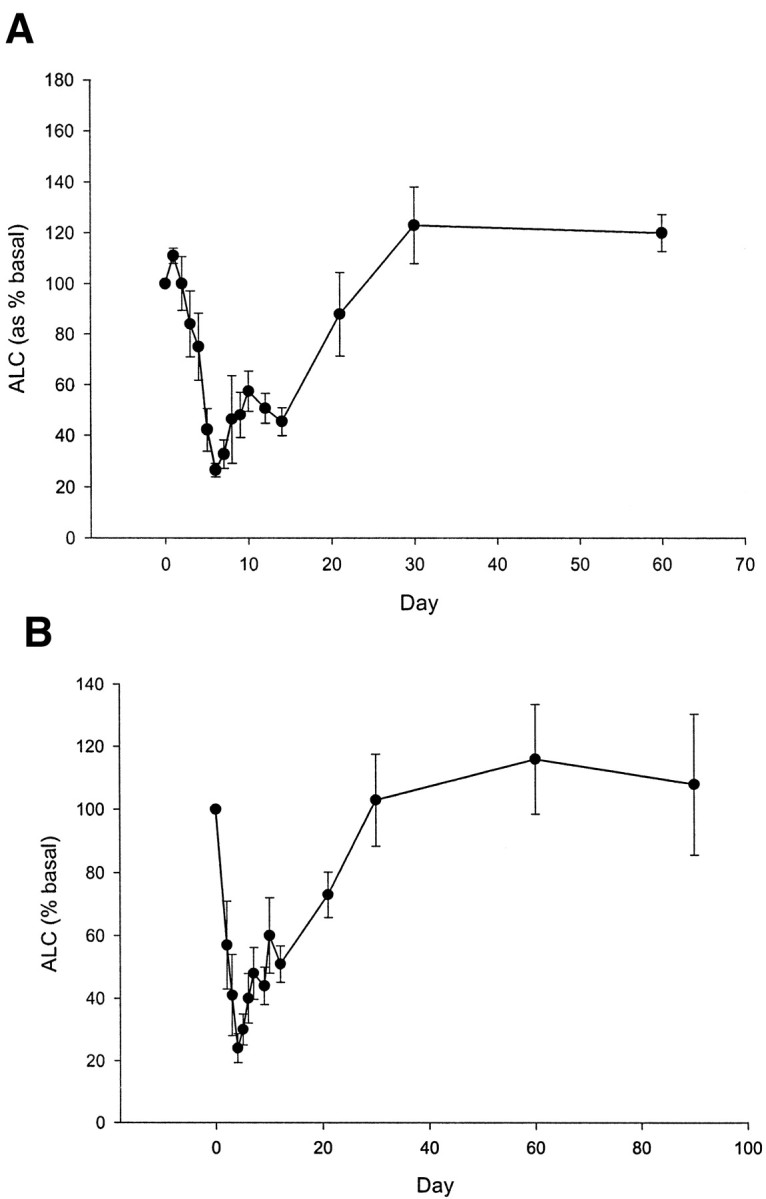
Absolute lymphocyte counts in patients with type 1 diabetes treated with hOKT3γ1(Ala-Ala). Twelve and nine patients received a 14-day (A) or 12-day (B) treatment protocol as described. The absolute lymphocyte counts in the peripheral blood are shown (means ± SE) expressed as a percentage of the absolute lymphocyte count before treatment.
The patients were followed for 2 years and were seen by their physicians and/or the study investigators at ∼3- to 6-month intervals. We observed no long-term side effects that we could relate to drug treatment. None of the patients developed an unusual infectious illness, and no changes in the number or types of circulating lymphocytes were detected.
Effects of drug treatment on C-peptide responses.
The subjects in both groups returned for a 4-h MMTT every 6 months. Patients in the drug treatment group displayed significantly higher C-peptide response to the MMTT. One year after enrollment, 15 of 21 drug-treated but only 4 of 19 control subjects maintained the same or an increased C-peptide response to a MMTT (Table 2, P < 0.01). After 1 year, the average C-peptide response to the MMTT was 97.1 ± 9.6% of the response at study entry in the drug-treated group, whereas it was 53.1 ± 7.6% of the response at entry in the control subjects (P = 0.001). Interestingly, after 6 months, the average MMTT response was 106 ± 8.6% of the response at study entry, suggesting improvement soon after drug treatment, which was seen in only 4 of 19 control subjects. At 12 months, 7 of 21 treated subjects had an increase in C-peptide responses that averaged 39 ± 12.4% and ranged between 10 and 100% above the AUC at study entry.
TABLE 2.
Changes in the C-peptide responses during a MMTT at 6 and 12 months
| 6 months* |
12 months* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Increase | No change | Decrease | Increase | No change | Decrease | |||||
| Drug | 9 | 6 | 6 | 7 | 8 | 6 | ||||
| Control | 2 | 2 | 15 | 1 | 3 | 15 | ||||
Data are n. The response was designated as unchanged if it was within 15% of the response at study entry.
P < 0.01.
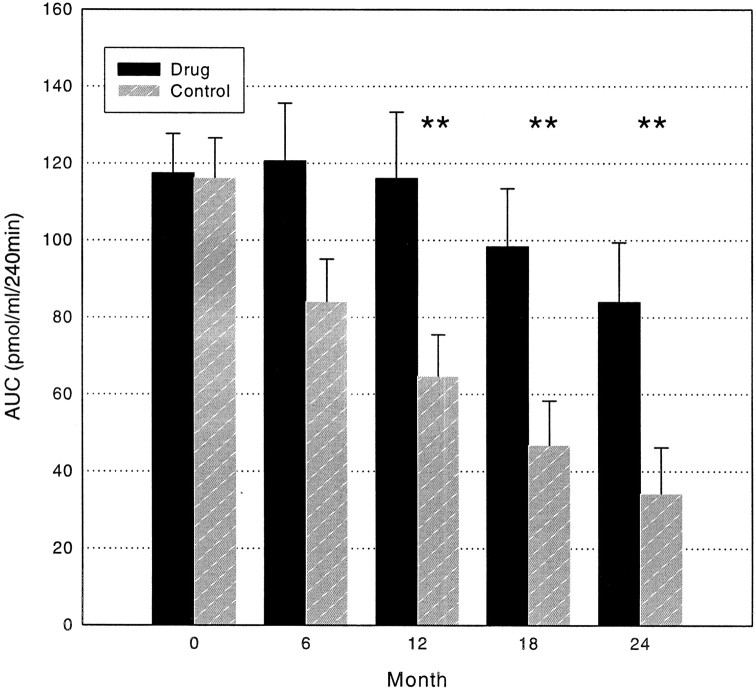
The clinical effects of drug treatment persisted even 2 years after study entry (Fig. 2). Using a mixed-effects ANCOVA model incorporating all of the data over the 2-year study period, a significant treatment effect was found (P = 0.002). The average AUC was significantly greater in the drug-treated group after 12, 18, and even at 24 months (P < 0.02 at 12, 18, and 24 months). The Diabetes Control and Complications Trial had suggested that a stimulated C-peptide of ≥0.2 pmol/ml was associated with improved metabolic control in patients with type 1 diabetes (25). The frequency of patients that met this threshold was significantly greater in the group receiving drug treatment (67%) than in the control subjects (26%) at 24 months (Table 3, P = 0.01). The greatest effect of drug treatment occurred in the first 6 months after drug treatment. A mixed-effects ANCOVA model did not show a statistical difference in the slopes of the change in the AUC after the initial improvement.
FIG. 2.
C-peptide responses to a MMTT in the control and drug-treated groups. The total AUC of the C-peptide during a 4-h MMTT is shown for the drug-treated and control groups (means ± SE). ▪, drug treated; ▪, control. **P < 0.02.
TABLE 3.
Number of subjects with stimulated C-peptide responses of >0.2 at 2 years after study entry
| >0.2 pmol/ml | <0.2 pmol/ml | |
|---|---|---|
| Drug* | 14 | 7 |
| Control | 5 | 14 |
P = 0.014.
Improvement in clinical metabolic parameters with drug treatment.
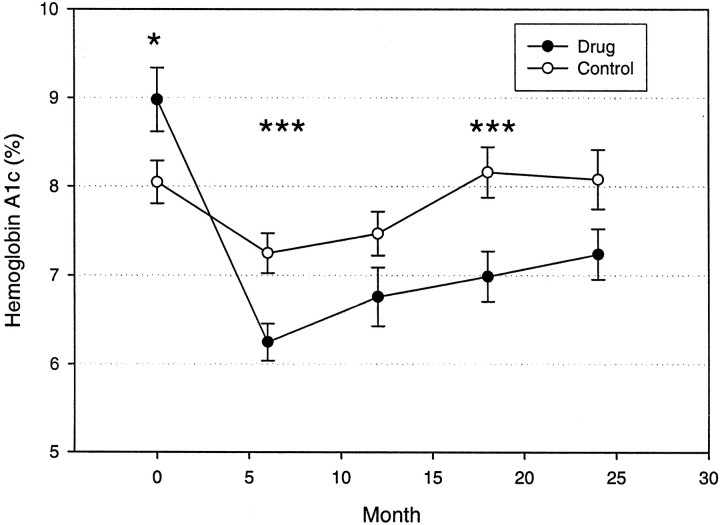
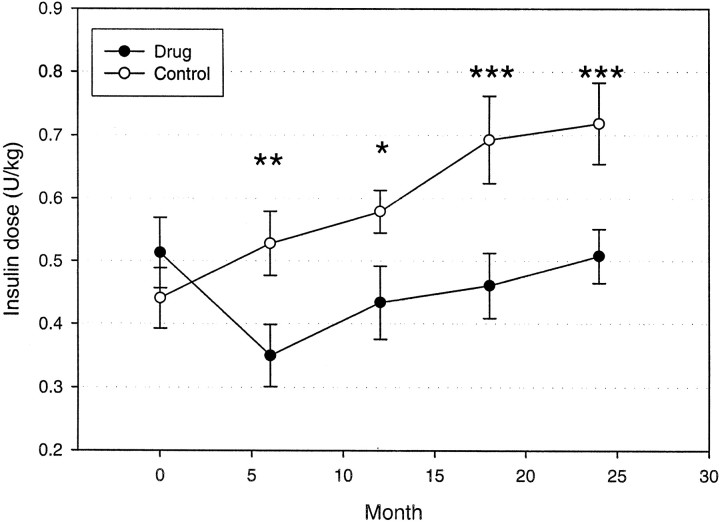
The HbA1c levels were significantly improved in the drug-treated group over the 2-year study period (P = 0.004) (Fig. 3). The average HbA1c remained under 7% out to 18 months in the drug-treated group, and the mean values were significantly lower than those for the control subjects at months 6 and 18. The improvement in the HbA1c levels was not due to the increased use of insulin in the drug-treated group because the drug treatment group actually used significantly lower doses of insulin despite the improved glycemic control (Fig. 4) (P = 0.001). The insulin dosage was significantly lower in the drug-treated group at months 6–24.
FIG. 3.
HbA1c levels in the control and drug-treated groups. The means ± SE for each group is shown. ○, control; •, drug treated. *P < 0.05, ***P < 0.01.
FIG. 4.
Insulin use in the control and drug-treated groups. The mean ± SE of the daily insulin dosage (units/kilogram) is shown. ○, control; •, drug treated. There was a significant effect of drug treatment on reduction in insulin usage. *P < 0.05, **P < 0.02, ***P < 0.01.
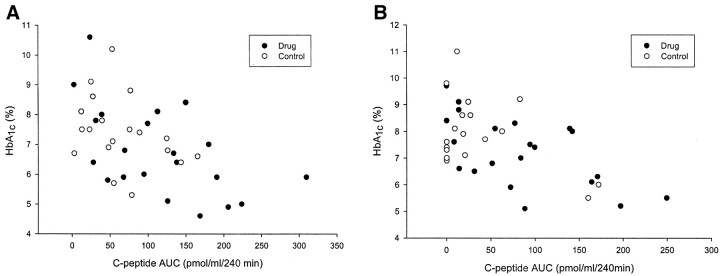
The approach to management of diabetes such as the use of insulin pumps and multiple injections of insulin was similar in the two groups. However, the C-peptide AUC was significantly correlated with the A1c response at each of the time points, suggesting that the improved HbA1c was associated with improved insulin responses (6 months: r = −0.518, P = 0.002; 12 months: r = −0.53, P = 0.003; 18 months r = −0.50, P = 0.001; 24 months r = −0.59, P < 0.001; Fig. 5). Thus, these data suggest that the improved HbA1c was associated with an improvement in the AUC of the C-peptide responses in the MMTTs.
FIG. 5.
Relationship between the C-peptide response (AUC) to a MMTT and HbA1c. The values from each subject at 6 (A) and 12 (B) months are shown. There was an inverse relationship between the C-peptide response and the HbA1c levels at each time point: 6 months, r = −0.52, P < 0.001; 12 months, r = −0.52, P < 0.001. ○, control; •, drug treated.
Predictors of clinical response to treatment.
The fasting C-peptide levels at study entry were similar in the patients who did and did not respond to drug treatment. In those that did respond, defined as maintained or increased C-peptide response to the MMTT, the fasting C-peptide at study entry was 0.213 ± 0.032 pmol/ml, and in those who did not, it was 0.192 ± 0.047 pmol/ml (P = 0.7). Likewise, the difference in the ages of the responders (14.8 ± 1.53 years) and nonresponders (11.7 ± 1.20 years) was not statistically significant (P = 0.24).
All of the subjects were positive for at least one autoantibody at the time of study entry. We measured the index of anti-GAD65, anti-ICA512, and insulin antibodies every 6 months to determine whether mAb treatment affected the levels of the autoantibodies or their isotypes. There was little change in the index of anti-GAD65 and anti-ICA512 antibodies or on the isotypes of these autoantibodies (not shown) over the 2-year study period in either of the two groups. In the study subjects overall, the insulin antibody indexes increased from an average of 0.754 ± 0.213 to 2.44 ± 0.604 after 24 months (P < 0.01), but the differences between the drug-treated and control groups were not statistically significant. The insulin autoantibody index was significantly lower in the drug-treated responders compared with the drug-treated nonresponders at 12 months (P = 0.01), but these differences were not persistent.
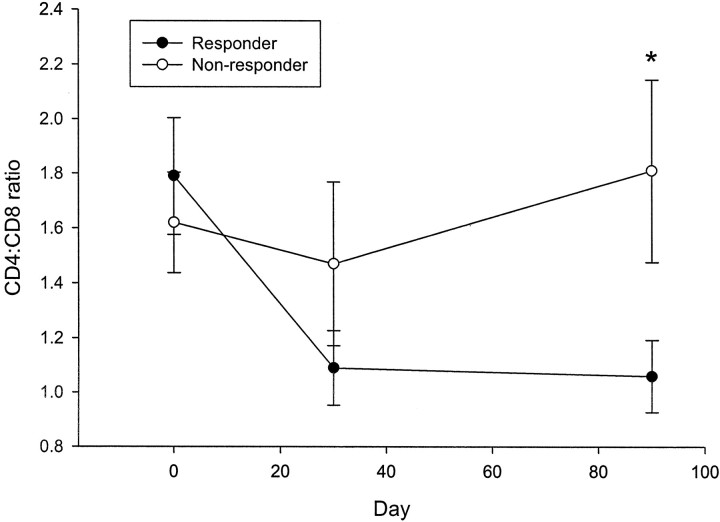
The clinical responses at 1 year could be predicted by the changes in the absolute number of CD4+ and CD8+ T-cells after drug therapy (Fig. 6). The mAb induced a transient reduction in the number of circulating T-cells with the start of drug therapy, but a recovery of the cell number occurred even while the drug was administered. However, at the time that the T-cells recovered, at months 1 and 3, there was an increase in the relative number of CD8+ T-cells compared with the baseline values in the clinical responders, which accounted for a decrease in the ratio of CD4 to CD8 T-cells in the clinical responders compared with the nonresponders (P = 0.011). The number of CD8+ cells in the drug-treated responders increased by 294 × 109/l (P = 0.01 compared with baseline), whereas in the nonresponders, the number of CD8+ cells changed insignificantly compared with the baseline number of cells (17 × 109/l, P = 0.63). The number of CD4+ T-cells was not significantly different in the responders or the nonresponders on day 90 compared with study entry.
FIG. 6.
CD4-to-CD8 T-cell ratios in clinical responders and nonresponders to drug treatment. Patients who received anti-CD3 mAb were classified as clinical responders or nonresponders based on the changes in the C-peptide response to a MMTT at 12 months compared with the response at study entry. The ratios of the number of CD4 to CD8 T-cells were calculated from measurements before treatment and at 30 and 90 days after drug administration. Clinical responders showed a reduction in the CD4-to-CD8 T-cell ratio (P < 0.02). ○, nonresponder (n = 6); •, responder (n = 15). *P < 0.05.
DISCUSSION
We have found that C-peptide responses to a mixed meal are significantly preserved for at least 2 years in patients with recent-onset type 1 diabetes who receive a single 12- or 14-day course of anti-CD3 mAb hOKT3γ1(Ala-Ala) treatment in a randomized controlled trial. Our study design did not involve placebo treatment of the control group because of difficulties in masking the treatment and the risk/benefit of placebo treatment in children. The effects of the mAb treatment are most dramatic for the 1st year after treatment when the C-peptide response is essentially unchanged compared with that found at diagnosis; but up to 2 years after diagnosis there is a significant improvement in C-peptide responses in patients treated with anti-CD3 mAb compared with control subjects. After 2 years, a greater proportion of the drug-treated patients retained clinically important insulin production compared with the untreated control subjects. Unlike other previously tested approaches to immune therapy of type 1 diabetes, the present results were achieved without continuous immunosuppressive medication, and there was no apparent long-term toxicity from the drug treatment.
The ability to make insulin is an important determinant of metabolic control in diabetes, and therefore interventions that lead to retention of insulin secretory capacity would be expected to improve glucose control. Consistent with this understanding, the improvement in the C-peptide responses was accompanied by an improvement in glucose control reflected by HbA1c level. Moreover, this was accomplished with reduced requirements for exogenous insulin despite similar usage of delivery systems including multiple daily doses and insulin pumps in the two groups. In the Diabetes Control and Complications Trial, improved glucose control was shown to mitigate the decline in C-peptide responses in patients with type 1 diabetes who were receiving intensive metabolic control (25). Because of this effect of glucose control per se on C-peptide responses, we reanalyzed our data using the HbA1c as a covariate and found that there still was a statistically significant effect of drug treatment on the C-peptide responses (P < 0.05; not shown). However, because insulin production, glucose control, and insulin usage are interrelated, we cannot precisely identify the contributions of metabolic control, different handling of insulin therapy because of the knowledge of treatment assignment to the effects on these parameters and the C-peptide responses that were seen in the drug treatment group (26). Nonetheless, our findings are consistent with the notion that increased insulin production may account for less dependence on exogenous insulin and improved metabolic control.
One year after study entry, 33% (7 of 21) of the drug-treated but only 5% (1 of 19) of the control subjects showed an increased C-peptide response to the mixed meal compared with the initial response (P < 0.05). A number of possible explanations can account for this finding including the effects of improved glycemic control, eliminating the functional effects of insulitis on insulin secretion, as well as an improvement in β-cell mass. However, we do not have a means of measuring β-cell mass directly and therefore can only rely on C-peptide response, a functional response, as an indirect reflection of β-cell mass. Studies with dynamic testing of islet function may help to resolve the basis for the changes in C-peptide responses in the future.
Similar to our previously reported findings, we could identify patients who showed a clinical response to the mAb by a relative increase in the number of circulating CD8+ T-cells after mAb treatment, instead of more conventional measures such as titers or isotypes of autoantibodies or even the fasting C-peptide at study entry. The number of subjects we studied in each group was small—a difference in autoantibody responses, particularly to insulin, might become apparent with a larger sample size. In addition, the changes in CD8+ T-cells that we found may reflect a direct biologic marker of the drug activity rather than individual differences in the immune responses of responders and nonresponders. In a previous report, we showed that the mAb had activating properties in vitro and in vivo, although compared with the parent anti-CD3 mAb OKT3, this modified molecule is markedly less mitogenic. Nonetheless, we found that CD4+ T-cells, taken from patients at the conclusion of drug treatment, produced interleukin-10, which could have immune regulatory effects in vivo (24). In preliminary studies, we have found that the mAb shows preferential activation of CD8+ T-cells in vitro, consistent with that seen with OKT3 (27). Thus, the differences in proliferative responses of CD4 and CD8+ T-cells to anti-CD3 mAb may account for the changes in CD8+ T-cells that we observed, but the relationship between these changes and the clinical effects that were seen require further study.
Unfortunately, we do not have a persistent biologic marker of the drug’s effect such as enumeration of regulatory T-cells or effector T-cells, and therefore we can only rely on the changes in biologic function as an indicator of recurrent autoimmunity. Our data from the 2nd year after drug treatment would suggest that repeated intervention(s) may be needed to sustain the clinical effects because there was a decline in the AUC in the drug-treated group after the 1st year. This is not surprising considering the need for repeated treatments with most immunotherapies and is consistent with a reduction in regulatory pathways and/or recovery of pathogenic effector cells or mechanisms with time. Repeated treatment may be needed, particularly in younger patients who continue to have an active thymus with continuous production of autoantigen reactive T-cells and/or disordered immune regulatory mechanisms. Thus, future studies will need to address a means of prolonging the effects of drug treatment either with a repeated treatment combination or, if precluded by anti-idiotype antibodies, with other immune agents and/or with other approaches to stimulate β-cell regeneration to restore a normal islet mass.
Acknowledgments
This work was supported by National Institutes of Health Grants R01-DK-57846, U19A146132, P30-DK-63608, M01-RR-00645, M01-RR-01271, and P60-DK-20595 and by Juvenile Diabetes Research Foundation Grant 4-1999-711 and Center Grant 4-1999-841.
We thank Dr. Jay Skyler for his reading and helpful comments on the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Stiller CR, Dupre J, Gent M, Jenner MR, Keown PA, Laupacis A, Martell R, Rodger NW, von Graffenried B, Wolfe BM: Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science 223: 1362–1367, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Skyler JS, Lorenz TJ, Schwartz S, Eisenbarth GS, Einhorn D, Palmer JP, Marks JB, Greenbaum C, Saria EA, Byers V: Effects of an anti-CD5 immunoconjugate (CD5-plus) in recent onset type I diabetes mellitus: a preliminary investigation. The CD5 Diabetes Project Team. J Diabetes Complications 7: 224–232, 1993 [PubMed] [Google Scholar]
- 3.Skyler JS, Rabinovitch A: Cyclosporine in recent onset type I diabetes mellitus: effects on islet beta cell function. Miami Cyclosporine Diabetes Study Group. J Diabetes Complications 6: 77–88, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Bougneres PF, Carel JC, Castano L, Boitard C, Gardin JP, Landais P, Hors J, Mihatsch MJ, Paillard M, Chaussain JL, et al.: Factors associated with early remission of type I diabetes in children treated with cyclosporine. N Engl J Med 318: 663–670, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Buckingham BA, Sandborg CI: A randomized trial of methotrexate in newly diagnosed patients with type 1 diabetes mellitus. Clin Immunol 96: 86–90, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bougneres PF, Landais P, Boisson C, Carel JC, Frament N, Boitard C, Chaussain JL, Bach JF: Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes 39: 1264–1272, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Parving HH, Tarnow L, Nielsen FS, Rossing P, Mandrup-Poulsen T, Osterby R, Nerup J: Cyclosporine nephrotoxicity in type 1 diabetic patients: a 7-year follow-up study. Diabetes Care 22: 478–483, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S: Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med 319: 599–604, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Eisenbarth GS, Srikanta S, Jackson R, Rabinowe S, Dolinar R, Aoki T, Morris MA: Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res 2: 271–276, 1985 [PubMed] [Google Scholar]
- 10.Chatenoud L, Thervet E, Primo J, Bach JF: Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 91: 123–127, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatenoud L, Primo J, Bach JF: CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 158: 2947–2954, 1997 [PubMed] [Google Scholar]
- 12.Herold KC, Bluestone JA, Montag AG, Parihar A, Wiegner A, Gress RE, Hirsch R: Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes 41: 385–391, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L: TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 9: 1202–1208, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Chatenoud L: OKT3-induced cytokine-release syndrome: prevention effect of anti-tumor necrosis factor monoclonal antibody. Transplant Proc 25: 47–51, 1993 [PubMed] [Google Scholar]
- 15.Chatenoud L: Humoral immune response against OKT3. Transplant Proc 25: 68–73, 1993 [PubMed] [Google Scholar]
- 16.Chatenoud L: Use of CD3 antibodies in transplantation and autoimmune diseases. Transplant Proc 26: 3191–3193, 1994 [PubMed] [Google Scholar]
- 17.Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, Hanna LS, Dolan KP, Parren PW, Bluestone JA, Jolliffe LK, Zivin RA: In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol 200: 16–26, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Alegre ML, Peterson LJ, Xu D, Sattar HA, Jeyarajah DR, Kowalkowski K, Thistlethwaite JR, Zivin RA, Jolliffe L, Bluestone JA: A non-activating “humanized” anti-CD3 monoclonal antibody retains immunosuppressive properties in vivo. Transplantation 57: 1537–1543, 1994 [PubMed] [Google Scholar]
- 19.Woodle ES, Xu D, Zivin RA, Auger J, Charette J, O’Laughlin R, Peace D, Jollife LK, Haverty T, Bluestone JA, Thistlethwaite JR Jr: Phase I trial of a humanized, Fc receptor nonbinding OKT3 antibody, huOKT3γ1(Ala-Ala) in the treatment of acute renal allograft rejection. Transplantation 68: 608–616, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Utset TO, Auger JA, Peace D, Zivin RA, Xu D, Jolliffe L, Alegre ML, Bluestone JA, Clark MR: Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J Rheumatol 29: 1907–1913, 2002 [PubMed] [Google Scholar]
- 21.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA: Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346: 1692–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Faber OK, Binder C, Markussen J, Heding LG, Naithani VK, Kuzuya H, Blix P, Horwitz DL, Rubenstein AH: Characterization of seven C-peptide antisera. Diabetes 27 (Suppl. 1): 170–177, 1978 [DOI] [PubMed] [Google Scholar]
- 23.Woo W, LaGasse JM, Zhou Z, Patel R, Palmer JP, Campus H, Hagopian WA: A novel high-throughput method for accurate, rapid, and economical measurement of multiple type 1 diabetes autoantibodies. J Immunol Methods 244: 91–103, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA: Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3γ1(Ala-Ala). J Clin Invest 111: 409–418, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual β-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. J Clin Endocrinol Metab 65: 30–36, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Madsbad S, Krarup T, Regeur L, Faber OK, Binder C: Effect of strict blood glucose control on residual B-cell function in insulin-dependent diabetics. Diabetologia 20: 530–534, 1981 [DOI] [PubMed] [Google Scholar]
- 27.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H: Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol 168: 1528–1532, 2002 [DOI] [PubMed] [Google Scholar]