Abstract
Endocytosis is a cellular process mostly responsible for membrane receptor internalization. Cell membrane receptors bind to their ligands and form a complex which can be internalized. We previously proposed that F-BAR protein initiates membrane curvature and mediates endocytosis via their binding partners. However, F-BAR protein partners involved in membrane receptor endocytosis and the regulatory mechanism remain unknown. In this study, we established a group of database mining strategies to explore mechanisms underlying receptor-related endocytosis. We identified 34 endocytic membrane receptors and 10 regulating proteins for vesicle formation in clathrin-dependent endocytosis (CDE), a major process of membrane receptor internalization. We found that F-BAR protein FCHSD2 (Carom) may facilitate endocytosis via 9 endocytic partners. Carom is highly expressed, along with highly expressed endocytic membrane receptors and partners, in endothelial cells and macrophages. We established 3 models of Carom-receptor complex and their intracellular trafficking based on protein-protein interaction and subcellular localization. We conclude that Carom may mediate receptor endocytosis and transport endocytic receptors to the cytoplasm for receptor signaling and lysosome/proteasome degradation, or to the nucleus for RNA processing, gene transcription and DNA repair.
Keywords: F-BAR proteins, Membrane receptor, Cellular trafficking, Nuclear translocation, Endocytosis
2. INTRODUCTION
Endocytosis is a cellular process by which molecules or substances are transported into the cell via cell membrane engulfment. Endocytosis is generally classified as phagocytosis and pinocytosis, which are distinguished by the size of the endocytic vesicles formed (Figure 1A & B) (1). Phagocytosis implies to the ingestion of large and solid particle (diameter 0.5–10μm) such as pathogens. Pinocytosis refers to internalization of various liquid via small endocytic vesicles and can be divided into four subtypes: macropinocytosis, clathrin-dependent, caveolae-dependent, and clathrin/caveolae-independent endocytosis based on clatherin or caveolae involvement (2). Pathogens or ligands induce endocytosis by binding to the cell membrane via receptor-dependent or -independent mechanisms, and then form phagosome or endocytic vesicle (Figure 1B & C). Endocytic vesicle may be coated with clathrin, caveolae or regulated by flotillin, Rho GTPase activating protein 26 (GRAF1), ADP-Ribosylation factor 6 (Arf6) and Ras homology family membrane A (RhoA). During phagocytosis, solid particle containing-phagosomes fuse with lysosomes (marked by lysosomal associated membrane protein (LAMP1)) and subjected to lysosomal degradation. In the process of pinocytosis, internalized vesicles are transported to early endosome (marked by Ras associated protein (Rab5)), which delivers the cargoes to three locations: 1) late endosome (marked by Ras associated protein (Rab7)) then lysosome for degradation, 2) recycling endosome (marked by Rab11) for signal transduction or receptor recycling to cell membrane, and 3) nucleus to regulate transcription factor and chromatin remolding (1–3).
Figure 1. Overview of endocytosis.
Endocytosis is a cellular process by which molecules or substances are transported into the cell via cell membrane engulfment. A. Classification of endocytosis Endocytosis is generally classified as phagocytosis and pinocytosis. Pinocytosis can be further divided into 4 subtypes; macropinocytosis, clathrin-dependent, caveolae-dependent, and clathrin/caveolae independent endocytosis based on clatherin or caveolae involvement. Most of the receptor-mediated endocytosis (REM) are processed via clathrin-dependent mechanism. B. Features of endocytosis. Features of endocytosis are summarized for the size of internalized particle, membrane domain localization and cargo content. C. Schematic diagram of endocytosis process. Pathogens and ligands induce endocytosis by binding to the cell membrane via receptor-dependent or -independent mechanism, and then form phagosome or endocytic vesicle which may be coated with clathrin/caveolae or regulated by flotillin, GRAF1, Arf6 and RhoA. Membrane–bounded F-BAR protein are linked to actin-associated proteins, can cause cell membrane curvature and facilitate clathrin-meditaed or caveolae-dependent endocytosis. During phagocytosis, cells bring in solid particles into phagosomes and then fuse with lysosomes (marked by LAMP1). During pinocytosis, internalized vesicles are transported to early endosome (marked by Rab5). The early endosome can send the cargoes to three locations: 1) late endosome (marked by Rab7) then lysosome for degradation, 2) recycling endosome (marked by Rab11) for signal transduction or recycling to plasma membrane, and 3)nucleus for transcription factor regulation or chromatin remolding machinery. Abbreviation: RTK, receptor tyrosine kinase; GPCR, G protein-coupled receptor; TFR, transferrin receptor; LDLR, low-density lipoprotein receptor; GPI, glycosylphosphatidylinositol; TGF-βR, transforming growth factor-beta receptor; IGF-IR, insulin-like growth factor I receptor; IL-2RB, interleukin 2 receptor beta; Rab, Ras associated protein; EEA1, Early Endosome Antigene 1; LAMP1, Lysosomal associated membrane protein 1;GRAF1, Rho GTPase Activating Protein 26; Arf6, ADP-Ribosylation Factor 6; RhoA, Ras Homolog Family Member A;
Membrane receptors are responsible for transducing external signals into the cell by receiving extracellular molecules. It is suggested that some of the cell membrane receptors bind to their ligands and form a complex which can be internalized and translocated to the cytoplasm or nucleus for signaling or degradation mostly via clathrin-dependent endocytosis (CDE) mechanism (4). For example, receptor tyrosine kinases (RTKs) and G-protein coupled receptors (GPCRs) can be internalized via directly interacting with adaptor protein AP2, epsin or intersectin and CDE-mediated mechanism (5, 6) However, molecular mechanisms regulating CDE signaling are not fully elucidated.
We previously proposed that F-BAR (Fes/CIP4 homology-BAR) protein initiates membrane curvature and mediate endocytosis via its binding partners (7, 8). Beyond F-BAR domain which initiates endocytosis, most of the proteins in this superfamily contain other domains, such as SH3 (Src homology-3) and SH2 (Src homology-2), which can recruit adaptor proteins to form complexes. Such F-BAR protein complexes participate in multiple steps of endocytosis, ranging from the assembly of endocytic vesicles and their scissions, F-actin polymerization and nucleation, etc. As a novel member of F-BAR protein, FCHSD2 (Carom) displays such structures and contains a F-BAR domain and two SH3 domains. Although this protein’s function hasn’t been fully addressed, it is predicted as a critical molecule in the process of CDE (Figure 2, Table 1). However, how Carom interacts with membrane receptor and facilitate receptor endocytosis is unknown.
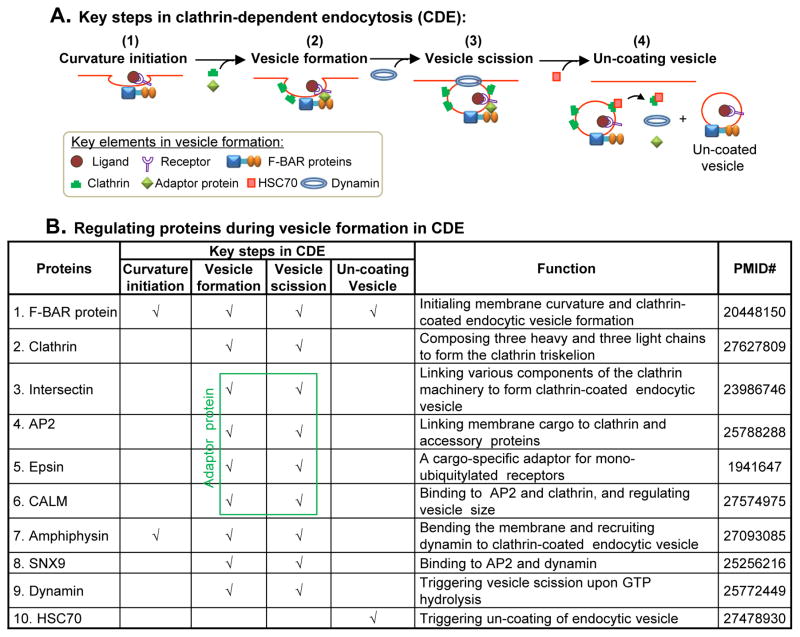
Figure 2. Key steps in clathrin-dependent endocytosis and its regulating proteins.
A. Key steps in vesicle formation in clathrin-dependent endocytosis (CDE). There are four steps during vesicle formation: (1) curvature initiation, (2) vesicle formation, (3) vesicle scission, and (4) un-coating vesicle. . At first, F-BAR protein binds to plasma membrane and initiates membrane curvature. F-BAR protein can recruit adaptor protein via its SH3 domain during vesicle formation. Clathrin are recruited directly from the cytosol to the site of adaptor-concentrated membrane to help the formation of coated vesicle. GTPase dynamin can then bind to the membrane and cause vesicle constriction, scission, and release. HSC70 binds to Clathrin, disassociates Clathrin, Intersectin and Dynamin from the vesicle and produces an un-coated endocytic vesicle containing the cargo molecules. B. Regulating proteins during vesicle formation in CDE. A group of proteins are involved in endocytic vesicle formation. F-BAR protein initiates membrane curvature and clathrin-coated endocytic vesicle formation. Adaptor proteins (Intersectin, AP2, Epsin, CALM) links various components of the clathrin machinery to the membrane and helps the formation of adaptor-concentrated clathrin-coated vesicle. Dynamin triggers vesicle scission upon GTP hydrolysis. HSC70 triggers un-coating of endocytic vesicle. Abbreviation: AP2, adaptor protein 2; SNX9, sorting nexin 9; HSC70, ATPase heat shock cognate 70; CALM, clathrin assembly lymphoid myeloid leukaemia; (N-)WASP/WAVE, Wiskott-Aldrich Syndrome Like.
Table 1. Classification of endocytic membrane receptor.
We selected 34 cell surface receptors from 259 genes related to endocytosis identified from Kegg pathway database (hsa04144, http://www.genome.jp/dbget-bin/www.bget?hsa04144) and classified them into three groups: 1)G-protein coupled receptor, 2) Receptor tyrosine kinase, and 3) Transmembrane receptor. Ligand and function of receptors are identified using Genecard database (http://ww.genecards.org). Endocytosis type are defined by literature search. Noted that most of the receptor-mediated endocytosis generally occurs via CDE. Abbreviation: Leuc, leukocyte; PLT, platelets; CDE, Clathrin-dependent endocytosis; CIE, Clathrin-independent endocytosis; Indt, Clathrin/caveolin-independent endocytosis;
| Gene (Symbol (Full name)) | Ligand | Function | Endocytosis type |
|---|---|---|---|
| G-protein coupled receptor (GPCR) | |||
| 1. ADRB1 (Adrenoceptor β 1) | Epinephrine, norepinephrine | Mediate catecholamines action | CDE |
| 2. ADRB2 (Adrenoceptor β 2) | Epinephrine, norepinephrine | Mediate catecholamines action | CDE |
| 3. ADRB3 (Adrenoceptor β 3) | Norepinephrine | Mediate catecholamines action | CDE |
| 4. CCR5 (Chemokine (C-C motif) receptor 5 ) | CCl3,CCl4,CCl5,CCl8,CCl13, CCl16 | Leuc trafficking, angiogenesis, apoptosis | CDE |
| 5. CXCR1 (Chemokine (C-X-C motif) receptor 1) | CXCl6,CXCl8 | Leuc trafficking, angiogenesis, apoptosis | CDE |
| 6. CXCR2 Chemokine (C-X-C motif) receptor 2) | CXCl1,CXCl2,CXCl3, CXCl5 CXCl6,CXCl7,CXCl8 | Leuc trafficking, angiogenesis, apoptosis | CDE |
| 7. CXCR4 (Chemokine (C-X-C motif) receptor 4) | CXCl14 | Leuc trafficking, angiogenesis, apoptosis | CDE |
| 8. F2R Coagulation factor II receptor) | Thrombin | PLT activation, vascular development | CDE |
| Receptor tyrosine kinase (RTK) | |||
| 9. CSF1R (Colony stimulating factor 1 receptor) | M-CSF,IL34 | Macrophage regulator | CDE |
| 10. EGFR (Epidermal growth factor receptor) | EGF | Proliferation, differentiation | CDE/CIE |
| 11. ERBB2 (Erb-b2 receptor tyrosine kinase 2) | EGF | Proliferation, differentiation | CDE |
| 12. ERBB3 (Erb-b2 receptor tyrosine kinase 3) | EGF | Proliferation, differentiation | CDE |
| 13. ERBB4 (Erb-b2 receptor tyrosine kinase 4) | EGF | Proliferation, differentiation | CDE |
| 14. FGFR1 (Fibroblast growth factor receptor 1) | FGF1,FGF2,FGF3,FGF6, FGF7 | Proliferation, differentiation | CDE/CIE |
| 15. FGFR2 (Fibroblast growth factor receptor 2) | FGF1,FGF4,FGF6,FGF7, FGF8 | Proliferation, differentiation | CDE/CIE |
| 16. FGFR3 (Fibroblast growth factor receptor 3) | FGF3,FGF4,FGF5,FGF6, FGF7 | Proliferation, differentiation | CDE/CIE |
| 17. FGFR4 (Fibroblast growth factor receptor 4) | FGF1,FGF3,FGF4,FGF5, FGF9 | Proliferation, differentiation | CDE/CIE |
| 18. FLT1 (Fms-related tyrosine kinase 1)/ VEGFR1 (Vascular endothelial growth factor receptor1) | VEGFA,VEGFB,PGF | Angiogenesis | CDE/CIE |
| 19. IGF1R (Insulin-like growth factor 1 receptor) | IGF1,IGF2 | Proliferation, differentiation | CDE/CIE |
| 20. IGF2R (Insulin-like growth factor 2 receptor) | IGF2,Transferrin | Proliferation, differentiation | CDE |
| 21. KDR (Kinase insert domain receptor)/ VEGFR2 (Vascular endothelial growth factor receptor2) | VEGFA,VEGFC | Proliferation, angiogenesis | CDE/CIE |
| 22. MET (Tyrosine-protein kinase met) | HGF | Proliferation, angiogenesis | CDE |
| 23. NTRK1 (Neurotrophic tyrosine kinase receptor type 1) | NGF | Differentiation | CDE |
| 24. PDGFRA (Platelet-derived growth factor α receptor) | PDGFC | Proliferation, differentiation, | CDE |
| 25. TGFBR1 (Transforming growth factor β receptor I) | TGF-β | Proliferation tumor transformation | CDE/CIE |
| 26. TGFBR2 (Transforming growth factor β receptor I) | TGF-β | Proliferation, tumor transformation | CDE/CIE |
| Transmembrane receptor (TMR) | |||
| 27. FOLR1 (Folate receptor 1) | Folic acid | Transport folic acid | CDE |
| 28. FOLR2 (Folate receptor 2) | Folic acid | Transport folic acid | CDE |
| 29. FOLR3 (Folate receptor 3 | Folic acid | Transport folic acid | CDE |
| 30. IL2RA (Interleukin 2 receptor α) | IL2 | Regulate immune system | Indt |
| 31. IL2RB (Interleukin 2 receptor β) | IL2,IL15 | Regulate immune system | Indt |
| 32. IL2RG (Interleukin 2 receptor γ) | IL2,IL-4,IL15 | Regulate immune system | Indt |
| 33. LDLR (Low density lipoprotein receptor) | LDL, ApoB100, ApoE, IDL | Transport lipid | CDE |
| 34. TFRC (Transferrin receptor) | Transferrin, HFE | Transport iron | CDE |
In the past ten years, bioinformatics analysis has emerged as an important tool for functional interpretation of genomics and proteomics information (9–11). In this study, we established a group of database mining strategies and performed intensive literature searches to explore mechanisms underlying receptor-related endocytosis. We identified endocytic membrane receptors and potential regulating proteins for vesicle formation and investigated the relationship of F-BAR protein Carom with endocytic membrane receptors and endocytic partners. We established models of endocytosis and Carom-mediated membrane receptor internalization.
3. MATERIALS AND METHODS
3.1. Identification of regulating protein in CDE, F-BAR protein potential in membrane receptor endocytosis, and prediction of Carom-receptor complex signaling (PubMed)
We searched through PubMed literature to summarize 10 important regulating proteins that take part in vesicle formation in CDE and F-BAR protein potential in membrane receptor endocytosis (Figure 2B, Table 2). In order to identify the cell signaling connection to Carom-receptor complex, we summarized 3 types of Carom-receptor complexes, predicted their intracellular trafficking based on their subcellular localization and the nuclear localization signal (NLS) (Table 4), and predict the Function of Carom-receptor complexes based on receptor signaling reported in the literature (Table 5).
Table 2. F-BAR proteins are involved in membrane receptor endocytosis.
F-BAR protein have 9 family members, each protein contains one F-BAR domain and other domains such as SH3,SH2, WW, and RhoGAP. Most of the F-BAR protein are involved in endocytosis and play important roles in membrane receptor trafficking/signaling cited by PMID#. Symbols listed in the framed box indicate representative domains. Abbreviation: F-BAR, Fes/CIP4 homology-BAR; FX, F-BAR extension; HR1, Protein kinase C-related kinase homology region 1; NPF, Asparagine proline phenylalanine; RhoGAP, Rho GTPase-activating protein; SH2, Src homology-2; SH3, Src homology-3; μHD, μ-homology domain; CIP4, Cdc42-interacting protein 4; FBP17, Formin binding protein 17; Toca-1, TOCA homolog 1; FCHO1-2 , FCH domain only 1-2; srGAP1-3, SLIT-ROBO Rho GTPase activating protein 1-3; PSTPIP1- 2, Proline-serine-threonine phosphatase-interacting protein 1-2; FCHSD1-2, FCH and double SH3 domains 1-2; NOSTRIN, Nitric oxide synthase traffic inducer; GAS7, growth arrest specific 7; Others refer to Table 1.
| F-BAR Proteins | Structure | Endocytosis | Roles in endocytosis | PMID# |
|---|---|---|---|---|
| 1. CIP4 subfamily | ||||
| CIP4 |
|
✓ | Required for EGFR trafficking /degradation | 19632321 |
| FBP17 | ✓ | Required for EGFR internalization | 19632321 | |
| Toca-1 | ✓ | Required for EGFR trafficking from endosomes | 19632321 | |
| 2. FCHOs subfamily | ||||
| FCHO1 |
|
✓ | Forming clathrin-coated vesicle | 20448150 |
| FCHO2 | ✓ | Required for LDLR endocytosis | 22323290 | |
| 3. srGAPs subfamily | ||||
| srGAP1 |
|
N/A | ||
| srGAP2 | N/A | |||
| srGAP3 | N/A | |||
| 4. PACSINs subfamily | ||||
| PACSIN1 |
|
✓ | Inhibiting endocytosis | 11082044 |
| PACSIN2 | ✓ | Required for EGFR translocated to endosomes | 23129763 | |
| PACSIN3 |
|
✓ | Inhibiting transferrin/TFRC complex endocytosis | 11082044 |
| 5. PSTPIPs subfamily | ||||
| PSTPIP1 |
|
✓ | Suppressing transferrin/TFRC complex endocytosis | 18480402 |
| PSTPIP2 |
|
N/A | ||
| 6. FCHSDs subfamily | ||||
| FCHSD1 |
|
✓ | Promoting F-actin polymerization and facilitate endocytosis | 23437151 |
| FCHSD2 (Carom) | ✓ | Stimulating F-actin polymerization and facilitate endocytosis | 23437151 | |
| 7. FES/FER subfamily | ||||
| FES |
|
N/A | ||
| FER | N/A | |||
| 8. NOSTRIN subfamily |
|
✓ | Assembling NOSTRIN-FGFR1-Rac1-Sos1 complex/regulate FGF signaling | 22751148 |
| 9. GAS7 subfamily |
|
N/A |
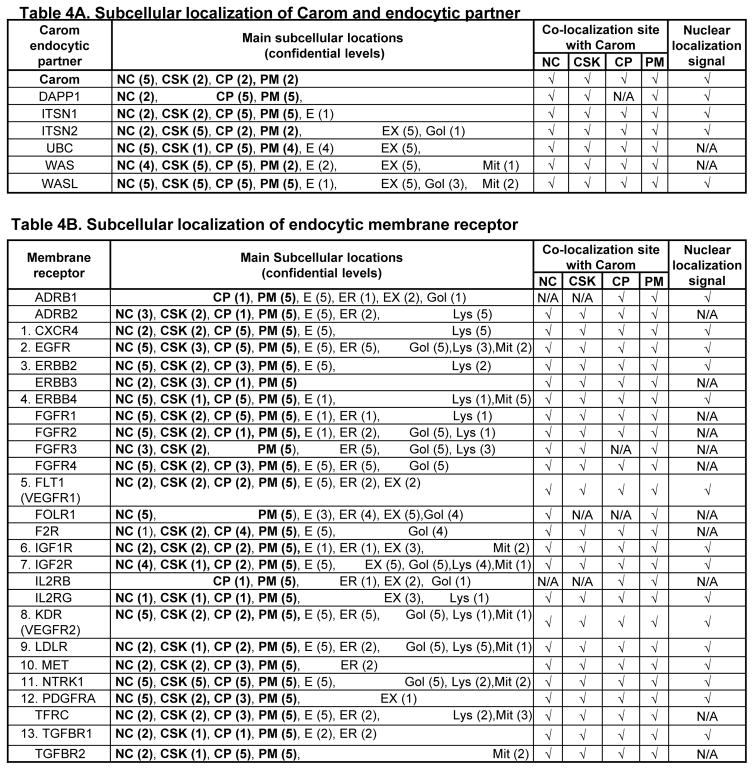
Table 4. Subcellular localization of Carom partner and membrane receptor, and co-localization with Carom (Compartments database/cNLS database).
Subcellular localization of Carom/partner and membrane receptors which can form 3 models Carom-receptor complex in Figure 4 were determined in Compartments database. A. Subcellular localization and co-localization of Carom with endocytic partner. B. Subcellular localization of membrane receptor and co-localization with Carom. Noted that most of the Carom partners and membrane receptors co-localize with Carom in PM, CP and NC. Some of Carom partner and membrane receptor contains NLS. 13 receptors can be potentially trans-localized to the nucleus because of the recognized NC localization and NLS, and labeled with numbers. Bolded words refer to molecules co-localized with Carom in subcellular sites as indicated in A. Numbers in parenthesis are confidence sores provided by Compartments database. Co-localization site with Carom are determined by present within the same cellular compartment and indicated by check marker (.). Nuclear localization signal are identified by using cNLS Mapper. Abbreviation: CP, cytoplasmic; CSK, cytoskeleton; E, endosome; ER, endoplasmic reticulum; EX, extracellular, Gol, golgi apparatus; Lys, lysosome; Mit, mitochondrion; NC, Nucleus; PM, plasma membrane, others refer to Table 2 and 3.
Table 5. Analysis of Carom:partner-receptor complex intracellular trafficking and function.
Carom-receptor complex function are predicted based on its binding partner and receptor signaling reported in the literature (PMID#) referred. We characterized 3 different Carom-receptor complex intracellular trafficking patterns: 1) only in PM, 2) PM to CP/CSK and 3) PM to NC. In three Carom-receptor complex models, the Carom-TGFBR1 and Carom:partner-ERBBs complex can be transferred to CP and CSK via endocytosis for signaling transduction and lysosomal degradation. They can also be transported into the nucleus and participate in RNA processing, transcription regulation and DNA repair because of the recognized NC localization and the detected NLS, except for Carom:DAPP1-ERBB3 and Carom:WAS-EGFR complex. The Carom:UBC-receptor complexes involves a large group of 24 receptors for ubiquitination and proteasome degradation by UBC which lacks of NLS. Abbreviation: refer to Table 1, 3 and 4.
| Carom-receptor complex models (Carom:Partner-receptor) | Predicted Carom complex trafficking
|
||||
|---|---|---|---|---|---|
| Only in PM | PM to CP | to NC | |||
|
| |||||
| (Function) | PMID# | Function | PMID# | ||
|
| |||||
| A. Carom-TGFBR1 complex | TGFBR1 signaling/ degradation | 21295082 | RNA processing | 22473997 | |
|
| |||||
|
B. Carom:partner-ERBBs complex
Carom:DAPP1-ERBB3 |
ERBB3 signaling/degradation | 22436610 | N/A | ||
|
| |||||
| Carom:ITSN1-ERBB2, ERBB4, EGFR | ERBB2/4, EGFR signaling/degradation | 23472148 | Transcriptional regulation/DNA repair | 26719328 | |
|
| |||||
| Carom:ITSN2-ERBB2, ERBB4, EGFR | ERBB2/4, EGFR signaling/degradation | 23472148 | Transcriptional regulation/DNA repair | 20670598 | |
|
| |||||
| Carom:WAS-EGFR | EGFR signaling/degradation | 23472148 | N/A | ||
|
| |||||
| Carom:WASL-EGFR | EGFR signaling/degradation | 23472148 | Transcriptional regulation/DNA repair | 22127113 | |
|
| |||||
| C. Carom:UBC-receptor complex | |||||
| Carom:UBC-ADRB1,ADRB2, CXCR4, EGFR, ERBB3, ERBB4, FGFR1, FGFR2, FGFR3, FGFR4, FOLTR1, FLT1 (VEGFR1), F2R, IGF1R, IGF2R,, IL2RB, IL2RG, KDR (VEGFR2), LDLR, MET, NTRK, PDGFRA, TFRC, TGFBR1, TGFBR2 | Ubiquitination, degradation | N/A | |||
3.2. Identification of endocytic membrane receptor and Carom potential endocytic partners (Kegg database, Genecard database and PubMed)
Thirty-four cell membrane receptors were selected from 259 genes related to endocytosis identified from Kegg pathway database (hsa04144, http://www.genome.jp/dbget-bin/www.bget?hsa04144) (Table 1). The ligand and function of receptors were identified using Genecard database (http://ww.genecards.org). A total of 26 binding partners for Carom protein were identified from previous study, which were established via Affinity Capture-MS, Affinity Capture-RNA, Affinity Capture-Western, Reconstituted Complex and Two-hybrid technologies (12, 13). Nine of 26 Carom endocytic partner were selected based on literature review and their function (13–15) (Table 3). Functions and binding domains of Carom partners were defined based on information obtained from Genecard database. Binding receptor were identified from NCBI Gene database and their corresponding regulation on receptor were determined by PubMed search (16–18).
Table 3. Identification of F-BAR protein Carom endocytic partner involved in endocytosis.
Nine Carom endocytic partners were selected based on literature review from 26 Carom partners we identified previously (Front Biosci (Landmark Ed), 2016; 21:856-72), which were established via Affinity Capture-MS, Affinity Capture-RNA, Affinity Capture-Western, Reconstituted Complex and Two-hybrid technologies. Functions and binding domains of Carom partners were defined based on information obtain from Genecard database. Binding receptor were identified from NCBI Gene database and its regulation on receptor were determined by PubMed search. All the Carom partners contain different domains such as SH2, PH, PDZ, EF and Ubiquitin which can recruit proteins to organize signaling complexes at cellular membranes. Noted that DAPP1 can bind to ERBB3, ITSN2/UBC/WAS/WASL bind to EGFR, and that ITSN1/UBC/UBD, VCP and WASL can regulate EGFR unbiquitilyation, degradation and endocytosis process. Abbreviation: CRIB, CDC42-Rac interactive binding; EF, EF hand; DH, DBL homology; AAA ATPase, ATPases associated with diverse cellular activities; PH, Pleckstrin homology; PDZ, PSD-95/Dlg-A/ZO-1; WH1/2, WASP-Homology 1/2; Others refer to Table 1. 1. Liu, S., et al., Analysis for Carom complex, signaling and function by database mining.
| Carom endocytic partner Symbol (Full name) | Interaction identification approach | Function | Domains on partner | Binding Receptor | Receptor regulation |
|---|---|---|---|---|---|
| 1. DAPP1 (B Lymphocyte Adapter Protein Bam32) | Two-hybrid | Regulates B-cell antigen receptor signaling | SH2,PH | ERBB3 | N/A |
| 2. GRASP (GRP1- Associated Scaffold Protein) | Two-hybrid | Regulate intracellular trafficking | PDZ | N/A | N/A |
| 3. ITSN1 (Intersectin 1) | Two-hybrid | Regulate endocytic vesicle formation | EF,DH,PH,C2 | N/A | EGFR ubiquitination |
| 4. ITSN2 (Intersectin 2) | Two-hybrid | Regulate endocytic vesicle formation | EF,DH,PH,C2 | EGFR | N/A |
| 5. UBC (Ubiquitin C) | Affinity Capture-MS | Regulate protein ubiquitination | Ubiquitin | EGFR | EGFR ubiquitination |
| 6. UBD (Ubiquitin D) | Affinity Capture-MS | Regulate protein ubiquitination | Ubiquitin | N/A | EGFR ubiquitination |
| 7. VCP (Valosin Containing Protein) | Co- Immunoprecipitation Two-hybrid | Regulate vesicle trafficking | AAA ATPase | N/A | EGFR degradation |
| 8. WAS (Wiskott-Aldrich Syndrome) | Two-hybrid | Regulate actin polymerization | WH1/2,PH,CRI B | EGFR | N/A |
| 9. WASL (Wiskott-Aldrich Syndrome Like) | Two-hybrid | Regulate actin polymerization | WH1/2,PH,CRI B | EGFR | EGFR endocytosis |
3.3. Cell expression profile of endocytic membrane receptor, Carom and Carom endocytic partner (Genevestigator database)
To investigate the expression profile of the endocytic membrane receptor, Carom and its endocytic partners in the cells, we used bioinformatics methods to gather extensive microarray information in the human primary cells. mRNA levels were obtained from microarray data available in the web site (https://www.genevestigator.com/gv/) and expressed as heat map (19) (Figure 3). The dark and light colour shading represents the relatively high and low expression levels of the endocytosis receptor in the different human primary cells. The three darker shadings represent higher levels of expression.
Figure 3. Endocytic membrane receptor, Carom and Carom endocytic partner expression profile in human primary cells.
A. Heat map of membrane receptor, Carom and Carom partner expression in human primary cells. mRNA levels are obtained from microarray data available in the web site (https://www.genevestigator.com/gv/) and expressed as heat map. Gradient bars indicate percent of expression potential. The dark and light color shadings represent relatively high and low expression levels, respectively. Dashed frames indicate body system and cells with relative high gene expression. B. Relationship of Carom, endocytic membrane receptor and partner expression in human circulatory and immune system cells. Noted that in aortic ECs, membrane receptor CXCR4, F2R, FLT1 (VEGFR1), KDR (VEGFR2), MET, IGF2R, LDLR and TFRC, and Carom endocytic partner GRASP, ITSN1-2, UBC, VCP and WASL are highly expressed. Carom is highly expressed in EC, LYM, MC, Mϕ and myoblast. Abbreviation: CMC, Cardiomyocyte; VSMC, vascular smooth muscle cell; EC, endothelial cell. LYM, lymphocyte; MC, Monocyte; Mϕ, Macrophage; GPCR, G-protein coupled receptor; RTK, Receptor tyrosine kinase; TRM, Transmembrane receptor; other refer to Table 1 and 3.
3.4. Predicted interaction of endocytic membrane receptor with Carom and its endocytic partners. (String database, NCBI database)
To generate an overview of whether Carom and its 9 endocytic partners may relate to membrane receptor functionality. We carried out an analysis of their protein-protein interactions by using String database (19, 20) (Figure 4). Solid lines indicate known interaction deposited in NCBI Gene database which was established based on Affinity Capture-MS, Affinity Capture-RNA, Affinity Capture-Western, Reconstituted Complex and Two-hybrid experimental data. Dashed lines indicate computational-predicted interaction in String database based on analyzing genomic information (‘genomic context’-methods) or from transferring associations/interactions between organisms (‘interolog’-transfer).
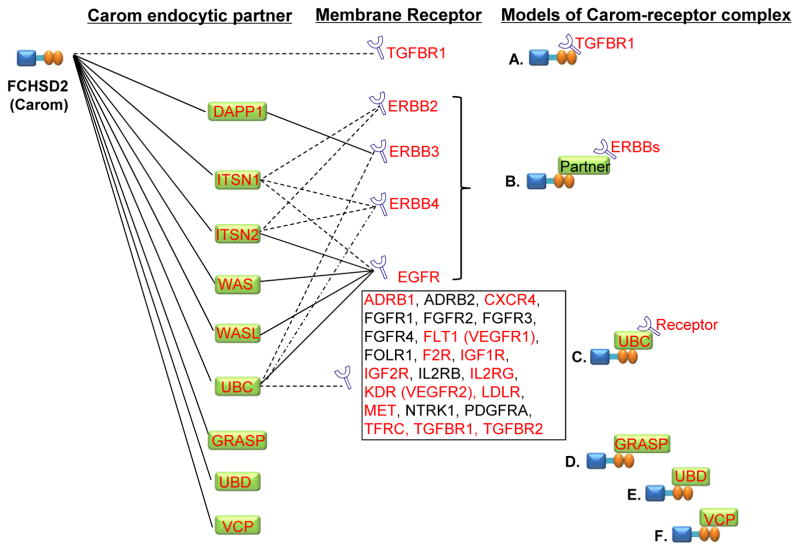
Figure 4. Models of Carom, endocytic partner and membrane receptor complexes.
Interaction of membrane receptor with Carom and Carom partner were identified via NCBI and String databases. Solid lines indicate known interaction deposited in NCBI Gene database which was established from affinity capture-MS, affinity capture-RNA, affinity capture-western, reconstituted complex and two-hybrid experimental data. Dashed lines indicate computational-predicted interaction in String database based on analyzing genomic information (‘genomic context’-methods) or from transferring associations/interactions between organisms (‘interolog’-transfer). Letters in red indicate genes with comparable high level of expression paralleled with high levels Carom in human cells identified in Figure 3B. Noted that Carom may directly bind to TGBR1 (model A), indirectly interact with EGFR, ERBB2, ERBB3 and ERBB4 via Carom partner DAPP1, ITSN1, ITSN2, WAS, and WASL (model B), bind to receptors through the partner UBC (model C), and directly interact with partner GRASP, UBD and VCP (model D, E and F). Abbreviation: refer to Table 1 and 3.
3.5. Subcellular localization analysis of Carom endocytic partner and membrane receptor, and co-localization with Carom (Compartments database, cNLS Mapper)
Subcellular localization of Carom, its endocytic partner and membrane receptors were determined using Compartments database established by manually curated literature, high-throughput screens, automatic text mining, and sequence-based prediction methods (20) (Table 4). Numbers are indicated confidential levels. Protein nuclear import generally involves a NLS, or interaction with carrier proteins (21). Nuclear localization and nuclear export signals are identified by analyzing protein sequence in cNLS Mapper. We predicted Carom-receptor complex intracellular trafficking from cell membrane into cytoplasm based on the analysis of co-localization of endocytic membrane receptor and partner in plasma membrane and cytoplasm (CP)/cytoskeleton (CSK). We predicted Carom-receptor complex translocation into the nucleus (NC) based on the analysis of co-localization of endocytic membrane receptor and partner in PM, NC and NLS.
4. RESULT
4.1. Identification of 34 endocytic membrane receptors
We identified 34 membrane receptors from 259 endocytosis-related genes selected from Kegg pathway database and classified them into three groups (Table 1). 1) 8 G-protein coupled receptor (GPCR): Adrenoceptor β1-3 (ADRB1-3), chemokine C-C motif receptor 5 (CCR5), chemokine C-X-C motif 1-2/4 (CXCR1-2/4) and Coagulation factor II receptor (F2R), 2) 18 tyrosine kinase receptors (RTK): Colony stimulating factor 1 receptor (CSF1R), Epidermal growth factor receptor (EGFR), Erb-b2 receptor tyrosine kinase 2-4 (ERBB2-4), Fibroblast growth factor receptor 1-4 (FGFR1-4), Fms-related tyrosine kinase 1/Vascular endothelial growth factor receptor 1 (FLT1 or VEGFR1), Insulin-like growth factor 1-2 receptor (IGF1-2R), Kinase insert domain receptor/Vascular endothelial growth factor receptor 2 (KDR or VEGFR2) , Tyrosine-protein kinase met (MET), Neurotrophic tyrosine kinase receptor type 1 (NTRK1), Platelet-derived growth factor α receptor (PDGFRA), Transforming growth factor β receptor 1-2 (TGFBR1-2), and 3) 8 transmembrane receptor (TRM): Folate receptor 1-3 (FOLR1-3), Interleukin 2 receptor α, β, γ (IL2RA, B, G), Low density lipoprotein receptor (LDLR) and Transferrin receptor (TFRC). It is known that endocytic membrane receptors can be internalized upon binding to its ligand and regulate various cellular functions including angiogenesis, proliferation, differentiation, and lipid/iron transport. The majority of these endocytic membrane receptors are internalized via CDE mechanism (22). Some of the receptors, such as EGFR, FGFR1-4, FLT1 (VEGFR1), IGF1R and TGFBR 1-2 can be internalized through both CDE and clathrin-independent endocytosis (CIE) (23). IL2R family appeared to be internalized by clathrin/caveolin-independent endocytosis (Indt) (Table 1) (24).
4.2. Key steps in vesicle formation in CDE and its regulating proteins
There are four steps during CDE process: 1) curvature initiation, 2) vesicle formation, 3) vesicle scission, and 4) un-coating vesicle (Figure 2A). A group of proteins are involved in regulating endocytic vesicle formation. At first, F-BAR protein binds to plasma membrane and initiates membrane curvature and clathrin-coated endocytic vesicle formation (25, 26). F-BAR protein also can recruit adaptor protein via its SH3 domain during vesicle formation. Clathrin are translocated to the site of adaptor-concentrated membrane from the cytosol via APs to form the clathrin-coated vesicle (2). The adaptor proteins link membrane cargo to clathrin and accessory proteins to form clathrin-coated endocytic vesicle. GTPase dynamin can then bind to the membrane and trigger vesicle scission and release upon GTP hydrolysis. Finally, ATPase heat shock cognate 70 (HSC70) binds to clathrin, disassociates clathrin, intersectin and dynamin from the vesicle and produces an un-coated endocytic vesicle containing the cargo molecules (27) (Figure 2B).
4.3. F-BAR protein participates in membrane receptor endocytosis
It is reported that F-BAR protein mediates membrane receptor endocytosis via initiating membrane curvature and endocytic vesicle formation in clathrin/caveolin-dependent endocytosis (Figure 1C) (8). F-BAR protein has 9 family members based on domain characterization. F-BAR protein contains one F-bar domain which binds to the cell membrane and other domains which interact with other molecules, such as Src homology-3 (SH3), Src homology-2 (SH2), protein kinase C-related kinase homology region 1 (HR1), F-BAR extension (FX), tyrosine kinase (Tyr-kinase), asparagine proline phenylalanine (NPF motif), μ-homology domain (μHD) and Rho GTPase-activating protein (RhoGAP) domains (Table 2). Six out of 9 F-BAR subfamily proteins are involved in membrane receptor endocytosis. CIP4 subfamily F-BAR proteins are required for EGFR internalization, trafficking and degradation (28). FCHO subfamily proteins are involved in LDLR endocytosis (29). PACSIN subfamily proteins participate in EGFR and transferrin/TFRC complex endocytosis (30). PSTPIP subfamily proteins can suppress transferrin/TFRC complex endocytosis (31). NOSTRIN subfamily protein is involved in assembling NOSTRIN-FGFR1-Rac1-Sos1 complex and regulating FGF signaling (28). FCHSDs subfamily proteins promote F-actin polymerization and membrane curvature which are key early events of endocytosis. However, endocytic receptors interacting with FCHSD protein have not been identified.
4.4. Identification of 9 Carom endocytic partners
The FCHSD2 gene encodes a protein termed as Carom, which is a novel membrane-associated protein with unknown function. Similar as most of the F-BAR family proteins, Carom binds to cell membrane via N-terminal F-BAR domain association with membrane phospholipids and bridges the membrane with cytoskeleton. It interacts with proline-rich proteins, such as adaptor and signaling proteins, via its C-terminal SH3 domains to form a functional complex at cellular membranes. Based on their endocytosis-related function reported in the literature, we selected 9 proteins from 26 Carom partners we previously identified, and termed them as Carom endocytic partners (Table 3) (32). It was reported that these Carom endocytic partners played important role in regulating actin polymerization, endocytic vesicle formation and protein ubiquitination. The Carom endocytic partner proteins contain different domains, such as SH2, Pleckstrin homology (PH), Ubiquitin, CDC42-Rac interactive binding (CRIB), and WASP (Wiskott–Aldrich syndrome protein)-Homology 1/2 (WH1/2) domain, which can recruit proteins to organize signaling complexes at cellular membranes. Carom partner can bind to receptors. We found that DAPP1 bind to ERBB3 and that ITSN2/UBC/WAS/WASL bind to EGFR from experimental data generated via affinity capture-MS, affinity capture-RNA, affinity capture-western, reconstituted complex and two-hybrid technologies, and deposited in NCBI database at the gene/interaction branch. It is well documented that Carom endocytic partner ITSN1/UBC/UBD, VCP and WASL regulate EGFR ubiquitination, degradation and endocytosis process (8, 28, 29, 33, 34).
4.5. Endocytic membrane receptor, Carom and its endocytic partner are differently expressed in human primary cells
We anticipate that the expression of Carom, their corresponding partners and endocytic receptors is comparable in the cells where Carom-organized receptor endocytosis take place, and examined their relevant expression in 12 human body systems and 10 primary cells (Figure 3A). Gene expression levels (mRNA) were obtained from microarray database (https://www.genevestigator.com/gv/). We found that human body systems do not have distinguished patterns of Carom, endocytic membrane receptors and partners’ expression. GPCR class endocytic membrane receptors had relative low levels of expression in most of the body system but highly expressed in immune system and in monocyte (MC), macrophage (Mϕ) and pancreatic islet cells. A few of RTK class receptors, such as EGFR, KDR (VEGFR2), MET, PDGFRA, appeared to be highly expressed in most of the body systems and in circulatory system cells, including cardiomyocyte (CMC), aortic vascular smooth muscle cell (VSMC), and aortic endothelial cell (EC). Cytokines and growth factor-related TRM class receptors had low levels of expression in most of body systems, but highly expressed in immune system (MC & Mϕ) and pancreatic islet cells. In contrast, TRM class receptors LDLR and TFRC, which transport lipid and iron into the cells, were highly expressed in all human body system and cells. Carom and its partners were expressed at medium levels in all body systems. In the circulatory system cells (Figure 3B), Carom was highly expressed in EC paralleled with highly expressed receptors (CXCR4, F2R, FLT1 (VEGFR1), KDR (VEGFR2), MET, IGF2R, LDLR and TFRC) and partners (GRASP, ITSN1-2, UBC, VCP and WASL). In LYM, Carom was highly expressed paralleled with highly expressed receptors (EGFR, ERBB3, MET, IGF1-2R, LDLR and TFRC) and partner (DAPP1, ITSN2 and WAS). In MC, highly expressed Carom was paralleled with receptors (CCR5, CXCR4, CSF1R, TGFBR1, IGF2R and FOLR3) and partners (DAPP1, ITSN2 and WAS). Mϕ had higher levels of Carom expression paralleled with large group of highly expressed receptors (CCR5, CXCR4, CSF1R, FLT1 (VEGFR1), TGFBR1, IGF2R, FOLR3 and TFRC) and partners (DAPP1, ITSN1-2, UBC, UBD, WAS and WASL), which is comparable with that in EC.
4.6. Carom may directly or indirectly bind to endocytic membrane receptor through partner protein
To generate models of Carom-receptor complexes for receptor endocytosis and signaling, we analyzed the interaction of Carom with membrane receptor and partners using information from NCBI experimental database and computational String database (Figure 4). We proposed 3 Carom-receptor complex models: A) Carom-TGFBR1 complex, in which Carom binds directly to TGFBR1, B) Carom:partner-ERBBs complex, in which Carom indirectly interacts with ERBB2, ERBB3, ERBB4, EGFR via Carom partner DAPP1, ITSN1, ITSN2, WAS, and WASL, C) Carom:UBC-receptor complex in which Carom may bind to 25 of 34 membrane receptors (ADRB1, ADRB2, CXCR4, EGFR, ERBB3, ERBB4, FGFR1, FGFR2, FGFR3, FGFR4, FLT1 (VEGFR1), FOLTR1, F2R, IGF1R, IGF2R, IL2RB, IL2RG, KDR (VEGFR2), LDLR, MET, NTRK1, PDGFRA, TFRC, TGFBR1, TGFBR2) through partner UBC. In addition, Carom directly interacts with partner GRASP, UBD and VCP (model D, E and F) and this aids in the transduction of their signals.
4.7. Carom co-localization with endocytic membrane receptors and partners in different cellular micro-compartment
Interacting complexes are more likely to be presented within the same cellular compartment. We analyzed subcellular localization of Carom-receptor complex proteins identified in Figure 4 using Compartments database from manually curated literature, high-throughput screens, automatic text mining, and sequence-based prediction methods. We found that Carom is located in 4 major cell compartments, PM, NC, CP and CSK with the highest confidential level in the NC (confidential level 5) (Table 4A). Carom endocytic partners (DAPP1, ITSN1/2, UBC, WAS and WASL) are distributed in multiple compartments and mostly co-localized with Carom in 4 major cell compartments, with the exception of DAPP1 which is not located in the CSK. Except for UBC and WAS, all Carom-receptor complex related partner proteins contain NLS.
Carom-related membrane receptors are localized in multiple cell compartments (Table 4B). While looking at the 4 Carom-existent cell compartments, most of the membrane receptors can co-localize with Carom, except that ADRB1 was only located on the PM and CP, and FOLR1 is not sited in CP and CSK. Interestingly, FGFR3 is located in the PM, CSK and NC, but not in CP. We identified 13 receptors which can be potentially trans-localized to the nucleus because of the recognized NC localization and the detected NLS.
4.8. Carom-receptor complex intracellular trafficking and function
We analyzed intracellular trafficking and function of Carom-receptor complexes identified in Figure 4. Three models of Carom-receptor complexes are listed as, A) Carom-TGFBR1, B) Carom:partner-ERBBs, and C) Carom:UBC-receptor (Table 5). Based on their co-localization in the subcellular compartment and NLS (Table 4), we characterized 3 different Carom-receptor complex intracellular trafficking patterns: 1) only in PM, 2) PM to CP/CSK, and 3) PM to NC. Among 13 potential nuclear trans-localized receptors identified (Table 4B), 9 receptors were found to interact with Carom via UBC which does not contain NLS (Table 4A). These 9 receptors were not justified as nuclear trans-localized receptors. The function of Carom-receptor complexes was determined based on signaling information related with its binding partner or receptor in literature.
As summarized in Table 5, the Carom-TGFBR1 complex can be transferred to CP and CSK via endocytosis for signaling transduction and lysosomal degradation (35). It can also be transported into the nucleus and participate in RNA processing (36). Carom:partner-ERBBs complex can be transferred to CP and CSK for signaling transduction and lysosomal degradation (37). Most of the Carom:partner-ERBBs complexes, except for Carom:DAPP1-ERBB3 and Carom:WAS-EGFR, can be transported into the nucleus to regulate transcription and DNA repair (38). The Carom:UBC-receptor complexes involve a large group of 24 receptors, including receptors for cytokine, growth factor, GPCR that are directed by UBC, which lacks NLS, and are subjected to ubiquitination and proteasome degradation (37, 39, 40). The Carom:UBC-FOLR1 complex may only stay in PM, because FOLR1 does not exist in CP and CSK.
5. DISCUSSION
Endocytotic trafficking of molecules is a highly regulated process involving multiple steps and molecules (Figure 1&2). In response to ligands stimulation, the BAR super family proteins can bind to cell membrane and bend to either positive or negative curvature. BAR proteins then recruit other adaptor proteins or accessory proteins to the deformed membrane to form endocytic vesicles. After endocytosis, cargos are destined to different subcellular organelles, including different endosome and lysosome.
Receptor trafficking is an important pathway for their signaling. The previous concept that receptor endocytosis would only contribute to its signal attenuation has already been challenged. Recent evidence demonstrated that receptor endocytosis and the following subcellular organelle redistribution regulate downstream signaling and gene regulation (41–44). As summarized in Table 1, at least 34 membrane receptors can be internalized via mostly CDE or CIE mediated endocytosis process, which contribute to their functions of regulating cell differentiation, proliferation, survival, angiogenesis, tumor transformation and immune regulation.
Recently, F-BAR proteins, a subfamily of BAR superfamily, have been identified as important coordinators that regulate endocytosis. In general, F-BAR proteins bind to the cell membrane via the association of F-BAR domain with membrane phospholipids. Through the SH3 domain, F-BAR proteins interact with WASP or GTPase dynamin to regulate the initiation and scission of the endocytic vesicle. We found out that at least 4 F-BAR protein subfamilies (CIP4, FCHO, PACSIN and NOSTRIN) are involved in the formation of endocytic vesicles and the assembly of endocytic complexes (Table 2) (7, 8). We listed 4 receptor endocytosis mechanisms, including CIP4 subfamily-related EGFR degradation, NOSTRIN subfamily-regulated FGFR signaling, FCHO2-regulated LDLR endocytosis and PACSIN3/PSTPIP1-regulated TFRC endocytosis. These findings presented fundamental mechanisms for F-BAR protein-mediated receptor endocytosis. F-BAR protein-mediated receptor endocytosis, although less studied, may play critical roles in growth control, angiogenesis and lipid metabolism.
The FCHSD subfamily has two members, FCHSD1 and FCHSD2, each containing of one F-BAR domain and two SH3 domains (Table 2). The biological function of FCHSD subfamily proteins may be related to F-actin polymerization based on their direct interaction with WASP in E. Coli to promote WASP-Arp2/3-dependent F-actin polymerization (7). WASP is known to bind to Arp2/3 complex, via its C-terminal, to nucleate actin filaments, which then elongate at their free barbed ends to induce F-actin polymerization (45, 46). FCHSD2 (Carom) is a newly identified FCHSD subfamily member with unknown function. It is suggested that acute myeloid leukemia (AML) patients with high Carom expression have increased leukemia chemoresistance. We have previously proposed that Carom may regulate membrane curvature, promote F-actin polymerization and recruit adaptor proteins via its partner in the process of CDE (25). Carom related membrane receptor and endocytosis have not been studied. We hypothesized that Carom may regulate receptor endocytosis via its partner proteins and identified 9 Carom endocytic partners (Table 3). We found that Carom partners DAPP1 can bind to ERBB3 and that ITSN2/UBC/WAS/WASL can bind to EGFR. ITSN1/UBC/UBD/VCP bind to both Carom and EGFR leading to EGFR ubiquitination and degradation (47). We hypothesize that Carom regulate EGFR and other receptor internalization and signaling via interaction with its endocytic partners.
In the efforts to explore the functional connection of Carom and related receptor, we examined cell type expression profile of Carom, its endocytic binding partners and membrane receptors in human tissues and primary cells (Figure 3). High level Carom expression was found paralleled with some highly expressed endocytosis-related membrane receptors and Carom partners in aortic endothelial cell, lymphocytes, monocytes and macrophages, which usually display robust endocytosis phenomena. These results indicate that Carom and its partners regulate endocytosis-related endothelial function and myeloid cell related innate immune function.
We further analyzed the subcellular localization of these proteins to search potential signal partners in cell organelles in Carom-related membrane receptors endocytosis (Table 4). We found that Carom is located in all major subcellular domains, including NC, CSK, CP and PM, which is a typical pattern of trafficking signal molecules. The co-localization relationship of Carom with different endocytic binding partners and membrane receptors is dynamic. It appears that Carom co-localizes with all membrane receptors and endocytic binding partners at plasma membrane, suggesting the critical role of Carom in the initial step of receptor endocytosis on plasma membrane. In the NC, Carom is co-localized with all partners and most of the receptors in the NC, except for ADRB1 and IL2RB. We identified NLS in Carom and hypothesize that Carom can be translocated into the nucleus and is responsible for taking the Carom:partner-receptor complexes into the nucleus, because that NC-localized receptors (FGFR1/2/3/4, ERBB3, FOLR1, F2R, TFRC, TGFBR2) and partners (UBC and WAS) do not have identified NLS. We found that except for FGFR3 and FOLR1, Carom and most of receptors and partners are also located in the CP, suggesting that a proportion of Carom:partner-receptor complexes can be disassociated from membrane structure and organelles, and released to the CP. The dynamic distribution of the component of Carom:partner-receptor complexes in various subcellular domain and organelles, including CSK, endosome (E), endoplasmic reticulum (ER), lysosome (Lys), mitochondrion (Mit) presented different intracellular trafficking pathways for Carom:partner-receptor complexes from CP to the NC.
Based on above findings, we presented three novel models for Carom-related receptor trafficking (Table 5 & Figure 5); Carom can A) directly bind to receptor (TGFBR1), B) indirectly binds to receptor through its partner (EGFR, ERBB and other yet characterized receptors) to initiate the formation of endocytic vesicle, and C3) facilitate membrane endocytosis through ubiquitination related proteins (UBC and UBD). Model C is likely responsible for endocytosis, ubiquitination and proteasome degradation of a large group of receptors. It is noticed that ubiquitin itself a sorting signal for membrane receptor endocytosis. There exist different sorting machineries that determine how receptors are selected by compartment specific ubiquitin-binding proteins and are delivered to cellular destination (48, 49). These three models suggest that Carom may play critical role in regulating intracellular trafficking and signaling of a large numbers of membrane receptors.
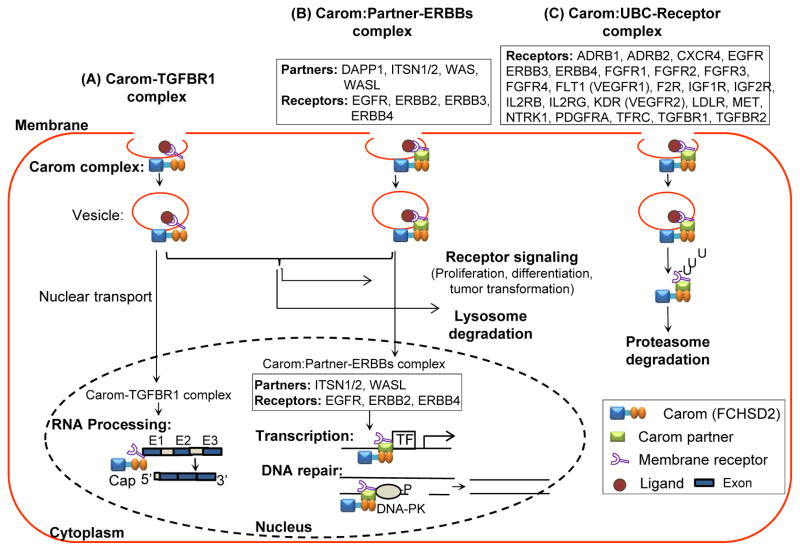
Figure 5. Hypothetic working model of Carom-mediated membrane receptor trafficking and endocytosis.
Carom can form 3 types of receptor complexes and mediate membrane receptor trafficking and endocytosis. A) Carom-TGFBR1 complex can enter nucleus via nuclear localization signal and facilitate RNA processing. B) Carom:Partner-ERBBs complexes that Carom associate with receptor via its partner, such as Carom:DAPP1-ERBB3; Carom:ITSN1-ERBB2, RBB4, EGFR; Carom:ITSN2-ERBB2, ERBB4, EGFR; Carom:WAS-EGFR; Carom:WASL-EGFR (details in table 5). Carom:Partner-ERBBs complex (Carom:ITSN1-ERBB2, RBB4, EGFR; Carom:ITSN2-ERBB2, ERBB4, EGFR; Carom:WASL-EGFR) can be transported into nucleus, bind to transcriptional factor and promote transcription, or bind to damaged DNA to facilitate DNA repair via activating DNA-PK. Both Carom-TGFBR1 and Carom:Partner-ERBBs complexes can facilitate receptor signaling in the cytoplasm leading to proliferation, differentiation and tumor transformation, and can be degradated in lysosome. C) Carom:UBC-Receptor complexes may bind to its partner UBC which further bind to 21 membrane receptor and facilitate ubiquitination and proteasome degradation. Abbreviation: DNA-PK, DNA-protein kinase, others refer to Table 1 and 3.
Transforming growth factor β (TGF-β) plays a critical role in embryogenesis and adult tissue development by regulating cell proliferation, differentiation, and migration (50). It is suggested that TGFBR1 plays an important regulatory role in TFG-β signaling via CDE to promote TGF-β-induced Smad activation and transcriptional regulation or Caveolae-dependent endocytosis to facilitate the degradation of TGF-β (35). We proposed a novel Carom-TGFBR1 complex for TGF-β signaling through database mining (Table 5). ERBBs family contains EGFR, ERBB2, ERBB3 and ERBB4. It is suggested that after ligand binding ERBBs are internalized mainly through CDE, which is followed by receptor activation or lysosomal degradation (51–53). Our data suggest that Carom can bind to ERBBs via its partners DAPP1, ITSN1/2, WAS and WASL, and such interaction complexes contribute to receptor endocytosis and control of signaling (Table 5). UBC gene encodes polyubiquitin-C protein, which is involved in the regulation of CDE and protein ubiquitination (54–56). It is reported. Through our analysis, we anticipate that Carom may mediate those 24 membrane receptor transport into cytoplasm and cytoskeleton via interacting with UBC for ubiquitination. As mentioned above, Carom may regulate receptor endocytosis through specific sorting machinery for individual receptor.
It is suggested that certain endocytic proteins translocate to the nucleus in response to extracellular signals which may affect gene transcription and chromatin remodeling machinery (57–59). The mechanism by which endocytic proteins enter the nucleus is based on NLS or interaction with carrier proteins (21). (Table 4). Carom and its endocytic partners (DAPP1, ITSN1-2 and WASL) were identified to have NLS through protein sequence analysis. Therefore, we propose that Carom-TGFBR1 and Carom:partner-ERBBs complexes may transport from the membrane to the nucleus to activate TGFBR1 and ERBB signaling for RNA processing, gene transcription or DNA repair (36, 60) (Table 5).
Traditional concept recognizes that the purpose of endocytosis of membrane receptors is to terminate receptor mediated signaling. However, it is now recognized that receptor internalization, especially for RTK families, is highly regulated via various mechanism. For example, EGFR and FGFR employ different molecular mechanisms for nuclear translocation (60, 61). Unlike EGFR which displays NLS, FGFR is translocated into nucleus from early endosome (62). EGFR endocytosis is required for optimal activation of sub-populations of signal transducers (63). EGFR endocytosis and post-endocytic traffic display versatile pathways and such traffic can lead to different cellular behaviors, such as proliferation, survival, tumorigenesis and DNA repair (Figure 5). Different KDR (VEGFR2) trafficking pathways via different subcellular compartments effect different cellular behaviors, ranging from proliferation, migration, tubulogenesis and blood vessel formation (42, 64). The proposed 3 models in Figure 5 presented a simple network. Studies to define the subcellular localization of Carom:partner-receptor complexes in early and late endosome, lysosome or trans Golgi, nucleus should provide strong evidence and discover relevant molecular mechanisms.
A more complex regulatory network could be involved in regulating Carom-mediated receptor endocytosis and trafficking to the nucleus, especially considering the two SH3 domains which can associate with many other adaptor proteins. SH3 signaling may lead to phosphorylation of the receptor and adaptor proteins. The detailed mechanisms of surface receptors translocation to nucleus are largely unveiled. Whether the nuclear translocated receptors come from receptors embedded in endosomes are under debate and requires experimental clarification (61, 65).
The combinations of various bioinformatics tools employed in the study is very powerful for the identification of protein complexes involving complicated intracellular trafficking mechanism. While more and more online large databases become available, it is possible to develop model systems regulating important biological process and to predict molecular targets. The identified mechanistic model system can be important guidance for future experimental science and may lead to the discovery of novel mechanism for human disease and therapeutic targets.
6. CONCLUSIONS
In this study, we identified 34 endocytic membrane receptors and 9 Carom endocytic partners and established their expression profiles in human primary cells. We established 3 models of Carom-receptor complexes and their intracellular trafficking based on protein-protein interaction and subcellular localization. We propose that F-BAR protein Carom may mediate receptor endocytosis and transport endocytic receptors to the cytoplasm for receptor signaling and lysosome/proteasome degradation, or to the nucleus for RNA processing, gene transcription and DNA repair.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) Grants number: HL67033, HL77288, HL82774, HL110764 and HL117654 (HW); HL9445, HL108910 and HL116917 (XFY). The authors declare that they have no conflict of interest.
Abbreviation
- CIP4
CDC42-interacting protein 4
- CRIB
CDC42-Rac interactive binding
- CP
Cytoplasmic
- CSK
cytoskeleton
- ECM
Extracellular matrix
- F-actin
Filamentous actin
- F-BAR
Fes/CIP4 homology-Bin/Amphiphysin/Rvs
- FCHO
FCH only
- FCHSD
FCH and double SH3 domain proteins
- FER
FES related
- FX
F-BAR extension
- GAS7
Growth arrest-specific 7
- HR1
Protein kinase C-related kinase homology region 1
- NOSTRIN
Nitric oxide synthase traffic inducer
- N-WASP
Neural Wiskott-Aldrich syndrome protein
- NC
Nucleus
- PACSIN
Protein kinase C and casein kinase 2 substrates in neurons
- PH
Pleckstrin homology
- PM
plasma membrane
- RhoGAP
Rho GTPase-activating protein
- SH2
Src homology-2
- SH3
Src homology-3
- srGAP
Slit-Robo GTPase-activating protein
- μHD
μ-homology domain
- VCA
Verprolin, cofilin, acidic
- WASP
Wiskott-Aldrich syndrome protein
- WAVE
WASP family verproline-homologous protein
- WH1/2
WASP-Homology 1/2
References
- 1.Du Toit A. Endocytosis. A new gateway into cells. Nat Rev Mol Cell Biol. 2015;16(2):68. doi: 10.1038/nrm3939. [DOI] [PubMed] [Google Scholar]
- 2.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517–33. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 3.Cheng ZJ, Singh RD, Marks DL, Pagano RE. Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids. Mol Membr Biol. 2006;23(1):101–10. doi: 10.1080/09687860500460041. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar RC, Wendland B. Endocytosis of membrane receptors: two pathways are better than one. Proc Natl Acad Sci U S A. 2005;102(8):2679–80. doi: 10.1073/pnas.0500213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kourouniotis G, Wang Y, Pennock S, Chen X, Wang Z. Non-Ligand-Induced Dimerization is Sufficient to Initiate the Signalling and Endocytosis of EGF Receptor. Int J Mol Sci. 2016;17(8) doi: 10.3390/ijms17081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poole DP, Bunnett NW. G Protein-Coupled Receptor Trafficking and Signalling in the Enteric Nervous System: The Past, Present and Future. Adv Exp Med Biol. 2016;891:145–52. doi: 10.1007/978-3-319-27592-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao H, Yin X, Cao Y, Jin Y, Wang S, Kong Y, Chen Y, Gao J, Heller S, Xu Z. FCHSD1 and FCHSD2 are expressed in hair cell stereocilia and cuticular plate and regulate actin polymerization in vitro. PLoS One. 2013;8(2):e56516. doi: 10.1371/journal.pone.0056516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328(5983):1281–4. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Gong R, Li X, Virtue A, Yang F, Yang IH, Tran AH, Yang XF, Wang H. Identification of novel pretranslational regulatory mechanisms for NF-kappaB activation. J Biol Chem. 2013;288(22):15628–40. doi: 10.1074/jbc.M113.460626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen NC, Yang F, Capecci LM, Gu Z, Schafer AI, Durante W, Yang XF, Wang H. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J. 2010;24(8):2804–17. doi: 10.1096/fj.09-143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF. IL-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. PLoS One. 2012;7(3):e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iioka H, Macara IG. Detection of RNA-Protein Interactions Using Tethered RNA Affinity Capture. Methods Mol Biol. 2015;1316:67–73. doi: 10.1007/978-1-4939-2730-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW, Luga V, Przulj N, Robinson M, Suzuki H, Hayashizaki Y, Jurisica I, Wrana JL. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307(5715):1621–5. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- 14.Kotlyar M, Pastrello C, Pivetta F, Lo Sardo A, Cumbaa C, Li H, Naranian T, Niu Y, Ding Z, Vafaee F, Broackes-Carter F, Petschnigg J, Mills GB, Jurisicova A, Stagljar I, Maestro R, Jurisica I. In silico prediction of physical protein interactions and characterization of interactome orphans. Nat Methods. 2015;12(1):79–84. doi: 10.1038/nmeth.3178. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Hunter T. Degradation of activated protein kinases by ubiquitination. Annu Rev Biochem. 2009;78:435–75. doi: 10.1146/annurev.biochem.013008.092711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galovic M, Xu D, Areces LB, van der Kammen R, Innocenti M. Interplay between N-WASP and CK2 optimizes clathrin-mediated endocytosis of EGFR. J Cell Sci. 2011;124(Pt 12):2001–12. doi: 10.1242/jcs.081182. [DOI] [PubMed] [Google Scholar]
- 17.Jones RB, Gordus A, Krall JA, MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439(7073):168–74. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- 18.Tong J, Taylor P, Peterman SM, Prakash A, Moran MF. Epidermal growth factor receptor phosphorylation sites Ser991 and Tyr998 are implicated in the regulation of receptor endocytosis and phosphorylations at Ser1039 and Thr1041. Mol Cell Proteomics. 2009;8(9):2131–44. doi: 10.1074/mcp.M900148-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bornke F. Corrigendum: The complex becomes more complex: protein-protein interactions of SnRK1 with DUF581 family proteins provide a framework for cell- and stimulus type-specific SnRK1 signaling in plants. Front Plant Sci. 2014;5:693. doi: 10.3389/fpls.2014.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang X, Hu X, Yang X, Fan Y, Li Y, Hu W, Liao Y, Zheng MC, Peng W, Gao L. Predicting diabetes mellitus genes via protein-protein interaction and protein subcellular localization information. BMC Genomics. 2016;17(Suppl 4):433. doi: 10.1186/s12864-016-2795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyrzynska B, Pilecka I, Miaczynska M. Endocytic proteins in the regulation of nuclear signaling, transcription and tumorigenesis. Mol Oncol. 2009;3(4):321–38. doi: 10.1016/j.molonc.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosesson Y, Shtiegman K, Katz M, Zwang Y, Vereb G, Szollosi J, Yarden Y. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J Biol Chem. 2003;278(24):21323–6. doi: 10.1074/jbc.C300096200. [DOI] [PubMed] [Google Scholar]
- 23.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6(2):112–26. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 24.Basquin C, Trichet M, Vihinen H, Malarde V, Lagache T, Ripoll L, Jokitalo E, Olivo-Marin JC, Gautreau A, Sauvonnet N. Membrane protrusion powers clathrin-independent endocytosis of interleukin-2 receptor. EMBO J. 2015;34(16):2147–61. doi: 10.15252/embj.201490788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Xiong X, Zhao X, Yang X, Wang H. F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases. J Hematol Oncol. 2015;8:47. doi: 10.1186/s13045-015-0144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarsch IK, Daste F, Gallop JL. Membrane curvature in cell biology: An integration of molecular mechanisms. J Cell Biol. 2016;214(4):375–87. doi: 10.1083/jcb.201604003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sousa R, Liao HS, Cuellar J, Jin S, Valpuesta JM, Jin AJ, Lafer EM. Clathrin-coat disassembly illuminates the mechanisms of Hsp70 force generation. Nat Struct Mol Biol. 2016;23(9):821–9. doi: 10.1038/nsmb.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, Troglio F, Mukhopadhyay A, Everingham S, Kwok E, Scita G, Craig AW. F-BAR-containing adaptor CIP4 localizes to early endosomes and regulates Epidermal Growth Factor Receptor trafficking and downregulation. Cell Signal. 2009;21(11):1686–97. doi: 10.1016/j.cellsig.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Mulkearns EE, Cooper JA. FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis. Mol Biol Cell. 2012;23(7):1330–42. doi: 10.1091/mbc.E11-09-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Kreuk BJ, Anthony EC, Geerts D, Hordijk PL. The F-BAR protein PACSIN2 regulates epidermal growth factor receptor internalization. J Biol Chem. 2012;287(52):43438–53. doi: 10.1074/jbc.M112.391078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper KM, Bennin DA, Huttenlocher A. The PCH family member proline-serine-threonine phosphatase-interacting protein 1 targets to the leukocyte uropod and regulates directed cell migration. Mol Biol Cell. 2008;19(8):3180–91. doi: 10.1091/mbc.E08-02-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Xiong X, Thomas SV, Xu Y, Cheng X, Zhao X, Yang X, Wang H. Analysis for Carom complex, signaling and function by database mining. Front Biosci (Landmark Ed) 2016;21:856–72. doi: 10.2741/4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacevic I, Hu J, Siehoff-Icking A, Opitz N, Griffin A, Perkins AC, Munn AL, Muller-Esterl W, Popp R, Fleming I, Jungblut B, Hoffmeister M, Oess S. The F-BAR protein NOSTRIN participates in FGF signal transduction and vascular development. EMBO J. 2012;31(15):3309–22. doi: 10.1038/emboj.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modregger J, Ritter B, Witter B, Paulsson M, Plomann M. All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J Cell Sci. 2000;113(Pt 24):4511–21. doi: 10.1242/jcs.113.24.4511. [DOI] [PubMed] [Google Scholar]
- 35.Bizet AA, Liu K, Tran-Khanh N, Saksena A, Vorstenbosch J, Finnson KW, Buschmann MD, Philip A. The TGF-beta co-receptor, CD109, promotes internalization and degradation of TGF-beta receptors. Biochim Biophys Acta. 2011;1813(5):742–53. doi: 10.1016/j.bbamcr.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 36.Chandra M, Zang S, Li H, Zimmerman LJ, Champer J, Tsuyada A, Chow A, Zhou W, Yu Y, Gao H, Ren X, Lin RJ, Wang SE. Nuclear translocation of type I transforming growth factor beta receptor confers a novel function in RNA processing. Mol Cell Biol. 2012;32(12):2183–95. doi: 10.1128/MCB.00320-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alwan HA, van Zoelen EJ, van Leeuwen JE. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J Biol Chem. 2003;278(37):35781-90. doi: 10.1074/jbc.M301326200. [DOI] [PubMed] [Google Scholar]
- 38.Wang YN, Hung MC. Nuclear functions and subcellular trafficking mechanisms of the epidermal growth factor receptor family. Cell Biosci. 2012;2(1):13. doi: 10.1186/2045-3701-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12(23):3663–74. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long X, Nephew KP. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J Biol Chem. 2006;281(14):9607–15. doi: 10.1074/jbc.M510809200. [DOI] [PubMed] [Google Scholar]
- 41.Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24(1):26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horowitz A, Seerapu HR. Regulation of VEGF signaling by membrane traffic. Cell Signal. 2012;24(9):1810–20. doi: 10.1016/j.cellsig.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balogh P, Katz S, Kiss AL. The role of endocytic pathways in TGF-beta signaling. Pathol Oncol Res. 2013;19(2):141–8. doi: 10.1007/s12253-012-9595-8. [DOI] [PubMed] [Google Scholar]
- 44.Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2013;5(5):a017459. doi: 10.1101/cshperspect.a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee WL, Bezanilla M, Pollard TD. Fission yeast myosin-I, Myo1p, stimulates actin assembly by Arp2/3 complex and shares functions with WASp. J Cell Biol. 2000;151(4):789–800. doi: 10.1083/jcb.151.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padrick SB, Doolittle LK, Brautigam CA, King DS, Rosen MK. Arp2/3 complex is bound and activated by two WASP proteins. Proc Natl Acad Sci U S A. 2011;108(33):E472-9. doi: 10.1073/pnas.1100236108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okur MN, Ooi J, Fong CW, Martinez N, Garcia-Dominguez C, Rojas JM, Guy G, O'Bryan JP. Intersectin 1 enhances Cbl ubiquitylation of epidermal growth factor receptor through regulation of Sprouty2-Cbl interaction. Mol Cell Biol. 2012;32(4):817–25. doi: 10.1128/MCB.05647-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piper RC, Dikic I, Lukacs GL. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb Perspect Biol. 2014;6(1) doi: 10.1101/cshperspect.a016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci. 2012;125(Pt 2):265–75. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- 50.Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MP, Pedersen LB, Benmerah A, Andersen CY, Larsen LA, Christensen ST. TGF-beta signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep. 2013;3(6):1806–14. doi: 10.1016/j.celrep.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 51.Sak MM, Breen K, Ronning SB, Pedersen NM, Bertelsen V, Stang E, Madshus IH. The oncoprotein ErbB3 is endocytosed in the absence of added ligand in a clathrin-dependent manner. Carcinogenesis. 2012;33(5):1031–9. doi: 10.1093/carcin/bgs128. [DOI] [PubMed] [Google Scholar]
- 52.Henriksen L, Grandal MV, Knudsen SL, van Deurs B, Grovdal LM. Internalization mechanisms of the epidermal growth factor receptor after activation with different ligands. PLoS One. 2013;8(3):e58148. doi: 10.1371/journal.pone.0058148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roepstorff K, Grovdal L, Grandal M, Lerdrup M, van Deurs B. Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem Cell Biol. 2008;129(5):563–78. doi: 10.1007/s00418-008-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhen M, Heinlein R, Jones D, Jentsch S, Candido EP. The ubc-2 gene of Caenorhabditis elegans encodes a ubiquitin-conjugating enzyme involved in selective protein degradation. Mol Cell Biol. 1993;13(3):1371–7. doi: 10.1128/mcb.13.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogel MR, Jaitovich A, Ridge KM. The role of the ubiquitin proteasome pathway in keratin intermediate filament protein degradation. Proc Am Thorac Soc. 2010;7(1):71–6. doi: 10.1513/pats.200908-089JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev Cell. 2012;23(6):1129–40. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, Sali A, Rout MP. The molecular architecture of the nuclear pore complex. Nature. 2007;450(7170):695–701. doi: 10.1038/nature06405. [DOI] [PubMed] [Google Scholar]
- 58.Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) mediates transforming growth factor beta (TGFbeta)-stimulated fibronectin synthesis through TGFbeta-activated kinase 1 and activation of the JNK pathway. J Biol Chem. 2005;280(27):25920–7. doi: 10.1074/jbc.M501150200. [DOI] [PubMed] [Google Scholar]
- 59.Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, Yang R, Fan F, Chen X, Pei G, Ma L. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123(5):833–47. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Brand TM, Iida M, Li C, Wheeler DL. The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov Med. 2011;12(66):419–32. [PMC free article] [PubMed] [Google Scholar]
- 61.Wang YN, Lee HH, Lee HJ, Du Y, Yamaguchi H, Hung MC. Membrane-bound trafficking regulates nuclear transport of integral epidermal growth factor receptor (EGFR) and ErbB-2. J Biol Chem. 2012;287(20):16869–79. doi: 10.1074/jbc.M111.314799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bryant DM, Wylie FG, Stow JL. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by E-cadherin. Mol Biol Cell. 2005;16(1):14–23. doi: 10.1091/mbc.E04-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274(5295):2086–9. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 64.Jopling HM, Odell AF, Pellet-Many C, Latham AM, Frankel P, Sivaprasadarao A, Walker JH, Zachary IC, Ponnambalam S. Endosome-to-Plasma Membrane Recycling of VEGFR2 Receptor Tyrosine Kinase Regulates Endothelial Function and Blood Vessel Formation. Cells. 2014;3(2):363–85. doi: 10.3390/cells3020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Domingues I, Rino J, Demmers JA, de Lanerolle P, Santos SC. VEGFR2 translocates to the nucleus to regulate its own transcription. PLoS One. 2011;6(9):e25668. doi: 10.1371/journal.pone.0025668. [DOI] [PMC free article] [PubMed] [Google Scholar]