Abstract
Background:
While body mass index (BMI), a modifiable parameter, and knee morphology, a nonmodifiable parameter, have been identified as risk factors for anterior cruciate ligament (ACL) rupture, the interaction between them remains unknown. An understanding of this interaction is important because greater compressive axial force (perhaps due to greater BMI) applied to a knee that is already at an increased risk because of its geometry, such as a steep lateral posterior tibial slope, could further increase the probability of ACL injury.
Purpose:
To quantify the relationship between BMI and select knee morphological parameters as potential risk factors for ACL injury.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
Sagittal knee magnetic resonance imaging (MRI) files from 76 ACL-injured and 42 uninjured subjects were gathered from the University of Michigan Health System’s archive. The posterior tibial slope (PTS), middle cartilage slope (MCS), posterior meniscus height (PMH), and posterior meniscus bone angle (MBA) in the lateral compartment were measured using MRI. BMI was calculated from demographic data. The association between the knee structural factors, BMI, and ACL injury risk was explored using univariate and multivariate logistic regression.
Results:
PTS (P = .043) and MCS (P = .037) significantly predicted ACL injury risk. As PTS and MCS increased by 1°, odds of sustaining an ACL injury increased by 12% and 13%, respectively. The multivariate logistic regression analysis, which included PTS, BMI centered around the mean (cBMI), and their interaction, showed that this interaction predicted the odds of ACL rupture (P = .050; odds ratio, 1.03). For every 1-unit increase in BMI from the average that is combined with a 1° increase in PTS, the odds of an ACL tear increased by 15%.
Conclusion:
An increase in BMI was associated with increased risk of ACL tear in the presence of increased lateral posterior tibial slope. Larger values of PTS or MCS were associated with an increased risk of ACL tear.
Keywords: knee, ligament, ACL, BMI, anatomy, injury prevention
Anterior cruciate ligament (ACL) tears are debilitating, especially for athletes and physically active individuals. They are burdensome in terms of rehabilitation time, treatment cost,4,14 and, most important, an increased risk of developing osteoarthritis within 10 to 15 years of injury.12,15 Injury prevention is the most efficacious intervention strategy.2 While various intervention programs do exist, the rate of ACL injury remains significant, as shown by injury rates in elite collegiate athletes over an 8-year period (2004-2012) compared with an earlier 16-year review (1988-2004).1 Clearly, improving currently available intervention programs is a worthy goal.7,20
Novel intervention strategies depend on the accurate identification of risk factors, both modifiable and nonmodifiable. Knowledge of nonmodifiable risk factors is important for patients, athletes, clinicians, and coaches, but of greater importance are those risk factors that can be modified. Such factors may have the greatest potential for intervention to decrease injury risk. Of particular interest is the interaction between modifiable and nonmodifiable risk factors, which remains unknown.
A number of nonmodifiable anatomic ACL injury risk factors have been identified to date. For example, an increase in lateral posterior tibial slope (PTS) or middle cartilage slope (MCS) has been associated with an increased risk of ACL tear.3,5,8,9,13,18,22,26 Similarly, a decrease in the lateral meniscal height in the posterior compartment (PMH) can increase the risk of ACL injury in females while a decrease in meniscal bone angle (MBA) can increase ACL injury risk in males.21,22 There is also evidence of a significant positive association between body mass index (BMI), a measure of weight in relation to height, and ACL injury risk.6,16,24 Although the association between these morphological factors and ACL injury risk has been studied, how these anatomic factors may interact with BMI to affect injury risk is unknown.
This interaction between the nonmodifiable knee morphological ACL risk factors and BMI, a modifiable factor, is also important if one considers how PTS mechanically increases one’s risk of sustaining an ACL injury. When an axial compressive force is applied to the knee joint, an anterior shear force and an internal tibial torque is produced due to mechanical coupling induced by the geometry of the tibial and femoral surfaces and their mechanical interaction. The posteriorly directed tibial slope causes the axial compressive force to have an anterior shear force component, and the steeper lateral compared with medial tibial slope19 produces internal tibial rotation because the axial force will cause the lateral side of the femur to slide posteriorly on the steeper slope of the lateral tibial plateau to a greater degree than on the medial tibial plateau. It is well accepted that anterior tibial translation and internal tibial rotation increases ACL strain and thus ACL injury risk.17,25 Therefore, the combination of a greater axial knee compressive force from greater body weight and/or greater BMI with a greater lateral posterior tibial slope, all else being equal, will increase ACL strain and most likely ACL injury risk. A similar argument can be made for MCS, PMH, and MBA. A body weight– or BMI-related increase in compression forces applied to a knee that is already at an increased risk could further increase the risk of ACL injury. Consequently, it is of interest to assess the interaction between BMI and the aforementioned knee morphological parameters.
The aim of this study, therefore, was to quantify the relationship between BMI and the 4 selected knee morphologic parameters, listed above, as potential risk factors for ACL injury. Based on the mechanical principles considered, we hypothesized that increased BMI in the presence of increased PTS or MCS would increase the risk of ACL injury; likewise, increased BMI in the presence of decreased PMH or MBA would result in increased risk of ACL injury.
Methods
A total of 118 knee magnetic resonance image (MRI) series from 76 subjects with a complete disruption of the ACL (grade 3) via a noncontact mechanism (36 females, 40 males; mean age, 24.6 ± 7.1 years; mean BMI, 26.4 ± 4.1 kg/m2) and 42 controls (21 females, 21 males; mean age, 26.5 ± 8.3 years; mean BMI, 26.2 ± 5.3 kg/m2) were obtained from the University of Michigan Health System. Given that no patients with partial ACL injury (grades 1-2) were included in this study, the terms “ACL tear” or “ACL injury” will henceforth refer to a complete disruption of the ACL, unless otherwise stated. Subjects were identified via an institutional review board–exempt, retrospective search of the University of Michigan Health System’s electronic health record database that included BMI and demographic data. Control subjects were chosen based on absence of ligamentous, meniscal, and articular cartilage tearing (Table 1) and skeletal maturity (between 15 and 40 years).
TABLE 1.
MRI Diagnoses of Control Subjects as Determined by Radiologists and Orthopaedic Surgeonsa
| Diagnosis | Subjects, n |
|---|---|
| Normal | 11 |
| Cyst, including Baker cyst | 5 |
| Meniscal injury, nontear | 5 |
| Patellar tendon–lateral femoral condyle friction syndrome/Hoffa fat pad | 4 |
| Patellar dislocation | 4 |
| MCL sprain | 1 |
| Patellar tendinosis | 2 |
| Suprapatellar fat pad syndrome | 2 |
| Semimembranous tendinosis | 2 |
| Joint effusion | 1 |
| Bipartite patella | 1 |
| Patellar subluxation | 1 |
| Femoral bone contusion | 1 |
| Tripartite patella | 1 |
| LCL sprain | 1 |
aLCL, lateral collateral ligament; MCL, medial collateral ligament; MRI, magnetic resonance imaging.
The height and weight of the subjects were measured at the time of their first visit to the University of Michigan clinics; hence, these measures were not self-reported. They were used to quantify BMI (weight in kg/height in m2).
Sagittal-plane MRIs were obtained using several MRI systems within the University of Michigan but with the same clinical protocol for knee imaging. The University of Michigan Health System’s protocol for knee imaging utilized a knee coil and a neutral knee position in the MRI scanner. The sagittal plane images were used for measurements (field of view, FH 160 mm; voxel size, AP 0.6 mm and FH 0.6 mm; slice thickness/gap: 3 mm/0.3 mm; number of slices, 29; fold over direction: AP; flip angle, 90°; scan time, 3 minutes 45 seconds). All knee geometry measurements were performed using OsiriX (version 6.5.1; open source, www.osirix-viewer.com).
The circle method described by Hudek et al11 was used to find the tibial longitudinal axis for measurement of knee geometries PTS, MCS, PMH, and MBA. This method involved 2 steps. The first step was finding the central sagittal-plane image that contains the posterior cruciate ligament attachment on the tibia, the intercondylar eminence, and the anterior and posterior tibial cortices both displaying a concave shape. The second step was finding the tibial axis by drawing 2 overlapping circles on the proximal tibia (Figure 1). The first circle was proximal to the second circle and incorporated the anterior, posterior, and proximal portions of the tibia. The center of the second (distal) circle was positioned on the most inferior portion of the first (proximal) circle; the distal circle incorporated the anterior and posterior tibial cortices. A line connecting the center of each circle defined the longitudinal axis of the tibia.
Figure 1.
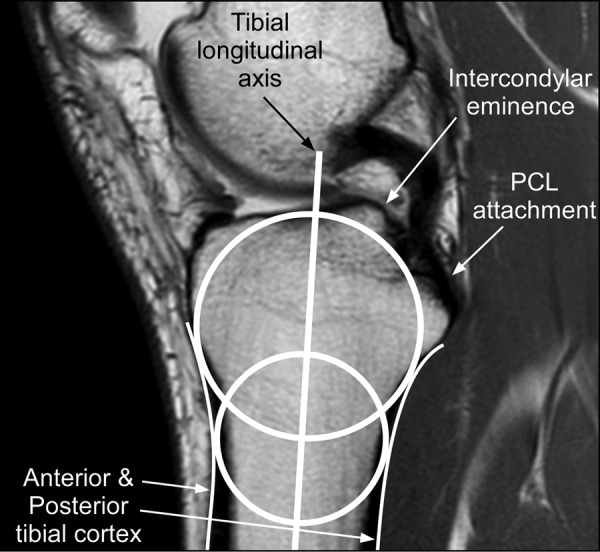
Midsagittal image defined by the presence of the posterior cruciate ligament (PCL) attachment, the intercondylar eminence, and concave anterior and posterior tibial cortex. The tibial longitudinal axis was found by drawing 2 overlapping circles: 1 proximal and 1 distal.
A second sagittal plane image, corresponding to the center of the lateral tibial condyle, was identified for measurement of the 4 knee geometric parameters PTS, MCS, MBA, and PMH. On that image, a line (L1) connecting the superior-anterior and superior-posterior cortices3 was drawn (Figure 2A). Specifically, this line connected the most anteriorly positioned superior point to the most posterior point of the superior tibial cortical surface that allowed the line to remain on the cortical surface, thus without going through the tibia. PTS was defined as the difference between 90° and the angle made between the tibial longitudinal axis and L1 (Figure 2A.). A second line (L2) along the superior surface of the wedge-shaped posterior meniscus was drawn.21 MBA was defined as the angle between L1 and L2 (Figure 2B). A third line (L3) that joins the most superior portions of the anteriorly and posteriorly located prominences of the middle articular cartilage surface21 was drawn. These anterior and posterior prominences were defined as the intersection of the femoral and tibial cartilage surfaces located anteriorly and posteriorly in the middle portion (sagittal plane) of the cartilage surface, respectively.22 MCS was defined as the difference between 90° and the angle made between the tibial longitudinal axis and L3 (Figure 2C). A fourth line (L4) was drawn from the most superior point of the posterior meniscus to the point at which the posterior meniscus intersected the middle articular cartilage.22 L4 was drawn so that it was parallel to the tibial longitudinal axis while still connecting the 2 aforementioned points. PMH was defined as the length of L4 (Figure 2D).
Figure 2.
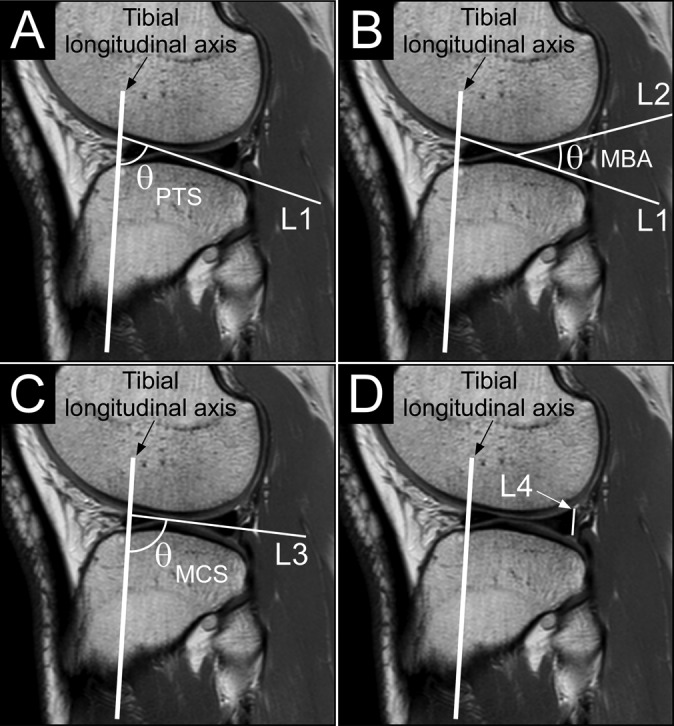
Examples of the various knee structural measurements. (A) The posterior tibial slope (PTS) was defined as the difference between 90° and the angle (θ) between the longitudinal axis of the tibia and a line (L1) that connects the superior-anterior and superior-posterior cortices of the proximal tibia. (B) The meniscal bone angle (MBA) was defined as the angle (θ) between L1 and a line (L2) that lies along the superior surface of the wedge-shaped posterior meniscal cartilage. (C) The middle cartilage slope (MCS) was defined as the difference between 90° and the angle (θ) between the longitudinal axis of the tibia and a line (L3) that joins the most superior portions of the anteriorly and posteriorly located prominences of the middle articular cartilage surfaces. (D) The posterior meniscal height (PMH) was defined as the length of a line (L4) parallel to the longitudinal axis of the tibia and connects the top of the posterior meniscal cartilage and the point at which the posterior meniscus intersects the middle articular cartilage.
The observer making the measurements was blinded to the state of the ACL (tear or no tear) after the midsagittal plane and central lateral tibial condyle images were found. This was achieved by deleting all unnecessary images from the MRI sequence. The observer was presented with only the central sagittal and central lateral tibial condyle images for each subject in a random order. The ACL, either torn or intact, was not viewable in either of these images.
The means, standard deviations, and 95% CIs were computed for each measurement. Univariate logistic regressions were used to analyze the association between risk of ACL tear and the variables PTS, MCS, MBA, PMH, BMI, height, and weight. Only variables found to significantly predict ACL injury, via the aforementioned univariate analyses, were further analyzed with multivariate logistic regressions. Multivariate logistic regression analyses were performed to predict ACL injury risk with BMI, each significant knee geometry variable, and their interaction as predictor variables to assess the relationship between each geometry variable and BMI. These multivariate logistic regressions were repeated with height as well as with weight instead of BMI as a predictor variable to investigate the individual contributions of these components of BMI to injury risk. When computing interaction variables, BMI, height, and weight were centered around the mean (cBMI, cHeight, cWeight) to decrease multicollinearity between the interaction variable and its effects.
Intraobserver reliability was examined using intraclass coefficients (ICCs). The observer made 2 series of measurements on MRIs from a subset of subjects (n = 10) 3 months apart. The ICC values of PTS (0.776), MCS (0.980), MBA (0.904), and PMH (0.860) were all considered to have good to excellent reliability, as all values exceeded 0.75.
Results
Participant height, weight, BMI, and knee morphological data are presented in Table 2. Results of univariate logistic analyses revealed that PTS (P = .043) and MCS (P = .037) were significant predictors of ACL injury while PMH (P = .072), MBA (P = .246), BMI (P = .424), height (P = .141), and weight (P = .277) were not significant predictors (Table 2). As PTS and MCS increased by 1°, there was an associated increased risk of sustaining an ACL injury of 12% and 13%, respectively.
TABLE 2.
BMI, Knee Morphological Data, and Results From Univariate Logistic Regression Modelsa
| Mean ± SD | P Value | Odds Ratio (95% CI) | |
|---|---|---|---|
| Height, m | .141 | 17.536 (0.386-797.669) | |
| ACL tear | 1.75 ± 0.10 | ||
| No ACL tear | 1.72 ± 0.09 | ||
| Weight, kg | .277 | 1.012 (0.990-1.035) | |
| ACL tear | 81.2 ± 17.5 | ||
| No ACL tear | 77.5 ± 17.9 | ||
| BMI, kg/m2 | .429 | 0.973 (0.908-1.042) | |
| ACL tear | 26.4 ± 4.1 | ||
| No ACL tear | 26.2 ± 5.3 | ||
| PTS, deg | .043 | 1.118 (1.003-1.247) | |
| ACL tear | 6.7 ± 3.9 | ||
| No ACL tear | 5.4 ± 3.4 | ||
| MCS, deg | .037 | 1.125 (1.007-1.254) | |
| ACL tear | 4.4 ± 3.7 | ||
| No ACL tear | 2.9 ± 3.3 | ||
| MBA, deg | .246 | 0.949 (0.868-1.037) | |
| ACL tear | 28.6 ± 4.1 | ||
| No ACL tear | 29.6 ± 4.6 | ||
| PMH, mm | .072 | 0.692 (0.463-1.033) | |
| ACL tear | 6.3 ± 0.9 | ||
| No ACL tear | 6.5 ± 1.0 |
aACL, anterior cruciate ligament; BMI, body mass index; MBA, meniscus bone angle; MCS, middle cartilage slope; PMH, posterior meniscus height; PTS, posterior tibial slope.
A multivariate logistic regression model that included PTS, cBMI, and their interaction (PTS * cBMI) was found to significantly predict ACL injury risk (P = .040) (model 1, Table 3). The odds ratios (ORs) for PTS and PTS * cBMI were 1.12 and 1.03, respectively. Specifically, for every 1° increase in PTS there was an 11% increase in the associated odds of tearing the ACL, keeping cBMI constant. Interpretation of the interaction term is that for a 1-unit increase in BMI from the mean in combination with a 1° increase in PTS, the associated odds of an ACL tear increased by 15% (ie, ORPTS × ORPTS * cBMI = 1.12 × 1.03) when compared with a 0° increase in PTS at the same BMI. Predicted increases in ACL injury risk associated with other combinations of increases in PTS and BMI can be found in Table 4. The 2 additional multivariate logistic regression models aimed at exploring the interactions between cHeight and PTS and cWeight and PTS, and thus, to explore the individual contributions of height and weight instead of BMI, were not found to significantly predict risk of ACL tear (models 2 and 3, Table 3). It is worth mentioning, however, that the model that included PTS, cWeight, and PTS * cWeight did approach significance (P = .055).
TABLE 3.
Multivariate Logistic Regression Models Predicting Anterior Cruciate Ligament Injury Risk
| Predictor Variables | P Value | Odds Ratio |
|---|---|---|
| Model 1a | ||
| PTS | .061 | 1.12 |
| cBMI | .140 | 0.88 |
| PTS * cBMI | .050 | 1.03 |
| Model 2b | ||
| PTS | .049 | 1.12 |
| cHeight | .754 | 3.07 |
| PTS * cHeight | .497 | 1.42 |
| Model 3c | ||
| PTS | .045 | 1.13 |
| cWeight | .348 | 0.83 |
| PTS * cWeight | .055 | 1.06 |
| Model 4d | ||
| MCS | .037 | 1.13 |
| cBMI | .707 | 0.98 |
| MCS * cBMI | .395 | 1.19 |
| Model 5e | ||
| MCS | .020 | 1.15 |
| cHeight | .288 | 32.98 |
| MCS * cHeight | .904 | 1.08 |
| Model 6f | ||
| MCS | .029 | 1.14 |
| cWeight | .812 | 1.04 |
| MCS * cWeight | .345 | 1.03 |
aModel 1: Results of the multivariate logistic regression model including posterior tibial slope (PTS), body mass index centered around the mean (cBMI), and the interaction variable (PTS * cBMI).
bModel 2: Results of the multivariate logistic regression model including PTS, height centered around the mean (cHeight), and the interaction variable (PTS * cHeight).
cModel 3: Results of the multivariate logistic regression model including PTS, weight centered around the mean (cWeight), and the interaction variable (PTS * cWeight).
dModel 4: Results of the multivariate logistic regression model including middle cartilage slope (MCS), cBMI, and the interaction variable (MCS * cBMI).
eModel 5: Results of the multivariate logistic regression model including MCS, cHeight, and the interaction variable (MCS * cHeight).
fModel 6: Results of the multivariate logistic regression model including MCS, cWeight, and the interaction variable (MCS * cWeight).
TABLE 4.
Predicted Increases in ACL Injury Riska
| PTS, degc | |||
|---|---|---|---|
| BMI, kg/m2 b | +1 | +2 | +3 |
| +0 | 12 | 25 | 40 |
| +1 | 15 | 29 | 45 |
| +2 | 19 | 33 | 49 |
| +3 | 22 | 37 | 54 |
| +4 | 26 | 41 | 58 |
| +5 | 30 | 45 | 63 |
aValues are expressed as percentages. ACL, anterior cruciate ligament; BMI, body mass index; PTS, posterior tibial slope.
bOne-unit increases in BMI from the mean BMI (26.3 kg/m2).
cOne-degree increases in PTS from the mean PTS (6.2°).
A multivariate regression model that included MCS, cBMI, and their interaction (MCS * cBMI) was not found to significantly predict ACL rupture risk (P = .132). However, the model showed significance of MCS in predicting tear (P = .037) but no significance of the interaction variable (P = .395) or cBMI (P = .707) (model 4, Table 3). The odds ratio for MCS was 1.13. As MCS increased by 1°, the associated odds of experiencing an ACL tear increased 14%, when accounting for BMI. There was no significant effect of BMI. The 2 additional multivariate logistic regression models that investigated the interactions between cHeight and MCS and cWeight and MCS were not found to significantly predict ACL injury risk (models 5 and 6, Table 3).
Discussion
This research demonstrates the important role that BMI played in determining the risk of ACL injury in these subjects. This is the first demonstration of how an individual’s BMI can combine with their knee morphology to increase their risk of sustaining an ACL injury. Since BMI is a modifiable factor, it presents an opportunity to improve ACL injury prevention strategies.
Our results showed that BMI is associated with an increase in the odds of ACL injury in the presence of an increased lateral posterior tibial slope. In other words, BMI appears to exacerbate the positive relation between PTS and ACL injury risk. Additional analyses revealed that it is mainly weight that is driving this significant relationship between BMI and PTS and its association with ACL injury risk. This is a key finding because BMI can be quantified easily from measures of height and weight, which are standard elements of most athletic and medical assessments, and can be modified via weight loss/gain. Although PTS is not readily modifiable, it may be beneficial to screen for this contributing factor to ACL injury risk because of the modulating role played by BMI within this PTS–ACL injury risk relationship. This is especially significant in college athletics, an environment in which many athletes, including those in sports where noncontact ACL injuries are common, are encouraged to increase weight, and consequently, BMI, to increase sports performance. ACL prevention efforts could target individuals with both an increased PTS and BMI. Whether these individuals should be advised to decrease their weight as a strategy to limit their risk of sustaining an ACL injury is to be determined. For one, it is unknown whether greater weight due to greater lean body mass or greater fat body mass, or an increase in the combination of these mass types, has different effects on injury risk when combined with an increased PTS. This is because BMI only accounts for overall weight but not where the extra weight lies. It is possible that an increase in fat body mass increases one’s risk of ACL injury in combination with a steep PTS, while an increase in lean body mass may not affect one’s risk or might even decrease it. The interaction of weight, BMI, and lean body mass (eg, muscle) may be complex in terms of ACL injury risk. Increasing muscle mass is often the goal of many athletes with the anticipation of increased strength and power, both of which may help prevent knee injuries.10 The increase in weight due to muscle gain may need to be balanced against its increased ACL injury risk when combined with “risky” structural factors (PTS) identified herein. On the other hand, it may only be an increase in fat body mass that is detrimental. This may explain why the interaction of weight and PTS and its association with ACL injury risk did not quite reach significance. Many questions, such as what kind of weight (lean vs fat body mass) and how much weight gain is hazardous, remain unanswered and could be the target of future research. Regardless, a high BMI appears to be an important modifiable ACL risk factor in the presence of an increased PTS.
As lateral PTS and lateral MCS increased, irrespective of BMI, the odds of experiencing an ACL tear also increased in the present study. These findings concur with results from previous research that suggest increases in PTS5,8,9,13,18,26 and MCS5,21 increase the risk for ACL injury. In the present study, PTS and MCS appeared to have similar effects on the odds of sustaining an ACL rupture (variables had odds ratio of 1.12 and 1.13, respectively).
Recent literature21,22 suggests that PMH and MBA are significant risk factors in females and males, respectively. However, PMH and MBA were not shown to be significant ACL risk factors in this study. The discrepancy in these findings may be explained by a variety of factors. First, the methods used to measure both PMH and MBA have not been validated using the method of Hudek et al11 for obtaining the tibial longitudinal axis. The measurement methods were based on the work of Sturnick et al21,22 but could not be duplicated exactly due to the lack of higher resolution MRIs. This is also a study strength, however, because lower resolution MRIs, as are often acquired clinically, can be used to predict ACL risk. Second, the effects of these 4 knee anatomic parameters on ACL injury risk could not be calculated independently for the female and male subjects due to the small sample size. Our combined analysis probably masked the sex-specific effects of PMH and MBA on injury risk previously reported.21,22 Further method development and sex-specific analysis may be necessary to achieve the results obtained by Sturnick et al.21,22
This study has several limitations. First, the retrospective nature of this study does not allow us to account for any changes in knee morphology after injury, such as tibiofemoral articular thickness changes,23 which may affect measures of the middle cartilage slope.3 Although our results pertaining to this measure should be interpreted with caution, similar results have been reported using the uninjured knee of patients with injured ACLs.22 Furthermore, there is no evidence that sustaining an ACL injury modifies the other 3 knee morphological factors we measured or BMI. Second, a selection bias may have existed in our control group because it consisted of patients who had obtained an MRI due to complaints of knee pain, which might have altered the morphologic features measured in this study. However, many (26%) of these MRIs were interpreted as normal by radiologists and clinicians. None of them showed ligament or meniscal pathology. Given the varied nature of the diagnoses, they are unlikely the source of the significant associations between ACL injury risk and PTS, MCS, and BMI found herein. Some other underlying knee pathologies, however, could have been present and thus confounded our results. Third, the ACL-injured group and the control group may have differed in terms of their exposure to ACL injury risk. This is unknown, however, given the retrospective nature of this study. Fourth, the clinical MR images available for measurement were of lower resolution than those used in prior studies3,5,21,22 to measure knee geometries such as PTS. This, however, may also be a strength of the study. If the measurement of knee geometries such as PTS is implemented in clinical practice, clinicians will not have access to the high-resolution MRIs often used in research settings. Fifth, although careful efforts were made by the MRI technicians to ensure that each patient’s knee was in a neutral position (0° of knee flexion) during the MRI scan, slight variations in knee flexion angles may have occurred. We have no reason to believe, however, that these variations were more than minimal or that they would have biased 1 group, thereby accounting for the significant results we reported. Last, these clinical MR images were obtained from various systems within the University of Michigan Health System, which may have added variability to our data. This variability, however, appears to be insignificant in terms of the differences in the knee morphologic parameters found between groups.
Conclusion
The effect of an increase in lateral PTS on ACL injury risk is affected by BMI. An increase in BMI was found to be associated with an increase in the risk of ACL tear in the presence of an increased lateral PTS. An increase in lateral PTS or MCS was associated with an increased risk of an ACL tear, irrespective of BMI.
Acknowledgment
The authors thank Elizabeth Sibilsky Enselman (University of Michigan); University of Michigan Consulting for Statistics, Computing, and Analytics Research; and the University of Michigan MedSport Department of Radiology.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding for this study was provided by United States Public Health Service grant R01 AR054821 and the University of Michigan Medical School Student Biomedical Research Program.
Ethical approval for this study was waived by the University of Michigan Medical School Institutional Review Board.
References
- 1. Agel J, Rockwood T, Klossner D. Collegiate ACL injury rates across 15 sports: National Collegiate Athletic Association Injury Surveillance System data update (2004-2005 through 2012-2013). Clin J Sport Med. 2016;26:518–523. [DOI] [PubMed] [Google Scholar]
- 2. Alentorn-Geli E, Mendiguchía J, Samuelsson K, et al. Prevention of anterior cruciate ligament injuries in sports. Part I: systematic review of risk factors in male athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22:3–15. [DOI] [PubMed] [Google Scholar]
- 3. Beynnon BD, Hall JS, Sturnick DR, et al. Increased slope of the lateral tibial plateau subchondral bone is associated with greater risk of noncontact ACL injury in females but not in males: a prospective cohort study with a nested, matched case-control analysis. Am J Sports Med. 2014;42:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brophy RH, Wright RW, Matava MJ. Cost analysis of converting from single-bundle to double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37:683–687. [DOI] [PubMed] [Google Scholar]
- 5. Dare DM, Fabricant PD, McCarthy MM, et al. Increased lateral tibial slope is a risk factor for pediatric anterior cruciate ligament injury: an MRI-based case-control study of 152 patients. Am J Sports Med. 2015;43:1632–1639. [DOI] [PubMed] [Google Scholar]
- 6. Evans KN, Kilcoyne KG, Dickens JF, et al. Predisposing risk factors for non-contact ACL injuries in military subjects. Knee Surg Sports Traumatol Arthrosc. 2012;20:1554–1559. [DOI] [PubMed] [Google Scholar]
- 7. Grimm NL, Jacobs JC, Jr, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43:2049–2056. [DOI] [PubMed] [Google Scholar]
- 8. Hashemi J, Chandrashekar N, Gill B, et al. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90:2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hashemi J, Chandrashekar N, Mansouri H, et al. Shallow medial tibial plateau and steep medial and lateral tibial slopes: new risk factors for anterior cruciate ligament injuries. Am J Sports Med. 2010;38:54–62. [DOI] [PubMed] [Google Scholar]
- 10. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27:699–706. [DOI] [PubMed] [Google Scholar]
- 11. Hudek R, Schmutz S, Regenfelder F, Fuchs B, Koch PP. Novel measurement technique of the tibial slope on conventional MRI. Clin Orthop Relat Res. 2009;467:2066–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louboutin H, Debarge R, Richou J, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee. 2009;16:239–244. [DOI] [PubMed] [Google Scholar]
- 13. Marouane H, Shirazi-Adl A, Adouni M, Hashemi J. Steeper posterior tibial slope markedly increases ACL force in both active gait and passive knee joint under compression. J Biomech. 2014;47:1353–1359. [DOI] [PubMed] [Google Scholar]
- 14. Mather RC, 3rd, Koenig L, Kocher MS, et al. ; MOON Knee Group. Societal and economic impact of anterior cruciate ligament tears. J Bone Joint Surg Am. 2013;95:1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neuman P, Englund M, Kostogiannis I, Friden T, Roos H, Dahlberg LE. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am J Sports Med. 2008;36:1717–1725. [DOI] [PubMed] [Google Scholar]
- 16. Nilstad A, Andersen TE, Bahr R, Holme I, Steffen K. Risk factors for lower extremity injuries in elite female soccer players. Am J Sports Med. 2014;42:940–948. [DOI] [PubMed] [Google Scholar]
- 17. Oh YK, Lipps DB, Ashton-Miller JA, Wojtys EM. What strains the anterior cruciate ligament during a pivot landing? Am J Sports Med. 2012;40:574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ristić V, Maljanović MC, Pericin B, Harhaji V, Milankov M. The relationship between posterior tibial slope and anterior cruciate ligament injury. Med Pregl. 2014;67:216–221. [DOI] [PubMed] [Google Scholar]
- 19. Simon RA, Everhart JS, Nagaraja HN, Chaudhari AM. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. J Biomech. 2010;43:1702–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stevenson JH, Beattie CS, Schwartz JB, Busconi BD. Assessing the effectiveness of neuromuscular training programs in reducing the incidence of anterior cruciate ligament injuries in female athletes: a systematic review. Am J Sports Med. 2015;43:482–490. [DOI] [PubMed] [Google Scholar]
- 21. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sturnick DR, Van Gorder R, Vacek PM, et al. Tibial articular cartilage and meniscus geometries combine to influence female risk of anterior cruciate ligament injury. J Orthop Res. 2014;32:1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tourville TW, Johnson RJ, Slauterbeck JR, Naud S, Beynnon BD. Assessment of early tibiofemoral joint space width changes after anterior cruciate ligament injury and reconstruction: a matched case-control study. Am J Sports Med. 2013;41:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31:831–842. [DOI] [PubMed] [Google Scholar]
- 25. Zantop T, Herbort M, Raschke MJ, Fu FH, Petersen W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am J Sports Med. 2007;35:223–227. [DOI] [PubMed] [Google Scholar]
- 26. Zeng C, Yang T, Wu S, et al. Is posterior tibial slope associated with noncontact anterior cruciate ligament injury? Knee Surg Sports Traumatol Arthrosc. 2016;24:830–837. [DOI] [PubMed] [Google Scholar]


