Abstract
Inflammation in the developing preterm lung leads to disrupted airway morphogenesis and chronic lung disease in human neonates. However the molecular mechanisms linking inflammation and the pathways controlling airway morphogenesis remain unclear. Here we show that IL-1β released by activated fetal lung macrophages is the key inflammatory mediator that disrupts airway morphogenesis. In mouse lung explants, blocking IL-1β expression, post-translational processing, and signaling each protected the formation of new airways from the inhibitory effects of E. coli LPS. Consistent with a critical role of IL-1β, mice expressing a gain of function Nlrp3 allele and subsequent overactive inflammasome activity displayed abnormal saccular stage lung morphogenesis and died soon after birth. While the early stage fetal lung appeared capable of mounting an NF-κB mediated immune response, airway formation became more sensitive to inflammation later in development. This period of susceptibility coincided with higher expression of multiple inflammasome components, which could therefore increase the capability to release bioactive IL-1β. Macrophages from Nlrp3 gain of function mice also expressed higher levels of more mature cell surface markers, additionally linking inflammasome activation with macrophage maturation. These data identify developmental expression of the inflammasome and IL-1β release by fetal lung macrophages as key mechanisms and potential therapeutic targets for neonatal lung disease.
Introduction
During normal breathing, the lung remains in continuous contact with the atmosphere. Both adults and young children repeatedly inhale airborne pathogens and injurious agents. However, newborn infants are far more susceptible to lung infection, inflammation, and injury. The particular susceptibility of neonatal lungs to disease suggests developmental immaturity of the lung innate immune system (1, 2). In preterm infants, infection and inflammation within the developing lung prevents normal airway morphogenesis and causes bronchopulmonary dysplasia (BPD) (3–5). The incidence of BPD in preterm infants remains very high, as disease-specific therapies are lacking (6, 7). Developing new therapeutic strategies for BPD may therefore require better understanding of lung innate immunity and how immune activation disrupts normal developmental pathways.
Inhaled microbes and particles contact multiple cell types in the airways (8, 9). However, alveolar macrophages are the primary initial sites of particle and microbial phagocytosis and subsequent inflammatory activation (10, 11). Even in the immature lung where many macrophages reside in the lung interstitium, macrophages are the major cells that respond to microbial products and release inflammatory mediators (12). Airway epithelia, vascular endothelia, and mesenchymal cells are downstream of macrophage activation and contribute to amplify or suppress the innate immune response (13–16). These additional cell populations are also targets of macrophage-derived soluble inflammatory mediators. In BPD pathogenesis, macrophage-derived inflammatory mediators appear to activate a second wave of innate immune signaling and NF-κB activation in target mesenchymal and epithelial cells (17–19). Inflammatory signaling in these target cells disrupts normal airway branching morphogenesis, causing simplified saccular airway and alveolar structures (20). Therefore production and release of paracrine inflammatory mediators connects macrophage activation and abnormal lung development.
Among the soluble inflammatory mediators released by lung macrophages, IL-1β appears to play a key role in neonatal lung injury (21). Transgenic IL-1β overexpression inhibits normal fetal lung morphogenesis (22). Interestingly, the effects of IL-1β on morphogenesis are more pronounced during the saccular stages of fetal lung development (23). Similarly, we previously observed that macrophage activation early in lung development did not have the same inhibitory effects on morphogenesis as during the later stages (12). These data suggested that the effects of IL-1β on lung morphogenesis were developmentally regulated, either at the level of IL-1β release or IL-1β signaling in target lung cells. Recent studies have also linked IL-1β signaling to hyperoxia-induced defects in alveolar development in neonatal mice (24). Therefore IL-1β may represent a biological link between lung injury, inflammation, and abnormal development.
Activation of NF-κB stimulates IL-1β transcription (25–27). IL-1β is then translated as a propeptide, which remains intracellular and lacks the ability to activate IL-1 receptors. Release of bioactive IL-1β requires post-translational cleavage by functional inflammasome complexes (28, 29). Inflammasomes typically contain intracellular Nod-like receptors (i.e. NLRP3, NLRC4), the linkage/adaptor protein ASC, and caspase enzymes (i.e. CASP1, CASP4/11) that actually perform substrate cleavage (30, 31). Here we use complementary mouse models to demonstrate that IL-1β is the key cytokine that connects activation of the initial inflammatory response in fetal lung macrophages and abnormal saccular airway branching morphogenesis. Consistent with this mechanism, functional inflammasome activity was required and sufficient for preventing normal saccular airway formation. Interestingly, expression of multiple inflammasome components was developmentally regulated, potentially representing the key mechanism that determines the developmental stage at which lung morphogenesis becomes vulnerable to inflammatory stimuli.
Methods and Materials
Reagents
The following antibodies were used in explant cultures and were obtained from R&D Systems: anti-IL1β, anti-TNFα, goat IgG, and rat IgG. Gel-purified Escherichia coli LPS (O55:B5) was purchased from Sigma-Aldrich. The Caspase-1 Inhibitor I (Ac-Tyr-Val-Ala-Asp-CHO; YVAD) was purchased from Calbiochem. The following antibodies were used for immunoblotting and immunofluorescence: rat anti-CD68 (Acris), rabbit anti-caspase 1 p10 (Santa Cruz Biotechnology), rabbit anti-NALP1/NLRP1 (Santa Cruz Biotechnology), rabbit anti-ASC (Abcam), and rabbit anti-Cryopyrin (NLRP3, Santa Cruz Biotechnology). ProLong Gold with DAPI mounting media and Alexa-conjugated secondary antibodies (AF488, AF555) were obtained from Invitrogen. The nuclear stain DRAQ5 was purchased from Thermo Scientific. Fetal lung mesenchymal cells were treated with recombinant IL1β (rIL1β, R&D Systems), recombinant TNFα (rTNFα, R&D Systems), recombinant IL18 (rIL18, MBL International Corporation), and Pam3Cys-Ser-(Lys)4 (Millipore). Luciferase activity was measured with a luciferase assay system from Promega. Cells were cultured in media from Corning’s Cellgro and 10% FBS (Thermo Scientific). DNase I from bovine pancreas type IV (DNase) and collagenase from clostridium histolyticum type XI were purchased from Sigma-Aldrich.
Mouse strains and lung explant culture
Animal experiments were approved by the Institutional Animal Care and Use Committees at Vanderbilt University and the University of California, San Diego. C57Bl/6 mice obtained from Harlan Laboratories were used as wild-type controls. IL1b−/−, LysM-Cre-NLRP3L351P, and IKFM mouse strains were developed and described previously (12, 32, 33). To generate LysM-Cre-NLRP3L351P mutants, homozygous LysM-Cre mice were crossed with heterozygous NLRP3L351P mice. LysM-Cre-NLRP3L351P offspring have macrophages with constitutively active NLRP3 inflammasome. IKFM mice (12, 34) are a doxycycline inducible model obtained by crossing Csf1r–rtTA mice with the Tet-O-cIKKβ strain. When treated with doxycycline, macrophages in IKFM mice express constitutively active IKKβ. Doxycycline-containing water (2g/L) was given to pregnant dams to induce transgene expression in IKFM embryos. The morning of plug identification was defined as embryonic day 0 (E0). Fetal lung explant isolation and culture has previously been described (12, 17, 20). Briefly, E15 fetal lungs were isolated, minced into approximately 1 mm3 cubes, and cultured on an air-liquid interface using clear Transwell inserts (Corning). Brightfield images of explants were taken at 24h and 72h of culture. Saccular airway branching was quantified as the number of new peripheral branches formed between 24h and 72h. For luciferase activity in NGL explants, explants were isolated and homogenized in reporter lysis buffer (Promega). Activity was measured using a luciferase assay system (Promega).
Fetal lung macrophage isolation
Isolation of fetal lung macrophages has previously been described (35). Briefly, fetal lungs were dissected and enzymatically digested with DNase (30ug/ml) and collagenase (0.7 mg/ml). Cell suspensions were collected following passage through a 70-µm cell strainer. Adult alveolar macrophages were isolated by bronchoalveolar lavage as previously described (36).
RNA isolation and real-time PCR measurement
RNA isolation, cDNA synthesis, and real-time PCR were performed on whole lung or isolated macrophages using standard techniques. Tissue and cells were stored and homogenized (TissueRuptor, Qiagen) in TRIzol (Invitrogen). After total RNA isolation, first strand cDNA was synthesized using an oligo(dT) primer and Superscript III (Invitrogen). Real time PCR was performed using either unlabeled oligonucleotides with SYBR Green or TaqMan probes. PCR was performed using either an iQ5 or CFX96 thermocycler (Bio-Rad). Gene expression comparisons were represented using 2−ΔΔCt method. Experiments were performed at least three independent times. Data between groups were analyzed by comparing ΔCT values using a multiple regression analysis model or the Mann-Whitney U test.
Immunoblotting
Whole lung tissue was snap frozen following dissection and stored at −80 °C. Samples were thawed in the presence of RIPA buffer (150 mM NaCl; 25 mM TRIS-HCl pH 8.0; 1 % Triton X-100, 0.5 % sodium deoxycholate; 0.1 % SDS) containing protease inhibitors (cOmplete protease inhibitor cocktail, Roche) and homogenized using a mechanical tissue disruptor. Following centrifugation, soluble protein was quantified and 10 µg of protein/sample was separated by SDS/PAGE. Immunoblotting was performed using standard techniques. For primary macrophages, cells were washed and lysed in RIPA buffer with protease inhibitors while on ice. Cell lysates were centrifuged and soluble protein was quantified, separated by SDS/PAGE, and immunoblotted using standard techniques. Immunoblots were visualized by enhanced chemiluminescence or by using fluorescently labeled secondary antibodies.
Tissue processing, immunolabeling, imaging, and morphometry
Fetal mouse lung tissue was dissected free of surrounding tissues and fixed in 4% paraformaldehyde (Electron Microscopy Sciences). For embedding and sectioning, fixed samples were washed and processed using standard protocols through a sucrose gradient for embedding into OCT media (Tissue-Tek) for frozen sectioning or a dehydrating series for embedding into paraffin. For immunofluorescence on frozen sections, Alexa-conjugated, species-specific secondary antibodies were used for visualization and DRAQ5 used to label nuclei. Confocal images were acquired using a Leica TCS SPE laser-scanning confocal microscope (Leica microsystems). Eosin (Ricca Chemical) and Mayer’s hematoxylin (Dako) were used on paraffin sections for H&E staining. Serial lung H&E sections were measured for fractional lung volumes of tissue and airspace. Intersection points of a grid overlaid on H&E images were defined as tissue or airspace within ImageJ (NIH). Images from explants were obtained from an inverted Olympus IX8I microscope with a Hamamatsu Orca ER CCD camera and Slidebook software or a Touptek Toupcam CCD camera with Toupview software. Fluorescent NGL explant GFP images were taken on an EVOS Cell Imaging System.
Flow Cytometry
The following antibodies were used for flow cytometry: CD11b–V450, CD86-Pe-Cy7, Gr1 PerCP-Cy5.5, and CD45 allophycocyanin-Cy7 (BD Biosciences); CD204-AF647, CD68-FITC, and CD206-PE (AbD Serotec); F4/80-PE (Invitrogen); and CD11c–Pe-Cy5 (BioLegend). Viable cells were selected using the LIVE/DEAD Fixable Blue Dead Cell Stain Kit (Invitrogen). For flow cytometry, fetal and adult lungs were digested to a single cell suspension with DNase and collagenase. Red blood cells were lysed with ACK lysing buffer (Invitrogen). On ice, 3% FBS was used to block cells for 15 min, followed by incubation with cell surface antibodies for 30–60 min. Intracelluar antibodies were incubated with cells for 30 min on ice after using the Intracellular Fixation and Permeabilization Kit (eBioscience). Flow cytometry measurements were conducted on a BD LSR II (BD Biosciences). The gating strategy: Doublets were excluded based on FSC-A against FSC-H followed by SSC-A against SSC-H. Cells negative for LIVE/DEAD Fixable Blue Stain defined viable cells. Further gating was for CD45+ and CD68+ cells, which were separated into F4/80lo and F4/80hi cells along with CD11b expression (35).
Results
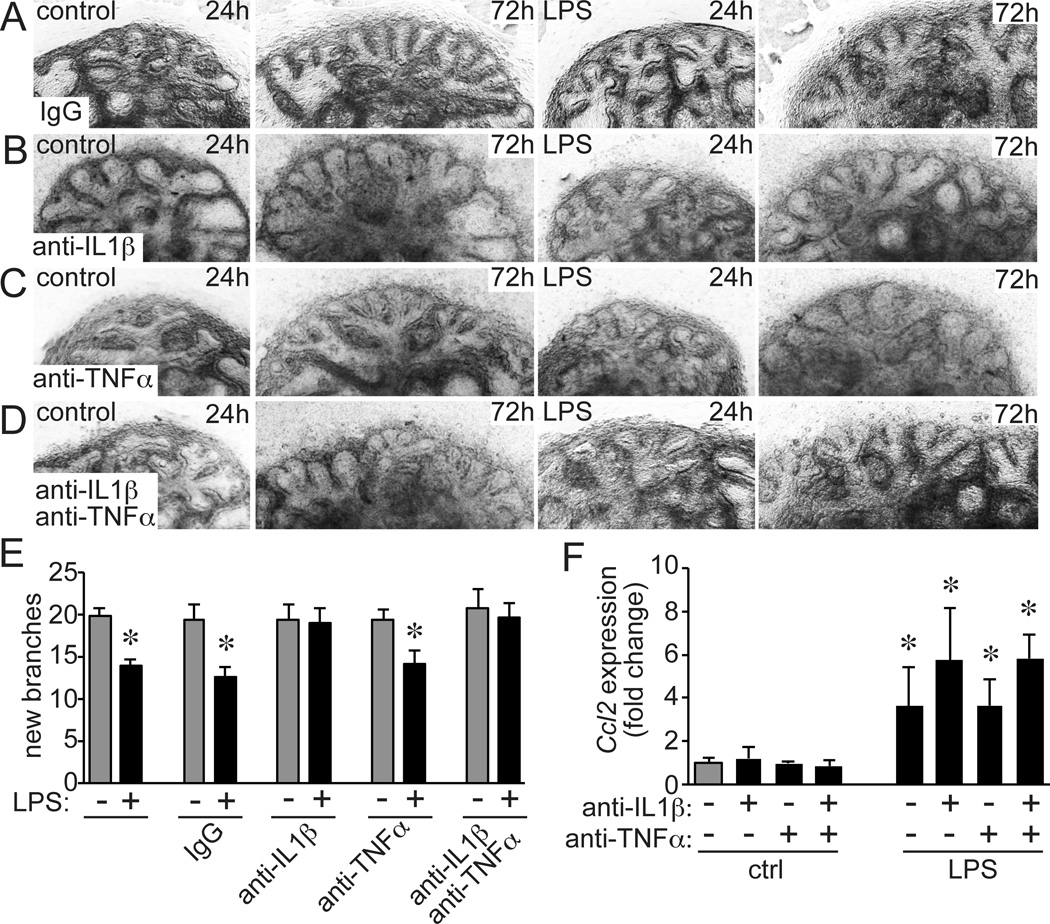
Soluble inflammatory mediators released by macrophages disrupt expression of key developmental genes in lung epithelia and adjacent mesenchyme (12, 18, 37). Our previous data showed IL-1β and TNFα activated NF-κB in target mesenchymal cells and altered developmental gene expression (15). To test if these mediators linked macrophage activation and abnormal airway morphogenesis, we added neutralizing antibodies against IL-1β and TNFα to cultured fetal mouse lung explants (Fig. 1A–E). As previously demonstrated (20), adding E. coli LPS to E15 fetal mouse lung explants reduced the formation of new peripheral airways over a 3 day period (Fig. 1A). Including anti-IL-1β in the culture media prevented this decrease and allowed normal airway branching (Fig. 1B). However, anti-TNFα antibodies had no effect (Fig. 1C). Including both anti-IL1β and anti-TNFα antibodies gave similar protection as anti-IL1β alone (Fig. 1D). These initial experiments suggested that IL-1β signaling was required for LPS to prevent normal airway morphogenesis. The protective effects of anti-IL-1β did not appear to be due to overall inhibition of inflammatory signaling, as LPS induced similar CCL2 expression in control IgG and anti-IL-1β-treated explants (Fig. 1F).
Figure 1.
Inhibiting IL-1β protects fetal airway development from the effects of LPS. E15 fetal lung explants from C57BL/6 mice were cultured in the presence of LPS (250 ng/ml) along with (A) nonimmune IgG, (B) neutralizing antibodies against IL-1β (10 ng/ml), (C) neutralizing antibodies against TNFα (10 ng/ml), or (D) both IL-1β and TNFα antibodies. Brightfield images from representative explants illustrate airway formation between 24–72 h of culture. (E). LPS inhibits airway formation, but antibodies against IL-1β prevented this inhibition (*P < 0.05, n = 18–53). Anti-TNFα had no effect. (F). Antibodies against IL-1β and TNFα did not prevent the initial inflammatory response, as measured by Ccl2 expression (*P < 0.05, n = 5).
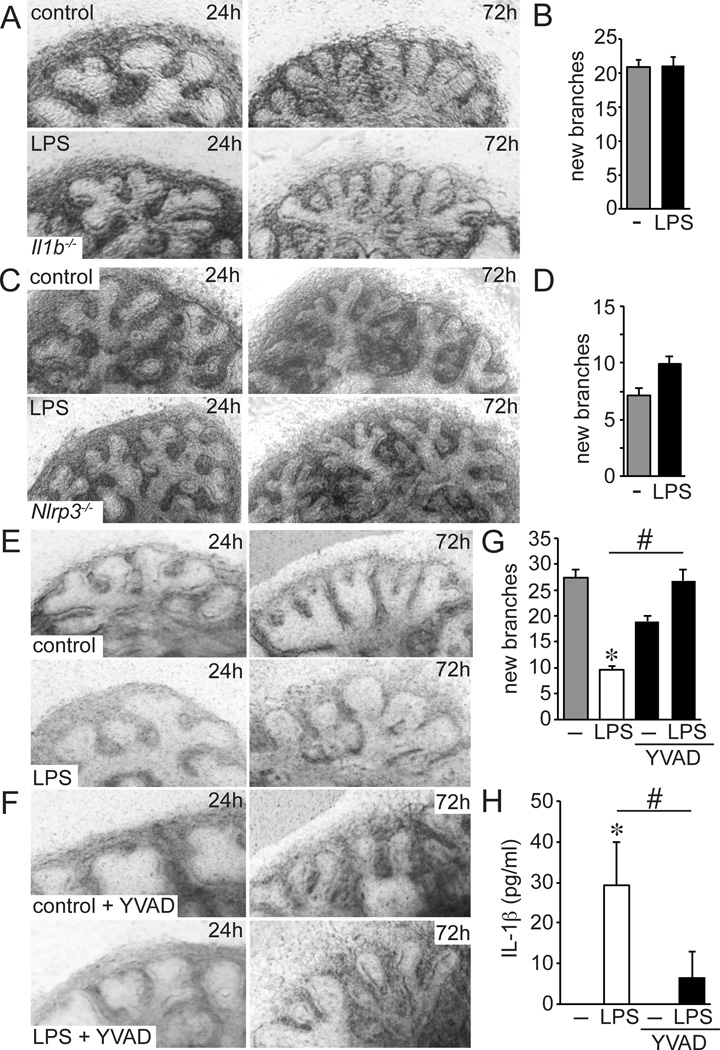
To further test the requirement of IL-1β, we measured the effects of LPS on airway morphogenesis using fetal lung explants from Il1b−/− mice (Fig. 2A,B). LPS did not affect airway branching in Il1b−/− mutant lung explants, additionally demonstrating that IL-1β is required for LPS to inhibit airway formation. Inflammatory activation in macrophages produces IL-1β precursor peptide that is cleaved by inflammasome-associated caspases and then secreted (38). The Nod-Like Receptor NLRP3 is among the key inflammasome components involved in IL-1β processing and secretion. LPS did not inhibit the formation of new airway branches in explants from Nlrp3−/− mice, consistent with the requirement of NLRP3-mediated IL-1β processing (Fig. 2C,D). To test the role of inflammasome-associated Casp1 activity, we treated fetal lung explants with the peptide-based Casp1 inhibitor Ac-Tyr-Val-Ala-Asp-CHO (YVAD) (39). As seen in Figure 2E–H, adding YVAD to the explant media both prevented IL-1β release following LPS treatment and preserved formation of new airways around the explant periphery.
Figure 2.
Inflammasome activation and IL-1β inhibit fetal lung airway morphogenesis. (A–D). LPS did not inhibit airway branching in E15 explants from Il1b−/− mice (n = 49–56) or Nlrp3−/− mice (n = 44–50). (E–H). Treating E15 fetal lung explants from C57BL/6 mice with the caspase-1 inhibitor Ac-Tyr-Val-Ala-Asp-CHO (YVAD, 1 µM) inhibited IL-1β release (H) and protected fetal lung explants from the effects of LPS (G). (*P < 0.05, #P < 0.01, n = ≥ 16 explants from 3 different litters).
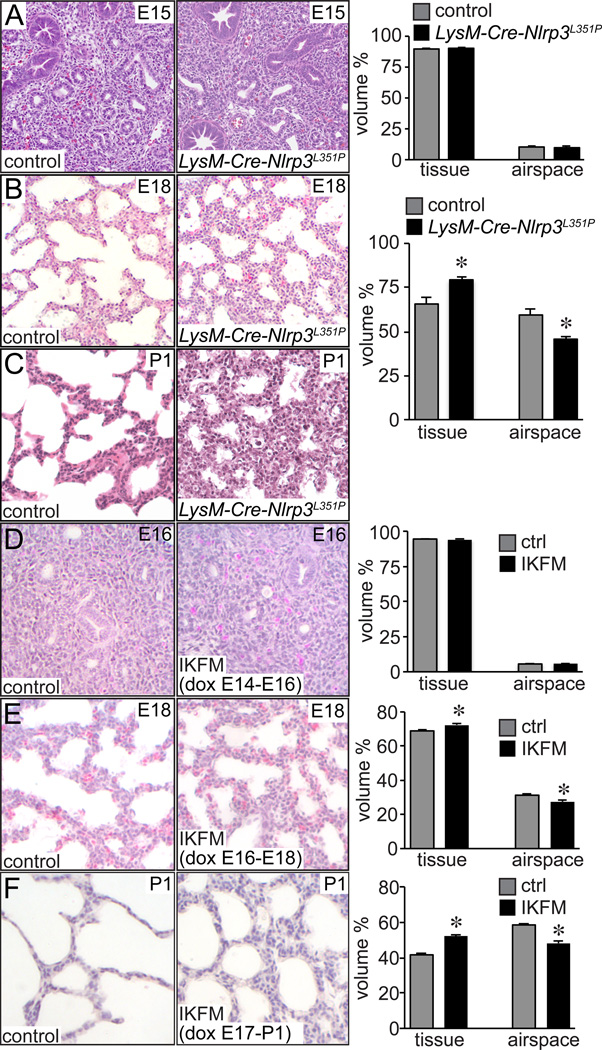
Because we suspected inflammasome-processed IL-1β as a key component in inhibiting normal lung development, we next examined lung morphology in mice expressing a gain of function mutation in the inflammasome receptor Nlrp3 (Nlrp3L351P). The LysM-Cre transgene was used to express this gain of function Nlrp3 allele in myeloid cells including fetal lung macrophages. While we did not measure differences between LysM-Cre-Nlrp3L351P and littermate controls at E15, we did observe abnormal airway morphogenesis defects in E18 fetal transgenic animals (Fig. 3A,B). In E18 transgenic lungs, distal airspaces were less expanded and appeared more simplified. Possibly as a consequence, the interstitial mesenchyme between airspaces appeared thicker. Similar structural defects appeared to persist in newborn LysM-Cre-Nlrp3L351P lungs (Fig. 3C). The majority of the newborn transgenic animals died soon after delivery. The changes in LysM-Cre-Nlrp3L351P lung morphogenesis and subsequent perinatal lethality closely resembled the phenotype we observed in mice expressing an inducible, constitutively active IKKβ in macrophages (IKFM) (12). Collectively, these data demonstrated that inflammasome activity and subsequent IL-1β release play key roles linking inflammation and abnormal fetal airway morphogenesis.
Figure 3.
Macrophage activation inhibits late stage airway morphogenesis. (A–C). Abnormal lung morphogenesis in LysM-Cre-NLRP3L351P mice. Lungs from LysM-Cre-NLRP3L351P mice and littermate controls were fixed, processed, and sectioned. Representative images from H&E stained sections from E15 (A), E18 (B) and postnatal day 1 (P1, C) mice are shown. Lung morphometry measurements at right showed no difference in mutant and control lungs at E15, but increased lung interstitial tissue volume and reduced airspace volume in E18 LysM-Cre-NLRP3L351P lungs (*P < 0.01, n = 16). Low viability in P1 LysM-Cre-NLRP3L351Pmice prevented analysis at postnatal time points. (D–F). To measure stage-dependent effects of macrophage activation on lung development, pregnant IKFM mice were provided with drinking water containing doxycycline (2 g/L) for 48 h periods from (D) E14-E16, (E) E16-E18, or (F) E17-P1. Following 48 h of treatment, lungs from IKFM and littermate control embryos were fixed, processed, and imaged for lung morphometry measurements. Representative images are shown. E16 control and IKFM lungs did not have measurable differences in airspace formation. However IKFM lungs contained more simplified airspaces and thicker lung mesenchyme at E18 and P1. (*P < 0.01, n = 94–126.)
Macrophage activation did not alter airway morphogenesis during early embryonal stages of lung development (before E16) (12). We hypothesized that inflammasome activity and IL-1β release were developmentally regulated, with higher inflammasome activity in more mature lungs. To further define the points during development when macrophage activation can influence lung morphogenesis, we used the inducible IKFM mouse model. In these mice, doxycycline induces expression of constitutively active IKKβ in Csf1r–expressing macrophages. Giving pregnant dams doxycycline from E14-E16 had no apparent effect on fetal lung airway morphology, but doxycycline from E16-E18 caused reduced airway branching and expansion in IKFM embryos compared to littermate controls (Fig. 3D,E). Even more significant changes were seen in newborn IKFM pups following doxycycline treatment from E17-P1 (Fig. 3F). These data suggested NF-κB activation in macrophages was more likely to disrupt lung morphogenesis during the canalicular to saccular stage of development.
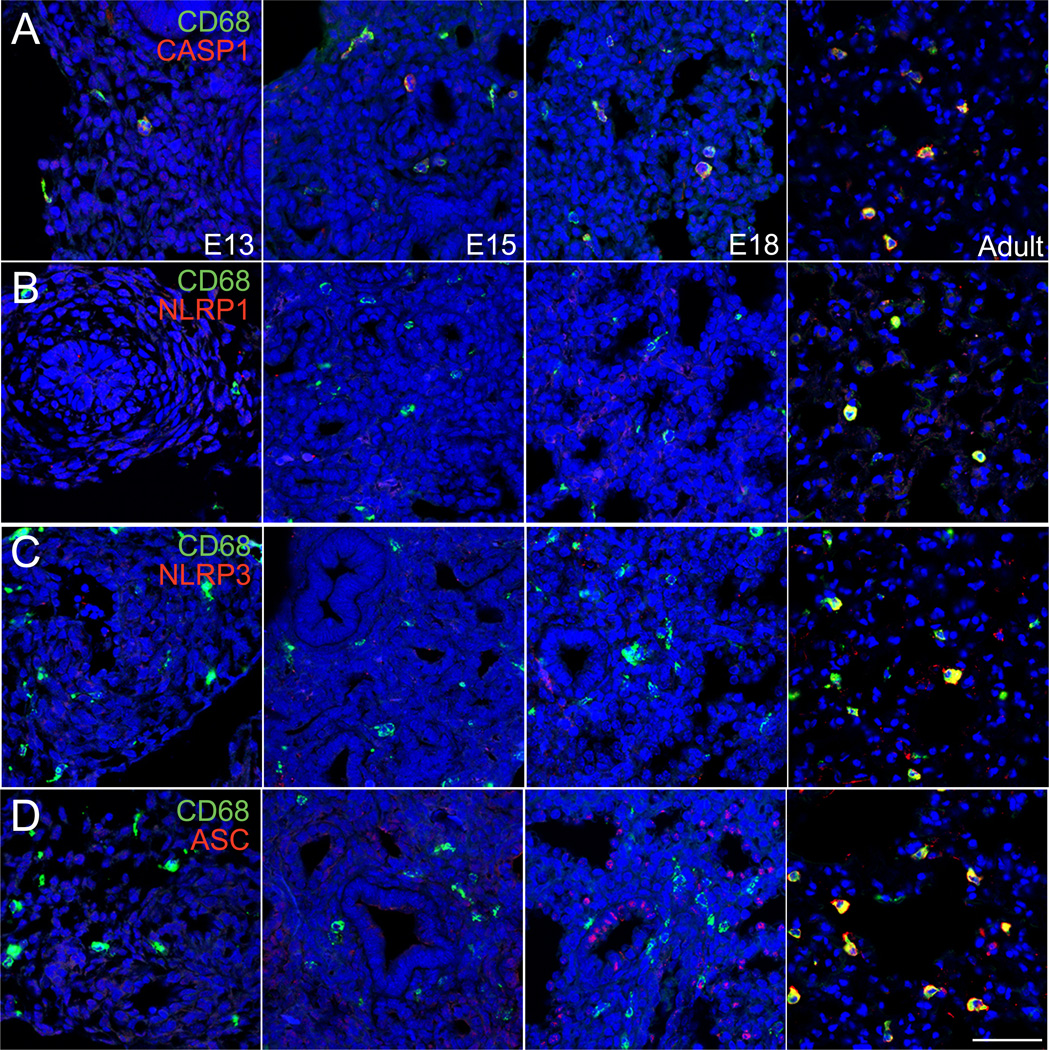
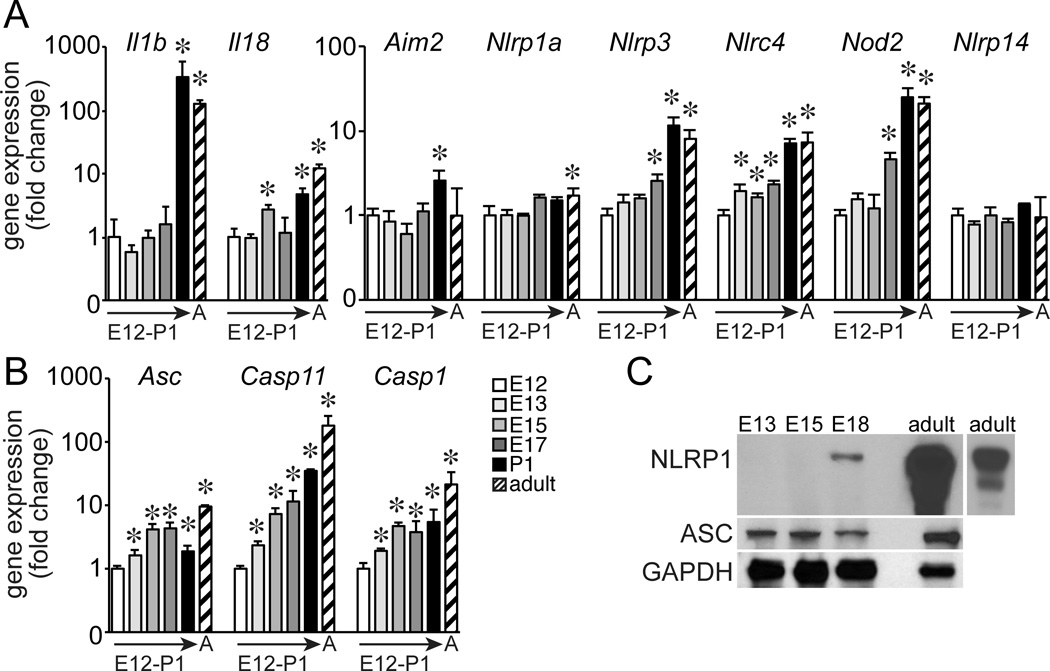
Based on this increased susceptibility in more mature lungs, we measured temporal expression of inflammasome components throughout lung development by immunostaining (Fig. 4). Casp1 expression was detected in CD68+ macrophages throughout development, with increased immunostaining intensity in adult lung macrophages. However, NLRP1, NLRP3, and ASC were detected only in adult lung macrophages and not in fetal lung macrophages. Lower levels of expression were occasionally detected, including some ASC expression in epithelial cells at E18. Gene expression of inflammasome components was also developmentally regulated. The inflammasome substrates IL-1β and IL-18 were most highly expressed in postnatal and adult lung (Fig. 5A). Additional inflammasome components were more varied, with Nlrp3, Nlrc4, and Nod2 all highest in postnatal and adult lung (Fig. 5A). Expression of Asc, Casp1, and Casp4 steadily increased during development with highest expression in adult lung (Fig. 5B). Immunoblotting of total lung homogenates also demonstrated these differences, with developmental expression of NLPR1 only detectable after E18 but ASC present as early as E13 (Fig. 5C). While the patterns of expression were not uniform for all inflammasome genes, multiple components were clearly developmentally regulated with increased expression in more mature lungs.
Figure 4.
Developmental regulation of inflammasome component expression in the lung. Frozen sections of E13, E15, E18, and adult mouse lung were immunostained with antibodies against (A) CASP1, (B) NLRP1, (C) NLRP3, and (D) ASC (each indicated in red). Macrophages were immunolabeled with anti-CD68 (green). Nuclei were labeled with DRAQ5 (blue). Images were obtained by laser scanning confocal microscopy. Scale bar, 50 µm.
Figure 5.
Changes in inflammasome expression throughout lung development. (A–B). Gene expression of inflammasome substrates and components in E12, E13, E15, E17, P1, and adult lung. (*P < 0.05, n = 4.) Gapdh expression used as internal control. Values normalized to E12 samples and expressed as fold change using the 2−ΔΔCT formula. (C). Immunoblotting of lung homogenates failed to detect NLRP1 protein expression before E18. NLRP1 expression was highly abundant in adult lung samples (lighter exposure of adult lung homogenate shown at right). ASC protein expression was detected by immunoblotting from E13-E18. GAPDH expression was measured as a protein loading control.
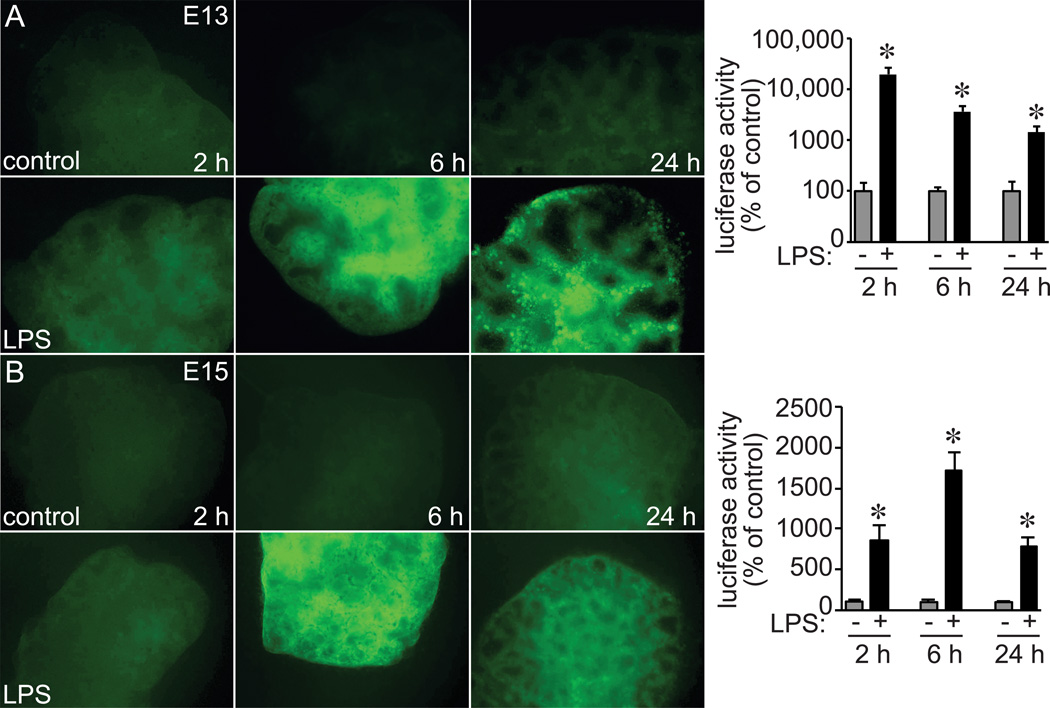
In addition to inflammasome expression, the NF-κB-mediated response to microbial products and TLR agonists could also be developmentally regulated. To test this possibility, we added LPS to fetal lung explants from E13 and E15 NF-κB-GFP-Luciferase (NGL) reporter mouse (Fig. 6). Within 6 h, LPS induced GFP reporter expression throughout both E13 and E15 lungs. Quantitative measurement of NF-κB-dependent luciferase activity also demonstrated the response in E13 and E15 lungs, with higher relative levels in E13 lungs. Therefore, the TLR4-NF-κB axis was clearly functional as early as E13 and not likely responsible for the developmental changes in how inflammatory activation affected lung morphogenesis.
Figure 6.
LPS induced NF-κB activation in early stage fetal mouse lung explants. (A) E13 and (B) E15 lung explants from NGL reporter mice were treated with LPS for up to 24 h. (A,B). GFP reporter expression was imaged by fluorescence microscopy and detected following LPS treatment in both E13 and E15 explants (representative images shown). NF-κB reporter expression was also quantified by luciferase activity in parallel experiments. Each condition and time point normalized to total protein and compared to untreated controls (*P < 0.05, n = 6).
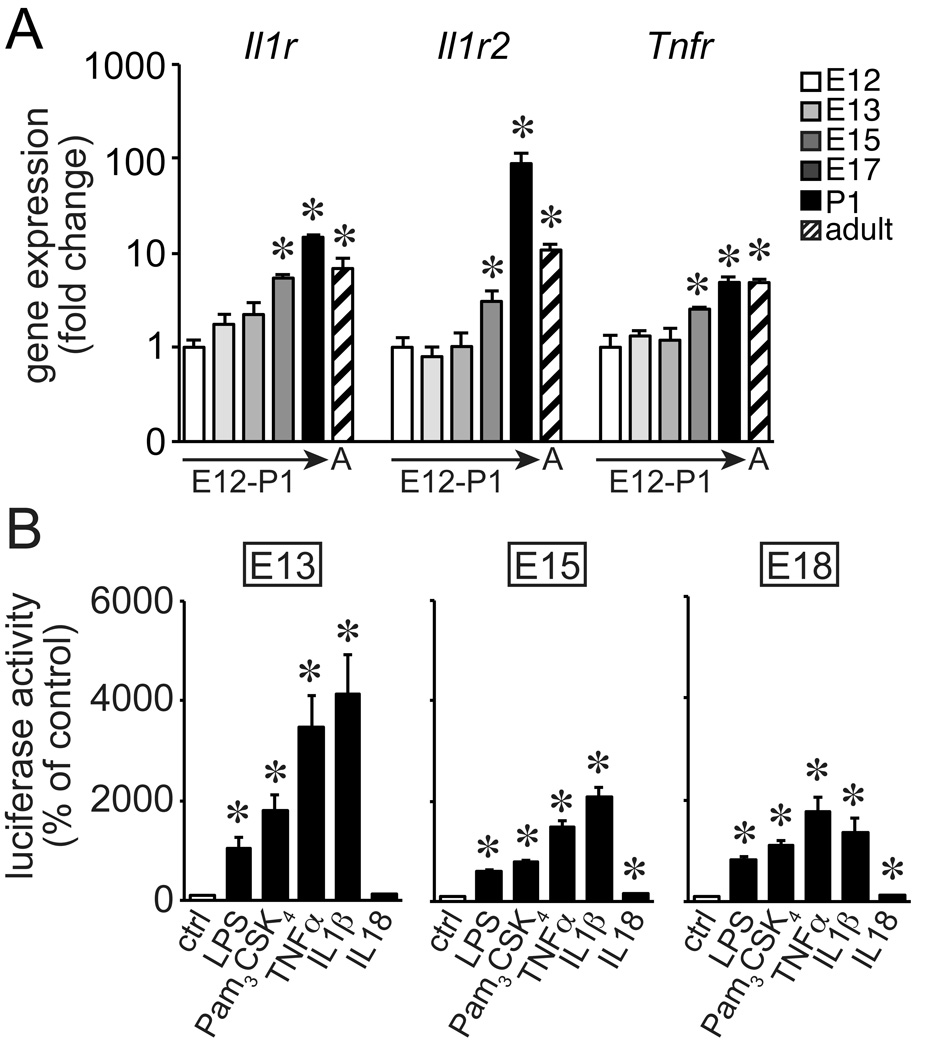
TLR activation and inflammatory mediators released from macrophages inhibit expression of key developmental genes in fetal lung mesenchymal cells (12, 15). These mesenchymal cells could become more sensitive to soluble mediators during the later stages of development. Real time PCR using mRNA from total lung tissue did demonstrate increased expression of the cytokine receptors Il1r, Il1r2, and Tnfr mRNAs in more mature lungs (Fig. 7A). However, primary lung mesenchyme isolated from E13, E15, and E18 NGL mice demonstrated similar NF-κB activation when treated with LPS, the TLR2 agonist Pam3CSK4, TNFα, or IL-1β (Fig. 7B). IL-18 had a more modest effect at each time point. The response of early stage fetal lung mesenchyme to innate immune signals suggested that inflammasome expression and function was more likely to determine when inflammation affected lung development.
Figure 7.
Fetal lung mesenchymal cells respond to inflammatory activators. (A). Expression of the cytokine receptors Il1r, Il1r2, and Tnfr was developmentally regulated in fetal lung tissue as measured by real-time PCR. (*P < 0.05, n = 4.) Gapdh expression used as internal control. Values normalized to E12 samples and expressed as fold change using the 2−ΔΔCT formula. (B) Primary fetal lung mesenchymal cells were isolated from E13, E15, or E18 NGL reporter mice. Cells were then treated with LPS (250ng/ml), Pam3Cys-Ser-(Lys)4 (1ug/ml), TNFα (10ng/ml), IL-1β (10ng/ml), or IL-18 (10 ng/ml). NF-κB activated reporter expression was measured by luciferase assay (*P < 0.05, n = 6–9).
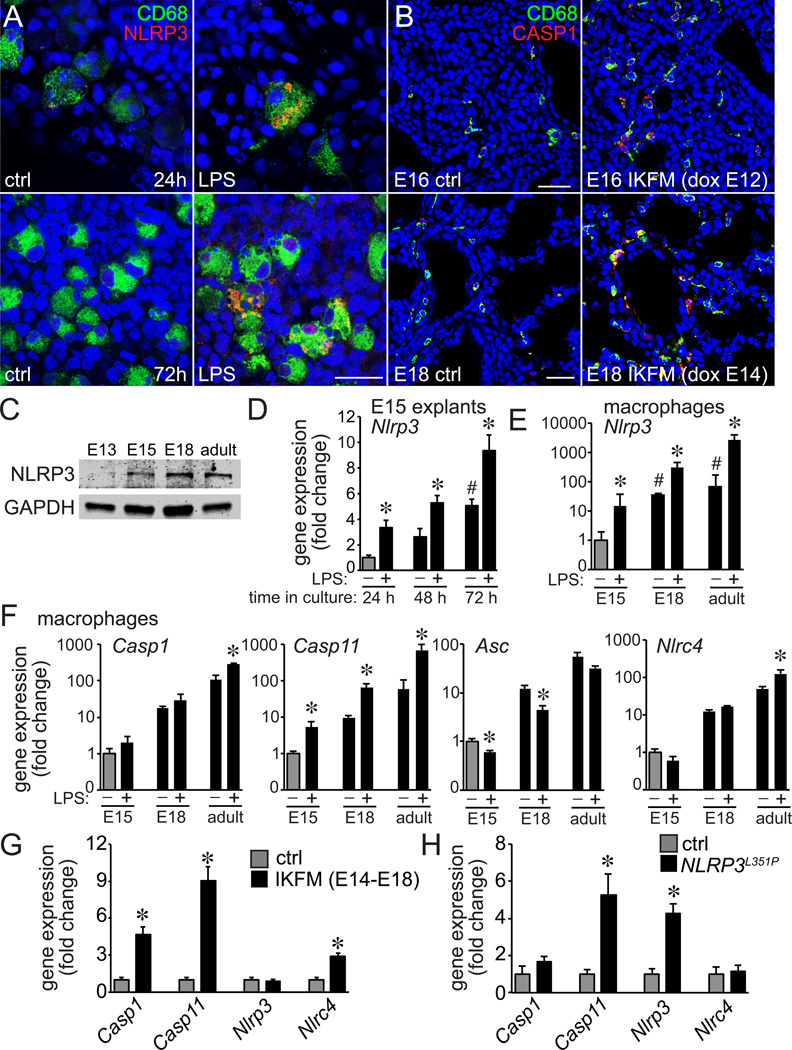
In previous studies, IKKβ activation accelerated macrophage maturation (35). To test if activation could also increase inflammasome expression, we measured NLRP3 expression in LPS-treated E15 fetal lung explants. LPS did increase NLRP3 immunostaining but the expression remained restricted to CD68+ macrophages (Fig. 8A). We obtained similar results following transgenic IKKβ activation, with increased CASP1 expression in CD68+ IKFM lung macrophages compared to control littermates (Fig. 8B). Immunoblotting showed NLRP3 expression appeared to be higher in more mature lung macrophages (Fig. 8C). Culturing E15 lung explants allowed us to measure developmental changes in vitro. Nlrp3 expression increased in explants cultured for 72 h under control conditions (Fig. 8D). Adding LPS to the media further increased Nlrp3 expression at each time point tested.
Figure 8.
Macrophage activation increased expression of inflammasome components. (A). E15 fetal lung explants cultured with LPS for 24h and 72h were immunostained with antibodies against NLRP3 (red), CD68 (green), and imaged by confocal microscopy. LPS exposure increased NLRP3 speck formation in CD68+ macrophages. (B). To measure the effects of transgenic macrophage activation on inflammasome expression in IKFM mice, pregnant dams were give doxycycline from E12-E16 or E14-E18. IKFM and littermate control fetal lungs were isolated at both time points, immunostained using antibodies against CASP1 (red), CD68 (green), and imaged by confocal microscopy. IKFM CD68+ macrophages expressed higher levels of CASP1 compared to littermate controls. Scale bar, 30 µm in (A) and 100 µm in (B). (C). NLRP3 expression was measured by immunoblotting in lung macrophages isolated from E13, E15, E18, and adult mice. GAPDH expression included as a loading control (10 µg/lane). (D). E15 fetal lung explants were cultured in the absence or presence of LPS (250 ng/ml) for 24–72 h. Nlrp3 gene expression was measured by real-time PCR at each time point. Nlrp3 expression increased over time in control explants (# P < 0.01 compared to 24 h control; n = 4). In addition, LPS increased Nlrp3 expression further at each time point (* P < 0.05, n = 4). (E,F) Macrophage were isolated from E15, E18, and adult mouse lungs and treated with LPS (250 ng/ml) for 4 h. Following treatment, expression of indicated inflammasome components were measured by real-time PCR. (*P < 0.05, n = 3). (G). Expression of inflammasome components in E18 IKFM macrophages compared to littermate controls as measured by real-time PCR (*P < 0.05, n = 4). (H). Expression of inflammasome components in E18 LysM-Cre-NLRP3L351P lung macrophages as measured by real-time PCR. (*P < 0.05, n = 7).
To test if macrophage activation could increase inflammasome component expression in a cell autonomous manner, we isolated and cultured primary macrophages from E15, E18, and adult lungs (Fig. 8E,F). LPS caused small increases in Casp1 expression that only reached statistical significance in adult macrophages (P = 0.02). However LPS stimulated larger increases in Casp11 expression in E15, E18, and adult cells. The effects of LPS on other inflammasome components were variable; LPS increased Nlrp3 expression at each time point but only increased Nlrc4 in adult cells. In addition, LPS actually decreased Asc mRNA levels (Fig. 8F). Therefore while expression of some inflammasome components could clearly be induced by inflammatory activation in a cell autonomous manner, the effects were variable and gene specific. Transgenic macrophage activation also had variable effects on inflammasome components, with increased Casp1 and Casp11 expression in IKFM macrophages but only increased Casp11 expression in LysM-Cre-Nlrp3L351P cells (Fig. 8G,H). Nlrc4 expression was increased in IKFM but not in LysM-Cre-Nlrp3L351P macrophages.
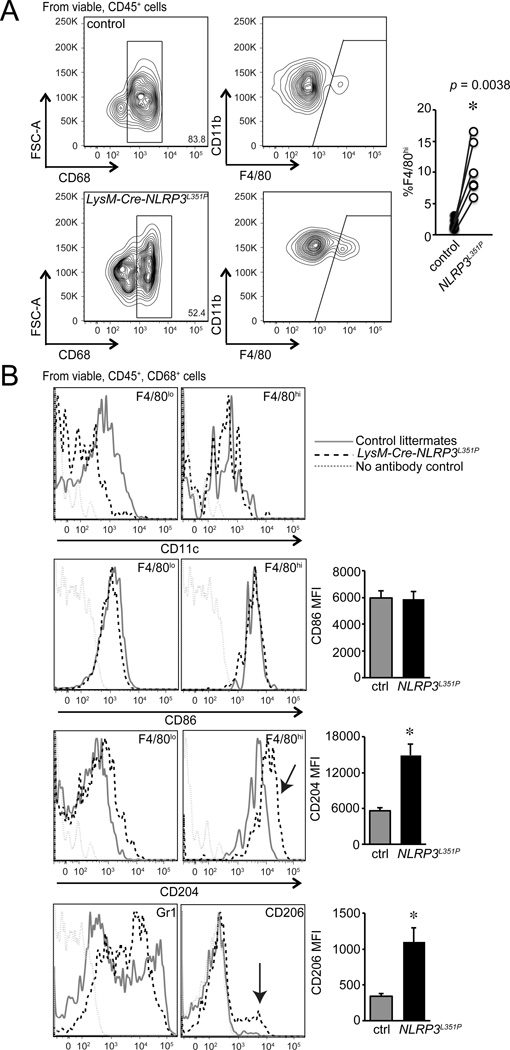
As fetal lung macrophages mature, cells gain F4/80 expression with similar to slightly lower CD11b expression (F4/80hi) (40). We previously demonstrated that IKKβ activation increased the percentage of F4/80hi macrophages in the fetal lung, consistent with accelerated alveolar macrophage maturation (35). To test if inflammasome activation could similarly increase fetal lung macrophage maturation, we used flow cytometry to identify different macrophage subtypes within LysM-Cre-Nlrp3L351P lungs (Fig. 9). Among live, CD45+, CD68+ macrophages, fetal LysM-Cre-Nlrp3L351P mice had higher percentages of F4/80hi macrophages compared to littermate controls (Fig. 9A). In addition, these F4/80hi macrophages expressed CD204 and CD206, consistent with alveolar macrophage maturation (Fig. 9B). These results suggested a positive feedback mechanism linking inflammasome expression, function and macrophage maturation.
Figure 9.
Inflammasome activation increased markers of fetal lung macrophage maturation. (A). Single cell suspensions from E18 LysM-Cre-NLRP3L351P and control littermate lungs were generated by enzymatic digestion and analyzed by flow cytometry. After doublet exclusion and selection for viable, CD45+, CD68+ macrophages, cells were gated for CD11b and F4/80 expression. LysM-Cre-NLRP3L351P mice (open circles) had increased percentages of F4/80hi macrophages compared to littermate controls (filled circles). Each separate litter is indicated in the graph at right. (B). LysM-Cre-NLRP3L351P macrophages had increased CD204 expression in the F4/80hi subpopulation and increased numbers of CD206+F4/80hi macrophages compared to control littermates. LysM-Cre-NLRP3L351P F4/80hi macrophages had similar CD86 and CD11c expression. (*P < 0.01, n = 7.)
Discussion
Our data clearly show the important role of IL-1β in connecting inflammation to defective airway development. While LPS induces expression of many different inflammatory mediators, blocking IL-1 signaling, inhibiting inflammasome-mediated IL-1β cleavage, and deleting the IL-1β gene each protected fetal mouse lung airway branching from the inhibitory effects of LPS. While other released inflammatory mediators can affect different developmental processes (41), the effects of LPS on formation and expansion of new saccular airways appears directly tied to IL-1β. Whether the importance of IL-1β over other inflammatory mediators is due to relative abundance, proximity to receptors on target cells, or other unique aspects of IL-1β biochemistry remains undetermined. The requirement of inflammasome-mediated proteolytic IL-1β cleavage identifies an intriguing potential therapeutic target for protecting the developing preterm lung.
Relevant to the critical role of IL-1β, we measured developmental regulation of expression of multiple inflammasome components. While immature fetal lung macrophages are capable of responding to LPS and activating NF-κB, the reduced expression of inflammasome components would prevent the release of bioactive IL-1β. The developmental increases in inflammasome expression and/or function may explain why NF-κB activation early in lung development fails to produce dramatic changes in lung morphogenesis (12). We showed here that even E13 lung mesenchymal cells responded to NF-κB dependent inflammatory mediators. However, lower macrophage inflammasome activity in early embryonal lungs could protect the adjacent lung mesenchyme. The transcriptional mechanisms regulating inflammasome component gene expression during development are not yet clear. Interestingly, several inflammasome genes were much higher in newborn and adult macrophages while other components were more consistently expressed throughout development. How inflammasome gene expression parallels the normal development of macrophages and other myeloid lineages also warrants further investigation.
Environmental microbial exposures could also drive inflammasome gene expression in maturing lung macrophages. Following delivery, newborns quickly become colonized with diverse populations of microbes (42). Recent work has implicated inflammasome function in determining how host-microbe interactions result in resistance to infection or tolerance to colonization (43). While inflammasome-microbiome dynamics have primarily been studied in the context of intestinal colonization, microbiota in the lung can clearly induce inflammasome component expression (44). Exposure to colonizing microbes could therefore contribute to the increased inflammasome component expression in newborn and adult lung macrophages.
Previously we demonstrated that increased IKKβ activity and NF-κB activation increased expression of mature alveolar macrophage markers (35). Here we obtained similar results with increased percentages of F4/80hi macrophages in mice expressing gain of function inflammasome alleles. Fetal lung LysM-Cre-Nlrp3L351P macrophages expressed higher levels of CD204 and CD206, both of which are expressed on more mature alveolar macrophages. Our data also suggest that IL-1β may function in a cell autonomous manner in fetal lung macrophages to accelerate the maturation process. Recent studies have reported similar mechanisms linking inflammatory mediators with macrophage differentiation in the lung (45, 46). In addition to cytokine effects on resident macrophages, recruitment of fetal monocytes into the developing lung may also lead to changes in lung innate immunity. We speculate that IL-1β-mediated maturation could influence macrophage function in response to repetitive or chronic inflammatory stimuli. Persistent presence of IL-1β may also influence macrophage-mediated wound healing following lung injury.
Identifying inflammasome expression, function, and IL-1β release as important mechanisms linking inflammation and abnormal development opens new therapeutic possibilities for neonatal lung disease. Blocking IL-1β signaling or inflammasome function in specific cell populations could prevent the inhibitory effects of inflammation on lung development seen in patients with BPD. Subsequent studies will need to test if therapeutic strategies should focus on macrophages as the primary sites of inflammation and IL-1β release or the mesenchymal and epithelial cells targeted by IL-1β. Additional experiments will also need to determine if accelerated macrophage maturation following inflammation is beneficial or detrimental to the developing lung innate immune system. Preventing inflammation-induced expression of inflammasome components in immature macrophages could be protective for the developing lung. However, the accelerated expression of inflammasomes in the immature lung could be necessary for proper killing of inhaled pathogens and removal of aerosolized particles.
Acknowledgments
We thank Chen Xie for his technical assistance and Rinat Zaynagetdinov for his helpful advice. We thank our colleagues at Vanderbilt University and the University of California, San Diego, Rady Children’s Hospital, San Diego for their helpful comments and critical review of the manuscript.
Grant support: This work was supported by the Department of Veterans Affairs (to T.S.B.), National Institutes of Health Grants HL097195 (to L.S.P. and T.S.B.), HL086324 (to L.S.P), HL116358 (to L.S.P and T.S.B.), and HL069765 (to A.N.H.)
References
- 1.Maisey HC, Doran KS, Nizet V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert reviews in molecular medicine. 2008;10:e27. doi: 10.1017/S1462399408000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nissen MD. Congenital and neonatal pneumonia. Paediatric respiratory reviews. 2007;8:195–203. doi: 10.1016/j.prrv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Bose CL, Laughon MM, Dammann CE. Bronchopulmonary Dysplasia and Inflammatory Biomarkers in the Premature Neonate. Arch Dis Child Fetal Neonatal Ed. 2008 doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari V. Postnatal inflammation in the pathogenesis of bronchopulmonary dysplasia. Birth defects research. Part A, Clinical and molecular teratology. 2014;100:189–201. doi: 10.1002/bdra.23220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunzmann S, Collins JJ, Kuypers E, Kramer BW. Thrown off balance: the effect of antenatal inflammation on the developing lung and immune system. American journal of obstetrics and gynecology. 2013;208:429–437. doi: 10.1016/j.ajog.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth defects research. Part A, Clinical and molecular teratology. 2014;100:145–157. doi: 10.1002/bdra.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali Z, Schmidt P, Dodd J, Jeppesen DL. Bronchopulmonary dysplasia: a review. Archives of gynecology and obstetrics. 2013;288:325–333. doi: 10.1007/s00404-013-2753-8. [DOI] [PubMed] [Google Scholar]
- 8.Wolff CH. Innate immunity and the pathogenicity of inhaled microbial particles. International journal of biological sciences. 2011;7:261–268. doi: 10.7150/ijbs.7.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sznitman J. Respiratory microflows in the pulmonary acinus. Journal of biomechanics. 2013;46:284–298. doi: 10.1016/j.jbiomech.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Hiraiwa K, van Eeden SF. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators of inflammation. 2013;2013:619523. doi: 10.1155/2013/619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal NR, King LS, D'Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. American journal of physiology. Lung cellular and molecular physiology. 2014;306:L709–L725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, Yull FE, Prince LS. NF-kappaB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol. 2011;187:2740–2747. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller-Redetzky HC, Suttorp N, Witzenrath M. Dynamics of pulmonary endothelial barrier function in acute inflammation: mechanisms and therapeutic perspectives. Cell and tissue research. 2014;355:657–673. doi: 10.1007/s00441-014-1821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowley JE, Johnson JR. Pericytes in chronic lung disease. International archives of allergy and immunology. 2014;164:178–188. doi: 10.1159/000365051. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin JT, Carver BJ, Plosa EJ, Yamamoto Y, Miller JD, Liu JH, van der Meer R, Blackwell TS, Prince LS. NF-kappaB activation limits airway branching through inhibition of Sp1-mediated fibroblast growth factor-10 expression. J Immunol. 2010;185:4896–4903. doi: 10.4049/jimmunol.1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner JL, Steele C. Innate receptors and cellular defense against pulmonary infections. Journal of immunology. 2014;193:3842–3850. doi: 10.4049/jimmunol.1400978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prince LS, Okoh VO, Moninger TO, Matalon S. Lipopolysaccharide increases alveolar type II cell number in fetal mouse lungs through Toll-like receptor 4 and NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2004;287:L999–L1006. doi: 10.1152/ajplung.00111.2004. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin JT, Smith RJ, Halloran BA, Day TJ, Kelly DR, Prince LS. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L550–L558. doi: 10.1152/ajplung.00329.2006. [DOI] [PubMed] [Google Scholar]
- 19.Kallapur SG, Kramer BW, Nitsos I, Pillow JJ, Collins JJ, Polglase GR, Newnham JP, Jobe AH. Pulmonary and systemic inflammatory responses to intra-amniotic IL-1alpha in fetal sheep. American journal of physiology. Lung cellular and molecular physiology. 2011;301:L285–L295. doi: 10.1152/ajplung.00446.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prince LS, Dieperink HI, Okoh VO, Fierro-Perez GA, Lallone RL. Toll-like receptor signaling inhibits structural development of the distal fetal mouse lung. Dev Dyn. 2005;233:553–561. doi: 10.1002/dvdy.20362. [DOI] [PubMed] [Google Scholar]
- 21.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 22.Bry K, Whitsett JA, Lappalainen U. IL-1beta disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol. 2007;36:32–42. doi: 10.1165/rcmb.2006-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backstrom E, Hogmalm A, Lappalainen U, Bry K. Developmental stage is a major determinant of lung injury in a murine model of bronchopulmonary dysplasia. Pediatr Res. 2011;69:312–318. doi: 10.1203/PDR.0b013e31820bcb2a. [DOI] [PubMed] [Google Scholar]
- 24.Nold MF, Mangan NE, Rudloff I, Cho SX, Shariatian N, Samarasinghe TD, Skuza EM, Pedersen J, Veldman A, Berger PJ, Nold-Petry CA. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14384–14389. doi: 10.1073/pnas.1306859110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiscott J, Marois J, Garoufalis J, D'Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Molecular and cellular biology. 1993;13:6231–6240. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saccani S, Pantano S, Natoli G. Modulation of NF-kappaB activity by exchange of dimers. Molecular cell. 2003;11:1563–1574. doi: 10.1016/s1097-2765(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 27.Scheibel M, Klein B, Merkle H, Schulz M, Fritsch R, Greten FR, Arkan MC, Schneider G, Schmid RM. IkappaBbeta is an essential co-activator for LPS-induced IL-1beta transcription in vivo. The Journal of experimental medicine. 2010;207:2621–2630. doi: 10.1084/jem.20100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1beta secretion. Cytokine & growth factor reviews. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunological reviews. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 32.Shornick LP, De Togni P, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Ferguson TA, Chaplin DD. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. The Journal of experimental medicine. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, Boyle DL, Firestein GS, Horner AA, Soroosh P, Watford WT, O'Shea JJ, Kastner DL, Hoffman HM. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connelly L, Barham W, Onishko HM, Chen L, Sherrill TP, Zabuawala T, Ostrowski MC, Blackwell TS, Yull FE. NF-kappaB activation within macrophages leads to an anti-tumor phenotype in a mammary tumor lung metastasis model. Breast cancer research : BCR. 2011;13:R83. doi: 10.1186/bcr2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stouch AN, Zaynagetdinov R, Barham WJ, Stinnett AM, Slaughter JC, Yull FE, Hoffman HM, Blackwell TS, Prince LS. IkappaB kinase activity drives fetal lung macrophage maturation along a non-M1/M2 paradigm. Journal of immunology. 2014;193:1184–1193. doi: 10.4049/jimmunol.1302516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. American journal of respiratory cell and molecular biology. 2013;49:180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamin JT, Gaston DC, Halloran BA, Schnapp LM, Zent R, Prince LS. The role of integrin alpha8beta1 in fetal lung morphogenesis and injury. Dev Biol. 2009;335:407–417. doi: 10.1016/j.ydbio.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccioli P, Rubartelli A. The secretion of IL-1beta and options for release. Seminars in immunology. 2013;25:425–429. doi: 10.1016/j.smim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. The Journal of biological chemistry. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 40.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. The Journal of experimental medicine. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller JD, Benjamin JT, Kelly DR, Frank DB, Prince LS. Chorioamnionitis Stimulates Angiogenesis in Saccular Stage Fetal Lungs Via CC Chemokines. Am J Physiol Lung Cell Mol Physiol. 2010 doi: 10.1152/ajplung.00414.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayres JS. Inflammasome-microbiota interplay in host physiologies. Cell Host Microbe. 2013;14:491–497. doi: 10.1016/j.chom.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anthony D, McQualter JL, Bishara M, Lim EX, Yatmaz S, Seow HJ, Hansen M, Thompson M, Hamilton JA, Irving LB, Levy BD, Vlahos R, Anderson GP, Bozinovski S. SAA drives proinflammatory heterotypic macrophage differentiation in the lung via CSF-1R–dependent signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.14-250332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huaux F, Lo Re S, Giordano G, Uwambayinema F, Devosse R, Yakoub Y, Panin N, Palmai-Pallag M, Rabolli V, Delos M, Marbaix E, Dauguet N, Couillin I, Ryffel B, Renauld JC, Lison D. IL-1alpha induces CD11b alveolar macrophage proliferation and maturation during granuloma formation. The Journal of pathology. 2014 doi: 10.1002/path.4487. [DOI] [PubMed] [Google Scholar]