Abstract
Background
Cardiovascular disease burden and treatment patterns among patients with familial hypercholesterolemia (FH) in the United States remain poorly described. In 2013, the FH Foundation launched the Cascade Screening for Awareness and Detection (CASCADE) of FH Registry to address this knowledge gap.
Methods and Results
We conducted a cross-sectional analysis of 1295 adults with heterozygous FH enrolled in the CASCADE-FH Registry from 11 US lipid clinics. Median age at initiation of lipid-lowering therapy was 39 years, and median age at FH diagnosis was 47 years. Prevalent coronary heart disease was reported in 36% of patients, and 61% exhibited 1 or more modifiable risk factors. Median untreated low-density lipoprotein cholesterol (LDL-C) was 239 mg/dL. At enrollment, median LDL-C was 141 mg/dL; 42% of patients were taking high-intensity statin therapy and 45% received >1 LDL-lowering medication. Among FH patients receiving LDL-lowering medication(s), 25% achieved an LDL-C <100 mg/dL and 41% achieved a ≥50% LDL-C reduction. Factors associated with prevalent coronary heart disease included diabetes mellitus (adjusted odds ratio 1.74; 95% confidence interval 1.08–2.82) and hypertension (2.48; 1.92–3.21). Factors associated with a ≥50% LDL-C reduction from untreated levels included high-intensity statin use (7.33; 1.86–28.86) and use of >1 LDL-lowering medication (1.80; 1.34–2.41).
Conclusions
FH patients in the CASCADE-FH Registry are diagnosed late in life and often do not achieve adequate LDL-C lowering, despite a high prevalence of coronary heart disease and risk factors. These findings highlight the need for earlier diagnosis of FH and initiation of lipid-lowering therapy, more consistent use of guideline-recommended LDL-lowering therapy, and comprehensive management of traditional coronary heart disease risk factors.
Keywords: coronary artery disease, familial hypercholesterolemia, genetic heart disease, low-density lipoprotein cholesterol, statin therapy
Heterozygous familial hypercholesterolemia (from here on designated FH) is a genetic disorder characterized by elevated low-density lipoprotein cholesterol (LDL-C), a markedly elevated risk of atherosclerotic cardiovascular disease (ASCVD), and a 50% chance of inheritance among offspring.1 Recent epidemiological2, 3 and genetic studies4, 5 support a prevalence of FH of ≈1 in 200 in the general community; if these prevalence figures hold true for the US population, as many as 1.5 million Americans may have FH, a substantially higher figure than suggested by earlier estimates.6 Despite data confirming that prompt detection and treatment of FH reduces the risk of premature coronary heart disease (CHD) and death,7 the majority of FH patients worldwide remain unidentified,3 and, of those diagnosed, most fail to receive appropriate treatment.2
Several mandates have been issued to address the vast detection and treatment gaps in FH.3, 8 The 2013 American College of Cardiology/American Heart Association (ACC/ AHA) cholesterol guidelines highlighted individuals with an LDL-C ≥190 mg/dL as the first of 3 key patient groups for whom statin initiation is recommended for primary prevention and the only primary prevention population for whom high-intensity statin therapy is universally recommended.9 Countries, including the Netherlands10 and the United Kingdom,11 have instituted nationwide programs to identify, treat, or track individuals with FH. In the United States, the landmark Make Early Diagnoses—Prevent Early Deaths (MEDPED) registry, active from 1989 to 2004, established LDL-C thresholds for the diagnosis of FH and elucidated risk factors for CHD in FH patients.12, 13 However, the registry no longer remains active, and contemporary diagnostic and treatment patterns, comorbidities, and cardiovascular disease status of FH patients in the United States remain poorly described, which has been highlighted as a major research gap in a scientific statement from the American Heart Association.14 To characterize the contemporary features and treatment of FH patients in the United States, the FH Foundation15—a nonprofit research and advocacy organization—launched the Cascade Screening for Awareness and Detection (CASCADE) FH Registry, a national initiative to increase FH awareness, characterize trends in treatment, and monitor clinical and patient-reported outcomes.16 Here, we aim to describe the characteristics and treatment patterns of adult FH patients enrolled in the CASCADE-FH Registry.
Methods
Study Design
The current analysis focused on adult FH patients enrolled at participating clinical sites in the CASCADE-FH Registry.16 To be included in CASCADE-FH, all patients must have had at least one office visit at a participating lipid clinic within the past 5 years with FH diagnosed based on existing clinical or genetic diagnostic methods.16 Briefly, diagnostic criteria included—but were not limited to—Simon Broome, Dutch Lipid Clinic Network, and MEDPED. No single diagnostic method was required, largely because no consensus diagnostic criteria exist in the United States. Exclusion criteria included any secondary cause of hypercholesterolemia (eg, hypothyroidism, nephrotic syndrome, and cholestasis).14
After excluding patients below 18 years (n=202), with homozygous FH (n=43, defined as a clinical or genetic diagnosis of homozygous FH, untreated LDL-C >500 mg/dL, or use of mipomersen or lomitapide), and with missing statin dosage information (n=25), we conducted a cross-sectional analysis of 1295 adult FH patients who were enrolled in the CASCADE-FH Registry prospectively through 11 participating lipid clinics between September 2013 and April 2015 (n=436) or for whom retrospective data were abstracted by reviewing historical medical charts (n=859; Figure in the Data Supplement).16 Institutional review boards at each site reviewed and approved the protocol. Signed informed consent was required for all prospectively enrolled patients, and a waiver was approved for retrospective data abstraction. CHD was defined as any prior diagnosis of CHD, including myocardial infarction (MI) or coronary revascularization. ASCVD was defined as any prior diagnosis of CHD, stroke, transient ischemic attack, or peripheral artery disease. Clinical and laboratory data including diagnoses of CHD or ASCVD were abstracted from patient medical records and entered by trained research staff.
Statistical Analysis
Characteristics of the study population are presented as frequencies and percentages for categorical variables and median (interquartile range) for continuous variables. We defined untreated total cholesterol and LDL-C levels as the highest documented values before initiation of drug therapy or occasionally when a patient was on a drug holiday. Treated lipid levels were defined as the most recent values available at the time of inclusion into the CASCADE-FH Registry among patients on LDL-lowering medication(s). Entry lipid levels were defined as the most recent values available at the time of inclusion into the CASCADE-FH Registry, regardless of treatment status. We evaluated the association between patient characteristics and prevalent CHD abstracted at baseline in a multivariable logistic regression model with generalized estimating equations to account for site variation, adjusting for age, diabetes mellitus, current smoking, hypertension, untreated total cholesterol, and low high-density lipoprotein cholesterol (<40 mg/dL in men and <50 mg/dL in women). We compared the use of high-intensity and low- or moderate-intensity statin therapy, as well as each individual statin therapy used at baseline, the number of lipid-lowering therapies used, and treatment with lipoprotein apheresis, stratified by statin use groups using Chi-square tests. We evaluated the association between patient characteristics and LDL-C goal attainment in 2 separate logistic regression models with generalized estimating equations to account for site variation, where goal attainment is defined as treated LDL-C <100 mg/dL or a ≥50% reduction compared with untreated LDL-C.3, 8, 17 Patients not taking LDL-lowering drug therapy were excluded from both analyses of LDL-C goal attainment; in addition, patients without data on untreated LDL-C were also excluded from models of goal attainment based on a ≥50% reduction in LDL-C. Multivariable models were adjusted for all covariates with P < 0.05 in unadjusted models. Odds ratios for age at enrollment and untreated LDL-C were modeled per 10-year and 10-mg/dL increase, respectively; all other factors were modeled as binary variables.
Results
Demographics, clinical, and lipid/lipoprotein characteristics of the cohort are shown in Table 1 and Table I in the Data Supplement. The median age at enrollment was 57 years; 59% were female; and 80% were white. With regard to FH diagnosis, formal diagnostic criteria (ie, Dutch Lipid Clinic Network, Simon Broome, or MEDPED criteria) were reported in 43% of cases; 3% of all cases had genetic confirmation; and the remainder of patients were diagnosed without the use of formal diagnostic criteria or genetic confirmation (clinically diagnosed). Tendon xanthoma was reported among 19% of cases. Median ages at the times of initiation of lipid-lowering therapy and the specific diagnosis of FH were 39 and 47 years, respectively. Initiation of lipid-lowering therapy and FH diagnosis occurred before age 30 years in 17% and 22% of patients, respectively. Median untreated and treated LDL-C levels were 239 and 134 mg/dL, respectively.
Table 1.
Demographics, Clinical, and Lipid/Lipoprotein Characteristics of Adults With Heterozygous Familial Hypercholesterolemia Enrolled in the CASCADE-FH Registry
| All Subjects | |
|---|---|
| Demographics | |
| Age at enrollment, y, median (IQR) | 57 (43–66) |
| Female, % | 59.3 |
| Ethnicity, % | |
| White | 80.0 |
| Black | 7.0 |
| Hispanic | 2.9 |
| Other | 10.2 |
| FH history | |
| Age at FH diagnosis, y, median (IQR), n=1232 | 47 (31–59) |
| Age at initiation of LDL-lowering therapy, y, median (IQR), n=677 | 39 (25–50) |
| Family history of premature MI, %, n=938 | 45.0 |
| LDL-C, mg/dL, median (IQR) | |
| Untreated, n=888 | 239 (211–294) |
| Treated, n=1084 | 134 (100–183) |
| Entry, mg/dL, n=1278 | 141 (103–197) |
| Cardiovascular risk factors | |
| Number of additional modifiable cardiovascular risk factors, %* | |
| 0 | 38.8 |
| 1 | 37.8 |
| 2 | 16.1 |
| 3 | 6.6 |
| 4 | 0.8 |
| Diabetes mellitus, %, n=1280 | 13.0 |
| Current smoker, %, n=1272 | 6.9 |
| Hypertension, %, n=1283 | 42.8 |
| Low HDLC (<40 mg/dL in men, <50 mg/dL in women), %, n=1285 | 31.0 |
| Obesity (body mass index >30 kg/m2), %, n=1223 | 31.5 |
| Body mass index, kg/m2, median (IQR), n=1223 | 27.3 (24.2–31.0) |
| Cardiovascular disease | |
| ASCVD, %, n=1273† | 37.9 |
| Age at onset, y, median (IQR) | 52 (42–61) |
| CHD, overall cohort, % | 35.9 |
| Age at onset, years, median (IQR) | 51 (42–61) |
| Stroke or TIA, %, n=1282 | 4.8 |
| Aortic valve disease, %, n=1284 | 3.0 |
Sample size for calculation of prevalence rates and medians is 1295 unless otherwise noted. ASCVD indicates atherosclerotic cardiovascular disease; CASCADE, Cascade Screening for Awareness and Detection; CHD, coronary heart diseasel; FH, familial hypercholesterolemia; HDLC, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; and MI, myocardial infarction.
Additional modifiable cardiovascular risk factors defined as diabetes mellitus, current smoker, hypertension, and low HDLC.
ASCVD includes any history of CHD, stroke, TIA, transient ischemic attack.
Sixty-one percent of study participants had at least 1 additional modifiable cardiovascular risk factor, defined as diabetes mellitus, current smoking, hypertension, or low high-density lipoprotein cholesterol (below 40 mg/dL for men and below 50 mg/dL for women; Table 1). Twenty-three percent of patients had ≥2 additional modifiable risk factors. A family history of premature MI was observed in 45%.
Prevalent ASCVD was reported in 38% of patients at entry (Table 1). The predominant ASCVD diagnosis was CHD, which was reported in 36% of patients (Table II in the Data Supplement). CHD was reported among 47% of men, with a median age of onset of 47 years, and among 29% of women, with a median age of onset of 55 years. In multivariate analysis, older age, male sex, family history of premature MI, diabetes mellitus, hypertension, and untreated total cholesterol were associated with increased odds of CHD at entry (Table 2).
Table 2.
Odds Ratios for Coronary Heart Disease Among Adults With Heterozygous Familial Hypercholesterolemia (n=1282)
| Characteristic | No CHD (N=833) | CHD (N=449) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|---|---|
| Age at enrollment, y† | 53 (36–62) | 63 (55–70) | 1.61 (1.55–1.68) | 1.49 (1.35–1.64) |
| Male | 278 (33.7%) | 243 (52.3%) | 2.13 (1.59–2.87) | 2.64 (1.56–4.46) |
| Family history of premature MI | 356 (57.6%) | 227 (70.9%) | 1.81 (1.32–2.48) | 1.84 (1.36–2.50) |
| Diabetes mellitus | 59 (7.2%) | 108 (23.5%) | 3.08 (2.04–4.64) | 1.74 (1.08–2.82) |
| Current smoking | 47 (5.8%) | 41 (9.0%) | 1.27 (0.08–2.02) | 1.13 (0.59–2.15) |
| Hypertension | 233 (28.4%) | 316 (68.3%) | 4.34 (3.70–5.09) | 2.48 (1.92–3.21) |
| Low HDLC‡ | 222 (27.1%) | 176 (37.8%) | 1.52 (1.27–1.83) | 1.45 (0.96–2.18) |
| Obesity | 221 (28.1%) | 164 (37.6%) | 1.35 (0.94–1.94) | 1.09 (0.66–1.80) |
| Untreated total cholesterol, mg/dL† | 324 (298–380) | 341 (294–400) | 1.02 (1.01–1.04) | 1.02 (1.00–1.03) |
| Untreated LDL-C, mg/dL† | 238 (211–291) | 242 (212–297) | 1.02 (1.00–1.04) | 1.00 (0.97–1.04) |
| Age at FH diagnosis, y† | 43 (25–56) | 55 (42–64) | 1.37 (1.26–1.48) | 0.92 (0.81–1.05) |
| Cholesterol years score, mg/dL,* y†, § | 25 393 (16 998–33 190) | 31 175 (24 790–38 445) | 1.04 (1.03–1.05) | 0.96 (0.93–0.99) |
| Age at initiation of lipid-lowering medication, y† | 36 (22–48) | 44 (33–53) | 1.31 (1.14–1.50) | 0.86 (0.70–1.07) |
| Statin use∥ | ||||
| None | 206 (24.8%) | 120 (25.8%) | Reference | Reference |
| Low- or moderate-intensity statin | 298 (35.9%) | 127 (27.3%) | 0.89 (0.71–1.11) | 0.86 (0.69–1.07) |
| High-intensity statin | 326 (39.3%) | 218 (46.9%) | 1.66 (1.05–2.63) | 1.49 (0.80–2.78) |
ACC indicates American College of Cardiology; AHS, American Heart Association; CHD, coronary heart diseasel; CI, confidence interval; FH, familial hypercholesterolemia; HDLC, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; OR< odds ratio
Adjusted for age at enrollment, diabetes mellitus, current smoking, hypertension, untreated total cholesterol, and low HDLC.
Median (IQR) shown. For age, OR shown per 10-year increment. For untreated total cholesterol and LDL-C, OR shown per 10-mg/dL increment. For modified cholesterol years score, OR shown per 1000-mg/dL years increment
Low HDLC defined as <40 mg/dL for men and <50 mg/dL for women.
Cholesterol-years score was calculated as [untreated total cholesterol×age at initiation of lipid-lowering therapy]+[baseline total cholesterol×(age at enrollment−age at initiation of lipid-lowering therapy)].
Statin intensity is defined according to the 2013 ACC/AHA Cholesterol Guidelines.
Treatment patterns of the overall cohort are detailed in Table 3. High-intensity and low- or moderate- intensity statin use (as defined by the 2013 ACC/AHA cholesterol guidelines9) were reported among 42% and 33% of patients, respectively. Among the 25% of patients not receiving statin treatment, reasons for the lack of statin use included intolerance or allergy (60%), patient preference (11%), physician preference (11%), pregnancy (3%), cost (1%), and clinical trial participation (1%). Lipoprotein apheresis was used in 6% of adults with FH and was more commonly used in patients not treated with statin therapy. Use of >1 lipid-lowering medication was reported in 45% of patients.
Table 3.
Lipid-Lowering Therapies Used Among Adults With Heterozygous FH (n=1295)
| Overall Cohort (n=1295) | Statin-Treated (n=969)* | Not Statin-Treated (n=326)† | P Value | |
|---|---|---|---|---|
| Statin intensity† | ||||
| High | 544 (42.0%) | 544 (56.1%) | … | … |
| Low/moderate | 425 (32.8%) | 425 (43.9%) | … | … |
| No statin | 326 (25.2%) | … | 326 (100%) | … |
| Statin | ||||
| Rosuvastatin | 475 (36.7%) | 475 (49.0%) | … | … |
| Atorvastatin | 334 (25.8%) | 334 (34.5%) | … | … |
| Simvastatin | 96 (9.9%) | 96 (9.9%) | … | … |
| Pitavastatin | 45 (3.5%) | 45 (4.6%) | … | … |
| Pravastatin | 25 (1.9%) | 25 (2.6%) | … | … |
| Fluvastatin | 8 (0.6%) | 8 (0.8%) | … | … |
| Lovastatin | 5 (0.4%) | 5 (0.5%) | … | … |
| Nonstatin | ||||
| Ezetimibe | 520 (40.2%) | 438 (45.2%) | 82 (25.2%) | <0.0001 |
| Bile acid sequestrant | 189 (15.0%) | 141 (15.0%) | 48 (15.0%) | 0.9997 |
| Niacin | 165 (13.1%) | 135 (14.4%) | 30 (9.4%) | 0.0234 |
| Fibrate | 64 (5.1%) | 44 (4.7%) | 20 (6.3%) | 0.2593 |
| Statin+ezetimibe | 438 (33.8%) | 438 (45.2%) | … | … |
| Lipid-lowering medications | <0.0001 | |||
| 0 | 196 (15.1%) | 0 (0.0%) | 196 (60.1%) | |
| 1 | 515 (39.8%) | 428 (44.2%) | 87 (26.7%) | |
| 2 | 389 (30.0%) | 353 (36.4%) | 36 (11.0%) | |
| 3+ | 195 (15.1%) | 188 (19.4%) | 7 (2.1%) | |
| Lipoprotein apheresis | 77 (6.1%) | 37 (3.9%) | 40 (12.4%) | <0.0001 |
FH indicates familial hypercholesterolemia.
Any statin dose.
Among the 326 patients not receiving statin treatment, reasons for the lack of statin use included intolerance or allergy (60%), patient preference (11%), physician preference (11%), pregnancy (3%), cost (1%), and clinical trial participation (1%).
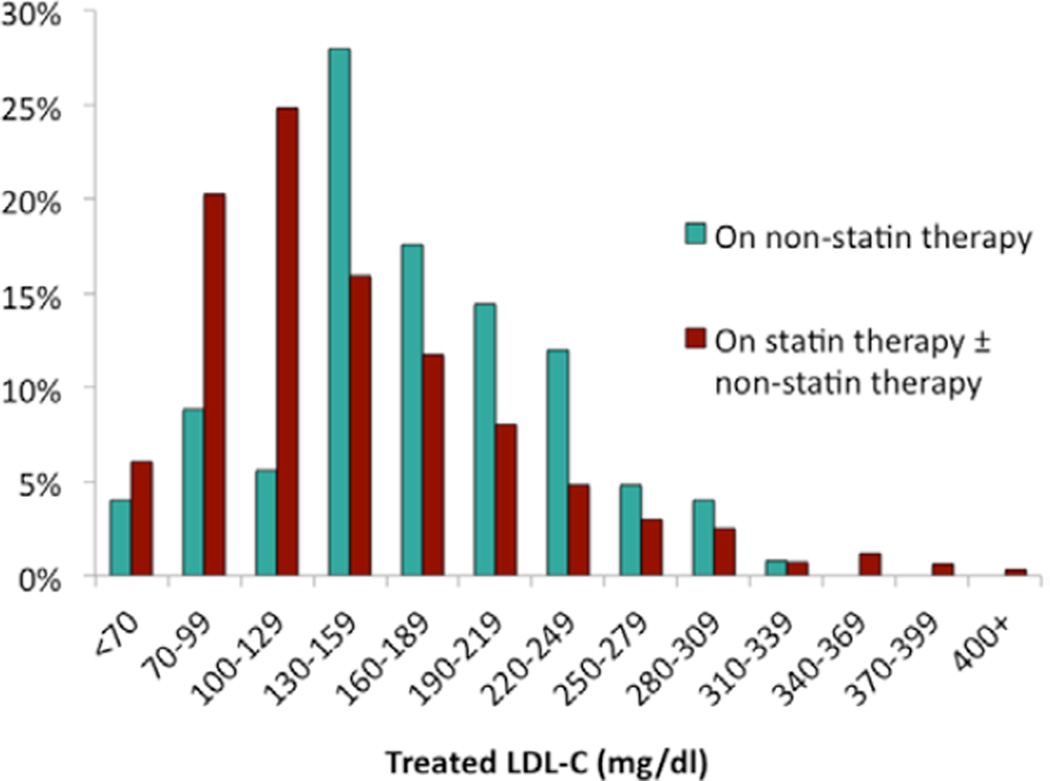
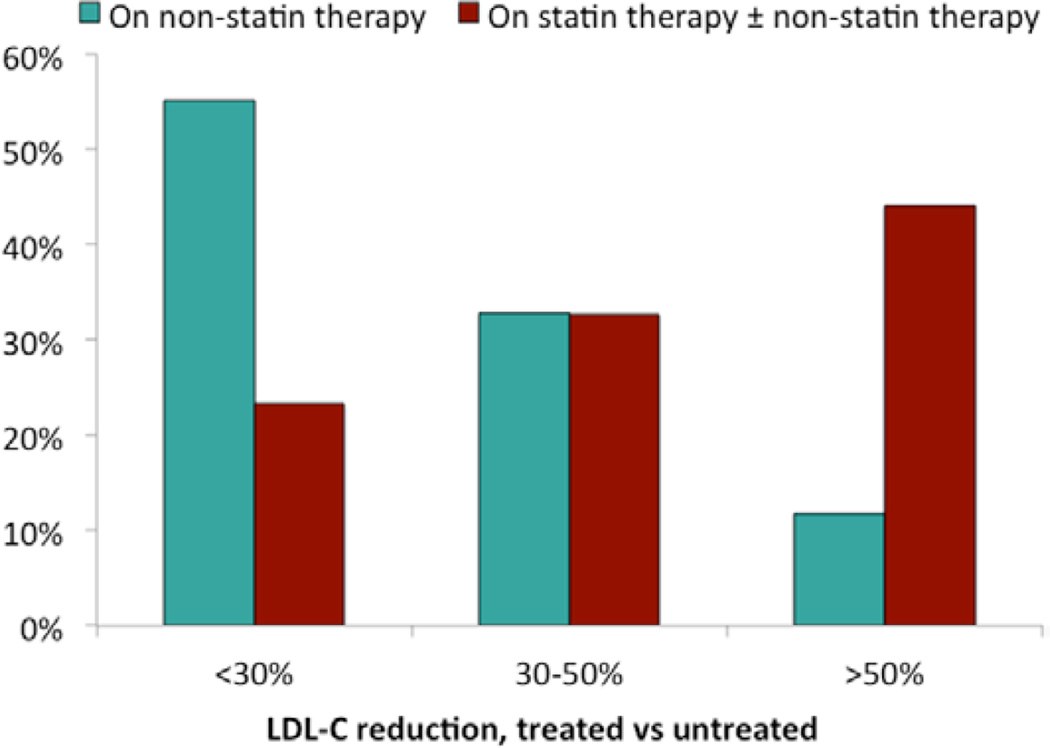
Distributions of treated LDL-C levels among 1084 patients on lipid-lowering medications and the associated LDL-C reductions from untreated values are shown in Table 4 and Figures 1 and 2. Twenty-five percent of patients had treated LDL-C <100 mg/dL, and 41% of patients achieved a ≥50% LDL-C reduction from untreated values. Older age, high-intensity statin therapy, and use of >1 LDL-lowering medication were associated with achieving a treated LDL-C <100 mg/dL (Table 5). Among 652 patients who were taking LDL-lowering medications and for whom untreated and treated LDL-C levels were available, older age, family history of premature MI, higher untreated LDL-C, high-intensity statin therapy, and use of >1 lipid-lowering medication were associated with achieving a ≥50% reduction in LDL-C (Table 6).
Table 4.
Treated LDL-C Levels and Magnitude of LDL-C Reductions Compared With Untreated Levels Among Adults With Heterozygous FH Taking LDL-Lowering Medications
| Overall Cohort | Statin-Treated | Not Statin-Treated | P Value* | |
|---|---|---|---|---|
| Treated LDL-C† | n=1084 | n=959 | n=125 | <0.0001 |
| <70 mg/dL | 63 (5.8%) | 58 (6.0%) | 5 (4.0%) | |
| 70–99 mg/dL | 205 (18.9%) | 194 (20.2%) | 11 (8.8%) | |
| 100–129 mg/dL | 245 (22.6%) | 238 (24.8%) | 7 (5.6%) | |
| 130–159 mg/dL | 188 (17.3%) | 153 (16.0%) | 35 (28.0%) | |
| 160–189 mg/dL | 135 (12.5%) | 113 (11.8%) | 22 (17.6%) | |
| ≥190 mg/dL | 248 (22.9%) | 203 (21.2%) | 45 (36.0%) | |
| LDL-C reduction‡ | n=652 | n=576 | n=76 | <0.0001 |
| ≥50% | 266 (40.8%) | 257 (44.6%) | 9 (11.8%) |
FH indicates familial hypercholesterolemia; LDL, low-density lipoprotein; and LDL-C, LDL cholesterol.
Chi-square test comparing statin users to statin nonusers.
n=1084 on LDL-lowering therapy for whom treated LDL-C values were available.
n=652 on LDL-lowering therapy for whom untreated and treated LDL-C values were available.
Figure 1.
Distribution of treated low-density lipoprotein cholesterol (LDL-C) levels by treatment status among adults with heterozygous familial hypercholesterolemia (FH) on LDL-lowering therapy (n=1084).
Figure 2.
Distribution of low-density lipoprotein cholesterol (LDL-C) reduction, comparing treated and untreated LDL-C levels, by treatment status among adults with heterozygous familial hypercholesterolemia (FH) on LDL-lowering therapy (n=652).
Table 5.
Odds Ratios for Treated LDL-C <100 mg/dL Among Adults With Heterozygous FH Taking LDL-Lowering Medications (n=1084)
| Characteristic | LDL-C ≥100 mg/dL (N=816) | LDL-C <100 mg/dL (N=268) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|---|---|
| Age at enrollment, years† | 55 (42–65) | 60 (49–67) | 1.23 (1.13–1.35) | 1.21 (1.01–1.45) |
| Male | 325 (40.1%) | 136 (50.7%) | 1.49 (1.32–1.69) | 1.24 (0.90–1.70) |
| Coronary heart disease | 273 (33.5%) | 122 (45.5%) | 1.55 (1.21–1.98) | 1.27 (0.91–1.79) |
| Family history of premature MI | 365 (61.9%) | 129 (66.5%) | 1.20 (0.77–1.88) | 1.89 (0.90–3.96) |
| Diabetes mellitus | 93 (11.5%) | 42 (15.8%) | 1.24 (0.63–2.43) | 1.10 (0.38–3.22) |
| Untreated LDL-C, mg/dL† | 245 (215–300) | 225 (197–270) | 0.95 (0.92–0.98) | 0.93 (0.90–0.97) |
| Confirmed FH mutation | 32 (3.9%) | 3 (1.1%) | 0.29 (0.06–1.38) | 0.17 (0.04–0.74) |
| High-intensity statin‡ | 374 (45.8%) | 162 (60.4%) | 4.23 (2.33–7.68) | 4.83 (2.24–10.45) |
| Low- or moderate intensity statin‡ | 333 (40.8%) | 90 (33.6%) | 2.37 (1.31–4.29) | 2.41 (0.93–6.20) |
| >1 lipid-lowering medication | 407 (49.9%) | 171 (63.8%) | 1.84 (1.46–2.32) | 1.86 (1.47–2.36) |
CHD indicates coronary heart disease; CI, confidence interval; FH, familial hypercholesterolemia; IQR, interquartile ratio; LDL, low-density lipoprotein; and LDL-C, LDL cholesterol; MI, myocardial infarction; and OR, odds ratio.
Adjusted for age, sex, untreated LDL-C, CHD, statin use, and use of >1 LDL-lowering medication.
Median (IQR) shown.
OR compared with no statin use.
Table 6.
Odds Ratios for ≥50% LDL-C Reduction, Comparing Treated and Untreated LDL-C Levels, Among Adults With Heterozygous FH Taking LDL-Lowering Medications (N=652)
| Characteristic | <50% LDL-C Reduction (N=386) |
≥50% LDL-C Reduction (N=266) |
Unadjusted OR (95% CI) |
Adjusted OR (95% CI)* |
|---|---|---|---|---|
| Age at enrollment, y† | 55 (40–65) | 57 (45–66) | 1.13 (1.00–1.28) | 1.27 (1.06–1.52) |
| Male | 131 (34.0%) | 117 (44.2%) | 1.52 (1.04–2.21) | 1.27 (0.84–1.92) |
| Coronary heart disease | 112 (29.0%) | 89 (33.5%) | 1.24 (0.91–1.68) | 0.63 (0.32–1.24) |
| Family history of premature MI | 152 (53.5%) | 145 (71.1%) | 2.12 (1.16–3.87) | 1.91 (1.09–3.34) |
| Diabetes mellitus | 37 (9.7%) | 28 (10.5%) | 1.10 (0.64–1.91) | 0.78 (0.42–1.46) |
| Untreated LDL-C, mg/dL† | 230 (208–270) | 265 (224–324) | 1.10 (1.07–1.12) | 1.06 (1.02–1.11) |
| Confirmed FH mutation | 10 (2.6%) | 13 (4.9%) | 1.97 (1.08–3.58) | 0.70 (0.31–1.54) |
| High-intensity statin‡ | 142 (36.8%) | 170 (63.9%) | 9.00 (2.76–29.32) | 7.33 (1.86–28.86) |
| Low- or moderate-intensity statin‡ | 177 (45.9%) | 87 (32.7%) | 3.68 (1.02–13.33) | 3.76 (0.79–17.91) |
| >1 lipid-lowering medication | 150 (38.9%) | 179 (67.3%) | 3.20 (2.51–4.10) | 1.80 (1.34–2.41) |
CI indicates confidence interval; FH, familial hypercholesterolemia; IQR, interquartile ratio; LDL, low-density lipoprotein; and LDL-C, LDL cholesterol; MI, myocardial infarction; and OR, odds ratio.
Adjusted for current age, sex, untreated LDL-C, family history of premature MI, confirmed FH mutation, statin use, and use of >1 LDL-lowering medication.
Median (IQR) shown.
OR compared with no statin use.
Discussion
Among adult FH patients enrolled in the US CASCADE-FH Registry, the prevalence of CHD was 47% in men and 30% in women, which is 5 to 7 times higher than the age-matched general US population.18 LDL-C goal attainment, defined as a treated LDL-C <100 mg/dL or a ≥50% LDL-C reduction, was poor, and achievement of the more aggressive goal of LDL-C <70 mg/dL was minimal. Our cross-sectional analysis also demonstrated late initiation of lipid-lowering therapy, delayed diagnosis of FH, limited use of high-intensity statin therapy and combination LDL-lowering therapy, and a substantial burden of traditional modifiable risk factors. Underdiagnosis and undertreatment of FH, which lead to missed opportunities to reduce early-onset cardiovascular morbidity and mortality, have been highlighted by recent reviews15 and guidelines,1, 3, 8, 17 including the 2013 ACC/AHA cholesterol guidelines9 and the AHA Scientific Statement on FH.14 Unfortunately, limited data have been available to determine whether FH patients in the United States are currently being identified and treated appropriately. Our analysis of data from the CASCADE-FH Registry provides the initial characterization of a large and contemporary cohort of adult patients with a clinical diagnosis of FH enrolled at 11 sites across the United States. These data quantify gaps in care and identify potential next steps to improve the management of FH patients.
Our findings are consistent with prevalence rates of CHD in FH observed in other countries2, 19 and historical US FH cohorts12, 20 (Tables III and IV in the Data Supplement). In 1974, Stone et al reported CHD in 30% of 289 FH patients followed at the National Institutes of Health.20 In an analysis of the MEDPED registry published in 2001, early-onset CHD was observed in 26% of 262 FH patients.12 The median ages of onset of CHD among men and women enrolled in the CASCADE-FH registry were 47 and 55 years, respectively, compared with a mean age of 42 and 62 years in the aforementioned 1974 National Institutes of Health study,20 and early-onset CHD, defined as onset before age 55 in men and before 65 in women, still occurred in 36% of men and 12% of women in the CASCADE-FH Registry.
Our study reaffirms the importance of traditional modifiable cardiovascular risk factors in adults with FH. At least one additional modifiable cardiovascular risk factor was identified in 61% of study participants (Table 1). Diabetes mellitus and hypertension were each significantly associated with the presence of CHD in multivariate analyses (Table 2). These findings are largely consistent with previous studies (Table V in the Data Supplement)12, 21, 22 and reinforce the importance of comprehensive preventive care to minimize cardiovascular risk in FH.
Given the inherited nature of FH and the fact that atherosclerotic risk parallels cumulative exposure to LDL-C levels, it is striking that both the diagnosis of FH and the initiation of lipid-lowering therapy in adult FH patients enrolled in the CASCADE-FH Registry occurred late in life, at median ages of 47 and 39 years, respectively. The discrepancy in these ages indicates that recognition of hypercholesterolemia— prompting initiation of drug therapy—frequently preceded the specific diagnosis of FH. Perhaps contributing to these issues, we identified a lack of uniformity in the use of diagnostic strategies among US physicians. A need exists for consensus formal diagnostic criteria for FH patients in the United States. In contrast to some European countries, such as the Netherlands, where national programs that include genetic screening and counseling have led to a high frequency of therapy early in life, only 3% of patients had genetic diagnostic testing in this cohort of academic lipid clinics. The lack of genetic testing means we cannot exclude the possibility that the CASCADE-FH population contains some individuals with polygenic hypercholesterolemia. More importantly, this reflects the fact that genetic testing remains underused in the United States, with no consensus on standard of care and variable reimbursement policies from payers. Recent guidelines from the American Academy of Pediatrics,22a National Lipid Association,1, 8 European Atherosclerosis Society,3, 23 and the International FH Foundation17 recommend assessment for FH beginning at 5 to 10 years and in the US universal screening of cholesterol levels by ages 9 to 11 years and age 2 years for children of parents with FH or a strong family history of premature CHD. This guidance is based on the good discriminatory ability of lipid testing to correctly classify FH in children, which is attenuated with age.24
Recommendations for lipid testing in family members of patients with premature CHD have existed since 1992,25 for universal screening of lipids beginning at age 20 years since 1988,26 and universal screening at age 9 to 11 years since 2011.27 The 2013 ACC/AHA adult cholesterol guidelines provided a class IB recommendation for statin initiation for patients aged ≥21 years with LDL-C ≥190 mg/dL.9 All statins are FDA-approved for treatment of children with FH beginning at the age of 8 years for pravastatin and 10 years for other statins. Pediatric statin trials demonstrate excellent safety and efficacy with follow-up of 2 years.28
Even with appropriate diagnosis of FH and initiation of LDL-lowering therapy, a minority of treated adult FH patients in the CASCADE-FH Registry achieved guideline-based goals of an LDL-C <100 mg/dL (25%) or a ≥50% reduction in LDL-C (41%). The magnitude of this treatment gap is similar to those observed in recent studies reported from France,29 the Netherlands,30 Spain,31 and the United Kingdom32 (Table IV in the Data Supplement). Two of the most potent predictors of LDL-C goal attainment were use of high-intensity statin therapy and use of >1 LDL-lowering medication, the latter previously identified in a study by Mata et al (Table VI in the Data Supplement).12, 21, 31 At the time of CASCADE-FH registry enrollment, fewer than half of the patients in our study were taking either a high-intensity statin or combination therapy, suggesting that these 2 interventions may represent readily available means to improve success achieving LDL-C targets, although patient tolerance is a limiting factor in some cases.
Elements of family history are incorporated into most diagnostic criteria for FH, and guidelines recommend completing a 4-generation pedigree for all FH patients.1, 3, 8 In our study, a family history of premature MI was reported in only 45% of adult FH patients. Several reasons may account for the low reported family history of early-onset CHD. First, the CASCADE-FH Registry restricted the definition of premature CHD to early-onset MI, similar to the Simon Broome criteria, excluding other forms of clinical CHD, such as coronary revascularization and angina. Second, data pertaining to family history of premature MI was unknown for 28% of study participants. Whether this gap is present because of patients’ lack of knowledge or a failure on behalf of healthcare providers to elicit and accurately record this information remains uncertain. Third, the availability of statins over the past 3 decades likely improved the cardiovascular health of more than a generation of FH patients, attenuating the value of a family history of premature CHD in diagnosing FH. These limitations notwithstanding, in our study, a family history of premature MI was significantly associated with prevalent CHD and achievement of a ≥50% LDL-C reduction.
In the CASCADE-FH Registry, the prevalence of diabetes mellitus was 13%, similar to the prevalence estimate of 12% reported for an age-matched general US population.33 In contrast, a recent cross-sectional analysis performed in the Netherlands reported a significantly lower prevalence of diabetes mellitus among patients with genetically confirmed FH compared with unaffected relatives, with an adjusted odds ratio of 0.49 (95% confidence interval 0.41–0.58).34 Several factors may explain this discrepancy. One explanation for this may be because of a referral bias to specialty lipid clinic of complex patients with FH and diabetes mellitus. Alternatively, findings from the Dutch cohort suggesting that FH patients may be less prone to the development of diabetes mellitus may not be generalizable to the US population. The greater body mass index (27.3 versus 23.5 kg/m2), higher prevalence of non-white populations (20% versus a small minority), and older age (57 versus 38 years) represent important risk factors for the development of diabetes mellitus that differentiate the US CASCADE-FH Registry study participants from the Dutch cohort. Third, the putative diabetogenic effect of statin therapy may have an influence.
To date, the role of genetic testing in FH—above and beyond phenotypic characterization through clinical history, physical examination, and LDL-C levels—remains debated. In the United States, genetic testing is rarely performed in routine clinical care, and insurance coverage is highly variable. Preliminary evidence largely derived from screening programs in the Netherlands35 and the United Kingdom36 suggests that a comprehensive approach to FH inclusive of genetic testing facilitates diagnosis, improves treatment, and enhances cascade screening. Based on these data, national guidelines from the United Kingdom, Australia, and the Netherlands support genetic testing for FH. On the contrary, recommendations for genetic testing are absent from recent guidelines provided by the European Society of Cardiology and the US National Lipid Association. Importantly, there is a notable lack of data regarding the downstream outcome of genetic testing for FH in US populations, where different healthcare delivery systems, patient and provider attitudes, and mutation heterogeneity may affect the success of genetic testing. To better answer the question regarding the clinical utility of genetic testing in FH in the United States, a randomized trial is currently underway comparing genetic testing and cholesterol testing alone.37 End points include effects on cascade screening, LDL control, lipid-lowering therapy, and downstream costs.
Limitations
Our analysis of the diagnosis, management, and outcomes of adult FH patients from 11 lipid specialty centers may not be generalizable to the broader US FH population. To our knowledge, the only published study to examine FH patients in a community setting is a 2012 Danish analysis of 502 FH patients identified from the large Copenhagen General Population Study.2 Despite similar median untreated lipid levels, mean ages, and prevalence rates of CHD (Table II in the Data Supplement), the Danish cohort exhibited higher on-treatment LDL-C levels compared with the CASCADE-FH Registry (182 versus 134 mg/dL). This suggests that our findings, restricted to experienced lipid clinics, may well underestimate the existing treatment gap in the United States. Increasing the number of institutions participating in the CASCADE-FH Registry (as of August 2015, 17 sites are actively enrolling) along with efforts to leverage large electronic health databases to identify patients with FH will yield more generalizable results. The latter approach, which includes the FH Foundation’s Flag, Identify, Network, Deliver (FIND) FH initiative and the Electronic Medical Records and Genomics (eMERGE) Network, may help identify a larger number and more diverse cohort of FH patients.
Although our study identified suboptimal use of high-intensity statin or combination therapy, the reasons for not pursuing these treatments, particularly in younger individuals, remain uncertain. Despite universal recommendations for statin therapy in adult FH patients,1, 3, 8, 17 1 out of 4 patients in our study were not taking statins at the time of registry enrollment, largely because of statin intolerance. The high proportion of statin nonusers in the CASCADE-FH Registry may reflect referral bias to participating specialty lipid centers, where patients who are intolerant to statins and other lipid-lowering therapies are more likely to be referred.
Data regarding medication adherence, clinic follow-up, and reasons for limited use of high-intensity statin therapy or combination lipid-lowering therapy are not available at this time. The CASCADE-FH Registry will add information regarding intolerance to lipid-lowering therapy and incorporate several measures of patient-reported medication adherence during follow-up data collection. In addition, the registry will monitor changes in medication use over time, including switching and discontinuation of lipid-lowering therapy.
Because of the cross-sectional nature of our data, associations between various characteristics and prevalent CHD or LDL-C goal attainment are hypothesis-generating and do not establish causality. Despite the high prevalence of CHD at baseline, our data may in fact underestimate the burden of CHD among adults with FH because of survival bias. The lack of an association between CHD and cholesterol years may reflect our reliance on patient self-report to document life-long LDL-C levels, and so this result must be interpreted with caution.
Finally, we cannot rule out the inclusion of phenocopies, such as familial combined hyperlipidemia or familial polygenic hypercholesterolemia. Additional analysis of patients diagnosed using existing clinical criteria and via genetic testing will be instructive in this regard. Of note, triglyceride levels were not particularly elevated, arguing against the inclusion of a large number of patients with familial combined hyperlipidemia. Nongenetic factors that may be causing elevations in LDL-C include age, postmenopausal status, diet, and obesity. For example, diets high in saturated fats, trans fats, and cholesterol may cluster in families and incorrectly suggest inheritance. Of note, such patients are also at increased risk of premature CHD and are, therefore, indicated for aggressive lipid-lowering therapy.
Conclusions
This cross-sectional analysis of the FH Foundation’s multicenter CASCADE-FH Registry provides the initial characterization of contemporary diagnostic and treatment patterns and current cardiovascular disease and risk factor burden among a large cohort of adult FH patients in the United States. The prevalence of CHD was high, and LDL-C goal attainment, defined as a treated LDL-C <100 mg/dL or a ≥50% LDL-C reduction, was low. Early diagnosis of FH and initiation of lipid-lowering therapy, use of high-intensity statin therapy and combination of LDL-lowering therapy, comprehensive management of traditional modifiable risk factors, and careful elicitation of a family history of premature CHD are suggested as opportunities to improve the care of FH patients and may represent an area that would benefit from the investigation of whether performance indices would improve care. Follow-up of CASCADE-FH Registry study participants is ongoing to prospectively examine treatment patterns and clinical outcomes.
Supplementary Material
CLINICAL PERSPECTIVE.
This cross-sectional analysis provides the initial characterization of contemporary diagnostic and treatment patterns and current cardiovascular disease and risk factor burden among 1295 adult heterozygous familial hypercholesterolemia (FH) patients in the United States from the FH Foundation’s multicenter Cascade Screening for Awareness and Detection (CASCADE)-FH Registry. The prevalence of coronary heart disease was high (36%) in FH patients, which is 5 to 7 times higher than the age-matched general population. Low-density lipoprotein cholesterol goal attainment, defined as a treated low-density lipoprotein cholesterol <100 mg/dL or a ≥50% low-density lipoprotein cholesterol reduction, was low (25% and 41%, respectively). The presence of diabetes mellitus and hypertension in FH patients were associated with significantly increased risk of coronary heart disease (adjusted odds ratio 1.74 and 2.48, respectively). Early diagnosis of FH and initiation of lipid-lowering therapy, use of high-intensity statin therapy and combination low-density lipoprotein-lowering therapy, comprehensive management of traditional modifiable risk factors, and careful elicitation of a family history of premature coronary heart disease are suggested as opportunities to improve the care of FH patients and may represent an area that would benefit by the investigation of whether performance indices would improve care.
Acknowledgments
We acknowledge the assistance of the following individuals for enrolling patients into the Cascade Screening for Awareness and Detection Familial Hypercholesterolemia (CASCADE-FH) Registry: Tracey Sikora, Kristen Dilzell, Anna Raper, Joyce Ross (University of Pennsylvania); Chandna Vasandani (UT Southwestern); Aleks Pavlovic (Stanford University); Misty Hale and Beth Medor (Vanderbilt University); Jill Rose (Oregon Health and Science University).
Sources of Funding
This study was supported by grants from Amgen, AstraZeneca, Pfizer, Regeneron, Sanofi, and Aegerion. Joshua W. Knowles is supported by an American Heart Association (AHA) National Innovative Research Grant 15IRG222930034. Zahid Ahmad is supported by an National Institutes of Health grant K23 HL114884.
Footnotes
The Data Supplement is available at http://circgenetics.ahajournals.org/lookup/suppl/doi:10.1161/CIRCGENETICS.116.001381/-/DC1.
The other authors report no conflicts.
Disclosures
E.M. deGoma has declared the following: research grants from Amgen, Pfizer, Regeneron, Sanofi (all Modest); other research support from Aegerion, Kaneka (all Modest); Consultant/Advisory Board for Sanofi (Modest). Z.S. Ahmad has declared the following: research grants from the National Institutes of Health, Regeneron (Modest); Honoraria from Genzyme, Sanofi (Modest); consultant/advisory board for Genzyme (Modest). E. O’Brien has declared research grants from NHLBI, PCORI, Merck, Pfizer, Novartis, BMS, GSK, Janssen; consultant/advisory board (modest) for Portola Pharmaceuticals. Y. Pokharel has declared research grant support from the American Heart Association (Significant). D.J. Rader has declared consultant/advisory board for Aegerion, Alnylam, Sanofi (all Modest), and other significant interest from Aegerion. P.M. Moriarty has declared research grant support from Amgen, Kowa, Eli Lilly, Novartis, Sanofi, Regeneron, Genzyme, Pfizer, Catabasis, Esperion, B. Braun, Kaneka (all Modest); honoraria from Amarin, Kowa, Aegerion (all Modest); consultant/advisory board for Regeneron, Duke Clinical Research Institute, Eli Lilly, Catabasis, B. Braun, Kaneka, Genzyme (all Modest). M.D. Shapiro has declared research grant support from National Institutes of Health, Amgen, Sanofi, Amarin, ISIS, Synageva, Merck (all Modest); consultant/advisory board for Synageva (Modest). M.F. Linton has declared research grant support from Merck, ISIS, Genzyme, Sanofi, Regeneron (all Modest); consultant/advisory board for Merck, Retrophin, Amgen (all Modest). C.M. Ballantyne has declared research grant from Eli Lilly, Amarin, Amgen, Esperion, Novartis, Pfizer, Regeneron, Sanofi, Takeda, National Institutes of Health, American Heart Association, American Diabetes Association (all Significant); consultant/advisory board for Amarin, Eli Lilly, Esperion, Genzyme, Matinas BioPharma, Novartis, Regeneron, Sanofi (all Modest); consultant/advisory board for Amgen, AstraZeneca, Merck, Pfizer (all Significant). D. Duffy has declared research grant support from Aegerion, Amgen, AstraZeneca, Forest, Pfizer, Regeneron (all Modest). S.J. Baum has declared consultant/advisory board for Aegerion, Genzyme, Sanofi, AstraZeneca, Merck, Regeneron (all Modest). M.T. Row has declared research grant support from Eli Lilly, Sanofi-Aventis, Daiichi-Sanko, Janssen Pharmaceuticals, Ferring Pharmaceuticals, American College of Cardiology, American Heart Association (all Modest); consultant/advisory board from PriMed, AstraZeneca, Boehringer-Ingelheim, Merck, Amgen, Elsevier (all Modest). J.W. Knowles has declared research grant support paid to institution, not individual from the American Heart Association, Amgen, Leducq Foundation (all Significant).
References
- 1.Hopkins PN, Toth PP, Ballantyne CM, Rader DJ. National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 suppl):S9–S17. doi: 10.1016/j.jacl.2011.03.452. [DOI] [PubMed] [Google Scholar]
- 2.Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97:3956–3964. doi: 10.1210/jc.2012-1563. [DOI] [PubMed] [Google Scholar]
- 3.Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do R, Stitziel NO, Won HH, Jørgensen AB, Duga S, Angelica Merlini P, et al. NHLBI Exome Sequencing Project. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518:102–106. doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjouke B, Kusters DM, Kindt I, Besseling J, Defesche JC, Sijbrands EJ, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J. 2015;36:560–565. doi: 10.1093/eurheartj/ehu058. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia: New insights in pathogenesis and treatment. In: Scrivner CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2000. [Google Scholar]
- 7.Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337 doi: 10.1136/bmj.a2423. a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson JG, Goldberg AC. National Lipid Association Expert Panel on Familial Hypercholesterolemia. Treatment of adults with familial hypercholesterolemia and evidence for treatment: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 suppl):S18–S29. doi: 10.1016/j.jacl.2011.03.451. [DOI] [PubMed] [Google Scholar]
- 9.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/ AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 10.Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ, Scheerder RL, Kastelein JJ. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet. 2001;357:165–168. doi: 10.1016/S0140-6736(00)03587-X. [DOI] [PubMed] [Google Scholar]
- 11.Wierzbicki AS, Humphries SE, Minhas R. Guideline Development Group. Familial hypercholesterolaemia: summary of NICE guidance. BMJ. 2008;337:a1095. doi: 10.1136/bmj.a1095. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins PN, Stephenson S, Wu LL, Riley WA, Xin Y, Hunt SC. Evaluation of coronary risk factors in patients with heterozygous familial hypercholesterolemia. Am J Cardiol. 2001;87:547–553. doi: 10.1016/s0002-9149(00)01429-6. [DOI] [PubMed] [Google Scholar]
- 13.Williams RR, Hunt SC, Schumacher MC, Hegele RA, Leppert MF, Ludwig EH, et al. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol. 1993;72:171–176. doi: 10.1016/0002-9149(93)90155-6. [DOI] [PubMed] [Google Scholar]
- 14.Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, et al. American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation. 2015;132:2167–2192. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 15.Knowles JW, O’Brien EC, Greendale K, Wilemon K, Genest J, Sperling LS, et al. Reducing the burden of disease and death from familial hypercholesterolemia: a call to action. Am Heart J. 2014;168:807–811. doi: 10.1016/j.ahj.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien EC, Roe MT, Fraulo ES, Peterson ED, Ballantyne CM, Genest J, et al. Rationale and design of the familial hypercholesterolemia foundation cascade screening for awareness and detection of familial hypercholesterolemia registry. Am Heart J. 2014;167:342 e317–349 e317. doi: 10.1016/j.ahj.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, et al. Integrated guidance on the care of familial hypercholesterolemia from the International FH Foundation. J Clin Lipidol. 2014;8:148–172. doi: 10.1016/j.jacl.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 19.Allard MD, Saeedi R, Yousefi M, Frohlich J. Risk stratification of patients with familial hypercholesterolemia in a multi-ethnic cohort. Lipids Health Dis. 2014;13:65. doi: 10.1186/1476-511X-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone NJ, Levy RI, Fredrickson DS, Verter J. Coronary artery disease in 116 kindred with familial type II hyperlipoproteinemia. Circulation. 1974;49:476–488. doi: 10.1161/01.cir.49.3.476. [DOI] [PubMed] [Google Scholar]
- 21.Jansen AC, van Aalst-Cohen ES, Tanck MW, Trip MD, Lansberg PJ, Liem AH, et al. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2400 patients. J Intern Med. 2004;256:482–490. doi: 10.1111/j.1365-2796.2004.01405.x. [DOI] [PubMed] [Google Scholar]
- 22.Chan DC, Ping J, Hooper AJ, Burnett JR, Bell DA, Bates TR, et al. Elevated lipoprotein (a), hypertension and renal insufficiency as predictors of coronary artery disease in patients with genetically confirmed heterozygous familial hypercholseterolemia. International J of Cardiology. 2015;201:633–638. doi: 10.1016/j.ijcard.2015.08.146. [DOI] [PubMed] [Google Scholar]
- 22a.Daniels SR, Greer FR. Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 23.Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, et al. European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36:2425–2437. doi: 10.1093/eurheartj/ehv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wald DS, Bestwick JP, Wald NJ. Child-parent screening for familial hypercholesterolaemia: screening strategy based on a meta-analysis. BMJ. 2007;335:599. doi: 10.1136/bmj.39300.616076.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National cholesterol education program (ncep): Highlights of the report of the expert panel on blood cholesterol levels in children and adolescents. Pediatrics. 1992;89:495–501. [PubMed] [Google Scholar]
- 26.Report of the national cholesterol education program expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. The expert panel. Arch Intern Med. 1988;148:36–69. [PubMed] [Google Scholar]
- 27.Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:133–140. doi: 10.1016/j.jacl.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Kusters DM, Avis HJ, de Groot E, Wijburg FA, Kastelein JJ, Wiegman A, et al. Ten-year follow-up after initiation of statin therapy in children with familial hypercholesterolemia. JAMA. 2014;312:1055–1057. doi: 10.1001/jama.2014.8892. [DOI] [PubMed] [Google Scholar]
- 29.Béliard S, Carreau V, Carrié A, Giral P, Duchêne E, Farnier M, et al. Improvement in LDL-cholesterol levels of patients with familial hypercholesterolemia: can we do better? Analysis of results obtained during the past two decades in 1669 French subjects. Atherosclerosis. 2014;234:136–141. doi: 10.1016/j.atherosclerosis.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Pijlman AH, Huijgen R, Verhagen SN, Imholz BP, Liem AH, Kastelein JJ, et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis. 2010;209:189–194. doi: 10.1016/j.atherosclerosis.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Mata N, Alonso R, Badimón L, Padró T, Fuentes F, Muñiz O, et al. Clinical characteristics and evaluation of LDL-cholesterol treatment of the Spanish Familial Hypercholesterolemia Longitudinal Cohort Study (SAFEHEART) Lipids Health Dis. 2011;10:94. doi: 10.1186/1476-511X-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadfield SG, Horara S, Starr BJ, Yazdgerdi S, Bhatnagar D, Cramb R, et al. Are patients with familial hypercholesterolaemia well managed in lipid clinics? An audit of eleven clinics from the Department of Health Familial Hypercholesterolaemia Cascade Testing project. Ann Clin Biochem. 2008;45(Pt 2):199–205. doi: 10.1258/acb.2007.007078. [DOI] [PubMed] [Google Scholar]
- 33.CDC. National Diabetes Statistics Report. 2014 [Google Scholar]
- 34.Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313:1029–1036. doi: 10.1001/jama.2015.1206. [DOI] [PubMed] [Google Scholar]
- 35.Wonderling D, Umans-Eckenhausen MA, Marks D, Defesche JC, Kastelein JJ, Thorogood M. Cost-effectiveness analysis of the genetic screening program for familial hypercholesterolemia in The Netherlands. Semin Vasc Med. 2004;4:97–104. doi: 10.1055/s-2004-822992. [DOI] [PubMed] [Google Scholar]
- 36.Nherera L, Marks D, Minhas R, Thorogood M, Humphries SE. Probabilistic cost-effectiveness analysis of cascade screening for familial hypercholesterolaemia using alternative diagnostic and identification strategies. Heart. 2011;97:1175–1181. doi: 10.1136/hrt.2010.213975. [DOI] [PubMed] [Google Scholar]
- 37.Rader DJ. A randomized trial of genetic testing in familial hypercholesterolemia. Global Familial Hypercholesterolemia Summit. 2015 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.