Abstract
Background
Peripheral artery disease (PAD) is highly prevalent and associated with significant morbidity and mortality, but sex-based differences are incompletely understood. We sought to define the associations between PAD and physical outcome measures, and to determine if these associations differed by sex in the Chronic Renal Insufficiency Cohort (CRIC).
Methods
Among 3,543 participants, we assessed the cross-sectional relationship between PAD severity defined by ankle brachial index (ABI) and 1) physical activity (MET-hours/week) 2) walking pace (slow vs medium/fast) and 3) physical function (12-item Short Form Health Survey [SF-12]).
Results
In a multivariable linear regression model, PAD severity was not associated with physical activity defined by total MET-hours/week in men or women (P=0.432). However, PAD severity was significantly associated with walking activity (P=0.037), although this relationship did not differ by sex (P=0.130). Similarly, PAD severity was significantly associated with walking pace (P<0.001), although this relationship did not differ by sex (P=0.086). In contrast, there was an independent association between PAD severity and SF-12 (P=0.018), with a significant interaction by sex (P<0.001).
Conclusions
These data suggest that tools used to evaluate the functional consequences of PAD should focus on walking activity and walking pace, as well as physical function, where sex-specific associations should be accounted for.
Keywords: ankle-brachial index, peripheral artery disease, physical activity, physical function
INTRODUCTION
Peripheral artery disease affects an estimated 8 million adults in the United States, a number which will continue to rise as the population ages.1,2 PAD remains a significant source of morbidity and mortality resulting in functional impairment, limb loss, as well as death.3 The burden of PAD is particularly relevant to the chronic kidney disease (CKD) population. Data from the National Health and Nutrition Examination Survey (NHANES) demonstrated that 24% of persons with CKD stage 3 or greater had PAD as defined by an ankle brachial index (ABI) < 0.9, which was significantly greater than the 4% prevalence in the group with normal renal function.4 Indeed, the National Kidney Foundation Task Force issued a statement suggesting that patients with CKD were in the highest risk category for subsequent cardiovascular events.5
Several survey instruments have been developed for the detection of PAD.6–8 While these survey instruments have been widely used for the diagnosis of PAD, the diagnosis can be mired by a lack of sensitivity and specificity. Prior literature has suggested that PAD is associated with a decline in physical function as well as physical activity.9,10 In the Women’s Health and Aging Study, asymptomatic women with an ABI<0.9 had a slower walking rate, poorer standing balance score, slower time to arise from a seated position, and fewer blocks walked per week after adjusting for age, smoking and other comorbidities. In the Walking and Leg Circulation Study (WALCS), an ABI<0.50 was associated with a shorter 6-minute walk distance, less physical activity, slower 4-m walking rate, and an inability to maintain a tandem stand for 10 seconds after adjusting for confounders.10 These data suggest that physical activity and function measures may be more sensitive than conventional questionnaires designed to detect circulatory insufficiency. Yet, the data on the utility and validity of these tests to measure degree of circulatory impairment is lacking.
Compounding the difficulty of identification of patients with PAD, prior epidemiologic studies suggest that the presentation of PAD differs in men and women. For example, data from cohort studies11,12 and large population studies suggest that women are typically older, have more atypical symptoms, and present with more advanced disease compared to men.13 Furthermore, collective data from large cohort studies have suggested worse outcomes in terms of rates of limb salvage, morbidity and mortality in women undergoing lower extremity revascularization.
Together, these data indicate a need to develop more robust strategies to evaluate PAD that will also account for sex differences in presentation. We leverage the strengths of a uniquely phenotyped cohort of individuals with CKD, the CRIC Study population, to determine if there is an association between PAD and physical activity, walking pace and physical function, and whether these associations differ between men and women. We hypothesized that circulatory impairment would be reflected in a corresponding decrease in these measures of physical outcome, and that their relationships with PAD severity would differ in men and women.
METHODS
Study design
The CRIC study design and methods have been described in detail previously.14 Briefly, CRIC is a prospective cohort study of 3,939 participants enrolled from June 2003 to August 2008 through 7 clinical centers in the United States (Ann Arbor and Detroit, Michigan; Baltimore, Maryland; Chicago, Illinois; Cleveland, Ohio; New Orleans, Louisiana; Philadelphia, Pennsylvania; and Oakland, California). Participants included men and women 21 to 74 years of age with mild to moderate CKD as defined by estimated glomerular filtration rate (eGFR). Age-based eGFR was used as an inclusion criterion for the study. Exclusion criteria included New York Heart Association class 3 and 4 heart failure, cancer and immunosuppressive therapies during the previous 6 months. Participants underwent detailed examinations including measurement of ankle-brachial index, undergoing the Multiethnic Study of Atherosclerosis (MESA) Typical Week Physical Activity Survey, ascertainment of walking pace and Short Form 12-item Health Survey (SF-12) scores (detailed below) at baseline and during annual in-person follow-up exams. The study was approved by the Institutional Review Board of each participating clinical center and the Scientific and Data Coordinating Center. Written informed consent was obtained from all participants.
For the purposes of this study, participants were excluded if they had a history of an amputation or a prior revascularization procedure (angioplasty or surgical bypass) because of uncertainty of how these procedures would affect physical activity and function. In this way, we limited the evaluation to those patients where their circulatory impairment could be directly compared to measures of physical outcome. Therefore, we focused on a subset of 3,543 participants for this analysis.
Primary outcome measures
Physical activity
Physical activity as measured by total MET (metabolic equivalent) -hours per week was derived from the MESA questionnaire. In brief, the MESA Typical Week Physical Activity Survey15 was based on the Cross-Cultural Activity Participation Study16 which quantitated the type, amount and intensity of physical activity experienced in a typical week in women of varying ethnicities. Activities captured in the survey included household chores, walking, conditioning exercises, leisure and occupational activities, which were further differentiated by light, moderate and heavy-intensity levels. These data were used to calculate total MET-hours/week spent on physical activity. Because prior studies have shown that certain summaries of physical activity (eg, time spent in moderate or heavy activity) are better associated with cardiorespiratory fitness17, we also considered the time (hours/week) spent in light, moderate, moderate/heavy, and heavy intentional exercise, as well as walking activity separately, as sub-categories of physical activity potentially associated with ABI.
Walking pace
Because the 6-minute walking distance has previously been reported to be associated with daily physical activity level18 and cardiorespiratory fitness19, walking pace (slow vs medium/fast) was also considered as a physical outcome measure.
Physical function
Physical function was measured via the physical component of SF-12, an abbreviated version of the SF-36 questionnaire which has been validated for assessing functional status, well-being and one’s overall perception of their health.20,21
PAD severity
The ankle brachial index (ABI) is the standard test utilized to diagnose PAD.22 After lying supine for 5 minutes, systolic blood pressure was measured in both arms using appropriately-sized cuffs. The systolic blood pressure for the dorsalis pedis artery and posterior tibial artery was measured for each leg using a Doppler probe. The ABI was determined by dividing the systolic blood pressure for each pedal artery by the higher systolic blood pressure of the brachial artery. In participants with functioning fistulae or arteriovenous grafts, the available contralateral brachial artery blood pressure was used. The lower ABI of the two limbs was used for each participant. We divided ABI into 4 categories and defined the degree of PAD as the following: (ABI<0.6-moderate-severe; 0.6≥ABI<0.9-mild; 0.9≥ABI<1.3-normal, ABI≥1.3-noncompressible). These categories are in accordance with previously published guidelines.23
Statistical analysis
Baseline demographic and clinical characteristics were summarized using mean and standard deviation for continuous variables and frequencies for categorical data, by sex. T-tests were used for between sex comparisons of continuous variables and chi-square tests were used for between sex comparisons of categorical variable frequencies. We assessed the association between PAD severity and measures of physical outcome as detailed below.
Physical activity measures
Linear regression was used to assess the association between ABI and physical activity (MET-hours/week). Traditional risk factors for atherosclerosis were considered as potential confounders in the multivariable models, including coronary artery disease, congestive heart failure, race, hypercholesterolemia, hypertension, smoking history, diabetes, eGFR, and body mass index.23,24 Any variable reaching a statistical significance of P<0.05 from the univariate analysis was considered for inclusion into the multivariable model, where a forward selection approach was undertaken using P = 0.25 as the cutoff for inclusion for all variables except for ABI, which was retained in the model. Effect modification by sex on the association between ABI and physical activity was of a priori interest, and was considered for inclusion in the selected model. This modeling approach was then repeated for the other 6 sub-categories of physical activity measures as described above.
Walking pace
Logistic regression was utilized to examine the relationship between PAD severity and walking pace (slow-<2mph, or medium/fast -≥2 mph). Any variable reaching a statistical significance of P<0.05 from the univariate analysis was considered for inclusion into the multivariable model, with model selection as described for physical activity. We then evaluated sex as an effect modifier in the association between ABI and walking pace.
Physical function measures
We examined the association between ABI and physical function as defined by the SF-12 score. Linear regression modeling was similar to that described above, for both model selection and inclusion of sex as an effect modifier for ABI.
All analyses were conducted using STATA version 12 (College Station, TX) and all tests were two-sided at the 0.05 significance level.
RESULTS
There were 3,543 participants included in this study. The mean (SD) for age of all participants at baseline was 57.4 (11.1) years and there were 54.0% men, 45.3% diabetics and 53.4% ever-smokers. 13.8% of the cohort had PAD at the time of study entry as defined by an ABI<0.90. Baseline demographics and clinical characteristics of the cohort stratified by sex are summarized in Table 1.
Table 1.
Baseline Characteristics of CRIC Participants by Sex, CRIC, United States, 2003- 2008
| Clinical Characteristic | Women N=1630 |
Men N=1913 |
P-value | |
|---|---|---|---|---|
| Age mean (SD) | 57.3 (11.2) | 57.5 (11.0) | 0.555 | |
| Race (overall) | <0.001 | |||
| White (%) | 42.1 | 49.8 | ||
| Black (%) | 47.4 | 37.5 | ||
| Other (%) | 10.5 | 12.7 | ||
| CHF (%) | 7.7 | 8.9 | 0.196 | |
| Diabetes (%) | 45.1 | 45.5 | 0.818 | |
| eGFR mL/min/1.73 m2, mean(SD ) | 44.7(17.5) | 46.7(16.5) | <0.001 | |
| Hypertension (%) | 83.4 | 86.9 | 0.003 | |
| CAD (%) | 15.7 | 22.3 | <0.001 | |
| Cigarette Smoking (overall) | <0.001 | |||
| Never (%) | 54.2 | 40.2 | ||
| Former (%) | 33.4 | 46.8 | ||
| Current (%) | 12.4 | 13.0 | ||
| Hypercholesterolemia (%) | 74.9 | 86.3 | <0.001 | |
| BMI mean(SD) | 33.0(8.9) | 31.0(6.3) | <0.001 | |
| Clinical center | <0.001 | |||
| ABI mean (SD) | 1.0(0.2) | 1.1(0.2) | <0.001 | |
| ABI category (overall) | <0.001 | |||
| ABI<0.6 (%) | 2.8 | 2.0 | ||
| ABI≥0.6<0.9 (%) | 12.8 | 10.2 | ||
| ABI ≥0.9<1.3 (%) | 80.3 | 81.0 | ||
| ABI ≥1.3 (%) | 4.1 | 6.7 | ||
ABI-Ankle Brachial Index; BMI-Body Mass Index; CAD-coronary artery disease; CHF-congestive heart failure; eGFR-estimated glomerular filtration rate
Association between ABI and physical activity (MET-hours/week)
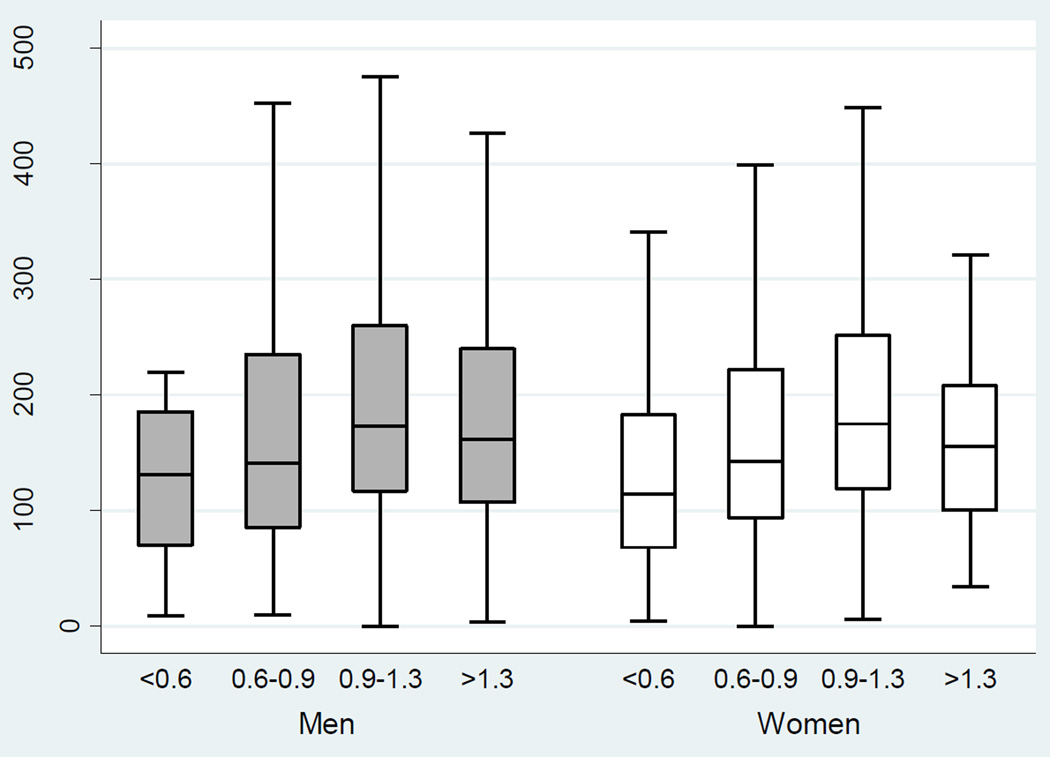
There was a significant association between severity of PAD and physical activity (total MET-hours/week) in the unadjusted model (P<0.001 for overall ABI, Table 3). With worsening PAD, there was a decrement in median physical activity score in men and women (Table 2; Figure 1). After adjusting for diabetes, smoking status, age, eGFR, congestive heart failure, race, clinical center and sex, however, there was no significant association between ABI and total MET-hours/week (P=0.432, Table 3). The adjusted relationship between ABI and total MET-hours/week also did not differ by sex (P=0.840).
Table 3.
Summary of Unadjusted and Adjusted association between Physical Activity (total MET hours/week) and Demographic variables, CRIC, United States, 2003–2008
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Clinical characteristic | β | SE | P-value | β | SE | P-value | |
| Intercept | 402.09 | 20.22 | <0.001 | ||||
| HTN | −30.19 | 7.03 | <0.001 | ||||
| Hypercholesterolemia | −24.09 | 6.36 | <0.001 | ||||
| Smoking (overall) | <0.001 | 0.190 | |||||
| Smoke (former) | −26.44 | 5.34 | <0.001 | −9.43 | 5.31 | 0.076 | |
| Smoke (current) | −1.47 | 7.87 | 0.852 | −1.33 | 7.69 | 0.862 | |
| Diabetes | −33.19 | 4.99 | <0.001 | −13.84 | 5.03 | 0.006 | |
| Age | −3.64 | 0.22 | <0.001 | −3.24 | 0.23 | <0.001 | |
| CHF | −46.08 | 9.00 | <0.001 | −23.39 | 9.12 | 0.010 | |
| CAD | −40.79 | 6.30 | <0.001 | −8.73 | 6.57 | 0.184 | |
| Race (overall) | 0.070 | 0.615 | |||||
| Black | −2.03 | 5.32 | 0.702 | 4.07 | 5.27 | 0.440 | |
| Other | −18.75 | 8.20 | 0.022 | −3.16 | 8.60 | 0.713 | |
| BMI | −0.70 | 0.32 | 0.031 | ||||
| eGFR | 1.38 | 0.14 | <0.001 | 0.63 | 0.16 | <0.001 | |
| Sex (women) | −11.13 | 5.01 | 0.026 | −14.78 | 4.97 | 0.003 | |
| ABI (overall) | <0.001 | 0.432 | |||||
| ABI<0.6 | −48.98 | 16.37 | 0.003 | −5.99 | 15.94 | 0.707 | |
| ABI≥0.6<0.9 | −35.40 | 7.87 | <0.001 | −5.62 | 7.77 | 0.469 | |
| ABI ≥1.3 | −23.11 | 10.92 | 0.034 | −16.15 | 10.57 | 0.127 | |
| Clinical center (overall) | <0.001 | <0.001 | |||||
ABI-Ankle Brachial Index; BMI-Body Mass Index; CAD-coronary artery disease; CHF-congestive heart failure; eGFR-estimated glomerular filtration rate; SE-standard error
Covariates were added using forward selection method
0.9≥ABI <1.3 used as the referent category
Table 2.
Summary of Physical Activity and Physical Function by ABI Category, Stratified by Sex, CRIC, United States, 2003–2008
| Physical Activity or Physical Function Variable |
Women | Men | P-value | |
|---|---|---|---|---|
|
Total MET- hours/week (median, IQR) |
167.5 (113.2, 246.5) | 167.4 (110.6, 256.1) | 0.546 | |
| ABI <0.6 | 114.4 (67.4, 182.6) | 131.03 (70.0, 185.6) | 0.536 | |
| ABI≥0.6<0.9 | 142.7 (93.8, 222.1) | 140.6 (85.4, 234.7) | 0.995 | |
| ABI ≥0.9<1.3 | 175.0 (118.4, 251.4) | 173.5 (116.5, 260.1) | 0.819 | |
| ABI ≥1.3 | 155.2 (99.9, 208.0) | 161.5 (106.5, 240.0) | 0.801 | |
|
SF-12 (median, IQR) |
39.9 (29.8, 50.6) | 46.5 (35.6, 53.5) | <0.001 | |
| ABI <0.6 | 38.7 (30.3, 46.6) | 32.8 (26.7, 43.5) | 0.128 | |
| ABI≥0.6<0.9 | 32.4 (25.9, 43.8) | 40.8 (31.3, 50.4) | <0.001 | |
| ABI ≥0.9<1.3 | 41.5 (30.3, 51.1) | 47.6 (36.4, 53.7) | <0.001 | |
| ABI ≥1.3 | 41.2 (30.0, 53.6) | 44.6 (34.9, 53.1) | 0.245 | |
ABI-Ankle Brachial Index; MET-metabolic equivalent; SF-12-Short form health study
Wilcoxon rank sum used for statistical comparison
P-value comparing women and men within a given ABI category
Figure 1.
Physical Activity versus ABI in Men versus Women in the Unadjusted Model, CRIC, United States, 2003–2008
MET-metabolic equivalent
Association between ABI and sub-categories of physical activity (MET-hours/week)
There were significant univariate associations between ABI and all six of our physical activity MET-hours/week subtype outcomes (light, moderate, heavy, moderate/heavy activity or intentional exercise, walking activity, all P<0.020). However, there was no independent association between ABI and physical activity defined as light, moderate, heavy, moderate/heavy activity, or intentional exercise. After adjusting for hypertension, smoking, age, race, eGFR, and BMI, there was an independent association between ABI and walking activity (P=0.037), but no effect modification by sex (P=0.130). The results of the univariable and multivariable analyses for walking activity are summarized in Table 4.
Table 4.
Summary of Unadjusted and Adjusted association between Walking Activity (walk-MET hours/week) and Demographic variables, CRIC, United States, 2003–2008
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Clinical characteristic | β | SE | P-value | β | SE | P-value | |
| Intercept | 53.33 | 5.80 | <0.001 | ||||
| HTN | −1.10 | 1.72 | 0.522 | 2.05 | 1.84 | 0.266 | |
| Hypercholesterolemia | −3.04 | 1.55 | 0.050 | ||||
| Smoking (overall) | <0.001 | 0.012 | |||||
| Former | −4.60 | 1.30 | <0.001 | −3.54 | 1.34 | 0.008 | |
| Current | 2.03 | 1.92 | 0.290 | 0.89 | 1.97 | 0.649 | |
| Diabetes | −4.00 | 1.22 | 0.001 | ||||
| Age | −0.36 | 0.05 | <0.001 | −0.31 | 0.06 | <0.001 | |
| CHF | −2.43 | 2.20 | 0.269 | ||||
| CAD | −4.59 | 1.54 | 0.003 | ||||
| Race (overall) | <0.001 | <0.001 | |||||
| Black | 3.99 | 1.29 | 0.002 | 4.66 | 1.36 | 0.001 | |
| Other | −3.48 | 2.00 | 0.081 | −2.55 | 2.17 | 0.240 | |
| BMI | −0.20 | 0.08 | 0.012 | −0.18 | 0.08 | 0.025 | |
| eGFR | 0.18 | 0.04 | <0.001 | 0.09 | 0.04 | 0.024 | |
| Sex (women) | −3.93 | 1.22 | 0.001 | −4.18 | 1.27 | 0.001 | |
| ABI (overall) | <0.001 | 0.037 | |||||
| ABI<0.6 | −7.08 | 3.99 | 0.076 | −4.36 | 4.02 | 0.278 | |
| ABI≥0.6<0.9 | −7.75 | 1.92 | <0.001 | −5.22 | 1.95 | 0.008 | |
| ABI ≥1.3 | −3.83 | 2.66 | 0.150 | −2.86 | 2.68 | 0.286 | |
| Clinical center (overall) | 0.005 | 0.166 | |||||
ABI-Ankle Brachial Index; BMI-Body Mass Index; CAD-coronary artery disease; CHF-congestive heart failure; eGFR-estimated glomerular filtration rate; SE-standard error
Covariates were added using forward selection method
0.9≥ABI <1.3 used as the referent category
Association between ABI and walking pace
ABI was associated with walking pace (P<0.001). As PAD worsened, the odds of having a slower walking pace increased (Table 5). After adjusting for hypercholesterolemia, congestive heart failure, coronary artery disease, diabetes, smoking status, age, race, eGFR, BMI, sex and clinical center, there remained an association between ABI and walking pace (P<0.001), although there was no significant interaction by sex, (P=0.086). The results of the univariable and multivariable analysis are summarized in Table 5.
Table 5.
Summary of Unadjusted and Adjusted Association Between Slow Walking Pace and Demographic variables, CRIC, United States, 2003–2008
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Clinical characteristic |
OR | 95% CI | OR | 95% CI |
| HTN | 2.11 | 1.73, 2.58 | ||
| Hypercholester olemia |
1.60 | 1.34, 1.90 | 1.15 | 0.94, 1.40 |
| Smoke (never) |
1.00 | Referent | 1.00 | Referent |
| Smoke (former) |
1.21 | 1.05, 1.39 | 1.09 | 0.93, 1.28 |
| Smoke (current) |
1.59 | 1.29, 1.96 | 1.53 | 1.21, 1.93 |
| Diabetes | 1.82 | 1.59, 2.08 | 1.16 | 0.99, 1.35 |
| Age | 1.02 | 1.02, 1.03 | 1.02 | 1.01, 1.03 |
| CHF | 2.33 | 1.81, 2.99 | 1.33 | 1.01, 1.76 |
| CAD | 1.90 | 1.60, 2.25 | 1.41 | 1.16, 1.72 |
| Race (White) |
1.00 | Referent | 1.00 | Referent |
| Race (Black) |
2.31 | 2.00, 2.67 | 1.80 | 1.53, 2.11 |
| Race (Other) |
1.20 | 0.96, 1.50 | 1.25 | 0.96, 1.63 |
| BMI | 1.08 | 1.07, 1.09 | 1.06 | 1.05, 1.07 |
| eGFR | 0.98 | 0.97, 0.98 | 0.99 | 0.98, 0.99 |
| Sex (Women) |
1.62 | 1.42, 1.85 | 1.55 | 1.33, 1.81 |
| ABI ≥0.9<1.3 | 1.00 | Referent | 1.00 | Referent |
| ABI<0.6 | 3.98 | 2.41, 6.55 | 2.44 | 1.44, 4.15 |
| ABI≥0.6<0.9 | 2.26 | 1.82, 2.80 | 1.49 | 1.18, 1.89 |
| ABI ≥1.3 | 1.22 | 0.91, 1.63 | 1.16 | 0.84, 1.59 |
ABI-Ankle Brachial Index; BMI-Body Mass Index; CAD-coronary artery disease; CHF-congestive heart failure; eGFR-estimated glomerular filtration rate
Covariates were added using forward selection method
Also adjusted for clinical center
Association between ABI and physical function
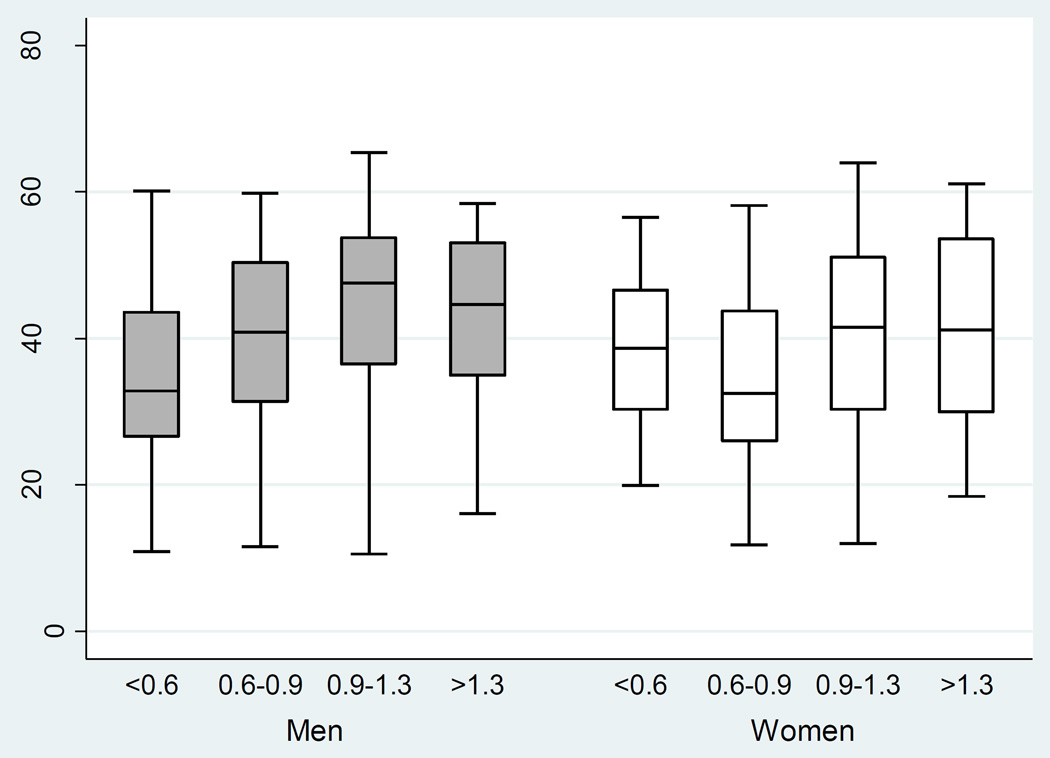
There was a significant univariate association between ABI and SF-12 score (P<0.001, Table 6). With worsening circulatory impairment, there was a decrement in median physical function score in men and women (Table 2; Figure 2). This remained significant after adjusting for congestive heart failure, coronary artery disease, diabetes, age, smoking status, race, sex, clinical center and eGFR, (P=0.018). Furthermore, the adjusted association between ABI and physical function differed by sex, (P<0.001). For men, ABI was significantly associated with SF-12 such that with mild circulatory impairment, there was a decrease in SF-12 score by a mean of 1.05 points (SE, 0.79) compared to those with normal ABI. In the presence of moderate-severe disease, there was a greater decrease in SF-12, by a mean of 6.03 points (SE, 1.66). For women, ABI was associated with SF-12 such that mild PAD was associated with a decrease in SF-12 score by a mean of 2.09 points (SE,0.78). Moderate-severe disease in women, paradoxically, was associated with a mean 2.80 point higher SF-12 score (SE,1.60). The results from the univariable and multivariable regression analyses are summarized in Table 6.
Table 6.
Summary of Unadjusted and Adjusted Association Between Physical Function (SF-12) and Demographic variables, CRIC, United States, 2003–2008
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Clinical characteristic | β | SE | P-value | β | SE | P-value | |
| Intercept | 58.59 | 1.58 | <0.001 | ||||
| HTN | −4.42 | 0.55 | <0.001 | ||||
| Hypercholesterolemia | −2.93 | 0.50 | <0.001 | ||||
| Smoking (overall) |
<0.001 | <0.001 | |||||
| Former | −2.06 | 0.42 | <0.001 | −1.32 | 0.38 | 0.001 | |
| Current | −3.65 | 0.62 | <0.001 | −3.38 | 0.56 | <0.001 | |
| Diabetes | −5.01 | 0.38 | <0.001 | −1.86 | 0.37 | <0.001 | |
| Age | −0.14 | 0.02 | <0.001 | −0.07 | 0.02 | <0.001 | |
| CHF | −8.42 | 0.69 | <0.001 | −4.20 | 0.66 | <0.001 | |
| CAD | −5.82 | 0.49 | <0.001 | −3.35 | 0.48 | <0.001 | |
| Race (overall) |
<0.001 | <0.001 | |||||
| Black | −3.92 | 0.41 | <0.001 | −1.49 | 0.38 | <0.001 | |
| Other | −2.40 | 0.63 | <0.001 | −1.62 | 0.62 | 0.009 | |
| BMI | −0.44 | 0.02 | <0.001 | −0.32 | 0.02 | <0.001 | |
| eGFR | 0.18 | 0.01 | <0.001 | 0.08 | 0.01 | <0.001 | |
| Sex (Women) |
−4.15 | 0.39 | <0.001 | −4.00 | 0.40 | <0.001 | |
| ABI (overall) |
<0.001 | 0.002 | |||||
| ABI<0.6 | −5.80 | 1.29 | <0.001 | −6.03 | 1.66 | <0.001 | |
| ABI≥0.6<0.9 | −5.17 | 0.61 | <0.001 | −1.05 | 0.79 | 0.184 | |
| ABI ≥1.3 | −0.44 | 0.84 | 0.603 | 0.10 | 0.94 | 0.916 | |
| Sex(Women)*ABI overall | <0.001 | ||||||
| Sex*ABI<0.6 | 8.82 | 2.29 | <0.001 | ||||
| Sex*ABI≥0.6<0.9 | −1.03 | 1.09 | 0.345 | ||||
| Sex*ABI≥1.3 | 1.37 | 1.57 | 0.381 | ||||
| Clinical center (overall) |
<0.001 | <0.001 | |||||
ABI-Ankle Brachial Index; BMI-Body Mass Index; CAD-coronary artery disease; CHF-congestive heart failure; eGFR-estimated glomerular filtration rate; SE-standard error
Covariates were added using forward selection method
0.9≥ABI <1.3 used as the referent category
Figure 2.
Physical Function versus ABI in Men versus Women in the Unadjusted Model, CRIC, United States, 2003–2008
SF-12-Short Form Health Survey
DISCUSSION
In this study, we demonstrate that ABI was not significantly associated with physical activity as measured by total MET-hours/week after adjustment for other sociodemographic and clinical factors. ABI was also not associated with other physical activity outcomes defined by MET-hours/week (light, moderate, heavy, moderate-heavy, intentional, exercise) in the multivariable models. PAD severity was associated with walking activity (MET-hours/week) and walking pace (slow-<2mph, or medium/fast -≥2 mph), but this relationship did not differ by sex.
PAD severity was independently associated with physical function, and this association differed by sex. In men, as PAD worsened, there was a corresponding decrease in SF-12 score. In women, on the other hand, those with mild PAD had a pronounced decrement of their SF-12 score, whereas those with moderate-severe PAD and a paradoxical increase in SF-12 score, reflecting the nonlinear association between ABI and SF-12 in women. The effect of PAD on SF-12 scores in men and women is clinically relevant, given that a 2–3 point difference has been shown to be clinically important.21 In a study of adults older than 45 years with chronic kidney disease, a decline in the eGFR from ≥ 90 mL/min/1.73 m2 to 15–29 mL/min/1.73 m2 was associated with a 3.6 point decrease in SF-12 score (from a score of 40.9 to a score of 37.3) in the adjusted model.25 Using data from the Medical Expenditure Panel Survey, the physical SF-12 score was 4.6 points lower in those with coronary heart disease compared to those without coronary heart disease.26
The results of this study have implications for improved strategies in evaluating for PAD. Specifically, walking activity (MET-hours/week) may be more appropriate to include in questionnaires than the total number of MET-hours expended per week. Indeed, prior studies have shown that general physical activity surveys lack sensitivity and specificity.27,28 Further, more specific measures of physical activity such as the 6-minute walk test have been performed with better validity and reliability, consistent with the findings from this study.9 The results from our study also suggest that incorporating walking pace would enhance the ability to detect PAD. Physical function via the SF-12 could also be incorporated in questionnaires evaluating for PAD, and would need to take into account the differing relationship between PAD severity and the decrement in functioning in men compared to women. Men experienced a decline in physical function with worsening categories of circulatory impairment, while women had a greater decline with mild disease, but paradoxically reported better function in the worst category of PAD. This could be interpreted in a few ways. Women could be more sensitive to circulatory impairment and experience a greater reduction in function at milder stages of PAD. In addition, the relatively greater functioning reported in women with moderate-severe PAD could be a result of social desirability bias, where there is a tendency of survey respondents to answer questions in a manner that will be viewed favorably by others.29 Further, women with severe PAD may have adapted to the reduction in circulation by avoidance of activities that exacerbate their symptoms.9 Lastly, there may be a biological explanation for why women may be less affected by moderate-severe PAD, compared to men. PAD is characterized histopathologically by an ischemic myopathy with calf muscle atrophy and fatty infiltration, or abnormal mitochondrial dysfunction.30 If women had less muscle atrophy in the moderate-severe stage of PAD, it could be reflected in relatively preserved function in severe stages of circulatory impairment. Overall, these nonlinear patterns suggest a more complex relationship between physical function and circulation in women, and deserve to be an area of future research.
The findings from this study are congruent with those found in the literature. One study compared the physical performance of patients with PAD to those without PAD via accelerometers as well as two general physical activity questionnaires, LTPAQ (Leisure Time Physical Activity Questionnaire) and PARQ (Stanford 7-day Physical Activity Recall Questionnaire ).27 Neither physical activity questionnaire detected a statistically significant difference between groups, despite a difference in accelerometer measurements between groups. These findings reaffirm the insensitivity of general physical activity surveys, and their lack of correlation with objective data. The significant relationship between ABI and walking activity and walking pace also parallels prior findings in the general population. The Walking and Leg Circulation Study (WALCS)10 showed that participants with an ABI<0.50 had a shorter 6-minute walking distance compared to those with an ABI of 1.10–1.50, as well as a slower 4-m walking velocity. Further, less than 40% of participants with an ABI<0.40 could walk continuously for 6 minutes compared to more than 95% of those with an ABI between 1.00 and 1.50. Thus, circulatory impairment is reflected in less walking activity as well as a slower walking pace, results that were also found in this cohort of patients with CKD, a finding that has not been reported previously.
The SF-12 Survey is a well-validated, general health questionnaire21 designed to monitor the impact of disease states over time31, or to assess the impact of different diseases on health status.32 The questions detect how one’s health may limit particular activities, such as climbing flights of stairs or work-related activities. PAD severity in this study was closely related to a decrease in functioning, analogous to prior studies. In the Women’s Health and Aging Study (WHAS)33, amongst community-dwelling women of ≥65 years of age, women with low ABI values were more likely to report an inability to walk ¼ of a mile or climb a flight of stairs without assistance, compared to women with normal ABI values. In the WALCS cohort, people with PAD had higher rates of mobility loss, compared to those without PAD.34 These studies reflect that ABI decrement is associated with a decrease in physical function, congruent with the results from our study. We additionally discovered that physical function was affected by circulation impairment in a more linear pattern in men, whereas in women, there was a nonlinear pattern.
There are some limitations to our study. Patients with CKD can have calcific, noncompressible vessels, which can lead to an overestimation of the ABI and misclassification. However, there was a low prevalence of ABI>1.3 (6.74% in men and 4.11% in women), suggesting that our main findings would not be different. This is a cross-sectional study, thus cause and effect cannot be determined, although it is more plausible to hypothesize that impaired circulation caused the decrease in walking activity, walking pace and physical function, rather than the converse. Additionally, the physical outcomes of physical activity, walking pace, and physical function were ascertained via self-report, which is subject to measurement error and social desirability bias and thus could alter associations between PAD and physical outcome. However, these questionnaires are well-validated surveys for the study of physical activity and physical function. Finally, the cohort is designed to study participants with CKD, and may not generalizable to the population at large. However, this is a rich dataset which lends important insight into a highly relevant population commonly afflicted by PAD.
We also note important strengths of this study. The near equal representation of the sexes, the rigorous determination of ABI, along with consistent administration of physical activity and function surveys represent key strengths in this study exploring the associations between PAD and decline in physical function and activity. These circumvent the pitfalls of earlier trials for PAD which reflected an underrepresentation of women35 and a general reluctance on the part of women to report leg symptoms to their medical providers. 36
In summary, in our cohort of 3,543 participants with CKD, a decrement in ABI was associated with a decline in walking activity and walking pace, as well as physical function, as defined by the SF-12. Furthermore, the decline in physical function differed in men versus women. Future studies should be devoted to developing tools to detect PAD that incorporate walking variables as well as physical function items, where sex-based differences should be accounted for.
Acknowledgments
This work was supported by a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this study was supported in part by the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003; Johns Hopkins University UL1 TR-000424; University of Maryland GCRC M01 RR-16500; Clinical and Translational Science Collaborative of Cleveland UL1TR000439; Michigan Institute for Clinical and Health Research UL1TR000433; University of Illinois at Chicago CTSA UL1RR029879; Tulane University Translational Research in Hypertension and Renal Biology P30GM103337; Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, and K01DK092353.
We would like to thank Sally Thompson for her administrative assistance.
Abbreviations
- ABI
ankle-brachial index
- BMI
body mass index
- CAD
coronary artery disease
- CRIC
Chronic Renal Insufficiency Cohort
- eGFR
estimated glomerular filtration rate
- MESA
Multi-Ethnic Study of Atherosclerosis
- PAD
peripheral artery disease
- SF-12
short-form health survey
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009 Jan 27;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004 Aug 10;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH. Peripheral arterial disease--epidemiological aspects. Vasc Med. 2001;6(3 Suppl):3–7. doi: 10.1177/1358836X0100600i102. [DOI] [PubMed] [Google Scholar]
- 4.O’Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2004 Jan 27;109(3):320–323. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Beto JA, Coronado BE, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998 Nov;32(5):853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 6.Fowkes FG. The measurement of atherosclerotic peripheral arterial disease in epidemiological surveys. Int J Epidemiol. 1988 Jun;17(2):248–254. doi: 10.1093/ije/17.2.248. [DOI] [PubMed] [Google Scholar]
- 7.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1(1):65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001 Oct 3;286(13):1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004 Jul 28;292(4):453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 10.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002 Jun 18;136(12):873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 11.Ballotta E, Gruppo M, Lorenzetti R, Piatto G, DaGiau G, Toniato A. The impact of gender on outcome after infrainguinal arterial reconstructions for peripheral occlusive disease. J Vasc Surg. 2012 Aug;56(2):343–352. doi: 10.1016/j.jvs.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Ortmann J, Nuesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg. 2012 Jan;55(1):98–104. doi: 10.1016/j.jvs.2011.07.074. [DOI] [PubMed] [Google Scholar]
- 13.Lo RC, Bensley RP, Dahlberg SE, et al. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2013 Sep 28; doi: 10.1016/j.jvs.2013.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003 Jul;14(7 Suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 15.Bertoni AG, Whitt-Glover MC, Chung H, et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009 Feb 15;169(4):444–454. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999 Jul-Aug;8(6):805–813. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 17.Delaney JA, Jensky NE, Criqui MH, Whitt-Glover MC, Lima JA, Allison MA. The association between physical activity and both incident coronary artery calcification and ankle brachial index progression: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2013 Oct;230(2):278–283. doi: 10.1016/j.atherosclerosis.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott MM, Ades PA, Dyer A, Guralnik JM, Kibbe M, Criqui MH. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg. 2008 Nov;48(5):1231–1237. doi: 10.1016/j.jvs.2008.06.050. 1237 e1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegrzynowska-Teodorczyk K, Rudzinska E, Lazorczyk M, et al. Distance covered during a six-minute walk test predicts long-term cardiovascular mortality and hospitalisation rates in men with systolic heart failure: an observational study. J Physiother. 2013 Sep;59(3):177–187. doi: 10.1016/S1836-9553(13)70182-6. [DOI] [PubMed] [Google Scholar]
- 20.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992 Jul 18;305(6846):160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med. 2012 Sep;79(9):651–661. doi: 10.3949/ccjm.79a.11154. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006 Sep;17(9):1383–1397. doi: 10.1097/01.RVI.0000240426.53079.46. quiz 1398. [DOI] [PubMed] [Google Scholar]
- 24.Hiramoto JS, Katz R, Weisman S, Conte M. Gender-specific risk factors for peripheral artery disease in a voluntary screening population. J Am Heart Assoc. 2014;3(2):e000651. doi: 10.1161/JAHA.113.000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClellan WM, Abramson J, Newsome B, et al. Physical and psychological burden of chronic kidney disease among older adults. Am J Nephrol. 2010;31(4):309–317. doi: 10.1159/000285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie J, Wu EQ, Zheng ZJ, Sullivan PW, Zhan L, Labarthe DR. Patient-reported health status in coronary heart disease in the United States: age, sex, racial, and ethnic differences. Circulation. 2008 Jul 29;118(5):491–497. doi: 10.1161/CIRCULATIONAHA.107.752006. [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Liu K, O’Brien E, et al. Measuring physical activity in peripheral arterial disease: a comparison of two physical activity questionnaires with an accelerometer. Angiology. 2000 Feb;51(2):91–100. doi: 10.1177/000331970005100201. [DOI] [PubMed] [Google Scholar]
- 28.Schorr EN, Treat-Jacobson D. Methods of symptom evaluation and their impact on peripheral artery disease (PAD) symptom prevalence: a review. Vasc Med. 2013 Apr;18(2):95–111. doi: 10.1177/1358863X13480001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams SA, Matthews CE, Ebbeling CB, et al. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol. 2005 Feb 15;161(4):389–398. doi: 10.1093/aje/kwi054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep. 2013 Apr;15(4):347. doi: 10.1007/s11886-013-0347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemingway H, Stafford M, Stansfeld S, Shipley M, Marmot M. Is the SF-36 a valid measure of change in population health? Results from the Whitehall II Study. BMJ. 1997 Nov 15;315(7118):1273–1279. doi: 10.1136/bmj.315.7118.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenfield S, Rogers W, Mangotich M, Carney MF, Tarlov AR. Outcomes of patients with hypertension and non-insulin dependent diabetes mellitus treated by different systems and specialties Results from the medical outcomes study. JAMA. 1995 Nov 8;274(18):1436–1444. [PubMed] [Google Scholar]
- 33.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women’s health and aging study. Circulation. 2000 Mar 7;101(9):1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 34.McDermott MM, Guralnik JM, Tian L, et al. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: the WALCS (Walking and Leg Circulation Study) J Am Coll Cardiol. 2009 Mar 24;53(12):1056–1062. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoel AW, Kayssi A, Brahmanandam S, Belkin M, Conte MS, Nguyen LL. Under-representation of women and ethnic minorities in vascular surgery randomized controlled trials. J Vasc Surg. 2009 Aug;50(2):349–354. doi: 10.1016/j.jvs.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins TC, Suarez-Almazor M, Bush RL, Petersen NJ. Gender and peripheral arterial disease. J Am Board Fam Med. 2006 Mar-Apr;19(2):132–140. doi: 10.3122/jabfm.19.2.132. [DOI] [PubMed] [Google Scholar]