Abstract
Background
How to optimally prescribe blood pressure, lipid and glucose-lowering treatments to adults with type 2 diabetes in low- and middle-income countries (LMICs) remains unclear.
Methods
We developed a microsimulation model to compare: (i) a “treat to target” (TTT) strategy, aiming to achieve target levels of biomarkers (blood pressure <130/80 mmHg, low-density lipoprotein <2.59 mmol/L, haemoglobin A1c <7%); with (ii) a “benefit-based tailored treatment” (BTT) strategy, aiming to lower estimated risk for complications (to a 10-year cardiovascular disease [CVD] risk <10%, and lifetime microvascular risk <5%) based on age, sex, and biomarker values. Data were obtained from cohorts in China, Ghana, India, Mexico, and South Africa, to span a spectrum of risk profiles.
Findings
TTT recommended treatment to many people at lower risk of diabetes complications, while BTT recommended treatment to fewer people at higher risk. BTT would be expected to avert 24% to 31% more complications than TTT, and be more cost-effective from a societal perspective (saving between $4 and $300 per DALY averted among the different countries simulated). Alternative treatment thresholds, matched by total cost or population size treated, did not change the comparative superiority of BTT, nor did titrating treatment using fasting plasma glucose (for areas without A1c testing). If insulin were unavailable, however, BTT was no longer significantly superior for preventing microvascular events, only for preventing CVD events.
Interpretation
A BTT strategy would be more effective and cost-effective than a TTT strategy in LMICs for prevention of both CVD and microvascular complications of type 2 diabetes. The superiority of the BTT strategy for averting microvascular complications, however, would be contingent on insulin availability.
Funding
Rosenkranz Prize for Healthcare Research in Developing Countries; U.S. National Institutes of Health (U54 MD010724, DP2 MD010478).
Keywords: type 2 diabetes, low- and middle-income countries, treatment guidelines, personalized medicine, cost-effectiveness
Introduction
Treatment for type 2 diabetes is required for an increasing number of people worldwide, as the prevalence of diabetes continues to rise.1 Treatment of type 2 diabetes in low- and middle-income countries, in particular, requires careful consideration of how to maximize the benefits of treatment for the largest number of patients within highly-constrained budgets.
Treating type 2 diabetes requires management of three principal, co-existing risk factors for morbidity and mortality: high blood pressure, dyslipidaemia, and poor glycaemic control.2–4 Treating these three risk factors has traditionally been guided by a “treat-to-target” (TTT) strategy focused on achieving specific levels of blood pressure, low-density lipoprotein cholesterol (LDL-C) and haemoglobin A1c.5 Recently, reflecting the concern that LDL-C levels are an imperfect marker for who benefits from statin treatment, U.S. practice guidelines have shifted towards a “benefit-based, tailored treatment” (BTT) strategy6—directing clinicians to prescribe statin treatment based on composite estimates of cardiovascular disease (CVD) risk, which incorporate numerous interrelated risk factors (e.g., age, sex, tobacco smoking, blood pressure, lipid profile) rather than LDL-C levels alone.7,8 A similar shift for blood pressure treatment decisions has been proposed,9,10 given accumulating evidence from randomized trials that composite estimates of CVD risk are a better predictor of benefits from blood pressure treatment than systolic or diastolic blood pressure values alone.11–13 Less well-studied, is the idea of extending a BTT strategy to glycemic control, as some patients experience greater microvascular risk reduction from the same decline in hemoglobin A1c.16,17 Whether TTT or BTT is a better treatment approach for averting both macrovascular and microvascular complications in people with type 2 diabetes remains unclear.
Treatment benefits for people with type 2 diabetes depend upon interactions between glycaemic control and blood pressure, lipids, and other co-morbidities.2–4,18 Hence, it remains unclear whether and under what circumstances a TTT approach or a BTT approach would provide more benefits to people with type 2 diabetes. Which treatment strategies are followed by clinicians will have profound implications for who receives treatment, how well treatment averts diabetes complications and related disability, and overall program cost and cost-effectiveness—all key considerations for government ministers evaluating whether and how to pay for treatment.19
Here, we sought to compare both the effectiveness and cost-effectiveness of TTT and BTT approaches for diabetes management in low- and middle-income countries. We developed a microsimulation model to compare the effectiveness and cost-effectiveness of therapy. Our model was informed by high-quality data from five countries classified as low- or middle-income by World Bank criteria,20 spanning a global spectrum of risk profiles: China, Ghana, India, Mexico, and South Africa.
Methods
Model structure
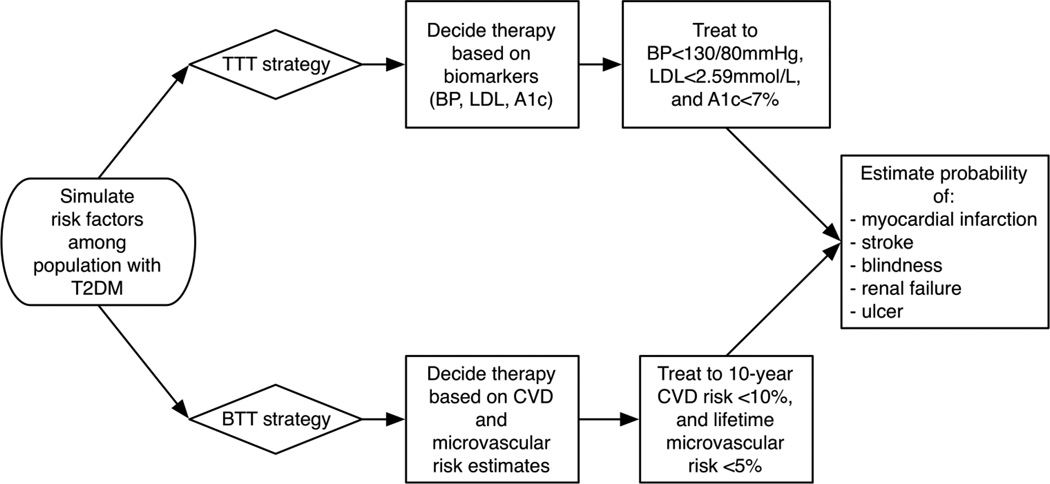
We constructed a microsimulation model (Figure 1) to simulate adults aged 20– 79 years old and their risks of five major diabetes complications: the CVD complications of myocardial infarction (MI) and stroke, and the microvascular complications of end-stage renal disease, blindness, and diabetic ulcer. We adopted a microsimulation approach over a more traditional Markov cohort approach, because a microsimulation captures the correlations between individual demographic characteristics, risk factor values, and complication rates—allowing us to account for important variations in risk within each country population (necessary for simulating the BTT approach), rather than only simulating the population average risk of complications.
Figure 1. Model diagram.
Legend: T2DM = type 2 diabetes mellitus; CVD = cardiovascular disease; TTT = treat-to-target strategy; BTT = benefit-tailored treatment strategy; BP = blood pressure; LDL-C = low-density lipoprotein; A1c = haemoglobin A1c.
We included parameters for: population projections by age, sex, and urban/rural residence by country from the United Nations (Appendix Table 1);21 age distribution and secular trends in type 2 prevalence from the International Diabetes Federation22 (Appendix Table 1); and risk factors for CVD and microvascular complications from cohorts including persons with type 2 diabetes in each country (particularly the WHO Study on Global Aging and Adult Health, or SAGE, 2007–2010),23 supplemented by a PubMed search to ensure a broad range of possible values were incorporated (Appendix Table 2). The estimated prevalence of type 2 diabetes was 9.3% in China, 2.2% in Ghana, 8.8% in India, 15.0% in Mexico, and 7.2% in South Africa, versus 14.3% in the US and 12.4% in the UK among the same age group.22,24,25 The SAGE study included N=3,993 in China; 3,938 in Ghana; 9,994 in India; 38,746 in Mexico; and 2,352 in South Africa. Within each country, we simulated a representative age-adjusted population of 100,000 people with type 2 diabetes.26 We then utilized the internationally-validated UKPDS OM2 equations27,28 to estimate the ethnicity-specific risk of each complication over time given a simulated individual’s risk factors, including systolic blood pressure, lipid profile, haemoglobin A1c, tobacco smoking, body mass index, and glomerular filtration rate. Age-, sex-, and country-specific mortality rates were incorporated from WHO estimates (Appendix Table 3). The Appendix text provides complete details on the model programming.
Comparative effectiveness of TTT and BTT
Under the TTT strategy, we simulated recommended treatment of people with type 2 diabetes with: (i) a statin with dose titration to achieve low-density lipoprotein <2.59 mmol/L (100 mg/dL);29 (ii) blood pressure agents to achieve a blood pressure <130/80 mmHg;14 (iii) metformin, a sulfonylurea and, if needed, insulin replacing the sulfonylurea to achieve a haemoglobin A1c <7%5 (see sensitivity analyses, below, for testing of alternate targets). We adopted medication choices following WHO guidelines (including NPH insulin as basal insulin, with regular insulin for prandial coverage as needed), and simulated dose effects on biomarkers and the risk of each complication based on meta-analyses of randomized trials (detailed in Appendix text).14,30–34 The TTT strategy attempts to achieve each of the targets independently, such that an individual person with normal BP would not require BP lowering treatment but may require only statin and glycemic treatment. Under the BTT strategy, we envisioned that CVD and microvascular risk could be assessed by risk tables (see Appendix Figure 1 for an example of a microvascular risk table), based on the UKPDS OM2 equations,27 similar to current WHO guideline charts displaying CVD risk by patient demographics and biomarker values.14 We simulated recommended treatment with: (i) statin treatment with simvastatin 40mg if an individual’s 10-year baseline CVD risk (combined risk of MI and stroke) was ≥10%;35 then (ii) subsequent addition of blood pressure agents to achieve a 10-year combined risk of MI and stroke ≥10% (provided blood pressure remained ≥110/55 mmHg for safety);36 and then (iii) glucose treatment with metformin, a sulfonylurea and, if needed, insulin replacing the sulfonylurea to achieve a lifetime combined risk of the major microvascular complications <5% (as long as fasting plasma glucose remained ≥3.33 mmol/L [60 mg/dL], for safety), with additional blood pressure agents and a statin prescribed to achieve the microvascular goal, if it was not achieved through glucose control alone (with blood pressure maintained ≥110/55 mmHg). Single-dose statin therapy, ordered first in the BTT protocol, was chosen because of prior work justifying this empiric dosing approach, and given limited availability of alternative statins and statin doses in low- and middle-income countries.35,37,38
Cost-effectiveness analysis
Per current cost-effectiveness analysis guidelines,39,40 we simulated the complete life-course of all persons alive or born who have or develop type 2 diabetes during the next 10 years (2016–2025).26,41 We integrated treatment costs from a societal perspective (regardless of payer), and expressed cost-effectiveness ratios as discounted costs over discounted disability-adjusted life-years (DALYs) at a 3% annual discount rate. We first calculated average cost-effectiveness ratios for both TTT and BTT, with “no treatment” as the comparator scenario, to correspond to WHO CHOICE guidelines, and given lack of data on current status-quo treatment access levels. We also calculated incremental cost-effectiveness ratios if TTT were switched to BTT at full availability/coverage. We calculated DALYs using disability weight values estimated by the Global Burden of Disease Project surveys,42 drug costs for therapy in all countries based on per-unit buyer cost estimates from the International Drug Price Indicator Guide,43 and assay and service cost data based on published field surveys (Appendix Table 4). We expressed all costs in 2016 U.S. Dollars. See Online Supplementary Material for Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.
Sensitivity analyses
We performed six sensitivity analyses: (i) varying the BTT glycaemic treatment threshold from a baseline of 5% lifetime microvascular risk to thresholds of 3% and 7% risk; (ii) changing the TTT glycaemic biomarker from haemoglobin A1c to fasting plasma glucose (target <7mmol/L, per WHO guidance)14 to reflect lack of A1c assay availability in some areas; (iii) setting the BTT treatment thresholds to produce the same total number of people treated as the TTT strategy within each country (where the number treated includes any form of treatment—any blood pressure agent, statin, or glycaemic control agent); (iv) simulating treatment if insulin were unavailable (i.e., only metformin and sulfonylurea available); (v) simulating outcomes if the BTT guidelines had 10% lower adherence from practitioners than the TTT scenario to reflect practitioner resistance to change from the conventional TTT approach, and (vi) simulating outcomes if combining the BTT approach for preventing CVD complications with the TTT approach for preventing microvascular complications.
In all scenarios, we performed discrete sensitivity analyses by repeating each country-specific simulation 10,000 times, repeatedly sampling with replacement from normal distributions constructed around all input parameter estimates to estimate 95% confidence intervals around the results. The analysis included uncertainty in the risk equation coefficients, to account for the imprecision and potential errors involved in risk estimation, and subsequent consequences for patient outcomes. We performed simulations in R (v. 3.2, The R Project for Statistical Computing, Vienna).
Results
Comparative effectiveness of TTT and BTT
As shown in Table 1, the TTT strategy would recommend treatment to a larger proportion of patients with type 2 diabetes than would the BTT strategy. While the TTT strategy would recommend at least one form of treatment (at least one blood pressure, lipid, or glycaemic control agent) to between 99.1% to 99.4% of persons with type 2 diabetes across the studied countries, the BTT strategy would recommend treatment to between 1.2% and 2.9% fewer people (between 96.4% and 98.1% of persons with type 2 diabetes). Yet the TTT strategy would recommend fewer medications per person treated, meaning that the BTT strategy would more intensely treat a smaller population of patients (3.0 to 3.3 medications on average per person for TTT across the studied countries, versus 3.7 to 3.8 for BTT).
Table 1.
Comparative effectiveness and cost-effectiveness of treat-to-target (TTT) and benefit-based tailored treatment (BTT) strategies for reducing type 2 diabetes complications in low- and middle-income countries. 95% confidence intervals in parentheses.
| China (type 2 diabetes prevalence ~9.3%) |
Ghana (type 2 diabetes prevalence ~2.2%) |
India (type 2 diabetes prevalence ~8.8%) |
Mexico (type 2 diabetes prevalence ~15.0%) |
South Africa (type 2 diabetes prevalence ~7.2%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TTT | BTT | TTT | BTT | TTT | BTT | TTT | BTT | TTT | BTT | |
| Pre-treatment patient characteristics: | ||||||||||
| Mean age at diagnosis (years) | 57.5 (53.6, 61.4) | 54.0(50.1,57.9) | 56.0(52.1,60.0) | 52.4 (48.5, 56.3) | 54.0(50.1,57.9) | |||||
| Prior history of CVD (%) | 21.4(10.7,42.8) | 16.4(8.2,32.8) | 17.1 (8.6,34.2) | 10.8(5.4,21.6) | 22.2(11.1,44.4) | |||||
| 10-year CVD risk (%) | 25.4 (4.4, 77.6) | 24.4 (4.4, 74.0) | 26.3 (4.7, 79.2) | 25.3 (4.3, 77.0) | 25.5 (4.5, 77.2) | |||||
| Haemoglobin A1c (%) | 8.2(6.2, 12.2) | 8.7(6.6, 13.0) | 8.4(6.3, 12.5) | 9.0(6.8, 13.4) | 8.7(6.6, 13.0) | |||||
| Systolic blood pressure (mmHg) |
141.1 (120.1, 165.0) | 144.4(119.9,173.8) | 143.7(119.2,173.3) | 143.0(118.7,173.3) | 143.1 (118.6,172.5) | |||||
| Low-density lipoprotein (mmol/L) |
2.9(1.8,4.2) | 3.0(1.9,4.3) | 3.1 (1.9,4.5) | 3.0(1.9,4.3) | 3.0(1.9,4.3) | |||||
| Lifetime microvascular risk (%) | 17.2(4.1,58.7) | 17.9(4.3,60.2) | 19.9(4.5,67.9) | 16.3(4.0,53.7) | 15.5(4.0,50.0) | |||||
| Treatment recommendations: | ||||||||||
| Adults with type 2 diabetes recommended any treatment, %: |
99.1 (99, 99.1) |
97.0 (97.0, 97.1) |
99.4 (99.3, 99.4) |
97.2 (97.1,97.2) |
99.3 (99.2, 99.4) |
98.1 (98,98.1) |
99.2 (99.2, 99.3) |
96.6 (96.4, 96.7) |
99.3 (99.2, 99.3) |
96.4 (96.4, 96.5) |
| Blood pressure treatment | 80.5 (80.3, 80.6) |
82.5 (82.2, 82.7) |
86.4 (86.3, 86.6) |
81.0 (80.9,81.1) |
85.4 (85.2, 85.5) |
84.4 (84.2, 84.5) |
84.1 (83.9, 84.2) |
82.3 (82.2, 82.5) |
84.1 (83.8, 84.3) |
82.7 (82.5, 82.8) |
| Lipid treatment | 75.2 (75.1,75.5) |
82.8 (82.5, 83.0) |
75.4 (75.2, 75.6) |
81.2 (81, 81.3) |
75.3 (75.1,75.6) |
84.5 (84.3, 84.7) |
75.3 (75.2, 75.5) |
82.5 (82.4, 82.7) |
75.3 (75.0, 75.5) |
82.9 ( 82.7, 83.0) |
| Glycaemic treatment | 80.7 (80.5, 80.9) |
92.8 (92.7, 92.9) |
80.7 (80.6, 80.9) |
93.8 (93.7, 94.0) |
80.7 (80.6, 80.9) |
95.1 (95, 95.2) |
80.7 (80.5, 80.9) |
92 (91.8, 92.1) |
80.8 (80.6,81.0) |
91.9 (91.7,92.0) |
| Insulin treatment | 13.8 (13.7,13.9) |
16.9 (16.7, 17.1) |
13.8 (13.7, 14.0) |
17.7 (17.5, 17.9) |
13.8 (13.7, 14.0) |
19.3 (19.1, 19.4) |
13.8 (13.5, 13.9) |
16.1 (15.9, 16.3) |
13.8 (13.7, 14.0) |
15.3 (15.2, 15.5) |
| number of medications, per person recommended treatment |
3.0 (3.0,3.1) |
3.8 (3.8, 3.8) |
3.3 (3.3, 3.3) |
3.7 (3.7, 3.7) |
3.2 (3.2, 3.3) |
3.8 (3.8, 3.8) |
3.2 (3.2, 3.2) |
3.7 (3.7, 3.8) |
3.2 (3.2, 3.2) |
3.8 (3.8, 3.8) |
| CVD events prevented per 100,000 people with type 2 diabetes from 10 years of treatment: | ||||||||||
| MI | 2098.4 (2005.4, 2146.1) |
3218.9 (3145.3, 3299.4) |
2206.5 (2133.2, 2268.7) |
3080.9 (3021.6, 3197.7) |
2290.4 (2229.6, 2356.0) |
3390.2 (3334.5, 3471.1) |
2201.1 (2125.5, 2311.4) |
3232.2 (3153.8, 3343.7) |
2200.8 (2111.3, 2309.9) |
3270.7 (3208.1, 3335.6) |
| Stroke | 1857.3 (1771.5, 1914.8) |
2841.4 (2813.1, 2882.8) |
1885.3 (1790.5, 1966) |
2595.1 (2539.0, 2677.6) |
2054.4 (1986.8, 2134.1) |
2967.7 (2857.3, 3085.0) |
1932.0 (1886.0, 1982.2) |
2784.0 (2731.7, 2822.7) |
1941.5 (1881.5, 1995.6) |
2850 (2791.2, 2906) |
| Microvascular events prevented per 100,000 people with type 2 diabetes from 10 years of treatment: | ||||||||||
| Blindness | 229.4 (214.2,240) |
303.5 (286.5, 327.1) |
244.1 (228, 264.8) |
305.7 (285.4, 328.8) |
238.2 (219.6, 270) |
310.1 (284.6, 329) |
225.2 (205.6, 242.9) |
295.9 (274.5, 336.8) |
222.3 (204.9, 243.7) |
295.1 (266.8, 319.9) |
| End-stage renal disease | 764(731.1, 799.2) |
881.7 (840, 920.7) |
833.5 (813.3, 862.9) |
955.0 (917.6, 989.4) |
973.7 (936.2, 1020.1) |
1110.5 (1065.5, 1155.8) |
705.4 (675.4, 736.4) |
810.3 (785.6, 871.6) |
590.0 (559, 614.6) |
687.5 (649.9, 731.5) |
| Ulcer | 265.3 (238.7, 286.0) |
260.4 (243, 284.4) |
261.8 (239.1, 295.5) |
245.1 (217.5, 262.8) |
220.6 (198.7, 256.0) |
200.1 (177.4, 218.7) |
306.1 (258.0, 345.1) |
291.3 (278.9, 307.9) |
411.9 (369.9, 442.6) |
389.7 (357.2, 425.7) |
| Deaths averted per 100,000 people with type 2 diabetes from 10 years of treatment: |
1409.8 (1346.0, 1454.3) |
2051.7 (2010.0, 2098.6) |
1467.9 (1409.6, 1522.3) |
1957.7 (1910.3, 2025.7) |
1596.8 (1545.0, 1657.7) |
2214.7 (2145.5, 2291.7) |
1439.3 (1394.2, 1493.5) |
2008.8 (1963.7, 2069.1) |
1404.5 (1351.1, 1457.3) |
2000.1 (1950.8, 2051.3) |
| Number needed to treat to prevent one CVD event |
25.0 (24.4, 26.2) |
16.0(15.7, 16.3) |
24.3 (23.5, 25.3) |
17.1 (16.5, 17.5) |
22.9(22.1, 23.5) |
15.4(15, 15.8) |
24.0(23.1, 24.7) |
16.1 (15.7, 16.4) |
24(23.1, 24.8) |
15.8(15.5, 16.1) |
| Number needed to treat to prevent one microvascular event |
64.0 (60.8, 67.8) |
57.1 (54.0, 60.1) |
74.2 (69.8, 77.6) |
64.5(61.5, 68.4) |
69.3 (64.3, 73.3) |
60.5 (57.6, 64.2) |
80.2 (74.9, 87.1) |
69.1 (63.8, 72) |
81.1 (76.3, 87.5) |
70.3 (65.4, 75.7) |
| Cost and cost-effectiveness: | ||||||||||
| Total costs, per capita per year, $US 2016 |
$891.1 ($876.6, $906.7) |
$886.8 ($871.2, $901) |
$725.4 ($717.4, $735.8) |
$724.6 ($717.7, $736.0) |
$952.6 ($946.8, $959.0) |
$952.5 ($946.3, $958.3) |
$1020.1 ($1005.3, $1035.6) |
$1012.4 ($997.8, $1028.1) |
$517.6 ($513.3, $522.7) |
$512.8 ($507.6, $517.3) |
| Total DALYs averted, per capita per year |
0.085 (0.083, 0.087) |
0.123 (0.121, 0.124) |
0.043 (0.042, 0.045) |
0.060 (0.059, 0.061) |
0.063 (0.062, 0.065) |
0.088 (0.087, 0.089) |
0.093 (0.091, 0.095) |
0.127 (0.125, 0.129) |
0.031 (0.031, 0.032) |
0.047 (0.046, 0.048) |
| $/DALYs averted (average cost- effectiveness, compared to no treatment) |
$10448.7 ($10353.1, $10514.3) |
$7215.9 ($7209.5, $7254.9) |
$16710.4 ($16327, $17094) |
$12038.8 ($11978.3, $12106.3) |
$15124.1 ($14800.2, $15385) |
$10769.1 ($10728.3, $10840.7) |
$11019.0 ($10897.4, $11062.4) |
$7941.9 ($7843.1, $7966) |
$16448.0 ($16168.6, $16728.4) |
$10928.4 ($10754.1, $11017.3) |
| Incremental cost-effectiveness of shifting from TTT to BTT |
$−113.2 (−126.5,−104.9) | $−47.1 (−57.1,−42.1) | $-4.0 (−4.5, −3.7) | $−226.5 (−256.7, −202.6) | $−300.0 (−342.9, −282.4) | |||||
The BTT strategy would be expected to avert more complications (between 24.4% and 30.5% more complications than TTT), and therefore had a significantly lower number needed to treat than did the TTT strategy (NNT to prevent a CVD event ranging from 15.4 to 17.1 for BTT, versus 22.9 to 25.0 for TTT across countries, and NNT to prevent a microvascular event ranging from 57.1 to 70.3 for BTT versus 64.0 to 81.1 for TTT, which was significantly different within each country, Table 1). The BTT strategy would be expected to avert significantly more complications for all outcomes measured (40% to 53% more myocardial infarctions; 38% to 53% more strokes; 25% to 33% more blindness cases; and 12% to 15% more end-stage renal disease cases), with the exception of the outcome of diabetic ulcer, for which there was no significant difference between the TTT and BTT strategies (Table 1). The proportion of complications averted by the TTT averaged 14% for myocardial infarctions, 17% for strokes, 6% for blindness, 6% for renal failure and 19% for ulcers; the proportion averted by BTT averaged 21% for myocardial infarctions, 26% for strokes, 9% for blindness, 7% for renal failure and 19% for ulcers.
Cost-effectiveness analysis
The BTT strategy had equivalent cost but significantly greater effectiveness than the TTT strategy in all countries studied, averting significantly more DALYs (Table 1). The absolute costs of both strategies varied from ~$500 to ~$1,000 per person per year among the studied countries, due to variations in medication costs (with 11% of total costs coming from blood pressure agents, statins, and glycaemic control agents, including 4% of total costs coming from insulin) and healthcare service costs (with 55% of total costs coming from microvascular disease management costs such as dialysis and ulcer treatment, and the remainder from CVD management costs of post-MI/stroke care). The BTT strategy averted between 0.016 and 0.038 more DALYs per person per year. In terms of average cost-effectiveness ratios compared to the “no treatment” strategy, the BTT strategy had an average cost-effectiveness of $7,200 to $12,000 per DALY averted across the studied countries, while the TTT strategy had an average cost-effectiveness that was always higher within each country, of $10,500 to $16,700 per DALY averted across the studied countries; the incremental cost-effectiveness ratio of shifting from TTT to BTT varied from −$4 to −$300 per incremental DALY averted across the studied countries, indicating a cost-savings from BTT (Table 1).
Patients treated similarly or differently by TTT and BTT
We compared those patients prevented from experiencing a complication by TTT versus by BTT, in terms of their pre-treatment characteristics, to identify whether any factors substantially differed between the groups, or whether any particular biomarkers could predict who would benefit more from one strategy versus the other. We did not observe any single biomarker to predict which persons would be prevented from a complication by TTT and not BTT, or vice versa. We observed that persons more likely to have a complication averted by TTT rather than BTT were substantially younger (by 6.2 years on average), and more likely to be female (19.8% more females) (Table 2). In general, those averted from a complication by TTT but not BTT were also of lower risk (4.0% lower 10-year CVD risk, 4.4% lower lifetime microvascular risk; Table 2).
Table 2.
Pre-treatment characteristics of patients averted from a diabetes complication by treat-to-target (TTT) and benefit-based tailored treatment (BTT). Standard deviations in parentheses.
| China | Ghana | India | Mexico | South Africa | |
|---|---|---|---|---|---|
| People prevented from a complication by both TTT and BTT | |||||
| Number (per 100,000 people with T2DM) |
3183.5(79.0) | 3301.3 (70.4) | 3547.9 (64.7) | 3252.2 (67.8) | 3239.8 (73.9) |
| Number (absolute pop. size) | 2,902,000 (72,000) | 11,000 (1,000) | 2,388,000 (44,000) | 401,000 (8,000) | 79,000 (2,000) |
| Age | 56.4 (2.2) | 56.4 (1.2) | 56.4 (2.0) | 56.5 (2.6) | 56.6 (2.3) |
| Sex (% Female) | 56.0(11.2) | 56.2 (6.7) | 56.6(11.3) | 55.9 (8.9) | 55.9 (8.9) |
| SBP(mmHg) | 144.2(2.9) | 151.5(3.1) | 133.9(2.4) | 144.2(2.2) | 143.4(2.3) |
| LDL-C (mmol/L) | 3.1 (0.1) | 3.2(0.1) | 3.3 (0.2) | 3.2(0.1) | 3.2(0.1) |
| A1c(%) | 8.6 (0.4) | 9.2 (0.2) | 8.8 (0.4) | 9.5 (0.4) | 9.2 (0.2) |
| Tobacco smoking (% current, both sexes) |
33.0 (4.7) | 9.2 (3.0) | 37.4 (5.4) | 24.3 (4.4) | 27.5 (4.6) |
| 10-year CVD risk (%) | 27.7 (3.3) | 26.3 (3.5) | 28.4 (2.8) | 27.5 (3.6) | 27.8 (3.6) |
| Lifetime microvascular risk (%) |
18.4(1.3) | 19.1 (2.5) | 21.1 (3.2) | 17.4(3.1) | 16.6(3.1) |
| People prevented from a complication by TTT but not BTT | |||||
| Number (per 100,000 people with T2DM) |
2030.9 (50.4) | 2129.9(45.4) | 2229.4 (40.7) | 2117.6(44.1) | 2126.7(48.5) |
| Number (absolute pop. size) | 1,851,000 (46,000) | 7,000(1,000) | 1,500,000(27,000) | 261,000 (5,000) | 52,000 (1,000) |
| Age | 51.3(1.9) | 51.4(2.1) | 51.3 (2.5) | 51.2(1.3) | 51.0 (2.3) |
| Sex (% Female) | 77.3 (7.4) | 77.4 (5.5) | 77.6 (5.3) | 77.3 (4.2) | 77.5 (7.4) |
| SBP(mmHg) | 138.1 (3.0) | 145.2(3.5) | 128.1 (3.3) | 138.2(3.2) | 137.5(2.3) |
| LDL-C (mmol/L) | 3.0 (0.2) | 3.1 (0.2) | 3.1 (0.2) | 3.1 (0.1) | 3.1 (0.1) |
| A1c(%) | 8.2 (0.5) | 8.7 (0.4) | 8.4 (0.5) | 9 (0.4) | 8.7 (0.5) |
| Tobacco smoking (% current, both sexes) |
32.2 (3.0) | 9.1 (1.9) | 36.9 (3.4) | 24.2 (2.9) | 27.1 (3.0) |
| 10-year CVD risk (%) | 16.9(2.3) | 16.1 (2.1) | 17.3(1.2) | 16.7(1.9) | 16.8(1.6) |
| Lifetime microvascular risk (%) |
11.0(0.6) | 11.4(1.4) | 12.5(1.2) | 10.5(1.7) | 10.1 (1.3) |
| People prevented from a complication by BTT but not TTT | |||||
| Number (per 100,000 people with T2DM) |
4322.4(100.3) | 3880.5 (70.7) | 4430.7 (92.6) | 4161.5(84.8) | 4253.2 (88.9) |
| Number (absolute pop. size) | 3,941,000(914,000) | 13,000(2,000) | 2,982,000 (623,000) | 514,000(105,000) | 103,000(22,000) |
| Age | 57.5 (8.6) | 57.3(11.7) | 57.1 (6.9) | 57.5(7.1) | 57.7(12.0) |
| Sex (% Female) | 57.4(4.1) | 57.6 (4.6) | 59 (2.3) | 57.1 (5.5) | 57 (5.3) |
| SBP(mmHg) | 134.0(3.2) | 139.7(3.9) | 123.7(3.6) | 133.4(3.5) | 132.7(3.1) |
| LDL-C (mmol/L) | 2.7 (0.3) | 2.8(0.1) | 2.9 (0.2) | 2.8(0.1) | 2.8 (0.2) |
| A1c(%) | 7.6 (0.4) | 8.1 (0.5) | 7.8 (0.4) | 8.3 (0.5) | 8.1 (0.4) |
| Tobacco smoking (% current, both sexes) |
32.8 (6.4) | 9.0 (3.5) | 37.3 (6.8) | 24.6 (5.8) | 27.6 (6.0) |
| 10-year CVD risk (%) | 21.0(7.1) | 19.7(9.1) | 21.1 (5.2) | 20.7(12.1) | 21.0(12.7) |
| Lifetime microvascular risk (%) | 15.5(6.2) | 16.0(5.6) | 17.7(10.7) | 14.6(4.3) | 13.9(7.1) |
To further compare the effectiveness and efficiency of pharmaceutical treatment among the two approaches, we compared risks and outcomes among those patients treated more intensively (with more medications) under the TTT strategy with those treated more intensively under the BTT strategy (Table 3). Both CVD and microvascular risks were lower among patients treated more intensively by TTT than by BTT (~12% lower 10-year CVD risk, and ~3% lower microvascular risk on average among those treated more intensively by TTT than by BTT). In terms of economic efficiency, the relative cost of treatment among persons treated more intensively by TTT was 2.5 to 3.7 times higher per DALY averted than among those treated more intensively by BTT, indicating lower efficiency of the TTT strategy per dollar spent in these subgroups of patients. Similarly, the pharmaceutical efficiency of TTT was 13.0% to 18.8% lower, with 0.2 to 0.5 fewer DALYs saved per 1,000 person-years of pharmaceutical treatment dispensed (Table 3).
Table 3.
Comparison of patients treated more intensively (i.e., with more medications) by treat-to-target (TTT) or by benefit-based tailored treatment (BTT) strategies for reducing type 2 diabetes complications in low- and middle-income countries. Standard deviations in parentheses.
| China | Ghana | India | Mexico | South Africa | |
|---|---|---|---|---|---|
| People treated with more medications by TTT | |||||
| % of people with type 2 diabetes | 26.8 (0.2) | 32.6 (0.2) | 28.1 (0.3) | 29.2 (0.2) | 28.4 (0.2) |
| Absolute number of people | 24,432,000(182,000) | 111,000 (1,000) | 18,912,000(202,000) | 3,604,000 (25,000) | 688,000 (5,000) |
| Age, years | 56.3 (9.8) | 59.8 (6.7) | 59.7 (6.6) | 59.7 (6.6) | 59.8 (6.6) |
| Female, % | 85.1 (6.3) | 85.3 (3.6) | 85.1 (3.2) | 84.8 (3.3) | 85.7 (5.8) |
| Smokers, % current, both sexes | 16.7(3.0) | 3.6(1.3) | 20.2 (2.8) | 11.4(2.1) | 13.1 (2.3) |
| Mean initial SBP, mmHg | 146.6(11.3) | 153.7(12) | 136.4(13.7) | 146.5(9.5) | 145.7(14.4) |
| Mean final SBP, mmHg | 129.9(8.3) | 129.9(8.3) | 129.9(8.3) | 129.9(8.3) | 129.9(8.3) |
| Mean initial LDL-C cholesterol, mmol/L | 3.1 (0.4) | 3.2 (0.5) | 3.3 (0.4) | 3.2 (0.4) | 3.2 (0.3) |
| Mean final LDL-C cholesterol, mmol/L | 2.6 (0.4) | 2.6 (0.4) | 2.6 (0.4) | 2.6 (0.4) | 2.6 (0.4) |
| Mean initial A1 c, % | 7.8 (0.5) | 8.3 (0.7) | 8 (0.6) | 8.6 (0.4) | 8.3 (0.7) |
| Mean final A1c, % | 6.9 (0.5) | 6.9 (0.5) | 6.9 (0.5) | 6.9 (0.5) | 6.9 (0.5) |
| Mean initial 10-year CVD risk, % | 12.3(7.0) | 12.5(6.4) | 13.3(4.5) | 12.6(4.2) | 12.7(5.5) |
| Mean final 10-year CVD risk, % | 7.8 (3.7) | 7.7 (3.4) | 8.2 (2.3) | 7.8 (2.5) | 7.9 (2.8) |
| Mean initial lifetime microvascular risk, % | 15.0(7.2) | 16(8.2) | 18.1 (10.4) | 14.0(5.8) | 13(6.9) |
| Mean final lifetime microvascular risk, % | 13.5(6.2) | 14.4(7.4) | 16.3(7.9) | 12.6(5.9) | 11.6(5.8) |
| $/DALY among treated | $20029.0(1079.0) | $38800.8(1461.4) | $30698.0(1815.4) | $21183.4(708.3) | $45026.1 (2744.6) |
| DALYs averted per 1000 patient-years of pharmacotherapy | 2.8 (6.4) | 1.3 (2.4) | 2 (2.3) | 2.9 (4.0) | 1.0(1.9) |
| People treated with more medications by BTT | |||||
| % of people with type 2 diabetes | 55.5 (0.2) | 48.2 (0.2) | 52.9 (0.2) | 52.1 (0.2) | 52.9(0.1) |
| Absolute number of people | 50,597,000(182,000) | 165,000(1,000) | 35,602,000(135,000) | 6,430,000 (25,000) | 1,282,000(2,000) |
| Age, years | 59.6 (6.6) | 54.1 (11.5) | 54.1 (10.4) | 54.7(10.7) | 53.9(10.8) |
| Female, % | 31.6(8.2) | 26.1 (6.7) | 31.0 (7.7) | 26.4 (7.3) | 34.1 (7.8) |
| Smokers, % current, both sexes | 42.7 (6.8) | 13.8(4.2) | 44.9 (7.0) | 26.1 (5.8) | 36.9 (6.5) |
| Mean initial SBP, mmHg | 137.9(8.5) | 144.5(12) | 127.8(8.4) | 137.7(9) | 136.9(7.8) |
| Mean final SBP, mmHg | 115.7(7.1) | 121.7(9.8) | 107.1 (11.5) | 115.7(8) | 115(8.3) |
| Mean initial LDL-C cholesterol, mmol/L | 2.9 (0.4) | 3.0 (0.4) | 3.1 (0.4) | 3.0 (0.5) | 3.0 (0.3) |
| Mean final LDL-C cholesterol, mmol/L | 2.1 (0.4) | 2.2 (0.4) | 2.2 (0.4) | 2.2 (0.5) | 2.2 (0.2) |
| Mean initial A1 c, % | 8.7 (0.9) | 9.3(1) | 8.9(1.2) | 9.5(1.5) | 9.3(1) |
| Mean final A1c, % | 6.8 (0.6) | 7.1 (1) | 6.9(1.1) | 7.4 (0.8) | 7.2 (0.8) |
| Mean initial 10-year CVD risk, % | 24.9(13) | 24.5 (8.5) | 25.8 (7.9) | 25.0 (9.3) | 25.1 (20) |
| Mean final 10-year CVD risk, % | 13.1 (6.5) | 12.9(4) | 13.5(3.4) | 13.1 (4.6) | 13.2(9.7) |
| Mean initial lifetime microvascular risk, % | 17.9(9.5) | 18.8(13.6) | 20.9(12.8) | 16.8(10.3) | 15.7(16.2) |
| Mean final lifetime microvascular risk, % | 13.5(7) | 13.8(9.8) | 15.8(10) | 12.4(10.2) | 11.4(9.3) |
| $/DALY among treated | $7892.8(198.7) | $12343.8(505.3) | $11402.6(220.8) | $8336.6(192.2) | $12039.4(289.6) |
| DALYs averted per 1000 patient-years of pharmacotherapy | 3.3 (8.5) | 1.6 (5.8) | 2.3 (3.9) | 3.4 (6.0) | 1.2(2.5) |
Sensitivity analyses
Altering the threshold for treating microvascular risk under the BTT strategy from the base case of 5% lifetime risk to 3% lifetime risk (Appendix Table 5) or to 7% lifetime risk (Appendix Table 6) did not significantly alter the comparative effectiveness or cost-effectiveness of BTT relative to TTT; the uncertainty range around risk calculations, costs and DALYs was larger than the incremental differences made by these slight adjustments to the BTT microvascular treatment threshold. Hence, the overall effectiveness and cost-effectiveness under these alternative treatment thresholds (3% or 7%) largely overlapped with the base case (5%), continuing to be significantly better than TTT even after accounting for the uncertainties in risk calculation and input parameter values through the discrete sensitivity analysis.
Using fasting plasma glucose (target <7mmol/L)14 rather than haemoglobin A1c to guide TTT treatment had no major effects on the comparative difference between TTT and BTT strategies (Appendix Table 7). The TTT strategy had slightly improved outcomes on average, likely due to the slightly greater glycaemic control implied by a fasting plasma glucose target of 7mmol/L versus an A1c of 7%, but the confidence intervals under this scenario marginally increased, given less consistency in how fasting plasma glucose correlated to long-term microvascular outcomes.
Adjusting the BTT treatment thresholds such that the same total population was treated in each country by BTT as by the TTT strategy (Appendix Table 8) did not appreciably change the comparative results, with the TTT strategy still being inferior in effectiveness and cost-effectiveness across all countries.
If insulin were unavailable, the total numbers needed to treat to prevent a microvascular event increased under both strategies (Appendix Table 9), and BTT remained comparatively more effective than TTT only for CVD outcomes but not for microvascular outcomes. TTT was superior to BTT in terms of averting more renal disease and ulcer complications when insulin was unavailable (by between 300 and 368 events per 100,000 people with diabetes). However, BTT remained significantly more cost-effective relative to ‘no treatment’ comparator due to the higher number of CVD events prevented (by between 1,800 and 2,100 events per 100,000 people with diabetes), which outweighed the smaller difference in microvascular events prevented. The proportion of major microvascular events prevented decreased by as much as 40% due to the lack of insulin availability, and overall costs increased because the high cost of treating preventable complications exceeded the cost of insulin and supplies, leading to a rise in average cost per DALY averted of $292 for TTT treatment and $1,048 for BTT treatment above the base case simulation. BTT was no longer cost-saving as compared to TTT, but the incremental cost-effectiveness ratio of switching from TTT to BTT would be highly cost-effective in all countries, varying from $840 to $1600 per DALY averted across the countries (less than GDP per capita).20
If a BTT guideline had 10% lower adherence from practitioners than TTT (Appendix Table 10), the strategy would still avert more CVD and microvascular complications at a population level, and maintain a lower average cost-effectiveness ratio than TTT as compared to the ‘no treatment’ comparator. We estimated that adherence would need to be 45% lower for BTT than TTT overall for the TTT strategy to become superior in effectiveness, and 30% lower overall for TTT to become superior in cost-effectiveness.
If combining the BTT approach for preventing CVD complications with the TTT approach for preventing microvascular complications (Appendix Table 11), the combined approach would have intermediate efficacy to the TTT and BTT outcomes, with less synergistic prevention of CVD events through averting microvascular disease (e.g., CVD events prevented by preventing kidney disease) than when adopting BTT for both CVD and microvascular disease prevention. As compared to using the BTT strategy for both CVD and microvascular prevention, using BTT for CVD and TTT for microvascular complications reduced the number of myocardial infarctions and strokes prevented by ~4% and the number of blindness and renal-failure complications prevented by ~3%, with no significant change in ulcer outcomes.
Discussion
We observed that a strategy focused on reaching target levels of biomarkers (a treat-to-target, or TTT, strategy) would be expected to avert 24% to 31% fewer complications of diabetes than a strategy focused on treating persons with a high estimated risk of complications (a benefit-based tailored treatment, or BTT, strategy). The TTT strategy was more costly and less effective ($3,100 to $5,500 more expensive per DALY averted), and less efficient in terms of the DALYs averted per dollar and the DALYs averted per pharmaceutical dispensed. Because of complex interactions between micro- and cardiovascular (e.g., renal disease modifies the risk of CVD complications), treatment to lower microvascular risk also affected CVD outcomes, which accentuated the superiority of the BTT strategy as compared to the TTT strategy. TTT was particularly inferior because the risk of any given diabetes complication was based on multiple simultaneous factors, rather than any single biomarker; hence, the TTT strategy was fundamentally unable to consistently direct therapy towards persons who would most benefit. However, the superiority of BTT treatment for preventing microvascular events may critically depend on the availability of insulin, which is unavailable in some low-and middle-income country settings. The TTT strategy would be superior to the BTT strategy if insulin were unavailable, in terms of preventing end-stage renal disease and blindness. Nevertheless, the BTT strategy remained more effective overall due to the 5-fold greater number of CVD cases averted, and hence was more cost-effective than the TTT strategy even in the absence of insulin. Overall, managing glycaemic control with insulin adds substantially to the complexities of patient care as well as to the logistics, and cost, of drug purchasing and distribution.44
Our analysis benefited from using equations that have been validated in ethnically-diverse populations,27,28 having data from recent nationally-representative WHO surveys providing consistent measurements of biomarkers,23 and using meta-analyses of international trials to inform estimates of relative risk reduction rather than assuming reversibility of risk. Yet the analysis has several important limitations. We had to assume that equations from the UK Prospective Diabetes Study would be relevant and capture risks among low- and middle-income country populations, using ethnic-specific parameters which capture variations in risk among persons of African and South Asian descent. Similar parameters are unavailable for the Chinese population, and it remains unclear whether that population would have significant variations in risk equations. It is improbable, however, that the availability of more population-specific parameters would alter the comparative benefits of the two strategies. We also lacked data on access or adherence to therapy, which would linearly scale our results to lower levels of impact; hence, we focused on how different guidelines would offer different treatment recommendations, and estimated the (large) degree to which adherence would have to be differentially worse in the BTT scenario to neutralize our finding of superior BTT outcomes. We found that the superiority of BTT to TTT was consistent for settings in which haemoglobin A1c testing was unavailable, but further work would need to determine what strategy would be optimal in settings without insulin access. Importantly, we lacked data on differential risk of hypoglycemia and other adverse events among the two approaches, or data on detailed treatment availability and quality. This will require further evaluation, noting that the BTT approach to glycemic control has not been directly clinically studied, the necessary next step in research after this comparative effectiveness modeling study. The risk of hypoglycemia may be higher under the BTT strategy given that it recommends more insulin treatment at a population level. We also focused on medications available on the WHO Essential Medicines list, which may be expanded pending cost-effectiveness evaluations of newer agents. Statin therapy may also become more accessible, and our estimates of CVD event outcomes would be subject to an additional 14% relative risk reduction if simvastatin 40mg daily were replaced by atorvastatin 40mg daily,35 further increasing the comparative effectiveness of the BTT approach.
In future work, researchers should also examine how importantly risk equation coefficients vary among diverse cohorts, and how both patients and clinicians in diverse settings respond to the prospect of shifting towards a BTT approach to therapy. Our results highlight the importance of further data collection for individual countries to determine optimal and cost-effective risk thresholds for therapy, as has occurred for statin therapy among people without diabetes in some high-income nations.45 Prior to such work, our current results indicate that a BTT strategy would be more effective and cost-effective than a TTT strategy overall, primarily by concentrating effective therapy among patients with high risk of CVD and microvascular complications.
Putting research in context
Two authors (SB and VS) independently conducted PubMed and Google Scholar searches for articles with the keywords “treatment targets”, “personalized treatment”, “risk-based therapy” or “benefit-based tailored therapy”, along with the keyword “diabetes” from 1980 through July 2016. Based on consensus discussion, we found four relevant papers on the subject of this study. Two of the papers involved prior simulation models of the U.S. population, and suggested that for lipid and blood pressure treatment, benefit-based tailored therapy would be more effective and cost-effective than treat-to-target therapy for patients without diabetes.35,46 One additional paper involved a simulation model of U.S. populations with type 2 diabetes, and reported that for most patients older than 50 years with an HbA1c level less than 9% receiving metformin therapy, additional glycaemic treatment usually offers modest benefits,47 supporting treatment based on a comprehensive consideration of risk rather than a universal target for A1c achievement. Finally, one additional paper involved a simulation of people without diabetes in low- and middle-income countries, and reported that a benefit-based tailored therapy approach to blood pressure treatment would be more effective and cost-effective than a treat-to-target approach for CVD prevention.36
By comparison to the existing literature, our current study offers a direct comparison of the treat-to-target and benefit-based tailored treatment strategies among persons with type 2 diabetes in low- and middle-income country populations. It specifically reveals that a benefit-based tailored treatment strategy for blood pressure, lipid, and glycaemic control would be more effective and cost-effective than a treat-to-target strategy for prevention of both CVD and microvascular complications of type 2 diabetes; however, the superiority of the benefit-based tailored treatment strategy for averting microvascular complications would necessitate insulin availability.
Supplementary Material
Acknowledgments
Data for this project were obtained from the World Health Organization Study on Global Aging and Adult Health (SAGE), which is supported by the United States National Institute on Aging’s Division of Behavioral and Social Research through interagency agreements and research grants (R01 AG034479), and the World Health Organization’s Department of Health Statistics and Information Systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None.
References
- 1.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L, Dicker D, Duan L, Erskine H, Feigin VL, Ferrari AJ, Fitzmaurice C, Fleming T, Graetz N, Guinovart C, Haagsma J, Hansen GM, Hanson SW, Heuton KR, Higashi H, Kassebaum N, Kyu H, Laurie E, Liang X, Lofgren K, Lozano R, MacIntyre MF, Moradi-Lakeh M, Naghavi M, Nguyen G, Odell S, Ortblad K, Roberts DA, Roth GA, Sandar L, Serina PT, Stanaway JD, Steiner C, Thomas B, Vollset SE, Whiteford H, Wolock TM, Ye P, Zhou M, Ãvila MA, Aasvang GM, Abbafati C, Ozgoren AA, Abd-Allah F, Aziz MI, Abera SF, Aboyans V, Abraham JP, Abraham B, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Aburto TC, Achoki T, Ackerman IN, Adelekan A, Ademi Z, Adou AK, Adsuar JC, Arnlov J, Agardh EE, Al Khabouri MJ, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allebeck P, Allen PJ, AlMazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Ameli O, Amini H, Ammar W, Anderson BO, Anderson HR, Antonio CA, Anwari P, Apfel H, Arsenijevic VS, Artaman A, Asghar RJ, Assadi R, Atkins LS, Atkinson C, Badawi A, Bahit MC, Bakfalouni T, Balakrishnan K, Balalla S, Banerjee A, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Basu S, Basu A, Baxter A, Beardsley J, Bedi N, Beghi E, Bekele T, Bell ML, Benjet C, Bennett DA, Bensenor IM, Benzian H, Bernabe E, Beyene TJ, Bhala N, Bhalla A, Bhutta Z, Bienhoff K, Bikbov B, Abdulhak AB, Blore JD, Blyth FM, Bohensky MA, Basara BB, Borges G, Bornstein NM, Bose D, Boufous S, Bourne RR, Boyers LN, Brainin M, Brauer M, Brayne CE, Brazinova A, Breitborde NJ, Brenner H, Briggs AD, Brooks PM, Brown J, Brugha TS, Buchbinder R, Buckle GC, Bukhman G, Bulloch AG, Burch M, Burnett R, Cardenas R, Cabral NL, Nonato IR, Campuzano JC, Carapetis JR, Carpenter DO, Caso V, Castaneda-Orjuela CA, Catala-Lopez F, Chadha VK, Chang JC, Chen H, Chen W, Chiang PP, Chimed-Ochir O, Chowdhury R, Christensen H, Christophi CA, Chugh SS, Cirillo M, Coggeshall M, Cohen A, Colistro V, Colquhoun SM, Contreras AG, Cooper LT, Cooper C, Cooperrider K, Coresh J, Cortinovis M, Criqui MH, Crump JA, Cuevas-Nasu L, Dandona R, Dandona L, Dansereau E, Dantes HG, Dargan PI, Davey G, Davitoiu DV, Dayama A, De la Cruz-Gongora V, de la Vega SF, De Leo D, Del Pozo-Cruz B, Dellavalle RP, Deribe K, Derrett S, Des Jarlais DC, Dessalegn M, deVeber GA, Dharmaratne SD, Diaz-Torne C, Ding EL, Dokova K, Dorsey ER, Driscoll TR, Duber H, Durrani AM, Edmond KM, Ellenbogen RG, Endres M, Ermakov SP, Eshrati B, Esteghamati A, Estep K, Fahimi S, Farzadfar F, Fay DF, Felson DT, Fereshtehnejad SM, Fernandes JG, Ferri CP, Flaxman A, Foigt N, Foreman KJ, Fowkes FG, Franklin RC, Furst T, Futran ND, Gabbe BJ, Gankpe FG, Garcia-Guerra FA, Geleijnse JM, Gessner BD, Gibney KB, Gillum RF, Ginawi IA, Giroud M, Giussani G, Goenka S, Goginashvili K, Gona P, de Cosio TG, Gosselin RA, Gotay CC, Goto A, Gouda HN, Guerrant RL, Gugnani HC, Gunnell D, Gupta R, Gupta R, Gutierrez RA, Hafezi-Nejad N, Hagan H, Halasa Y, Hamadeh RR, Hamavid H, Hammami M, Hankey GJ, Hao Y, Harb HL, Haro JM, Havmoeller R, Hay RJ, Hay S, Hedayati MT, Pi IB, Heydarpour P, Hijar M, Hoek HW, Hoffman HJ, Hornberger JC, Hosgood HD, Hossain M, Hotez PJ, Hoy DG, Hsairi M, Hu H, Hu G, Huang JJ, Huang C, Huiart L, Husseini A, Iannarone M, Iburg KM, Innos K, Inoue M, Jacobsen KH, Jassal SK, Jeemon P, Jensen PN, Jha V, Jiang G, Jiang Y, Jonas JB, Joseph J, Juel K, Kan H, Karch A, Karimkhani C, Karthikeyan G, Katz R, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Khader YS, Khalifa SE, Khan EA, Khan G, Khang YH, Khonelidze I, Kieling C, Kim D, Kim S, Kimokoti RW, Kinfu Y, Kinge JM, Kissela BM, Kivipelto M, Knibbs L, Knudsen AK, Kokubo Y, Kosen S, Kramer A, Kravchenko M, Krishnamurthi RV, Krishnaswami S, Defo BK, Bicer BK, Kuipers EJ, Kulkarni VS, Kumar K, Kumar GA, Kwan GF, Lai T, Lalloo R, Lam H, Lan Q, Lansingh VC, Larson H, Larsson A, Lawrynowicz AE, Leasher JL, Lee JT, Leigh J, Leung R, Levi M, Li B, Li Y, Li Y, Liang J, Lim S, Lin HH, Lind M, Lindsay MP, Lipshultz SE, Liu S, Lloyd BK, Ohno SL, Logroscino G, Looker KJ, Lopez AD, Lopez-Olmedo N, Lortet-Tieulent J, Lotufo PA, Low N, Lucas RM, Lunevicius R, Lyons RA, Ma J, Ma S, Mackay MT, Majdan M, Malekzadeh R, Mapoma CC, Marcenes W, March LM, Margono C, Marks GB, Marzan MB, Masci JR, Mason-Jones AJ, Matzopoulos RG, Mayosi BM, Mazorodze TT, McGill NW, McGrath JJ, McKee M, McLain A, McMahon BJ, Meaney PA, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen W, Melaku YA, Meltzer M, Memish ZA, Mensah G, Meretoja A, Mhimbira FA, Micha R, Miller TR, Mills EJ, Mitchell PB, Mock CN, Moffitt TE, Ibrahim NM, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montico M, Montine TJ, Moore AR, Moran AE, Morawska L, Mori R, Moschandreas J, Moturi WN, Moyer M, Mozaffarian D, Mueller UO, Mukaigawara M, Murdoch ME, Murray J, Murthy KS, Naghavi P, Nahas Z, Naheed A, Naidoo KS, Naldi L, Nand D, Nangia V, Narayan KM, Nash D, Nejjari C, Neupane SP, Newman LM, Newton CR, Ng M, Ngalesoni FN, Nhung NT, Nisar MI, Nolte S, Norheim OF, Norman RE, Norrving B, Nyakarahuka L, Oh IH, Ohkubo T, Omer SB, Opio JN, Ortiz A, Pandian JD, Panelo CI, Papachristou C, Park EK, Parry CD, Caicedo AJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pedraza LS, Pellegrini CA, Pereira DM, Perez-Ruiz FP, Perico N, Pervaiz A, Pesudovs K, Peterson CB, Petzold M, Phillips MR, Phillips D, Phillips B, Piel FB, Plass D, Poenaru D, Polanczyk GV, Polinder S, Pope CA, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Prasad NM, Qato D, Quistberg DA, Rafay A, Rahimi K, Rahimi- Movaghar V, Rahman SU, Raju M, Rakovac I, Rana SM, Razavi H, Refaat A, Rehm J, Remuzzi G, Resnikoff S, Ribeiro AL, Riccio PM, Richardson L, Richardus JH, Riederer AM, Robinson M, Roca A, Rodriguez A, Rojas-Rueda D, Ronfani L, Rothenbacher D, Roy N, Ruhago GM, Sabin N, Sacco RL, Ksoreide K, Saha S, Sahathevan R, Sahraian MA, Sampson U, Sanabria JR, Sanchez-Riera L, Santos IS, Satpathy M, Saunders JE, Sawhney M, Saylan MI, Scarborough P, Schoettker B, Schneider IJ, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Serdar B, Servan-Mori EE, Shackelford K, Shaheen A, Shahraz S, Levy TS, Shangguan S, She J, Sheikhbahaei S, Shepard DS, Shi P, Shibuya K, Shinohara Y, Shiri R, Shishani K, Shiue I, Shrime MG, Sigfusdottir ID, Silberberg DH, Simard EP, Sindi S, Singh JA, Singh L, Skirbekk V, Sliwa K, Soljak M, Soneji S, Soshnikov SS, Speyer P, Sposato LA, Sreeramareddy CT, Stoeckl H, Stathopoulou VK, Steckling N, Stein MB, Stein DJ, Steiner TJ, Stewart A, Stork E, Stovner LJ, Stroumpoulis K, Sturua L, Sunguya BF, Swaroop M, Sykes BL, Tabb KM, Takahashi K, Tan F, Tandon N, Tanne D, Tanner M, Tavakkoli M, Taylor HR, Te Ao BJ, Temesgen AM, Have MT, Tenkorang EY, Terkawi AS, Theadom AM, Thomas E, Thorne-Lyman AL, Thrift AG, Tleyjeh IM, Tonelli M, Topouzis F, Towbin JA, Toyoshima H, Traebert J, Tran BX, Trasande L, Trillini M, Truelsen T, Trujillo U, Tsilimbaris M, Tuzcu EM, Ukwaja KN, Undurraga EA, Uzun SB, van Brakel WH, van de Vijver S, Dingenen RV, van Gool CH, Varakin YY, Vasankari TJ, Vavilala MS, Veerman LJ, Velasquez-Melendez G, Venketasubramanian N, Vijayakumar L, Villalpando S, Violante FS, Vlassov VV, Waller S, Wallin MT, Wan X, Wang L, Wang J, Wang Y, Warouw TS, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Wessells KR, Westerman R, Wilkinson JD, Williams HC, Williams TN, Woldeyohannes SM, Wolfe CD, Wong JQ, Wong H, Woolf AD, Wright JL, Wurtz B, Xu G, Yang G, Yano Y, Yenesew MA, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis M, Yu C, Kim KY, Zaki ME, Zhang Y, Zhao Z, Zhao Y, Zhu J, Zonies D, Zunt JR, Salomon JA, Murray CJ. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015 doi: 10.1016/S0140-6736(15)60692-4. published online June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 3.Zoungas S, Chalmers J, Neal B, et al. Follow-up of Blood-Pressure Lowering and Glucose Control in Type 2 Diabetes. N Engl J Med. 2014;371:1392–1406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of Medical Care in Diabetes--2014. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 6.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-Year Risk Thresholds for Initiation of Statin Therapy for Primary Prevention of Cardiovascular Disease. JAMA. 2015;314:142–150. doi: 10.1001/jama.2015.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized Guidelines: The Potential for Increasing Quality and Reducing Costs. Ann Intern Med. 2011;154:627. doi: 10.7326/0003-4819-154-9-201105030-00008. [DOI] [PubMed] [Google Scholar]
- 10.Sussman J, Vijan S, Hayward R. Using Benefit-Based Tailored Treatment to Improve the Use of Antihypertensive Medications. Circulation. 2013;128:2309–2317. doi: 10.1161/CIRCULATIONAHA.113.002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SPRINT Research Group. Wright JT, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-Pressure Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374:2009–2020. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 13.The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. The Lancet. 2014;384:591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care in Low-Resource Settings. Geneva: WHO; 2013. [Google Scholar]
- 15.Hajifathalian K, Ueda P, Lu Y, et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 2015;3:339–355. doi: 10.1016/S2213-8587(15)00081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d4169. d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HAW, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75) Diabetologia. 2006;49:1761–1769. doi: 10.1007/s00125-006-0297-1. [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Bendavid E, Sood N. Health and Economic Implications of National Treatment Coverage for Cardiovascular Disease in India Cost-Effectiveness Analysis. Circ Cardiovasc Qual Outcomes. 2015;8:541–551. doi: 10.1161/CIRCOUTCOMES.115.001994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Bank. World Development Indicators 2014. Washington D.C.: World Bank Publications; 2015. [Google Scholar]
- 21.United Nations. World Population Prospects: The 2012 Revision. Geneva: UN; 2013. [Google Scholar]
- 22.International Diabetes Federation. Diabetes Atlas. Brussels: IDF; 2015. [Google Scholar]
- 23.Kowal P, Chatterji S, Naidoo N, et al. Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE) Int J Epidemiol. 2012;41:1639–1649. doi: 10.1093/ije/dys210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adult obesity and type 2 diabetes - Publications - GOV.UK. [(accessed Aug 23, 2016)]; https://www.gov.uk/government/publications/adult-obesity-and-type-2-diabetes.
- 25.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the united states, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 26.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–1933. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 28.Clarke P. Constructing diabetes model from individual level data. Stanford University; 2014. [Google Scholar]
- 29.American Diabetes Association. Dyslipidemia Management in Adults With Diabetes. Diabetes Care. 2004;27:s68–s71. doi: 10.2337/diacare.27.2007.s68. [DOI] [PubMed] [Google Scholar]
- 30.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 33.Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. The Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 34.Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008143.pub2. CD008143. [DOI] [PubMed] [Google Scholar]
- 35.Hayward RA, Krumholz HM, Zulman DM, Timbie JW, Vijan S. Optimizing statin treatment for primary prevention of coronary artery disease. Ann Intern Med. 2010;152:69–77. doi: 10.7326/0003-4819-152-2-201001190-00004. [DOI] [PubMed] [Google Scholar]
- 36.Basu S, Yudkin JS, Sussman JB, Millett C, Hayward RA. Alternative Strategies to Achieve Cardiovascular Mortality Goals in China and India: A Microsimulation of Target- Versus Risk-Based Blood Pressure Treatment. Circulation. 2016;133:840–848. doi: 10.1161/CIRCULATIONAHA.115.019985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendis S, Fukino K, Cameron A, et al. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ. 2007;85:279–288. doi: 10.2471/BLT.06.033647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. WHO Model List of Essential Medicines, 19th Edition. Geneva: WHO; 2015. [Google Scholar]
- 39.World Health Organization. Choosing interventions that are cost effective (WHO-CHOICE) Geneva: WHO; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—Explanation and Elaboration: A Report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. The Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 42.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. The Lancet. 2013;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Management Sciences for Health. International drug price indicator guide. Cambridge, MA: Management Sciences for Health; 2015. [(accessed Jan 28, 2015)]. http://www.msh.org/sites/msh.org/files/international-drug-price-indicator-guide.pdf. [Google Scholar]
- 44.Beran D, Ewen M, Laing R. Constraints and challenges in access to insulin: a global perspective. Lancet Diabetes Endocrinol. 2016;4:275–285. doi: 10.1016/S2213-8587(15)00521-5. [DOI] [PubMed] [Google Scholar]
- 45.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-Year Risk Thresholds for Initiation of Statin Therapy for Primary Prevention of Cardiovascular Disease. JAMA. 2015;314:142–150. doi: 10.1001/jama.2015.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sussman J, Vijan S, Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128:2309–2317. doi: 10.1161/CIRCULATIONAHA.113.002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234. doi: 10.1001/jamainternmed.2014.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.