Abstract
Purpose
To examine maternal smoking and body mass index (BMI) interactions in contributing to risk of oral clefts.
Methods
We studied 4935 cases and 10,557 controls from 6 population-based studies and estimated a pooled logistic regression of individual-level data, controlling for study fixed effects and individual-level risk factors.
Results
We found a significant negative smoking-BMI interaction, with cleft risk with smoking generally declining with higher BMI. For all clefts combined, the OR for smoking was 1.61 (95% CI: 1.39–1.86) at BMI 17 (underweight), 1.47 (95% CI: 1.34–1.62) at BMI 22 (normal weight), 1.35 (95% CI: 1.22–1.48) at BMI 27 (overweight), 1.21 (95% CI: 1.04–1.41) at BMI 33 (obese), and 1.13 (95% CI: 0.92–1.38) at BMI 37 (very obese). A negative interaction was also observed for isolated clefts and across cleft types but was more pronounced for cleft lip only and cleft palate only.
Conclusions
Our findings suggest that the risk of oral clefts associated with maternal smoking is largest among underweight mothers, although the smoking-BMI interaction is strongest for cleft lip only and cleft palate only. BMI was not protective for the effects of smoking; a clinically relevant increase in smoking-related cleft risk was still present among heavier women.
Keywords: Cleft lip, cleft palate, smoking, obesity, body weight, body mass index
Introduction
Oral clefts continue to be one of the most prevalent birth defects, affecting close to 1/700 births on average. Of all the environmental factors that might contribute to the risk of oral clefts, maternal smoking is the most well established (1) and was considered a causal factor for oral clefts in the 2014 US Surgeon General’s Report (2). Other factors such as body mass index (BMI) have also been supported in recent studies. Another consistent risk factor is BMI. Obese mothers have elevated risks of children with oral clefts compared with normal-weight mothers (3).
No prior study has examined whether the risk of oral clefts associated with smoking is moderated by BMI. There are several reasons why such an interaction may exist, although the direction of the interaction is theoretically ambiguous. On one hand, each of these risk factors may reinforce the effects of the other. Smoking may exacerbate metabolic problems (4, 5), while maternal obesity may modify the activity of drug metabolizing enzymes such as CYP1A1 (6). Furthermore, both maternal smoking and obesity increase the risk of placental insufficiency (7–9). Even though the placenta is not fully functional until after clefts form, early synergetic and adverse effects of these risk factors on placental function could potentially exacerbate the risk of clefts.
On the other hand, carrying one risk factor may simply reduce the relative importance of the other for cleft risk. For instance, the added risk of clefts due to smoking may be lower among obese mothers who themselves have a higher risk for clefting due to metabolic problems. There may also be mechanistic interactions that offset the risk of one factor in the presence of the other. Among smokers, the interactions between lipophilic carcinogens and DNA (DNA adducts) were reported to be lower with higher BMI (10). One may also hypothesize that, all else being equal, higher BMI provides a greater fat tissue volume for storing cigarette chemicals such as PAHs or dioxins, thus potentially delaying fetal exposure to their metabolites during the critical first few weeks of pregnancy when clefts form. Some support for an offsetting interaction in another context comes from observational studies reporting a lower lung cancer risk among smokers with increasing BMI (11). Similarly, other studies reported that smoking was associated with an elevated risk of postmenopausal breast cancer only in non-obese but not in obese women (12), and that smoking was related to higher cancer-related mortality among underweight young women than among women with higher weight (13).
We examined the interaction between maternal smoking and pre-pregnancy BMI as they influence the risk of oral clefts. Using data from a large international consortium of six population-based case-control studies, we evaluated whether the association of first-trimester smoking with oral clefts varies by BMI. Understanding this heterogeneity may help to accurately quantify the contributions of these risk factors to oral clefts.
Methods
Data
Our study combined samples from six population-based studies of oral clefts. Each study provided a sample of cases with oral clefts and controls and included detailed data on environmental risk factors during the first trimester through maternal interviews(1). Together the studies provide a sample of 4935 cases and 10557 controls. We did not exclude any case enrolled in the participating studies based on whether the cleft was isolated or non-isolated (occurring with other birth defects or syndromes), although alternative analyses focused on isolated clefts as described below. Cases with known syndromes were not enrolled in the NBDPS.
The Iowa Child Health Study (ICHS) identified cases with clefts through active, state-wide surveillance of births by the Iowa Registry for Congenital and Inherited Disorders between 1987 and 1991 and randomly selected non-malformed controls from Iowa birth certificates during the same time period providing a total sample of 280 cases and 293 controls (14). The Utah Child and Family Health Study (UCFHS) identified cases through state-wide surveillance and randomly selected controls from Utah birth certificates between 1995 and 2004 providing a total analytical sample of 557 cases and 658 controls (15). The National Birth Defects Prevention Study (NBDPS) identified cases through surveillance programs in 10 US states between 1997 and 2007 and randomly selected controls from birth certificates, providing a total analytical sample of 3277 cases and 7834 controls (16). Cases with clefts from Utah were enrolled beginning in 2005, with no overlap between the NBDPS and UCFHS. The Norway Facial Clefts Study (NFCS) identified cases with clefts born between 1996 and 2001 through national registries from centralized cleft repair centers. Controls were randomly selected from all Norwegian births in the same period, providing a total analytical sample of 559 cases and 754 controls (17). The Norwegian National Mother and Child Cohort Study (MoBa) enrolled a population-based cohort of about 100,000 pregnancies in 1999–2009 (18), from which births with clefts were selected as well as a random sample of controls for a total analytical sample of 280 cases and 293 controls. The Danish National Birth Cohort (DNBC) is also a population-based sample of 100,000 pregnancies from Denmark in 1997–2003, providing an analytical sample of 123 cases and 592 controls (19, 20).
Outcomes and Risk Factors
Because smoking has been shown to be related to the three cleft types (cleft lip, cleft lip with palate, and cleft palate only) both combined and individually (1), we first examined any cleft including isolated and non-isolated cases (i.e. occurring with other birth defects), and then isolated cases alone, in order to maximize the power of our analysis. We also considered each of the three cleft types separately, pooling isolated and non-isolated cases and then including only isolated cases. We evaluated any active maternal smoking during the first trimester of pregnancy. In additional analyses, we considered dose, separating smokers into non-smokers, low smokers (≤ 4 cigarettes/day) and moderate-to-heavy smokers (≥ 5 cigarettes per day) (1). We classified mothers based on their pre-pregnancy BMI [(weight in kg)/(height in m)2] into underweight (BMI<18.5), normal weight (18.5≤BMI<25), overweight (25≤BMI<30), and obese (BMI ≥30). Smoking and pre-pregnancy maternal height and weight were measured through maternal surveys using comparable questions across the studies (1).
Statistical Analysis
Our analysis was based on the pooled dataset of the six studies while accounting for any potential differences between the study populations and case-control ratios as noted below. We used logistic regression with case-control status as the outcome to examine the interaction between smoking and BMI. We estimated a logistic regression for case-control status that included any smoking (yes/no), BMI as a continuous measure, and their interaction. The model also adjusted for any maternal alcohol consumption and use of folic-acid-containing supplements in the first trimester, and age (≤18, 19–25, 26–30, 31–35, and ≥36 years), an indicator for low maternal education (incomplete high school versus higher education), and dummy variables representing the 6 contributing studies. These study-specific fixed effects account for differences among the study populations in oral cleft risk factors, prevalence, time effects, and case-to-control ratios (1, 3, 21). These fixed effects, as well as the balancing of cases and controls by year of birth within each study, ensured that time is not a confounder in the pooled analysis. We did not control for diabetes because it can be a consequence of obesity. Furthermore, prior work has shown that it did not meaningfully account for the associations of BMI with risk of oral clefts (3).
Results
Table 1 provides the counts of cases by cleft type as well as smoking rates and BMI distributions for cases and controls. The sample included 4935 cases with clefts (including 4041 cases with isolated clefts) and 10557 controls. Among cases, 1134 had cleft lip only, 2078 had cleft lip with palate, and 1723 had cleft palate only. As reported in prior studies (1, 3), smoking, underweight, and obesity rates were higher among cases than controls.
Table 1.
Cleft Type and First Trimester Smoking and Pre-Pregnancy BMI Distribution by Case-Control Status in Analytical Sample
| Cases | Controls | |||
|---|---|---|---|---|
| N | % | N | % | |
| Total Sample | 4935 | 100 | 10557 | 100 |
| Any Cleft | 4935 | 100 | - | - |
| Cleft Lip Only | 1134 | 23.0 | - | - |
| Cleft Lip with Palate | 2078 | 42.1 | - | - |
| Cleft Palate Only | 1723 | 34.9 | - | - |
| Any Cleft Isolated | 4041 | 100 | - | - |
| Cleft Lip Only | 1029 | 25.5 | - | - |
| Cleft Lip with Palate | 1740 | 43.1 | - | - |
| Cleft Palate Only | 1272 | 31.5 | - | - |
| Smoking | 4935 | 100 | 10557 | 100 |
| Yes | 1177 | 23.9 | 1908 | 18.1 |
| No | 3758 | 76.2 | 8649 | 81.9 |
| BMI Categories | 4935 | 100 | 10557 | 100 |
| Underweight | 308 | 6.2 | 565 | 5.4 |
| Normal weight | 2642 | 53.5 | 5821 | 55.1 |
| Overweight | 1136 | 23.0 | 2502 | 23.7 |
| Obese | 849 | 17.2 | 1669 | 15.8 |
Notes: Rates were rounded to 1 decimal.
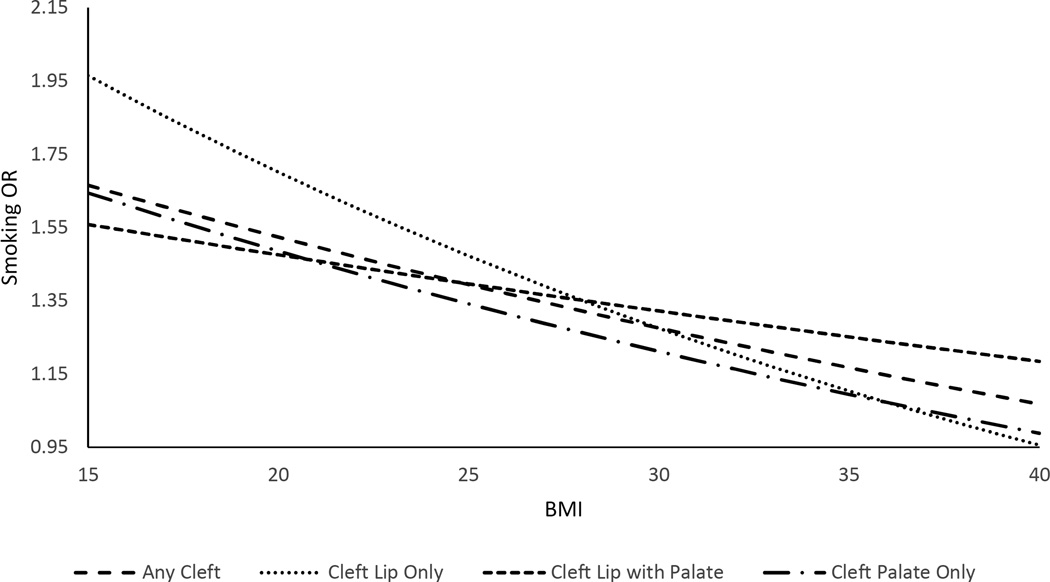
Table 2 presents the results from the logistic regressions examining interactions between smoking and BMI across the various cleft groups. Both smoking and BMI were associated with an increase in oral cleft risk on their own, although risks were not significantly elevated (at p<0.05) for certain cleft types. There was a significant negative interaction between the two risk factors, in that the increase in risks of any oral cleft with smoking declined as BMI increased. For any cleft (isolated and non-isolated), the ORs were 2.18 (95% CI: 1.49–3.17) for smoking, 1.13 (95% CI: 1.05–1.21) per 10 BMI units, and 0.84 (95% CI: 0.72–0.97) for the smoking-BMI interaction. Because the OR for the smoking-BMI interaction term is not directly interpretable, we derived the ORs for smoking at various BMI levels (Figure 1) to illustrate the magnitude of the interaction. The OR for smoking was 1.61 (95% CI: 1.39–1.86) at BMI 17 (underweight), 1.47 (95% CI: 1.34–1.62) at BMI 22 (normal weight), 1.35 (95% CI: 1.22–1.48) at BMI 27 (overweight), 1.21 (95% CI: 1.04–1.41) at BMI 33 (obese), and 1.13 (95% CI: 0.92–1.38) at BMI 37 (very obese). Similar results were observed for any isolated cleft.
Table 2.
Logistic Regression for Oral Clefts Including Interactions between First-Trimester Maternal Smoking and Pre-Pregnancy BMI
| Cleft Status | Smoking | BMI├ | Smoking × BMI├ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR† | 95% CI | OR† | 95% CI | OR† | 95% CI | P-Value | ||||
|
Isolated and non- Isolated Clefts | ||||||||||
| Any Cleft | 2.18 | 1.49 | 3.17 | 1.13 | 1.05 | 1.21 | 0.84 | 0.72 | 0.97 | 0.02 |
| Cleft lip Only | 3.03 | 1.53 | 6.00 | 1.09 | 0.96 | 1.24 | 0.75 | 0.57 | 0.99 | 0.04 |
| Cleft Lip with Palate |
1.84 | 1.11 | 3.04 | 1.13 | 1.02 | 1.24 | 0.90 | 0.74 | 1.09 | 0.28 |
| Cleft Palate Only | 2.23 | 1.27 | 3.93 | 1.17 | 1.05 | 1.29 | 0.82 | 0.65 | 1.02 | 0.07 |
| Isolated Clefts | ||||||||||
| Any Cleft | 2.26 | 1.51 | 3.36 | 1.11 | 1.03 | 1.19 | 0.84 | 0.72 | 0.98 | 0.03 |
| Cleft lip Only | 3.15 | 1.56 | 6.36 | 1.07 | 0.94 | 1.23 | 0.76 | 0.57 | 1.01 | 0.06 |
| Cleft Lip with Palate |
1.52 | 0.89 | 2.60 | 1.07 | 0.97 | 1.19 | 0.98 | 0.79 | 1.21 | 0.84 |
| Cleft Palate Only | 3.20 | 1.66 | 6.14 | 1.18 | 1.05 | 1.32 | 0.71 | 0.55 | 0.92 | 0.01 |
Notes:
Multivariable logistic regression model adjusted for, maternal education (less than high school degree versus higher education), maternal age (≤18, 19–25, 26–30, 31–35, 36–49yrs), any alcohol use (Yes/No) and folic acid use(Yes/No) as well as study dummy variables. A separate regression is estimated for each cleft group.
BMI is expressed in 10 units.
Figure 1. ORs for First-Trimester Maternal Smoking by Pre-Pregnancy BMI Derived from Logistic Regression Including Smoking-BMI Interaction.
Notes: ORs for smoking were estimated at BMI levels from 15 to 40 in increments of 1 unit. Isolated and non-isolated clefts are combined.
This negative interaction was more pronounced for cleft lip only and cleft palate only. The interaction was smaller and insignificant for cleft lip with cleft palate. For cleft lip only (combining isolated and non-isolated cases), the ORs were 3.03 (95% CI: 1.53–6.00) for smoking, 1.09 (0.96–1.24) for 10 BMI units, and 0.75 (0.57–0.99) for the smoking-BMI interaction. When evaluated at specific BMI levels, the smoking OR was 1.86 (95% CI: 1.44–2.39) at BMI 17, 1.61 (95% CI: 1.36–1.90) at BMI 22, 1.39 (95% CI: 1.17–1.65) at BMI 27, 1.17 (95% CI: 0.88–1.55) at BMI 33, and 1.04 (95% CI: 0.71–1.53) at BMI 37 (Figure 1). For cleft palate only (isolated and non-isolated), the comparable ORs were 2.23 (1.27–3.93), 1.17 (1.05–1.29) and o.82 (0.65–1.02); the smoking OR was 1.58 (95% CI: 1.27–1.96) at BMI 17, 1.43 (95% CI: 1.23–1.65) at BMI 22, 1.29 (95% CI: 1.12–1.48) at BMI 27, 1.14 (95% CI: 0.91–1.43) at BMI 33, and 1.05 (95% CI: 0.77–1.43) at BMI 37 (Figure 1). Results were similar when considering isolated clefts, but the smoking risk and interaction with BMI interaction were more pronounced for isolated cleft palate only.
Discussion
Maternal smoking is widely considered one of the most unequivocal environmental risk factors for oral clefts. Previous studies have focused mainly on average associations, with little exploration of possible heterogeneity in risk across other factors. We examined the heterogeneity in smoking associations with oral cleft risk by maternal BMI, another risk factor. We found that the increase in the risk of oral clefts associated with maternal smoking generally declines with increasing maternal BMI, with the largest risk from smoking among underweight mothers and the lowest risk among obese mothers. This is the first study to report this interaction for oral clefts. When examining cleft types separately, the trend was strongest for cleft lip only and cleft palate only, and much weaker for cleft lip with palate. This suggests further heterogeneity in the relationship between maternal smoking and BMI by cleft type. Our findings suggest that the relative importance of smoking and BMI generally declines as the risk from the other factor increases although this interaction has further heterogeneity with respect to cleft type. Such an interaction may occur because of reduction in DNA adducts with higher BMI among smokers (10), or potential reduction in fetal exposure to cigarette chemicals in the critical weeks of cleft formation with greater storage of these chemicals in maternal fat tissue. Our findings should not be interpreted as suggesting that smoking is harmless to the fetuses of obese women. Despite the ORs of smoking being much reduced among obese women, their magnitude was still clinically relevant (Figure 1).
Our study has several strengths including the large population-based samples and a pooled individual-level analysis that controls for several potential confounders. However, unmeasured confounding cannot be excluded as an alternative explanation for the interaction effect as noted above. For instance, it is possible that the observed interaction is capturing an overall nutritional effect with increasing BMI, such as reduced food insecurity, which has been linked to cleft palate (22). It is worth noting however that our results are robust to adjustments for the individual-level risk factors described above, which is reassuring when considering both unmeasured and residual confounding (Supplementary Table S1). Furthermore, there are alternative approaches to examine the interaction between smoking and BMI such as stratifying by BMI levels or evaluating combinations of smoking and BMI levels. These approaches, however, are less powerful than the above-described model that directly tests the smoking-BMI interaction. Nonetheless, these alternative models provide generally consistent results with our main model (Supplementary Tables S2 and S3), although power dramatically declines in these models because of the stratified samples and multiple smoking-BMI groups, thus limiting our ability to statistically test this interaction.
The findings highlight the complexity of pathways for risk of oral clefts. Ignoring potential interactions between risk factors may result in average risk estimates that are not representative across population subgroups defined by other risk factors. Examining these interactions can shed light on the population-level contributions of risk factors to oral clefts. Exposure to passive smoke has also been associated with increased risk of oral clefts(1). Future research examining interactions between maternal active smoking, passive smoke, and BMI may shed further light on the etiology of oral clefts. Also, investigations of the mechanisms through which interactions between smoking and BMI operate are needed to understand how these two common factors contribute to the etiology of oral clefts.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Dental and Craniofacial Research at the National Institutes of Health (grant 1 R01 DE020895). The authors are grateful to the Data Sharing Committee of the National Birth Defects Prevention Study for comments on earlier drafts of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kummet C, Moreno L, Wilcox A, et al. Passive Smoke Exposure as a Risk Factor for Oral Clefts – A Large International Population-Based Study. American journal of epidemiology. 2016 doi: 10.1093/aje/kwv279. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014. [Google Scholar]
- 3.Kutbi H, Wehby GL, Moreno LM, et al. Maternal Underweight and Obesity and Risk of Orofacial Clefts in a Large International Consortium of Population-Based Studies. International journal of epidemiology. 2016 doi: 10.1093/ije/dyw035. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canoy D, Wareham N, Luben R, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obesity research. 2005;13(8):1466–1475. doi: 10.1038/oby.2005.177. [DOI] [PubMed] [Google Scholar]
- 5.Lv J, Chen W, Sun D, et al. Gender-specific association between tobacco smoking and central obesity among 0.5 million Chinese people: the China Kadoorie Biobank Study. PloS one. 2015;10(4):e0124586. doi: 10.1371/journal.pone.0124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuBois BN, O'Tierney-Ginn P, Pearson J, et al. Maternal obesity alters feto-placental cytochrome P4501A1 activity. Placenta. 2012;33(12):1045–1051. doi: 10.1016/j.placenta.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta,. GA. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010. 2010 [Google Scholar]
- 8.Huang L, Liu J, Feng L, et al. Maternal prepregnancy obesity is associated with higher risk of placental pathological lesions. Placenta. 2014;35(8):563–569. doi: 10.1016/j.placenta.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Ditchfield A, Desforges M, Mills T, et al. Maternal obesity is associated with a reduction in placental taurine transporter activity. International Journal of Obesity. 2014 doi: 10.1038/ijo.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godschalk RW, Feldker DE, Borm PJ, et al. Body mass index modulates aromatic DNA adduct levels and their persistence in smokers. Cancer Epidemiology Biomarkers & Prevention. 2002;11(8):790–793. [PubMed] [Google Scholar]
- 11.Kabat GC, Kim M, Hunt JR, et al. Body mass index and waist circumference in relation to lung cancer risk in the Women's Health Initiative. American journal of epidemiology. 2008;168(2):158–169. doi: 10.1093/aje/kwn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J, Horn K, Ockene JK, et al. Interaction between smoking and obesity and the risk of developing breast cancer among postmenopausal women: the Women's Health Initiative Observational Study. American journal of epidemiology. 2011;174(8):919–928. doi: 10.1093/aje/kwr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitsantas P, Wu H. Body mass index, smoking, age and cancer mortality among women: a classification tree analysis. The journal of obstetrics and gynaecology research. 2013;39(8):1330–1338. doi: 10.1111/jog.12065. [DOI] [PubMed] [Google Scholar]
- 14.Munger RG, Romitti PA, Daack-Hirsch S, et al. Maternal alcohol use and risk of orofacial cleft birth defects. Teratology. 1996;54(1):27–33. doi: 10.1002/(SICI)1096-9926(199607)54:1<27::AID-TERA4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Munger RG, Tamura T, Johnston KE, et al. Oral clefts and maternal biomarkers of folate-dependent one-carbon metabolism in Utah. Birth defects research Part A, Clinical and molecular teratology. 2011;91(3):153–161. doi: 10.1002/bdra.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon PW, Rasmussen SA, Lynberg MC, et al. The National Birth Defects Prevention Study. Public health reports (Washington, DC : 1974) 2001;116(Suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox AJ, Lie RT, Solvoll K, et al. Folic acid supplements and risk of facial clefts: national population based case-control study. BMJ (Clinical research ed) 2007;334(7591):464. doi: 10.1136/bmj.39079.618287.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnus P, Irgens LM, Haug K, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) International journal of epidemiology. 2006;35(5):1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 19.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort--its background, structure and aim. Scandinavian journal of public health. 2001;29(4):300–307. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 20.Bille C, Olsen J, Vach W, et al. Oral clefts and life style factors--a case-cohort study based on prospective Danish data. European journal of epidemiology. 2007;22(3):173–181. doi: 10.1007/s10654-006-9099-5. [DOI] [PubMed] [Google Scholar]
- 21.DeRoo LA, Wilcox AJ, Lie RT, et al. Maternal alcohol binge-drinking in the first trimester and the risk of orofacial clefts in offspring: a large population-based pooling study. European Journal of Epidemiology. 2016:1–14. doi: 10.1007/s10654-016-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmichael SL, Yang W, Herring A, et al. Maternal food insecurity is associated with increased risk of certain birth defects. The Journal of nutrition. 2007;137(9):2087–2092. doi: 10.1093/jn/137.9.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.