Abstract
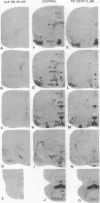
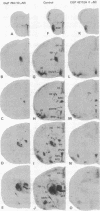
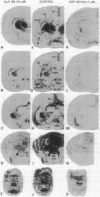
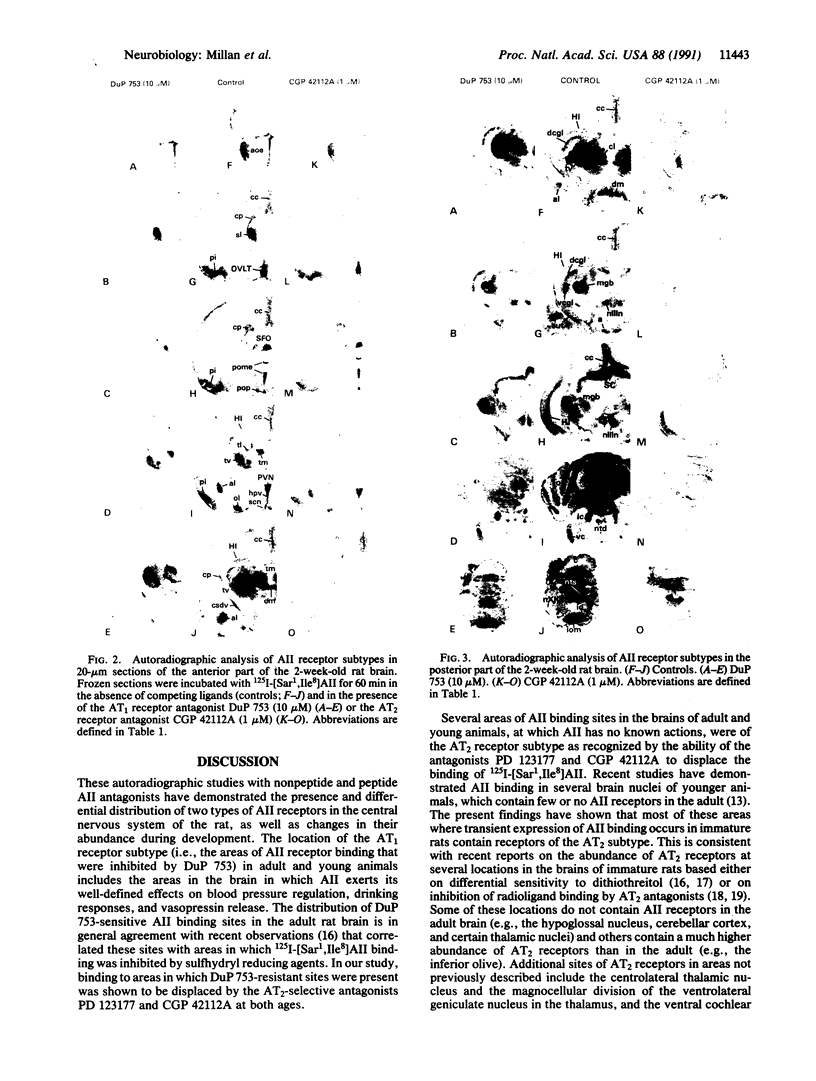
Angiotensin II (AII) receptor subtypes were analyzed in the brains of adult and 2-week-old rats by in vitro autoradiography with 125I-labeled [Sar1,Ile8]AII and competition studies with three AII antagonists: the nonpeptide antagonist, DuP 753, which is specific for AT1 receptors that mediate the calcium-inositol phospholipid signaling actions of AII; and nonpeptide (PD 123177) and peptide (CGP 42112A) antagonists that are selective for AT2 receptors of yet unknown function. In the adult rat brain, DuP 753 inhibited radioligand binding to the circumventricular organs and paraventricular nucleus but not to the lateral septum, subthalamic nucleus, and inferior olive. However, binding of 125I-labeled [Sar1,Ile8]AII in the latter regions was inhibited by the AT2 receptor antagonists PD 123177 and CGP 42112A. These areas showed similar displacement by the AT2 receptor subtype-specific antagonists in 2-week-old rats. In addition, radioligand binding at multiple sites of transient expression of AII receptors in 2-week-old rats, including several thalamic nuclei, the nuclei of the 3rd and 12th cranial nerves, geniculate bodies, cerebellum, and cingulate cortex, was displaced by the AT2 antagonists but not by DuP 753. These studies have demonstrated the presence of two AII receptor subtypes in the brain, one (AT1) in areas related to regulation of blood pressure, water intake, and pituitary hormone secretion, and one (AT2) whose function is not yet defined. The abundance and location of brain AT2 receptors in young animals, and the age-related changes in relative expression of the receptor subtypes, suggest that AII exerts specific actions according to the developmental stage of the central nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972 Aug;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Bergman H., Wichmann T., DeLong M. R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990 Sep 21;249(4975):1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bumpus F. M., Catt K. J., Chiu A. T., DeGasparo M., Goodfriend T., Husain A., Peach M. J., Taylor D. G., Jr, Timmermans P. B. Nomenclature for angiotensin receptors. A report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension. 1991 May;17(5):720–721. doi: 10.1161/01.hyp.17.5.720. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Mendelsohn F. A., Millan M. A., Aguilera G. The role of angiotensin II receptors in vascular regulation. J Cardiovasc Pharmacol. 1984;6 (Suppl 4):S575–S586. doi: 10.1097/00005344-198406004-00004. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., Herblin W. F., McCall D. E., Ardecky R. J., Carini D. J., Duncia J. V., Pease L. J., Wong P. C., Wexler R. R., Johnson A. L. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989 Nov 30;165(1):196–203. doi: 10.1016/0006-291x(89)91054-1. [DOI] [PubMed] [Google Scholar]
- Douglas J. G. Angiotensin receptor subtypes of the kidney cortex. Am J Physiol. 1987 Jul;253(1 Pt 2):F1–F7. doi: 10.1152/ajprenal.1987.253.1.F1. [DOI] [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Gomez R. A., Cassis L., Lynch K. R., Chevalier R. L., Wilfong N., Carey R. M., Peach M. J. Fetal expression of the angiotensinogen gene. Endocrinology. 1988 Nov;123(5):2298–2302. doi: 10.1210/endo-123-5-2298. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Catt K. J. Activation of dihydropyridine-sensitive calcium channels and biphasic cytosolic calcium responses by angiotensin II in rat adrenal glomerulosa cells. Endocrinology. 1988 Dec;123(6):2818–2826. doi: 10.1210/endo-123-6-2818. [DOI] [PubMed] [Google Scholar]
- Millan M. A., Jacobowitz D. M., Catt K. J., Aguilera G. Distribution of angiotensin II receptors in the brain of nonhuman primates. Peptides. 1990 Mar-Apr;11(2):243–253. doi: 10.1016/0196-9781(90)90077-i. [DOI] [PubMed] [Google Scholar]
- Millan M. A., Kiss A., Aguilera G. Developmental changes in brain angiotensin II receptors in the rat. Peptides. 1991 Jul-Aug;12(4):723–737. doi: 10.1016/0196-9781(91)90125-9. [DOI] [PubMed] [Google Scholar]
- Millan M., Aguilera G., Wynn P. C., Mendelsohn F. A., Catt K. J. Autoradiographic localization of brain receptors for peptide hormones: angiotensin II, corticotropin-releasing factor, and gonadotropin-releasing hormone. Methods Enzymol. 1986;124:590–606. doi: 10.1016/0076-6879(86)24041-0. [DOI] [PubMed] [Google Scholar]
- Naftilan A. J., Pratt R. E., Dzau V. J. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. I. Functions of angiotensin in the central nervous system. Annu Rev Physiol. 1987;49:413–435. doi: 10.1146/annurev.ph.49.030187.002213. [DOI] [PubMed] [Google Scholar]
- Pobiner B. F., Hewlett E. L., Garrison J. C. Role of Ni in coupling angiotensin receptors to inhibition of adenylate cyclase in hepatocytes. J Biol Chem. 1985 Dec 25;260(30):16200–16209. [PubMed] [Google Scholar]
- Raizada M. K., Phillips M. I., Gerndt J. S. Primary cultures from fetal rat brain incorporate [3H]-isoleucine and [3H]-valine into immunoprecipitable angiotensin II. Neuroendocrinology. 1983;36(1):64–67. doi: 10.1159/000123438. [DOI] [PubMed] [Google Scholar]
- Richards E. M., Hermann K., Sumners C., Raizada M. K., Phillips M. I. Release of immunoreactive angiotensin II from neuronal cultures: adrenergic influences. Am J Physiol. 1989 Sep;257(3 Pt 1):C588–C595. doi: 10.1152/ajpcell.1989.257.3.C588. [DOI] [PubMed] [Google Scholar]
- Rowe B. P., Grove K. L., Saylor D. L., Speth R. C. Angiotensin II receptor subtypes in the rat brain. Eur J Pharmacol. 1990 Sep 21;186(2-3):339–342. doi: 10.1016/0014-2999(90)90457-h. [DOI] [PubMed] [Google Scholar]
- Song K., Allen A. M., Paxinos G., Mendelsohn F. A. Angiotensin II receptor subtypes in rat brain. Clin Exp Pharmacol Physiol. 1991 Feb;18(2):93–96. doi: 10.1111/j.1440-1681.1991.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Sood P. P., Panigel M., Wegmann R. Co-existence of renin-like immunoreactivity in the rat maternal and fetal neocortex. Neurochem Res. 1989 Jun;14(6):499–502. doi: 10.1007/BF00964909. [DOI] [PubMed] [Google Scholar]
- Sood P. P., Panigel M., Wegmann R. The existence of renin-angiotensinogen system in the rat fetal brain: I. Immunocytochemical localization of renin-like activity at the 19th day of gestation. Cell Mol Biol. 1987;33(6):675–680. [PubMed] [Google Scholar]
- Sood P. P., Richoux J. P., Panigel M., Bouhnik J., Wegmann R. The existence of renin-angiotensinogen system in the rat fetal brain: II. Immunocytochemical localization of angiotensinogen in the telencephalon and the diencephalon. Cell Mol Biol. 1987;33(6):681–689. [PubMed] [Google Scholar]
- Speth R. C., Kim K. H. Discrimination of two angiotensin II receptor subtypes with a selective agonist analogue of angiotensin II, p-aminophenylalanine6 angiotensin II. Biochem Biophys Res Commun. 1990 Jun 29;169(3):997–1006. doi: 10.1016/0006-291x(90)91993-3. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Lynch D. R., Snyder S. H. Differential ontogeny of rat brain peptidases: prenatal expression of enkephalin convertase and postnatal development of angiotensin-converting enzyme. Brain Res. 1986 Oct;394(2):207–215. doi: 10.1016/0165-3806(86)90096-9. [DOI] [PubMed] [Google Scholar]
- Sumners C., Myers L. M., Kalberg C. J., Raizada M. K. Physiological and pharmacological comparisons of angiotensin II receptors in neuronal and astrocyte glial cultures. Prog Neurobiol. 1990;34(5):355–385. doi: 10.1016/0301-0082(90)90032-c. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K., Saavedra J. M. Differential development of angiotensin II receptor subtypes in the rat brain. Endocrinology. 1991 Jan;128(1):630–632. doi: 10.1210/endo-128-1-630. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K., Saavedra J. M. Increased dithiothreitol-insensitive, type 2 angiotensin II receptors in selected brain areas of young rats. Cell Mol Neurobiol. 1991 Apr;11(2):295–299. doi: 10.1007/BF00769042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitebread S., Mele M., Kamber B., de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989 Aug 30;163(1):284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]