Abstract
Background/Objectives:
Postoperative urinary retention (POUR) is a common event following surgical procedures. An increase in the number of elderly individuals who undergo hip fracture repair procedures is inevitable due to the aging of population. Postoperative urinary retention is associated with both early (infections, delirium) and late complications (urinary incontinence) of surgery. The objective of the current study is to direct attention to the less studied population of patients admitted to a geriatric rehabilitation ward following hip fracture repair who are at risk of POUR.
Design:
Prospective single-center cohort study.
Setting:
Academic tertiary hospital.
Measurement:
Postvoid bladder volume by ultrasonography (US).
Results:
Postvoid bladder volume was measured by US in 88 consecutive female patients on the morning following their admission to the geriatric rehabilitation department. The mean age of the patients was 82.5 ± 6.5 years, and the frequency of POUR (defined as postvoid bladder volume ≥200 mL) was 37.5%. The POUR (n = 33) and non-POUR (n = 55) groups were similar with respect to most demographic and disease states. Multivariable stepwise logistic regression revealed a significant effect for opioid use (relative risk [RR] = 8.0, P < .001) and for treatment with anticholinergic medication (RR = 1.3, P = .046). There was an unexpectedly high proportion of patients with asymptomatic urinary retention (29 of the 33 patients, 88%).
Conclusion:
The high incidence of asymptomatic POUR in elderly patients calls for the need for improved screening tools for early identification and treatment.
Keywords: postoperative, urinary retention, hip fractures
Introduction
Postoperative urinary retention (POUR) is a well-known complication of various surgical procedures, with a reported prevalence between 5% and 70%.1,2 This wide range reflects the variability of the definitions of POUR, the surgical interventions, and the patient populations.3 Postoperative urinary retention is associated with both early and late complications. The former includes the development of delirium4 and urinary tract infections,5 which may develop due to the stasis of urine in the bladder, and the need for urinary catheterization (a procedure that itself significantly increases the risk of infection). The latter includes the development of urinary incontinence attributable to overdistension of the bladder and collagen deposition in between the detrusor fibers that reduce its contractility.6 Various studies attempted to identify risk factors for the development of POUR in surgical patients. Risk factors can be defined as intrinsic (patient related, eg, age, medical background) and extrinsic (intervention related, eg, type of surgery, anesthesia, analgesics). Among the comorbidities suggested as being related to the increased risk of POUR are neurologic diseases (stroke, poliomyelitis, multiple sclerosis, spinal lesions) and peripheral neuropathies.7 The durations of surgery and anesthesia were also shown to correlate with POUR.8 The method of anesthesia or analgesia, that is, general anesthesia, conduction blockades (spinal, epidural, and combined spinal epidural anesthesia), and the specific drugs that had been administered (anesthetics, opioids, etc), can also affect POUR occurrence.2 The risk of POUR is highly affected by the size of the prostate. The International Prostate Symptom Score, a scoring system for the evaluation of prostate-related symptoms, has been used as a tool for predicting POUR but with conflicting results.9,10 In order to omit the effect of the prostate as a factor in the occurrence of POUR, several studies have excluded males altogether.
Hip fractures are a major concern in the geriatric population. Most of the patients who suffer a hip fracture will undergo a surgical intervention for reduction and fixation of the fractured femur bone. Subsequent to a corrective operation, the patients are exposed to early complications (delirium, infections, pain, venous thromboembolism) and late complications related to the accompanying functional decline. Many patients undergo a course of rehabilitation in order to avoid these late complications.
In the current study, we focused on female geriatric patients who underwent surgical repair for hip fracture. Unlike other studies11 that observed patients immediately following the surgical intervention, we decided to examine the patients at a later stage, specifically, upon admission to a geriatric rehabilitation ward. The aims of the study were to determine the prevalence and risk factors for POUR occurrence.
Methods
Study Participants
Inclusion criteria were female gender, age ≥65 years, and a score of ≥20 on the Mini-Mental State Examination (MMSE) in patients who were admitted to the rehabilitation ward following surgery for a hip fracture. Patients who were transferred to the rehabilitation ward with urinary catheter due to acute POUR were excluded. A total of 88 patients met all eligibility criteria and were enrolled between October 1, 2010, and July 31, 2011. Written informed consent for study participation was obtained from all participants according to procedures approved by the institutional review board of Tel Aviv Medical Center.
Assessment of POUR
The study participants were examined by the same physician on the day following their admission to the rehabilitation ward, after the first morning void. Urinary retention was defined as postvoid bladder volume on ultrasonography (US) ≥200 mL (data are also presented for US ≥ 400 mL). The patients diagnosed as having POUR were reexamined by US every 24 hours for 7 days. An indwelling catheter was inserted when the scan estimated the bladder volume to be >700 mL, whereupon a complete pelvic examination was conducted to rule out pelvic floor prolapse and urine was collected to be cultured. The clinical and demographic data were extracted from the participants’ medical records. Data regarding constipation were collected from the medical and nursing records at the orthopedic and geriatric departments. Diagnosis of constipation was based on specific physician or nurse diagnosis or the use of laxatives upon admission to the geriatric rehabilitation ward.
Statistical Analyses
Comparisons between patients with/without POUR were conducted with T tests or Wilcoxon rank sum tests for continuous parameters and χ2 or Fisher exact tests for categorical parameters, depending on the distribution of the data. Multivariable stepwise logistic regression, including statistically significant and/or clinically relevant variables, was used for variable selection. Relative risks (RRs) of significant parameters were estimated with Poisson regression with robust error variance.
Results
The characteristics of the 88 study participants are summarized in Table 1. The mean postvoid bladder volume was 247 ± 340 mL (median 140 mL). The frequency of POUR was 37.5% (33 of the 88 participants). The POUR and non-POUR groups were similar in most demographic and disease parameters: age (mean 82.5 ± 6.5 years), days in rehabilitation (26 ± 16), cognitive state as measured by MMSE (24 ± 3), comorbidities, and a panel of plasma chemistry markers. Days from surgery until bladder US assessment also did not significantly differ between the groups (13.5 ± 8.7 and 12.0 ± 4.1, P = .672 for POUR and non-POUR, respectively). Of note, the thyroid-stimulating hormone (TSH) and albumin levels were significantly lower in the POUR group (P = .02 and .017, respectively; Table 1). Additional analysis is presented for postvoid bladder volume on US ≥ 400 mL (Table 2). In addition to TSH and albumin, mean free T4 levels were significantly increased in the POUR group (1.6 ± 0.4 and 1.3 ± 0.3, P = .008 for POUR and non-POUR, respectively).
Table 1.
Patient Characteristics—Continuous Variables.a
| Characteristics | POUR (n = 33 Females) | Non-POUR (n = 55 Females) | P Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| Age | 83.8 ± 6.0 | 85.0 (71-94) | 81.7 ± 6.7 | 81.0 (65.0-99.0) | .154b |
| MMSE | 23.5 ± 2.6 | 23.0 (18.0-28.0) | 24.7 ± 3.1 | 25.0 (19.6-30.0) | .046b |
| Hgb (g/dL) | 10.5 ± 1.1 | 10.4 (8.5-13.8) | 10.9 ± 2.9 | 10.5 (8.6-31.0) | .718c |
| Albumin (g/L) | 32.3 ± 4.2 | 32.0 (22.0-43.0) | 34.6 ± 4.1 | 34.0 (25.0-44.0) | .017b |
| TSH (mu/L) | 3.5 ± 5.6 | 1.8 (0.03-31.0) | 5.0 ± 5.6 | 2.6 (0.6-25.6) | .020c |
| T4 (ng/dL) | 1.4 ± 0.4 | 1.4 (0.6-2.7) | 1.3 ± 0.2 | 1.4 (0.8-1.8) | .398c |
| CRP (mg/L) | 36.4 ± 24.6 | 31.2 (1.0-101.0) | 31.6 ± 21.7 | 27.7 (4.7-115.7) | .391c |
| B12 (pg/mL) | 513.2 ± 293.5 | 460 (250-2000) | 501.2 ± 310.5 | 393 (113-1749) | .298c |
Abbreviations: B12, vitamin B12; CRP, C-reactive protein; Hgb, hemoglobin; MMSE, Mini-Mental State Examination; POUR, postoperative urinary retention; SD, standard deviation; TSH, thyroid-stimulating hormone.
aPostoperative urinary retention defined as postvoid bladder volume ≥200 mL.
b T test.
cWilcoxon rank sum test.
Table 2.
Patient Characteristics—Continuous Variables.a
| Characteristics | POUR (n = 17 Females) | Non-POUR (n = 71 Females) | P Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| Age | 85.2 ± 4.4 | 86 (78-91) | 81.9 ± 6.8 | 82 (65-99) | .059b |
| MMSE | 23.6 ± 2.1 | 23.0 (21-28) | 24.4 ± 3.1 | 24.0 (18-30.0) | .339b |
| Hgb (g/dL) | 10.5 ± 0.8 | 10.3 (9.2-12.0) | 10.8 ± 2.6 | 10.5 (8.5-31.0) | .784c |
| Albumin (g/L) | 30.8 ± 4.1 | 31.0 (22.0-36.0) | 34.4 ± 4.0 | 34.0 (25.0-44.0) | .001b |
| TSH (mu/L) | 2.1 ± 1.0 | 1.7 (0.8-4.3) | 5.0 ± 6.1 | 2.5 (0.03-31.0) | .041c |
| T4 (ng/dL) | 1.6 ± 0.4 | 1.5 (1.1-2.7) | 1.3 ± 0.3 | 1.3 (0.6-1.8) | 0.008c |
| CRP (mg/L) | 35.7 ± 23.1 | 31.2 (6.9-98.0) | 32.9 ± 22.9 | 27.7 (1.0-115.7) | 0.584c |
| B12 (pg/mL) | 564.0 ± 393.3 | 460 (285-2000) | 491.7 ± 278.3 | 419 (113-1749) | 0.349c |
Abbreviations: B12, vitamin B12; CRP, C-reactive protein; Hgb, hemoglobin; MMSE, Mini-Mental State Examination; POUR, postoperative urinary retention; SD, standard deviation; TSH, thyroid-stimulating hormone.
aPostoperative urinary retention defined as postvoid bladder volume ≥400 mL.
b T test.
cWilcoxon rank sum test.
For the multivariable stepwise logistic regression analysis, we selected a group of significant and/or clinically relevant variables (age, albumin, cortisol, hemoglobin, folic acid, TSH, free T4, vitamin B12, opioids upon admission, anticholinergic medication upon admission, days from operation until bladder US, and preadmission bladder function). There was a significant group difference in opioid use (RR = 8.0, P < .001) and treatment with anticholinergic medications (RR = 1.3, P = .046) between the groups, where opioid and anticholinergic use increased POUR occurrence. Similar multivariable stepwise logistic regression analysis using POUR defined as bladder volume on US ≥ 400 mL resulted in significant group difference in opioid use (RR = 10.6, P = .022), treatment with anticholinergic medications (RR = 3.0, P < .001), TSH (RR = 0.6, P < .001), and albumin (RR = 0.9, P < .001). An unexpected high proportion of asymptomatic urinary retention was observed: 29 (88%) of the 33 patients with a bladder volume ≥200 mL were asymptomatic and 14 (82%) of the 17 patients with a bladder volume ≥400 mL were asymptomatic (Figure 1).
Figure 1.
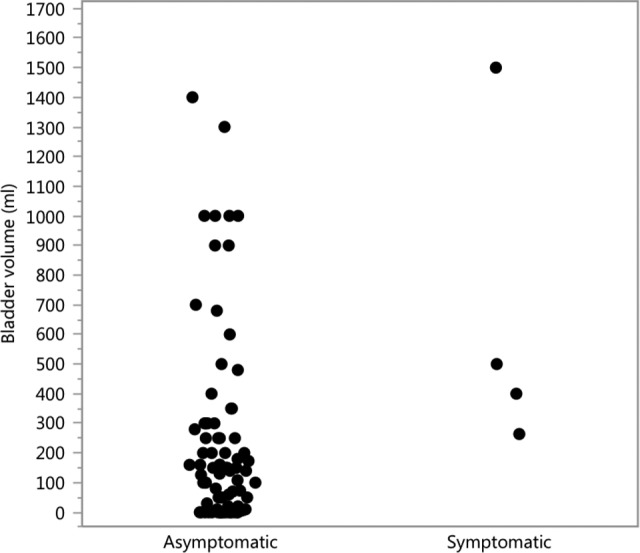
Postvoid bladder volume (mL) as measured on the morning following the patient’s admission to the rehabilitation ward. Asymptomatic = no symptoms of postoperative urinary retention (POUR) following hip surgery (n = 29); symptomatic = symptoms of POUR (n = 4).
Twenty (23%) participants were prescribed medications with anticholinergic properties: 10 were treated with selective serotonin reuptake inhibitors (SSRIs), 4 with mirtazapine, and 1 each with risperidone, baclofen, elatrol, maprotiline, trospium, and oxybutynin.
Discussion
Most studies on POUR are conducted on patients in the early postoperative period, typically during the first day following the operation. The use of a urinary catheter is common at this stage, and patients who were successfully weaned from the catheters are typically evaluated for urinary retention only in the event that there are characteristic symptoms, such as lower abdominal pain and a prolonged anuria state. In this work, we focused upon a period of time during which the effect of intraoperative factors on POUR has usually subsided. Prostate enlargement is ubiquitous among elderly males and a common risk factor for having urinary retention following both pelvic and nonpelvic operations. There is no equivalently obvious risk factor among women, and so the level of awareness of POUR among female patients is understandably much lower. Postoperative urinary retention should also be investigated among female patients who comprise most of the elderly patients admitted to our institute with hip fracture (70%). This information does not appear in the current literature. We therefore sought to determine the extent of its presence and its characteristics and to identify the main risk factors for its occurrence. We also considered that it may be associated with symptomatology for which other sources appeared not to be responsible. It is important to note that there is no established definition for urinary retention based on bladder volume. We arbitrarily defined urinary retention as a bladder volume ≥200 mL. Most studies define urinary retention as a postvoid bladder volume ≥200 mL, some use 400 mL as a cutoff, and others use even higher volumes12.
In our study, patients suffering from POUR had lower levels of TSH. Both POUR and non-POUR patients had mean levels of TSH in the normal range, and concomitant free T4 levels did not differ significantly between the groups. Of note, when POUR was defined based on a bladder volume of 400 mL, similar results were observed. Under this definition, free T4 levels increased in a statistically significant manner in the POUR group, yet the magnitude of change was clinically insignificant. Contrasting results were observed in a recent work by Justo et al,13 where hypothyroidism was associated with asymptomatic urinary retention; moreover, a direct correlation has been found between TSH serum values and postvoid residual urine. A plausible explanation was provided by Justo et al suggesting that hypothyroidism might be related to atonic bladder or fecal impaction, impairing bladder emptying. Although albumin levels were significantly lower in the POUR group compared with the non-POUR group, the magnitude of the discrepancy was subtle in clinical terms.
Opioids are commonly used during the intraoperative and the postoperative periods.14 In our study, 34 (39%) of the 88 participants received opioids on the day of admission to the rehabilitation ward (12.5 ± 6.2 days following hip surgery). This high proportion warrants increased awareness to the possibility of POUR in this population. Opioids are known to increase the risk of urinary retention by several mechanisms: (1) decreasing the sensation of bladder fullness by partially inhibiting the parasympathetic innervation of the bladder, (2) increasing the bladder sphincter tonus via sympathetic overstimulation, conferring increased resistance to the urine flow,15 and (3) opioid-induced constipation. Chart review showed that 20 patients were diagnosed with constipation upon admission, yet no significant correlation was found between the presence of constipation and POUR development (P = .25).
The use of drugs with anticholinergic properties is a well-known risk factor for various ominous side effects, mainly in the elderly population. While some drugs are prescribed for their anticholinergic effect (such as bladder relaxants for urge incontinence), most of the adverse effects are related to drugs prescribed for other pharmacologic properties, such as tricyclic antidepressants and neuroleptics.16 Moreover, anticholinergic medications contribute to urinary retention by inhibiting effective contraction of the urinary bladder. In our sample, 20 (23%) of the 88 participants were prescribed medications with anticholinergic properties, of which 4 are known to carry low anticholinergic activity (SSRIs, mirtazapine, and risperidone), and 4 are known to carry medium-to-high anticholinergic activity (baclofen, elatrol, maprotiline, trospium, and oxybutynin).17
Importantly, most of the POUR cases were asymptomatic (29 of the 33, 88%). Pavlin et al observed that asymptomatic POUR is highly prevalent after outpatient surgery, mainly in older patients.18 The high incidence of asymptomatic POUR in our elderly female patients during a relatively late postoperative period emphasizes the need for raising the level of awareness of POUR and selection of patients for a bladder scan. It is yet unclear whether early identification of asymptomatic POUR will result in better clinical outcome. Further studies would be needed to evaluate asymptomatic POUR identification and treatment at this setting.
Footnotes
Authors’ Note: Presented as a poster at the 2016 Scientific Meeting of the American Geriatrics Society, May 2016, Long Beach, California.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Oishi CS, Williams VJ, Hanson PB, Schneider JE, Colwell CW, Walker RH. Perioperative bladder management after primary total hip arthroplasty. J Arthroplasty. 1995;10(6):732–736. [DOI] [PubMed] [Google Scholar]
- 2. Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110(5):1139–1157. [DOI] [PubMed] [Google Scholar]
- 3. Dreijer B, Møller MH, Bartholdy J. Post-operative urinary retention in a general surgical population. Eur J Anaesthesiol. 2011;28(3):190–194. [DOI] [PubMed] [Google Scholar]
- 4. Waardenburg IE. Delirium caused by urinary retention in elderly people: a case report and literature review on the “cystocerebral syndrome”. J Am Geriatr Soc. 2008;56(12):2371–2372. [DOI] [PubMed] [Google Scholar]
- 5. Hooton TM, Bradley SF, Cardenas DD, et al. ; Infectious Diseases Society of America. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–663. [DOI] [PubMed] [Google Scholar]
- 6. Taylor JA, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006;54(12):1920–1932. [DOI] [PubMed] [Google Scholar]
- 7. Toyonaga T, Matsushima M, Sogawa N, et al. Postoperative urinary retention after surgery for benign anorectal disease: potential risk factors and strategy for prevention. Int J Colorectal Dis. 2006;21(7):676–682. [DOI] [PubMed] [Google Scholar]
- 8. Mulroy MF, Salinas FV, Larkin KL, Polissar NL. Ambulatory surgery patients may be discharged before voiding after short-acting spinal and epidural anesthesia. Anesthesiology. 2002;97(2):315–319. [DOI] [PubMed] [Google Scholar]
- 9. Sarasin SM, Walton MJ, Singh HP, Clark DI. Can a urinary tract symptom score predict the development of postoperative urinary retention in patients undergoing lower limb arthroplasty under spinal anaesthesia? A prospective study. Ann R Coll Surg Engl. 2006;88(4):394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elkhodair S, Parmar HV, Vanwaeyenbergh J. The role of the IPSS (International Prostate Symptoms Score) in predicting acute retention of urine in patients undergoing major joint arthroplasty. Surgeon. 2005;3(2):63–65. [DOI] [PubMed] [Google Scholar]
- 11. Tobu S, Noguchi M, Hashikawa T, Uozumi J. Risk factors of postoperative urinary retention after hip surgery for femoral neck fracture in elderly women. Geriatr Gerontol Int. 2014;14(3):636–639. [DOI] [PubMed] [Google Scholar]
- 12. Kaplan SA, Wein AJ, Staskin DR, Roehrborn CG, Steers WD. Urinary retention and post-void residual urine in men: separating truth from tradition. J Urol. 2008;180(1):47–54. [DOI] [PubMed] [Google Scholar]
- 13. Justo D, Schwartz N, Dvorkin E, Gringauz I, Groutz A. Asymptomatic urinary retention in elderly women upon admission to the Internal Medicine department: a prospective study [Published online May 13, 2016]. Neurourol Urodyn. [DOI] [PubMed] [Google Scholar]
- 14. Feldt KS, Ryden MB, Miles S. Treatment of pain in cognitively impaired compared with cognitively intact older patients with hip-fracture. J Am Geriatr Soc. 1998;46(9):1079–1085. [DOI] [PubMed] [Google Scholar]
- 15. Verhamme KM, Sturkenboom MC, Stricker BH, Bosch R. Drug-induced urinary retention: incidence, management and prevention. Drug Saf. 2008;31(5):373–388. [DOI] [PubMed] [Google Scholar]
- 16. Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508–513. [DOI] [PubMed] [Google Scholar]
- 17. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333–1341. [DOI] [PubMed] [Google Scholar]
- 18. Pavlin DJ, Pavlin EG, Fitzgibbon DR, Koerschgen ME, Plitt TM. Management of bladder function after outpatient surgery. Anesthesiology. 1999;91(1):42–50. [DOI] [PubMed] [Google Scholar]


