Abstract
Background:
Structural brain changes, potentially resulting from repetitive brain trauma (RBT), have been correlated with neurocognitive decline and increased symptom reporting in retired athletes.
Hypothesis:
In a cohort of retired National Football League (NFL) players, the relationships between 3 neuroimaging parameters, neuropsychological testing, and symptom scores will be significantly correlated.
Study Design:
Cross-sectional study.
Level of Evidence:
Level 3.
Methods:
Comprehensive magnetic resonance imaging was performed in 45 retired NFL players. Three neuroanatomical parameters were assessed by board-certified radiologists blinded to the purpose of the study: (1) the absence or presence of small or large cavum septum pellucidum, (2) a global mean score of fractional anisotropy (FA), and (3) the presence or absence of microhemorrhages. The subjects underwent a battery of 9 paper-and-pencil neuropsychological tests, a computerized neurocognitive test, and multiple symptom and depression scales. The associations among the neuroimaging results with these outcome measures were assessed utilizing Pearson, Spearman rank, and point-biserial correlations.
Results:
The 45 subjects (mean age, 46.7 ± 9.1 years) reported a mean 6.9 (±6.2) concussions and 13.0 (±7.9) “dings” in the NFL. Ten (22%) did not have a cavum septum pellucidum, while 32 (71%) had a small and 3 (7%) had a large one. Four (9%) had microhemorrhages. Global FA mean was 0.459 (±0.035). The majority (50.8%) of correlations among the neuroimaging parameters and neurocognitive/symptom scores fell below the threshold of “small” effect size (r < 0.10). The remaining (49.2%) correlations were between “small” and “medium” effect sizes (0.1 < r < 0.3). However, all correlations were statistically nonsignificant.
Conclusion:
There were minimal and statistically nonsignificant correlations among the neuroimaging, neurocognitive, and symptom scores examined in this cohort of NFL retirees.
Clinical Relevance:
Associating the severity of structural brain changes to neurocognitive performance and symptom burden after RBT is complex may involve other moderating variables or biomarkers, and demands further study.
Keywords: sport-related concussion, National Football League, chronic impairment, neuroimaging, neuroradiology, neuropsychological testing, cognitive function
The potential long-term effects of repetitive brain trauma (RBT) in American football have taken hold of the national spotlight.14,35 RBT may occur in the form of concussions and/or subconcussive impacts.6,43,54 While some reports support a causal link between neurocognitive decline and RBT,64,65 others have suggested that the evidence is incomplete and that a direct causal link cannot be made at the present time.11,26,27,62 Neuroimaging and neuropsychological testing represent evolving and commonly used modalities to quantify intracranial neurologic injury.
Many different neuroimaging modalities have been utilized to study brain injury. Routine magnetic resonance imaging (MRI) can assess various anatomical parameters, including cortical thinning34 and cavum septum pellucidum.20,44 Diffusion tensor imaging (DTI) is a noninvasive MRI method that uses the diffusion properties of water to generate measures of white matter structural integrity within the brain.68 In particular, fractional anisotropy (FA) and mean diffusivity (MD) measure the directionality and magnitude of water diffusion.68 Functional MRI can evaluate brain activity in the acute and chronic phases of injury,1,54,69 and positron emission tomography (PET) can identify protein structures, such as amyloid plaques, which may be suggestive of ongoing neurodegeneration.47
Utilizing these advanced neuroimaging techniques, several studies have correlated positive neuroimaging findings (thought to result from cumulative RBT) with neurocognitive decline.23,33,59,66,67,74 As a result, it has become widely accepted that RBT leads to structural brain pathology, which in turn can serve as either an in vivo or postmortem biomarker for clinical diagnoses of abnormal neurobehavioral changes or neurocognitive decline. However, this widely accepted paradigm warrants further study due to limitations inherent to studying RBT, including small sample sizes and the potential for selection bias.2,61
The purpose of this investigation was to assess the relationships among 3 neuroimaging findings (cavum septum pellucidum, FA global mean, and microhemorrhages) and neuropsychological test performance and symptom endorsement in a relatively large sample of retired National Football League (NFL) athletes. For this initial investigation, based on the prior literature examining retired NFL players,23,33,66,74 we accepted the hypothesis that the relationships between these neuroimaging parameters and neuropsychological test and symptom scores would be significantly associated, as evidenced by multiple statistically significant correlations.
Methods
Institutional review board approval was granted for the study, and oral and written consent for subjects who met the inclusion and exclusion criteria was obtained by the study coordinator. Data were collected in a confidential manner, deidentified, and aggregated into a Microsoft Excel (Microsoft Inc) spreadsheet.
The subjects included in the current study were originally recruited by Casson et al10 and have subsequently been restudied in a separate independent analysis.60 We summarize their methodology below.
Subject Recruitment and Inclusionary Criteria
Through the National Football League Players Association (NFLPA), 5000 retired players received recruitment letters explaining the purpose of the original study. The list of players was obtained through the NFL players’ union. A dedicated, confidential telephone number was provided for those who were interested in participating. Additionally, the study coordinator randomly selected names of players on file to call and ask whether they were interested in participation. During the study period, current subjects contacted former teammates, friends, or colleagues who were also retired NFL players who may have been interested in participating. Two former NFL players (4.4%) heard about the study in this manner and were included in the final cohort. Subjects were included only if they were aged 60 years or younger, retired from the NFL, and able to tolerate MRI scanning.
Exclusionary Criteria
Subjects were excluded if they reported meeting any of the following criteria: a history of (1) brain surgery; (2) brain tumor, stroke, multiple sclerosis, or seizures that began prior to entering the NFL (except febrile seizures); (3) HIV or AIDS; (4) significant head injury from automobile accidents or other nonathletic-related trauma to the head resulting in loss of consciousness and/or amnesia and/or hospitalization and/or neurosurgery; (5) concussion/mild traumatic brain injury (mTBI) post–NFL career with minutes of loss of consciousness or hospitalization; (6) open heart surgery, organ transplantation, or carotid artery surgery; (7) treatment with chemotherapy or radiation therapy for cancer affecting the brain or spinal cord; (8) renal failure requiring dialysis or liver failure resulting in cirrhosis or request for liver transplantation; (9) significant alcohol abuse and/or drug abuse in the past or present manifested by having been suspended by a league, arrested for DUI, or treated in a rehabilitation facility for drug or alcohol abuse; (10) daily use of an illegal drug; and (11) daily intake of more than 4 beers or more than 2 “hard liquor” drinks during the past 5 years.10,60
History-Taking Procedures, Demographics, and Exposure-Based Information
Medical, social, and familial histories were obtained from each subject. Extensive questioning included: (1) information related to football exposure such as position(s) played, number of concussions, number of “dings” (defined as “a momentary abnormal sensation in the head occurring immediately upon head impact, with complete resolution within a few seconds and no residual effects”10), and number of years played at each level; (2) social factors unrelated to football, including post–NFL career employment history, whether public assistance was ever received, annual income, housing status, history of domestic violence incidents, educational history, history of diagnosed attention-deficit hyperactivity disorder or learning disabilities; and (3) pre-NFL details of childhood social situations, including exposure to abuse and/or violence. Additional demographic factors were recorded, including date of birth, height, and weight. All histories were taken individually and in person.
Neuroradiological Outcome Measures
Every subject underwent MRI, consisting of baseline T1, T2, T2* gradient echo, and fluid attenuated inversion recovery (FLAIR) sequences. Susceptibility weighted imaging (SWI) and diffusion tensor imaging (DTI) sequences were also taken.
Cavum Septum Pellucidi
Anatomical MRI interpretations were performed and scored by 2 professor-level, board-certified neuroradiologists who were both blinded to the clinical data on the subjects. The absence or presence of a small or large cavum septum pellucidum was recorded qualitatively for each subject. The results were coded in an ordinal fashion (0 = absent, 1 = small, 2 = large).
Fractional Anisotropy Mean
DTI data were collected with 6 gradient directions uniformly spaced on the surface of a b = 1000 s/mm2 sphere (repetition time [TR]/echo time [TE], 6500 ms/100 ms; voxel size, 2 × 2 × 3 mm3; EPI [echo planar imaging] factor, 96; time duration, 7 minutes and 43 seconds). A global white matter FA mean analysis was performed for each subject using an approach known to be sensitive to mTBI.8
SWI Microbleeds
The SWI sequence consisted of a strongly susceptibility weighted, low bandwidth (80 Hz/pixel) 3D FLASH sequence (TR/TE, 50 ms/40 ms; FA, 15°), with first-order flow compensated in all 3 orthogonal directions.72 Mean acquisition time was 7 minutes and 42 seconds. Regions analyzed included the cerebral hemispheres and posterior fossa. A neuroradiologist and MR scientist, each with more than 3 decades of clinical experience, were blinded to the specifics of this study and reviewed these data. Potential lesions were confirmed by both, and the total number and volume of these hemorrhagic lesions (microbleeds) were quantified and analyzed utilizing developed software from the MRI Institute for Biomedical Research Signal Processing for NMR [SPIN]. SWI data were coded dichotomously as either the presence or absence of microbleeds.
Neuropsychological Testing and Depression Scale Outcome Measures
Paper-and-Pencil Neuropsychological Tests
A committee of 5 members of the National Academy of Neuropsychology (3 of whom were not affiliated with the NFL or any of its teams) constructed a battery of paper-and-pencil neuropsychological tests designed to measure the following cognitive domains: verbal and visual memory, executive functioning, psychomotor speed, sustained attention, working memory, and estimated premorbid verbal IQ. These tests included the following: the Test Of Memory Malingering (TOMM)71; the Brief Visuospatial Memory Test–Revised (BVMT-R) Sum of trials 1-3 and Delayed Recall7; the California Verbal Learning Test–Second Edition (CVLT-II), including short delay and long delay free recall15; Trail Making Tests A and B (Trails A and Trails B)50; Wechsler Adult Intelligence Scale–Third Edition (WAIS-3) Digit Symbol and Letter Number Sequencing subtests77; Controlled Word Association Test (COWAT; letters FAS)39; Category Fluency (Animals); and the Wechsler Test of Adult Reading (WTAR IQ).78 The paper-and-pencil neurocognitive tests were administered and scored in accordance with standard neuropsychometric procedures by a licensed, doctoral-level clinical neuropsychologist.
Immediate Postconcussion Assessment and Cognitive Testing (ImPACT)
ImPACT (ImPACT Applications, Inc),40 a commonly used computerized neurocognitive test for neurocognitive assessment in sport-related concussion, was also administered. ImPACT was designed to measure cognitive functioning across 6 testing modules assessing attention, memory, reaction time, and processing speed. Numerical composite scores are computed for the domains of verbal memory, visual memory, visual motor (processing) speed, reaction time, and impulse control. A registered nurse supervised all computerized testing sessions.
Depression and Symptom Scales
ImPACT also contains a section for demographic information and a symptom assessment scale, which includes 22 commonly reported concussion symptoms, each rated from 0 to 6 based on severity.41 In addition, the Beck Depression Inventory–II (BDI-II),5 Patient Health Questionnaire (PHQ),42,63 and Mini-Mental State Examination (MMSE)19 were also administered to measure current self-reported depressive and other neuropsychiatric symptoms in subjects.
Statistical Analyses
Deidentified data were imported into a Microsoft Excel spreadsheet. Descriptive statistics were calculated for all demographic, structural brain imaging, and neurocognitive/symptom scores as either a mean and standard deviation (SD) or counted as n (%). Each neurocognitive test or depression/neuropsychiatric symptom score was then correlated with structural imaging data by Pearson (FA mean), point-biserial (microbleeds), and Spearman rank (cavum septum pellucidum) correlations. In instances where outcome data were nonnormally distributed (as determined by Shapiro-Wilk tests for normality), Spearman rank correlations were used. All statistics were computed at the 95% confidence interval level via the statistical software program SPSS (version 22.0; IBM Corp).25
Results
Descriptive Statistics
Demographic and exposure-based characteristics pertaining to the 45 subjects analyzed in this study can be found in Table 1.
Table 1.
Demographic characteristics of 45 retired National Football League (NFL) players a
| Characteristic | Mean (±SD) or n (%) |
|---|---|
| Age, y | 46.7 (±9.1) |
| Height, in | 75.0 (±1.8) |
| Weight, lb | 254.9 (±45.9) |
| Body mass index | 31.4 (±4.8) |
| Learning disability | 10 (22.2) |
| NFL position | |
| Quarterback | 0 (0) |
| Runningback/fullback | 2 (4) |
| Wide receiver | 2 (4) |
| Tight end | 1 (2) |
| Offensive lineman | 9 (20) |
| Defensive lineman | 9 (20) |
| Safety/cornerback | 9 (18) |
| Linebacker | 14 (32) |
| Football experience, y | |
| Pre–high school | 2.5 (±2.3) |
| High school and college | 7.7 (±1.0) |
| NFL training camp | 6.8 (±3.2) |
| Played other contact sports | 5 (11) |
| Prior sport-related concussion | |
| Football | 9.0 (±6.9) |
| NFL | 6.9 (±6.2) |
| Prior sport-related “dings” | |
| Football | 14.9 (±7.9) |
| NFL | 13.0 (±7.9) |
| Nonsport-related head injury | |
| Concussion | 0.2 (±0.4) |
| Other head injury | 0.1 (±0.3) |
| Alcohol or drug suspension | 0 (0) |
Reprinted with permission from Solomon et al.60
Correlations Among Neuroimaging Findings, Neuropsychological Test Performance, and Depression and Symptom Scores
Interpretations of the distributions of neuropsychological testing, depression, and total symptom scores have been described in detail in a previous study.10 Outcomes are reprinted in Table 2. In general, these paper-and-pencil neuropsychological test scores were normally distributed. Shapiro-Wilk tests for normality demonstrated that for 4 (33%) of the 12 scores, BVMT delay (P = 0.02), Trails B (P = 0.016), FAS (P = 0.008), and WTAR IQ (P = 0.031) did not follow a pattern of normal distribution. Further, ImPACT reaction time (P < 0.001) and impulse control (P < 0.001) composite scores were not normally distributed. None of the symptom scale scores were normally distributed and displayed positively skewed distributions (ie, the mean was greater than the median) except for MMSE, which displayed negative skewness. For these outcome measures, Spearman rank correlations were utilized.
Table 2.
Structural brain imaging, neuropsychological testing, and depression and symptom scores in 45 retired National Football League players a
| Outcome | Mean (±SD) or n (%) |
|---|---|
| Structural brain imaging | |
| Cavum septum pellucidum | |
| Absent | 10 (22) |
| Small | 32 (71) |
| Large | 3 (7) |
| SWI microbleeds | 4 (9) |
| FA mean | 0.459 (±0.035) |
| Neuropsychological testing | |
| Brief visuospatial memory test—sum | 43.0 (±10.9) |
| Brief visuospatial memory test—delayed recall | 49.4 (±11.0) |
| California Verbal Learning Test—sum | 39.1 (±9.2) |
| California Verbal Learning Test—short delay free recall | 39.5 (±10.4) |
| California Verbal Learning Test—long delay free recall | 38.4 (±8.6) |
| Trails A | 51.1 (±10.8) |
| Trails B | 46.5 (±8.0) |
| Controlled Oral Word Association Test—FAS | 47.9 (±9.0) |
| Animals | 50.2 (±9.6) |
| Wechsler Adult Intelligence Scale—Digit Span | 47.7 (±8.9) |
| Wechsler Adult Intelligence Scale—Letter Numbering Sequence | 50.6 (±8.9) |
| Wechsler Test of Adult Reading—Intelligence Quotient | 98.6 (±12.3) |
| ImPACT verbal memory | 75.9 (±12.0) |
| ImPACT visual memory | 59.0 (±14.0) |
| ImPACT visual motor processing speed | 28.6 (±8.3) |
| ImPACT reaction time | 0.77 (±0.19) |
| ImPACT impulse control | 5.70 (±6.0) |
| Depression and symptom scales | |
| Beck Depressive Inventory–II | 9.71 (±9.6) |
| Mini-Mental State Examination | 28.5 (±1.3) |
| Patient Health Questionnaire | 26.5 (±19.9) |
| ImPACT Total Symptom Score | 20.0 (±21.3) |
FA, fractional anisotropy; ImPACT, Immediate Post-Concussion Assessment and Cognitive Testing; SWI, susceptibility weighted image; Trails A and B, Trail Making Tests A and B.
Reprinted with permission from Solomon et al.60
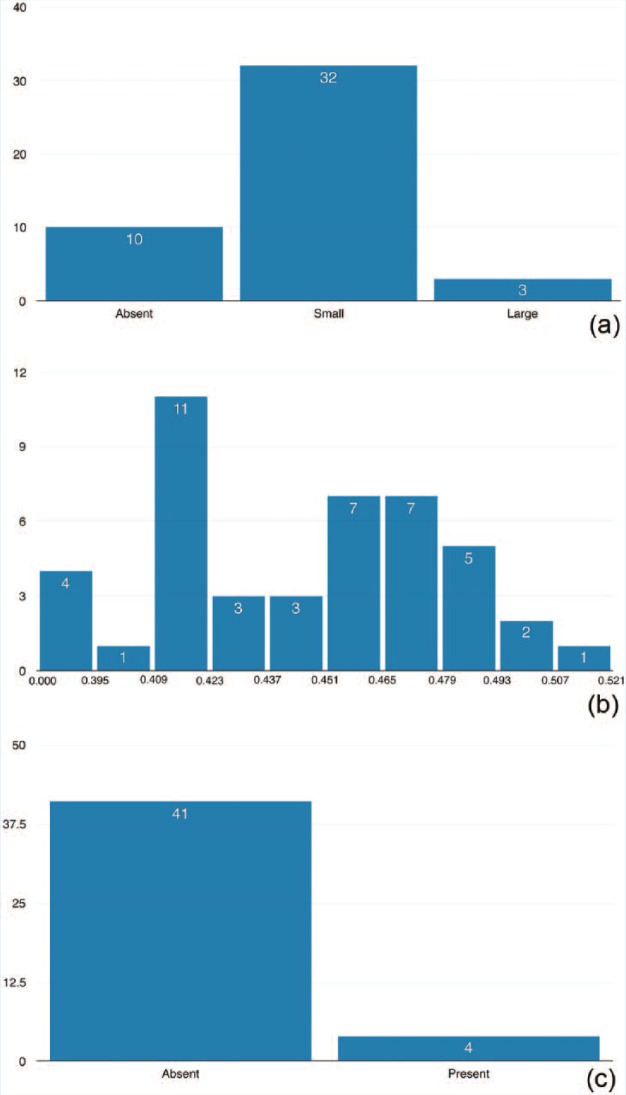
In terms of neuroradiological findings, 10 subjects (22%) did not have a cavum septum pellucidum, 32 (71%) had a small one, and 3 (7%) presented with a large one. Four (9%) of the subjects had microbleeds present, and average FA mean was 0.459 (±0.035). For 1 subject, FA mean was discarded for technical reasons, thus these correlations had 1 less degree of freedom (n = 44). The distributions of neuroimaging results are presented in Figure 1. Interpretations of the distribution of these neuroimaging findings have been described in a previous study.10
Figure 1.
Distribution of neuroimaging biomarkers of (a) cavum septum pellucidum, (b) fractional anisotropy, and (c) microbleeds in 45 retired National Football League players.
None of the correlations among neuroimaging and neurocognitive functioning reached a conventional level of statistical significance (Table 3). A post hoc adjustment for multiple comparisons was not needed as none of the values reached the a priori level of statistical significance (P = 0.05).
Table 3.
Overall correlations among structural brain imaging findings, neuropsychological testing performance, depressive and total symptom scores in retired National Football League athletes a
| Structural Brain Imaging |
|||
|---|---|---|---|
| SWI Microbleeds (n = 45) | FA Mean (n = 44) | Cavum Septum Pellucidum (n = 45) | |
| Neuropsychological testing | |||
| Brief visuospatial memory test sum | −0.077 (0.617) | 0.098 (0.528) | −0.043 (0.779) |
| Brief visuospatial memory test—delayed recall | −0.123 (0.419) | 0.011 (0.941) | 0.068 (0.657) |
| California Verbal Learning Test—sum | −0.236 (0.119) | −0.250 (0.101) | −0.172 (0.259) |
| California Verbal Learning Test—short delay free recall | −0.128 (0.403) | −0.178 (0.247) | −0.176 (0.247) |
| California Verbal Learning Test—long delay free recall | −0.113 (0.458) | −0.212 (0.168) | −0.154 (0.312) |
| Trails A | 0.035 (0.822) | 0.043 (0.780) | 0.042 (0.783) |
| Trails B | −0.021 (0.891) | 0.053 (0.732) | −0.077 (0.617) |
| Controlled Oral Word Association Test—FAS | 0.247 (0.102) | 0.146 (0.343) | 0.125 (0.412) |
| Animals | −0.048 (0.755) | 0.039 (0.803) | 0.089 (0.561) |
| Wechsler Adult Intelligence Scale—Digit Span | 0.020 (0.896) | 0.138 (0.371) | 0.196 (0.196) |
| Wechsler Adult Intelligence Scale—Letter Numbering Sequence | −0.046 (0.762) | 0.071 (0.647) | −0.110 (0.471) |
| Wechsler Test of Adult Reading—Intelligence Quotient | 0.027 (0.860) | −0.003 (0.982) | −0.106 (0.487) |
| ImPACT verbal memory | −0.235 (0.120) | −0.135 (0.381) | −0.108 (0.482) |
| ImPACT visual memory | 0.045 (0.768) | 0.033 (0.831) | −0.101 (0.508) |
| ImPACT visual motor processing speed | 0.063 (0.679) | 0.151 (0.329) | 0.011 (0.943) |
| ImPACT reaction time | 0.093 (0.542) | −0.014 (0.930) | −0.082 (0.593) |
| ImPACT impulse control | 0.024 (0.875) | −0.014 (0.926) | −0.109 (0.475) |
| Depression and symptom scales | |||
| Beck Depressive Inventory–II | 0.009 (0.953) | 0.048 (0.757) | 0.103 (0.502) |
| Mini-Mental State Examination | −0.257 (0.089) | −0.218 (0.156) | −0.132 (0.388) |
| Patient Health Questionnaire | 0.175 (0.251) | 0.231 (0.131) | 0.173 (0.256) |
| ImPACT Total Symptom Score | 0.106 (0.490) | 0.028 (0.855) | 0.056 (0.715) |
FA, fractional anisotropy; ImPACT, Immediate Post-Concussion Assessment and Cognitive Testing; SWI, susceptibility weighted image; Trails A and B, Trail Making Tests A and B.
Data are presented as r/rs (P value).
Cohen delineated correlative r values of 0.10, 0.30, and 0.50 as “small,” “medium,” and “large” effect sizes, respectively.12 According to these values, the majority (50.8%) of the correlations obtained in this study were below the threshold of a small effect size. The remaining (49.2%) fell in between small and medium effect sizes (Figure 2). While these reference values have served as the gold standard across many clinical health disciplines, effect sizes should always be interpreted in accordance with the measurements taken, data analyzed, and clinical importance of the study.16 In many instances, another understanding of effect size is the r2 value, which calculates the amount of variance observed in the outcome variable accounted for by the (independent) predictor variable. In the current study, the presence or absence of microhemmorhages accounted for between 0.04% and 6.10% of the variance observed across neurocognitive functioning and between 0.008% and 6.53% of the variance seen in reported symptoms. FA global mean values accounted for between 0.0009% and 6.25% of the variance observed across neurocognitive functioning and between 0.078% and 5.33% of the variance seen in reported symptoms. Last, the absence or presence of a small or a large cavum septum pellucidum accounted for between 0.17% and 3.84% of the variance observed across ranked neurocognitive functioning and between 0.31% and 2.99% of the variance observed in ranked reported symptoms. For the purposes of contextual reference, an independent variable accounting for 1% to 9% of the variance observed in the dependent variable could be classified as small to medium effect size.12
Figure 2.
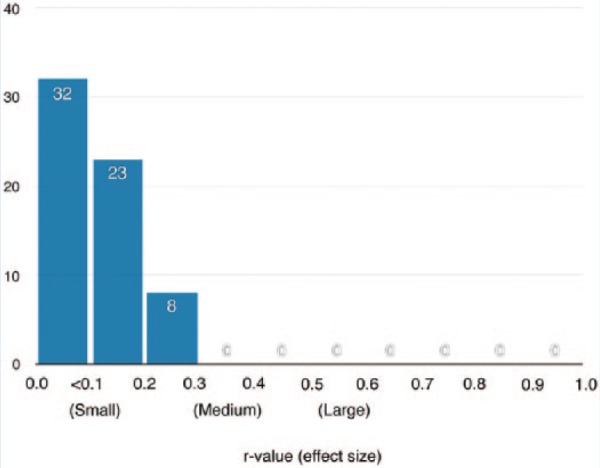
Frequency of absolute correlative r values and Cohen effect sizes among the 3 neuroimaging biomarkers and 63 neuropsychological testing and symptom endorsement outcome measures analyzed.
Discussion
Assessment of cavum septum pellucidum, FA global mean score, and microbleed data yielded no significant associations with several measure of neurocognitive functioning, including 9 paper-and-pencil tests (yielding 12 scores), a computerized neuropsychological test (yielding 5 scores), and 4 scales of depressive and other neuropsychiatric symptoms.
Cavum Septum Pellucidum
The prevalence of cavum septum pellucidum ranges widely, from as low as 0.73% to as high as 60.3% in pediatric and adults populations because of selection bias and different imaging and measuring techniques.9,18,37,48,51,53,57,58,70 However, this structural abnormality has been commonly associated with conditions such as schizophrenia, bipolar disorder, seizures, developmental/intellectual disabilities, stroke and cranial nerve abnormalities, and head trauma.31,51,53 After the publication of the classic 1973 neuropathological description of the cardinal features of chronic traumatic encephalopathy (CTE) in boxers by Corsellis et al,13 it is widely accepted that abnormalities of the septal region (large cavum septum pellucidum) can serve as structural hallmarks of CTE. Imaging studies have subsequently revealed that cavum septum pellucidum is highly prevalent in retired boxers.9,45 Recent neuropathology studies have reported large cavum septum pellicidum in some, but not all, cases of putative CTE in American football players.45 In a case-control study, retired professional football players had cavum septum pellucidum more frequently with a significantly higher grade and measured length than their age-/sex-matched counterparts.20 However, the current analysis yielded only 7% of players having a large cavum septum pellucidum. Regardless, this structural abnormality and its direct implications in neurobehavioral changes and neuropsychiatric functioning in retired football players remains to be clarified.
The most recent exploration on the role of septal abnormalities was in 2016; 72 former NFL players (age range, 40-69 years) were compared on neuroradiological and neuropsychological testing data to 14 unmatched former athlete controls who had played at least 2 years of noncontact sports at the collegiate level and had no history of concussion.33 After controlling for confounders (body mass index, age, years of education, and years playing football), cavum septum pellucidum was more frequent in the NFL group versus controls. In addition, the mean length of cavum septum pellucidum was greater in the NFL group than the control group. The results of cavum septum pellucidum length in the NFL group were then correlated with behavioral measures. The cavum septum pellucidum length was dichotomized into 2 groups (≥6 mm and ≤2 mm), and those in the ≥6-mm group displayed lower scores on 2 neurocognitive tests (NAB List A Immediate Recall and WRAT-4 Reading Test). Between-group differences across the other 22 outcome measures (91.7%) were nonsignificant. Effect sizes were not reported. The authors concluded that cavum septum pellucidum was associated with lower verbal memory and word pronunciation, but that true clinical significance is difficult to gauge without effect sizes.33 In addition, the WRAT-4 is most often used as a measure of premorbid cognitive ability, and its appropriateness in assessing the potential long-term effects of RBT is questionable.36
The results of the current study lend credence to the hypothesis that the ordinal ranking of cavum septum pellucidum may contribute only minimally to performance on a comprehensive battery of neurocognitive and neurobehavioral measures in retired NFL players. However, given the variable findings previously, future study is warranted.
Fractional Anisotropy
FA provides an index of white matter structure and integrity by measuring directionality of water motion.29,49 FA is sensitive to the acute phases of mTBI and has utility in studying the intermediate- and long-term structural effects of RBT.17 In patients without a history of head trauma (mean age, 27.5 years), global FA mean was 0.431.8 While direct comparisons are difficult to gauge because of possible age-related differences, in this cohort of retired NFL athletes, global FA mean was measured as 0.459 and significantly associated with number of NFL concussions.10
A small preliminary study of younger athletes who had sustained RBT found that white matter changes, while evident, were not correlated with cognitive test performance. Ten Division III collegiate football players and 5 nonathlete controls were examined during the preseason, immediate postseason, and 6 months after no-contact rest in the postseason.4 Decreased FA was observed between preseason and postseason and correlated with RBT, but white matter changes were not consistently associated with cognition or balance, as assessed by a computerized neurocognitive test (ImPACT) and the Balance Error Scoring System (BESS), respectively. While this is an important pilot study, the results are limited by sample size and a small battery of measurement instruments such that generalizing to a larger group would be premature. Similarly, 16 university ice hockey players with a history of concussion were compared with 18 players without a history of concussion and it was found that in the concussed group, there were specific cerebral regions (right posterior limb of the internal capsule, right corona radiata, and right temporal lobe) with significant increases in FA, which were not found in the nonconcussed athletes.56 However, these imaging features did not correlate with computerized neurocognitive (ImPACT) or Sport Consussion Assessment Tool–2 (SCAT2) scores (the latter including measures of balance and cognition, as well as symptoms).
When examining older, retired athletes, as in the current study, correlations have been found between FA and neurocognitive functioning. In a cohort of 26 retired NFL athletes, 5 had depression on BDI-II and 22 controls had FA data with BDI-II scores correlated.65 There were no significant differences in FA between healthy controls and nondepressed retired athletes. In the entire 26-athlete cohort, 4 white matter tracts (forceps minor, right uncinate fasciculus, right frontal aslant tract, and left superior longitudinal fasciculus) displayed depressed FA mean values that were significantly associated with increased total BDI-II scores. The 16 other (80%) white matter tracts displayed negative correlations with BDI-II scores but were statistically nonsignificant. In a separate cross-sectional analysis, 34 retired NFL athletes were compared with age-matched (mean age, 61.8 years) and estimated IQ-matched controls.23 The symptomatic retired athletes demonstrated widely distributed reductions of FA in frontal and parietal regions bilaterally as well as along the corpus callosum and in the left temporal lobe. Comparisons were made between cognitively impaired athletes (n = 10) and matched controls (n = 10) as well as between depressed athletes (n = 6) and matched controls (n = 6). Both comparisons showed widely distributed voxels with lower FA in the symptomatic (cognition or mood) athlete groups. Retired NFL players without cognitive impairment or depression did not demonstrate white matter abnormalities compared with controls. However, common to most studies analyzing retired NFL players, this study was small.
Last, 15 retired collegiate athletes (mean age, 60.9 years) with a history of concussion and who were otherwise clinically normal (no other comorbidities) were examined and compared with 15 age- and education-matched controls.74 The majority (70%) of these were former ice hockey players and the remainder (30%) were American football players, all university-level. The concussion group had decreases in FA in the frontoparietal networks and the frontal aspects of the corpus callosum. These white matter abnormalities were significantly associated with a decrease in episodic visual memory (delayed recall of the Taylor Complex Figure Test). They suggested that their results fit better with changes seen in normal aging, and that perhaps a history of concussion may cause these effects to be expedited, concluding, “the nature of interaction between ageing and a history of concussions involves a latent microstructural injury that leaves the brain more vulnerable to the deleterious effects of ageing.”74
In this analysis, FA global mean values accounted for 0.0009% to 6.25% of the variance observed across neurocognitive test performance and between 0.078% and 5.33% of the variance seen in reported symptoms. While rendering the strongest correlation across all outcome measures with CVLT-II sum of trials (r = −0.250, P = 0.101), this relationship was statistically nonsignificant and did not reach the traditional definition of medium effect size. Research supports a relationship between RBT and chronic white matter structural changes; however, its direct relationship to neurobehavioral changes and neuropsychiatric disorders remains limited, and at present, is without a proven causative relationship.
Microhemorrhages
In healthy adults (age range, 52.9-64.4 years), the prevalence of cerebral microhemorrhaging ranges from 3.1% to 6.4%,28,55,75 but is reported as high as 56.0% in patients who are hypertensive.38,76 While microhemorrhages or microbleeds can be attributed to amyloid angiopathy and/or hypertension, in otherwise healthy patients younger than 60 years, it is more likely the result of head trauma with a rotational shear mechanism of injury.3,10,21,32 A prior study examining these same retired NFL athletes reported that the presence of microbleeds was low (9%) but positively correlated with reported “number of dings” (“abnormal sensation in the head occurring immediately upon head impact, with complete resolution within a few seconds and no residual effects”).10 There have been higher rates of cerebral microhemorrhaging reported in boxers when compared with control subjects, yet these comparisons have rendered statistically nonsignificant results.22,24 In the current study, the presence of microbleeds was not associated with any neurocognitive test performance or neuropsychiatric symptom outcome measure and accounted for between 0.008% and 6.53% of the variance observed across these measures.
Direction for Future Study
Assessment of correlations among 3 structural imaging parameters with multiple indices of neurocognitive functioning and symptoms in a group of retired NFL players yielded nonsignificant results. Future study is needed to elucidate more completely the relationships among structural imaging, neurocognitive test performances, and neurobehavioral functioning in retired athletes exposed to RBT and in those with documented brain trauma. It may be the case that RBT can have a cumulative effect on some structural anatomical parameters, but these changes in relation to neurocognitive and neurobehavioral functioning remain unclear. Conceivably, comorbid health conditions and lifestyle factors may be moderating variables. Given the nature of studying retired professional athletes, most studies have suffered from small sample sizes, varying ages of subjects, and sample convenience. Age is a critical factor in metabolic and structural brain changes and must be taken into account when evaluating the results of various studies. Furthermore, caution must be employed in the interpretation of conclusions published, as some conclusions have been proclaimed as significant yet perhaps overgeneralized (ie, where a single neuropsychological test is deemed to be representative of an entire cognitive domain) when the results obtained may have in actuality been due to a variety of other factors not limited to imprecision of the measures utilized and/or by chance of multiple testing. For instance, across a large battery of neuropsychological tests, statistical significance might be obtained with only 1 or a small proportion of tests or subtests.33 Additionally, in this study, “total symptom score” was used as an outcome measure when others have suggested that a 2- or 3-factor analysis approach to symptom scores should be used.30,52
The current study is not without other limitations. This was a cross-sectional study of a relatively large NFL sample (45 subjects) but did not include a control group. In addition, the subjects in the current study were younger than 60 years, and therefore, these results cannot be generalized to retired NFL athletes older than 60 years. Although strict inclusion and exclusion criteria were utilized, subjects were not selected randomly, and the final sample was one of convenience, indicating selection bias as a possible confounder. In addition, variance in the structural parameters may have been truncated, as only 4 (9%) of the 45 players had microhemmorhages present. Although a shortcoming of most studies of sport-related concussion, concussion histories and other historical data were self-reported and not documented or corroborated by medical records. Only 3 neuroimaging outcome measures were examined (1 of which was recorded qualitatively), and other structural neuroimaging biomarkers implicated in RBT in prior studies (eg, lateral ventricle, hippocampal, and cortical volumes46,59,67,73) may be of greater value in assessing these presumed brain-behavior relationships. Additionally, there were 2 neuroradiologists who made neuroanatomical measurements. While they agreed on all measurements of cavum septum pellicidum, a formal interrater reliability procedure or calculation was not conducted. Finally, while nonsignificant, these results are measures of correlation and not causation, and should be taken in that light.
Conclusion
In a relatively large sample of retired NFL players, across 9 paper-and-pencil neuropsychological tests (yielding 12 measures), a computerized neuropsychological test (yielding 5 composite scores), and 4 scales of mood and other neuropsychiatric functions, the MRI measures of cavum septum pellucidum, FA mean, and microhemorrhaging accounted for between 0.0009% and 6.53% of the variance observed. While a well-respected and often-utilized paradigm, concluding that in vivo structural brain changes are the direct cause of specific neuropsychiatric symptomatology after chronic RBT is a complex endeavor and requires further study.
Future efforts in disentangling the relationships among neuroimaging parameters and neurocognitive functioning will require prospective studies of larger samples of retired professional football players across various age ranges, with clear inclusion/exclusion criteria; multimodal and comprehensive neuroimaging, neurocognitive, and neuropsychiatric test measures; access to actual historical concussion and medical records data; thorough personal biopsychosocial data; family history information; and perhaps genetic markers. Although highly aspirational, definitive conclusions will require studies of this magnitude and depth to address the matter empirically.
Acknowledgments
Examinations of the NFL retirees were funded by the National Football League (NFL) and coordinated with the NFL Players Association (NFLPA).
Footnotes
The following authors declared potential conflicts of interest: Gary S. Solomon, PhD, receives consulting fees from the Tennessee Titans (NFL), the Nashville Predators (NHL), and the athletic departments of several universities. In addition, he is a member of the ImPACT Scientific Advisory Board, receives reimbursement for expenses to board meetings, and also receives royalties from book sales. Ira R. Casson, MD, and David C. Viano, Dr Med, PhD, served as members of the NFL MTBI Committee from 1994 to 2009, and as co-chairs from 2007 to 2009. Dr. Casson has done consulting for law firms and insurance companies.
References
- 1. Abbas K, Shenk TE, Poole VN, et al. Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting-state functional magnetic resonance imaging study. Brain Connect. 2015;5:91-101. [DOI] [PubMed] [Google Scholar]
- 2. Antonius D, Mathew N, Picano J, et al. Behavioral health symptoms associated with chronic traumatic encephalopathy: a critical review of the literature and recommendations for treatment and research. J Neuropsychiatry Clin Neurosci. 2014;26:313-322. [DOI] [PubMed] [Google Scholar]
- 3. Ayaz M, Boikov AS, Haacke EM, Kido DK, Kirsch WM. Imaging cerebral microbleeds using susceptibility weighted imaging: one step toward detecting vascular dementia. J Magn Reson Imaging. 2010;31:142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bazarian JJ, Zhu T, Zhong J, et al. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS One. 2014;9:e94734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2nd ed. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 6. Belanger HG, Vanderploeg RD, McAllister T. Subconcussive blows to the head: a formative review of short-term clinical outcomes. J Head Trauma Rehabil. 2016;31:159-166. [DOI] [PubMed] [Google Scholar]
- 7. Benedict R. Brief Visuospatial Memory Test–Revised. Lutz, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 8. Benson RR, Meda SA, Vasudevan S, et al. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury.J Neurotrauma. 2007;24:446-459. [DOI] [PubMed] [Google Scholar]
- 9. Bogdanoff B, Natter HM. Incidence of cavum septum pellucidum in adults: a sign of boxer’s encephalopathy. Neurology. 1989;39:991-992. [DOI] [PubMed] [Google Scholar]
- 10. Casson IR, Viano DC, Haacke EM, Kou Z, LeStrange DG. Is there chronic brain damage in retired NFL players? Neuroradiology, neuropsychology, and neurology examinations of 45 retired players. Sports Health. 2014;6:384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castellani RJ, Perry G, Iverson GL. Chronic effects of mild neurotrauma: putting the cart before the horse? J Neuropathol Exp Neurol. 2015;74:493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 13. Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3:270-303. [DOI] [PubMed] [Google Scholar]
- 14. DeLamielleure J. Concussion in the National Football League: viewpoint of an elite player. J Law Med Ethics. 2014;42:133-134. [DOI] [PubMed] [Google Scholar]
- 15. Delis DCK, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2nd ed. San Antonio, TX: Psychological Corportation; 2000. [Google Scholar]
- 16. Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34:917-928. [DOI] [PubMed] [Google Scholar]
- 17. Eierud C, Craddock RC, Fletcher S, et al. Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 2014;4:283-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finke J, Koch G. Das cavi septi pellucidi: vorkommen und aussagewert: bericht uber 128 falle. Deutsch Z Nervenheilk. 1968;193:154-157. [PubMed] [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. Mini-Mental State Examination: Clinical Guide. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 20. Gardner RC, Hess CP, Brus-Ramer M, et al. Cavum septum pellucidum in retired American pro-football players. J Neurotrauma. 2016;33:157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haacke EM, DelProposto ZS, Chaturvedi S, et al. Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2007;28:316-317. [PMC free article] [PubMed] [Google Scholar]
- 22. Hahnel S, Stippich C, Weber I, et al. Prevalence of cerebral microhemorrhages in amateur boxers as detected by 3T MR imaging. AJNR Am J Neuroradiol. 2008;29:388-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hart J, Jr, Kraut MA, Womack KB, et al. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 2013;70:326-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hasiloglu ZI, Albayram S, Selcuk H, et al. Cerebral microhemorrhages detected by susceptibility-weighted imaging in amateur boxers. AJNR Am J Neuroradiol. 2011;32:99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. IBM SPSS Statistics [computer program]. Version 22: IBM Corp; 2013. [Google Scholar]
- 26. Iverson GL. Chronic traumatic encephalopathy and risk of suicide in former athletes. Br J Sports Med. 2014;48:162-165. [DOI] [PubMed] [Google Scholar]
- 27. Iverson GL, Gardner AJ, McCrory P, Zafonte R, Castellani RJ. A critical review of chronic traumatic encephalopathy. Neurosci Biobehav Rev. 2015;56:276-293. [DOI] [PubMed] [Google Scholar]
- 28. Jeerakathil T, Wolf PA, Beiser A, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35:1831-1835. [DOI] [PubMed] [Google Scholar]
- 29. Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239-254. [DOI] [PubMed] [Google Scholar]
- 30. Joyce AS, Labella CR, Carl RL, Lai JS, Zelko FA. The Postconcussion Symptom Scale: utility of a three-factor structure. Med Sci Sports Exerc. 2015;47:1119-1123. [DOI] [PubMed] [Google Scholar]
- 31. Kim MJ, Lyoo IK, Dager SR, et al. The occurrence of cavum septi pellucidi enlargement is increased in bipolar disorder patients. Bipolar Disord. 2007;9:274-280. [DOI] [PubMed] [Google Scholar]
- 32. Kirsch W, McAuley G, Holshouser B, et al. Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J Alzheimers Dis. 2009;17:599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koerte IK, Hufschmidt J, Muehlmann M, et al. Cavum septi pellucidi in symptomatic former professional football players. J Neurotrauma. 2016;33:346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koerte IK, Mayinger M, Muehlmann M, et al. Cortical thinning in former professional soccer players. Brain Imaging Behav. 2016;10:792-798. [DOI] [PubMed] [Google Scholar]
- 35. Korngold C, Farrell HM, Fozdar M. The National Football League and chronic traumatic encephalopathy: legal implications. J Am Acad Psychiatry Law. 2013;41:430-436. [PubMed] [Google Scholar]
- 36. Larrabee GJ, Rohling ML, Binder LM. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology. 2015;85:1007-1008. [PubMed] [Google Scholar]
- 37. Larroche JC, Baudey J. Cavum septi lucidi, cavum vergae, cavum veli interpositi: cavities of the median line. Anatomical and pneumoencephalographic study in the neonatal period [in French]. Biol Neonat. 1961;3:193-236. [PubMed] [Google Scholar]
- 38. Lee SH, Bae HJ, Ko SB, Kim H, Yoon BW, Roh JK. Comparative analysis of the spatial distribution and severity of cerebral microbleeds and old lacunes. J Neurol Neurosurg Psychiatry. 2004;75:423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lezak M. Controlled Word Association Test (COWAT). 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 40. Lovell MR. ImPACT 2007 (6.0) Clinical Interpretation Manual. Pittsburgh, PA: University of Pittsburgh Medical Center; 2007. [Google Scholar]
- 41. Lovell MR, Iverson GL, Collins MW, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13:166-174. [DOI] [PubMed] [Google Scholar]
- 42. Lowe B, Kroenke K, Herzog W, Grafe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9). J Affect Disord. 2004;81:61-66. [DOI] [PubMed] [Google Scholar]
- 43. Martini D, Eckner J, Kutcher J, Broglio SP. Subconcussive head impact biomechanics: comparing differing offensive schemes. Med Sci Sports Exerc. 2013;45:755-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCrory P. Cavum septi pellucidi—a reason to ban boxers? Br J Sports Med. 2002;36:157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mez J, Solomon TM, Daneshvar DH, Stein TD, McKee AC. Pathologically confirmed chronic traumatic encephalopathy in a 25-year-old former college football player. JAMA Neurol. 2016;73:353-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitsis EM, Riggio S, Kostakoglu L, et al. Tauopathy PET and amyloid PET in the diagnosis of chronic traumatic encephalopathies: studies of a retired NFL player and of a man with FTD and a severe head injury. Transl Psychiatry. 2014;4:e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakanno S, Hojo H, Kataoka K, Yamasaki S. Age related incidence of cavum septum pellucidum and cavum vergae on CT scans of pediatric patients. J Comput Assist Tomogr. 1981;5:348-349. [DOI] [PubMed] [Google Scholar]
- 49. Orr CA, Albaugh MD, Watts R, et al. Neuroimaging biomarkers of a history of concussion observed in asymptomatic young athletes. J Neurotrauma. 2016;33:803-810. [DOI] [PubMed] [Google Scholar]
- 50. Partington JE, Leiter RG. Partington’s Pathway Test. Psychol Serv Cntr Bull. 1949;1:9-20. [Google Scholar]
- 51. Pauling KJ, Bodensteiner JB, Hogg JP, Schaefer GB. Does selection bias determine the prevalence of the cavum septi pellucidi? Pediatr Neurol. 1998;19:195-198. [DOI] [PubMed] [Google Scholar]
- 52. Potter S, Leigh E, Wade D, Fleminger S. The Rivermead Post Concussion Symptoms Questionnaire: a confirmatory factor analysis. J Neurol. 2006;253:1603-1614. [DOI] [PubMed] [Google Scholar]
- 53. Rajarethinam R, Miedler J, DeQuardo J, et al. Prevalence of cavum septum pellucidum in schizophrenia studied with MRI. Schizophr Res. 2001;48:201-205. [DOI] [PubMed] [Google Scholar]
- 54. Robinson ME, Shenk TE, Breedlove EL, Leverenz LJ, Nauman EA, Talavage TM. The role of location of subconcussive head impacts in FMRI brain activation change. Dev Neuropsychol. 2015;40:74-79. [DOI] [PubMed] [Google Scholar]
- 55. Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52:991-994. [DOI] [PubMed] [Google Scholar]
- 56. Sasaki T, Pasternak O, Mayinger M, et al. Hockey Concussion Education Project, Part 3. White matter microstructure in ice hockey players with a history of concussion: a diffusion tensor imaging study. J Neurosurg. 2014;120:882-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schunk H. Congenital dilatations of the septum pellucidum. Radiology. 1963;81:610-618. [DOI] [PubMed] [Google Scholar]
- 58. Shaw CM, Alvord EC., Jr Cava septi pellucidi et vergae: their normal and pathogical states. Brain. 1969;92:213-223. [DOI] [PubMed] [Google Scholar]
- 59. Singh R, Meier TB, Kuplicki R, et al. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA. 2014;311:1883-1888. [DOI] [PubMed] [Google Scholar]
- 60. Solomon GS, Kuhn AW, Zuckerman SL, et al. Participation in pre-high school football and later life neurological, neuroradiological, and neuropsychological findings: a study of 45 retired NFL players. Am J Sports Med. 2016;44:1106-1115. [DOI] [PubMed] [Google Scholar]
- 61. Solomon GS, Sills A. Chronic traumatic encephalopathy and the availability cascade. Phys Sportsmed. 2014;42(3):26-31. [DOI] [PubMed] [Google Scholar]
- 62. Solomon GS, Zuckerman SL. Chronic traumatic encephalopathy in professional sports: retrospective and prospective views. Brain Inj. 2015;29:164-170. [DOI] [PubMed] [Google Scholar]
- 63. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737-1744. [DOI] [PubMed] [Google Scholar]
- 64. Stamm JM, Bourlas AP, Baugh CM, et al. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology. 2015;84:1114-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Strain J, Didehbani N, Cullum CM, et al. Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology. 2013;81:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Strain JF, Womack KB, Didehbani N, et al. Imaging correlates of memory and concussion history in retired National Football League athletes. JAMA Neurol. 2015;72:773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sundman M, Doraiswamy PM, Morey RA. Neuroimaging assessment of early and late neurobiological sequelae of traumatic brain injury: implications for CTE. Front Neurosci. 2015;9:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Terry DP, Adams TE, Ferrara MS, Miller LS. FMRI hypoactivation during verbal learning and memory in former high school football players with multiple concussions. Arch Clin Neuropsychol. 2015;30:341-355. [DOI] [PubMed] [Google Scholar]
- 70. Thieffry S, Lefebvre J, Leprintre MJ, Faure C, Masselin MS. Contribution a L’etude radiologique des malformations du plan sagittal interhemispherique a propos de 45 observations. Acta Radiol. 1958;50:242-252. [DOI] [PubMed] [Google Scholar]
- 71. Tombaugh TN. TOMM, Test of Memory Malingering. North Tonawanda, NY: Multi-Health Systems; 1996. [Google Scholar]
- 72. Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. 2003;227:332-339. [DOI] [PubMed] [Google Scholar]
- 73. Tremblay S, De Beaumont L, Henry LC, et al. Sports concussions and aging: a neuroimaging investigation. Cereb Cortex. 2013;23:1159-1166. [DOI] [PubMed] [Google Scholar]
- 74. Tremblay S, Henry LC, Bedetti C, et al. Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain. 2014;137:2997-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsushima Y, Tanizaki Y, Aoki J, Endo K. MR detection of microhemorrhages in neurologically healthy adults. Neuroradiology. 2002;44:31-36. [DOI] [PubMed] [Google Scholar]
- 76. Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke. 2006;37:550-555. [DOI] [PubMed] [Google Scholar]
- 77. Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed (WAIS-III). San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 78. Wechsler D. Wechsler Test of Adult Reading (WTAR). San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]