Abstract
Infections due to Cryptococcus are a leading cause of fungal infections worldwide and are acquired as a result of environmental exposure to desiccated yeast or spores. The ability of Cryptococcus to grow, mate, and produce infectious propagules in association with plants is important for the maintenance of the genetic diversity and virulence factors important for infection of animals and humans. In the Western United States and Canada, Cryptococcus has been associated with conifers and tree species other than Eucalyptus; however, to date Cryptococcus has only been studied on live Arabidopsis thaliana, Eucalyptus sp., and Terminalia catappa (almond) seedlings. Previous research has demonstrated the ability of Cryptococcus to colonize live plants, leaves, and vasculature. We investigated the ability of Cryptococcus to grow on live seedlings of the angiosperms, A. thaliana, Eucalyptus camaldulensis, Colophospermum mopane, and the gymnosperms, Pseudotsuga menziesii (Douglas fir), and Tsuga heterophylla (Western hemlock). We observed a broad-range ability of Cryptococcus to colonize both traditional infection models as well as newly tested conifer species. Furthermore, C. neoformans, C. deneoformans, C. gattii (VGI), C. deuterogattii (VGII) and C. bacillisporus (VGIII) were able to colonize live plant leaves and needles but also undergo filamentation and mating on agar seeded with plant materials or in saprobic association with dead plant materials. The ability of Cryptococcus to grow and undergo filamentation and reproduction in saprobic association with both angiosperms and gymnosperms highlights an important role of plant debris in the sexual cycle and exposure to infectious propagules. This study highlights the broad importance of plants (and plant debris) as the ecological niche and reservoirs of infectious propagules of Cryptococcus in the environment.
Introduction
Over 200 species of opportunistic fungi have been recognized as human pathogens over the last decade of which Candida, Aspergillus, and Cryptococcus species comprise the three most dominant opportunistic fungal infections worldwide [1]. Immunocompromised host status due to HIV/AIDS, diabetes, cancer, organ transplant, various prescribed medical treatments (immunosuppressive medications, steroids), and other genetic abnormalities are associated with increased risk of opportunistic fungal infections although infections in otherwise healthy individuals do occur less frequently [1, 2]. The development of new antifungals, highly active antiretroviral treatment (HAART), and combination therapy regimes have greatly increased long-term survival rates but infections due to opportunistic fungi remain difficult to control and frequently recur.
Cryptococcus is a dimorphic, environmental, basidiomycete that can occur as a yeast or in a filamentous/hyphal form that can cause fatal pulmonary and neurological infections if left untreated. Infections result from inhalation of infectious propagules (yeast or basidiospores) from the environment, which can have various length of incubation within the host prior to the development of overt disease. Progressive infections can disseminate, leading to systemic infections involving the brain and central nervous system that are often fatal, even with aggressive treatment. Historically, two major lineages, Cryptococcus neoformans and Cryptococcus gattii, of the pathogenic species complex were formerly described within the pathogenic species complex now delineated into seven species [3]. However, other species including C. laurentii, C. albidus, C. uzbekistanensis, C. adeliensis, C. curvatus, C. magnus, C. humicolus, C. luteolus, C. macerans, C. flavescens, and C. uniguttulatus have been associated with sporadic infections in immunocompromised individuals and other animals [4–7]. Cryptococcus is also associated with an ever increasing number of infections in seemingly healthy humans and animals in some regions of the world.
C. neoformans is now speciated into C. deneoformans (AFLP2, molecular type VNIV serotype D, and formerly C. neoformans), C. neoformans (molecular type VNI/AFLP1, VNII/AFLP1A/AFLP1B, and VNB/AFLP1; formerly C. neoformans var. grubii), and C. deneoformans and C. neoformans hybrids (VNIII/AFLP3). Five molecular types formerly recognized as C. gattii are currently described as five species, C. gattii (AFLP4, VGI, serotype B), C. deuterogattii (AFLP6, VGII, serotype B), C. bacillisporus (AFLP5, VGIII, serotype B and C), and C. tetragattii (AFLP7, VGIV, Serotype C) C. decagattii (AFLP10, VGIV). Rare interspecies hybrids have been identified between C. deneoformans, C. neoformans, C. gattii, and C. deuterogattii which are primarily isolated from clinical samples. Infections caused by C. neoformans are the most common worldwide and within the United States but in California C. bacillisporus VGIII is responsible for a majority of the naturally acquired cryptococcal infections in immunocompromised hosts, although VGI and VGII infections have also been reported [3, 8–11].
Infections associated with Cryptococcosis and Aspergillus are distinct from Candida because Candida is a natural component of the human microbiome, whereas Aspergillus and Cryptococcus are acquired from environmental exposure [12]. Infections initiated by Cryptococcus are unique from those initiated by Aspergillus because they require specific exposure to restricted ecological reservoirs, whereas spores of Aspergillus are ubiquitously dispersed in the air we breathe [13–15]. Periodic disease outbreaks of Cryptococcus, Coccidioides immitis/posadasii, Blastomyces dermatitidis, Histoplasma capsulatum, Sporothrix schenckii, or Apophysomyces trapeziformis are known to occur as a result of naturally occurring (earthquakes, tornadoes, or dust storms,) or human-initiated (construction, landscaping, or forestry practices) disturbances within specific ecological niches [16–26]. Therefore, the environmental reservoirs of Cryptococcus, the genetic diversity contained within these reservoirs, and the frequency of exposure to these reservoirs can greatly influence the risk of cryptococcal disease in immunocompromised individuals.
The environment plays an essential role as the exposure reservoir and breeding ground for the propagation and dispersal of infectious propagules (desiccated yeast and spores). Historically, C. deneoformans/C. neoformans was associated with pigeon and bird guano. Mating was first observed and described as the result of incubation on malt extract [27], sporulation agar [27], minimal medium minus thiamine agar [28], V8 agar [28], and more recently described on pigeon guano [29], in association with live plants [30], and on plant debris agars [31]. Cryptococcus gattii was distinguished from C. neoformans in the 1970’s and matings were observed and described on sporulation agar [32], V8 agar [33] and A. thaliana plants [30]. Laboratory matings of C. gattii (VGI, VGII, VGIII) are slower to progress and are less frequently observed than those involving C. neoformans isolates. Hybrid interspecies and intermolecular type matings and have been demonstrated in the laboratory but were associated with reduced viability [34, 35]. Less frequently, productive hybrid-matings can result in hybrid vigor, enhanced virulence, and increased antifungal resistance in hosts [34–36]. Opposite (MATα + MATa) and same-sex (MATα + MATα) matings were first described for C. neoformans/C. deneoformans and later identified in C. gattii (VGI, VGII, VGIII) [27, 37]. The accumulation of 40 years of research now illustrates that both C. neoformans, C. deneoformans, C. deuterogattii, C. bacillisporus, and C. gattii can complete their lifecycle in the environment. Whereas C. deneoformans is more frequently isolated from bird guano, C. gattii (VGI, VGII, VGIII, and VGIV) is more frequently isolate from trees, and C. neoformans is associated with both bird guano and trees. Filamentation, mating, and the production of spores in association with plants may enhance nutrient accumulation and dispersal in the environmental niche [38]. Therefore, the genetic drivers of pathogenicity and virulence are a direct result of interactions and selective forces Cryptococcus encounters in the environment. In Western Canada and the Western United States, Cryptococcus has been associated with many tree species, but to date Cryptococcus has only been studied on live A. thaliana, Eucalyptus sp., and Terminalia catappa (Almond) seedlings. However C. deuterogattii (VGII) is frequently associated with conifers in the Western USA and information pertaining to the growth of Cryptococcus in these naturally associated plant species is lacking [30, 31, 39–42]. We therefore sought to extend the known interaction of Cryptococcus with plant host plants encountered within the United States, including live conifers and saprobic matter to further understand the role of plants in the infectious lifecycle and virulence of Cryptococcus.
Methods
Arabidopsis thaliana infection model
Cryptococcus cells were subcultured twice in yeast peptone dextrose (YPD) broth at 25°C shaking at 180 rpm. Cells were collected by centrifugation, washed in ddH2O, and resuspended to OD600 = 0.1 (~ 107 cells/mL) in autoclaved ddH2O. Four whole leaves of three- to four-week old soil-grown A. thaliana plants (Col-0 wildtype, jar1-1, or npr1-1) were infiltrated with a suspension of Cryptococcus cells. Plants were incubated in 16 hours light at 24°C and 8 hours of dark at 20°C, or 12 hours of light and 12 hours of darkness.
One leaf per plant was harvested, homogenized in ddH2O, and plated onto YPD and Niger seed agar to analyze colony forming units (CFUs). Plates were incubated at 30°C for two to five days and CFUs were counted.
Conifer, Mopane, and Eucalyptus infection models
Mopane (Colophospermum mopane, B and T World Seeds: http://b-and-t-world-seeds.com) and Eucalyptus seedlings (Eucalyptus camaldulensis, B and T World Seeds: http://b-and-t-world-seeds.com) were soil-grown from seed. Two- to four-year-old, non-sterile, soil-grown Douglas fir (Pseudotsuga menziesii) and Western hemlock (Tsuga heterophylla) seedlings were purchased from Musser Forest Inc. (https://www.musserforests.com). Cryptococcus cells were subcultured twice in YPD broth at 25°C with the appropriate drug selection nourseothricin [NAT 10 mg/L], neomycin [NEO 10 mg/L], or hygromycin [HYG 10 mg/L] respective to strain genotype (30°C, 180 rpm shaking). Cryptococcus cells were then collected by centrifugation, washed in ddH2O, and resuspended to OD600 = 0.1 (~ 107 cells/mL) in autoclaved ddH2O.
Individual and mating mixtures of C. deneoformans, C. neoformans or C. gattii, C. deuterogattii, or C. bacillisporus were inoculated on three different branches or leaves of the same individual plant and replicated on three individual plants. For Mopane, Eucalyptus, Douglas fir, and Eastern hemlock experiments multiple leaves/branches of the same plant were used for different treatments, starting from top, ddH2O control, 3 branches for Cryptococcus inoculation (Strain 1, Strain 2, Strain 1 + Strain 2), and remaining branches served as inoculated controls. 20 μL of a OD600 = 0.1 Cryptococcus cell suspension (~2.0 x 105 cells) were drop inoculated onto the adaxial surface of two to three Mopane or Eucalyptus leaves. Three individual branch tips of Douglas fir or Western hemlock seedlings (composed of multiple needles and emerging buds) were dipped into the OD600 = 0.1 cell suspension, excesses drips were blotted off with sterile Kimtech Science* KIMWIPES*, and allowed to air dry in a BSLIII hood. Additionally, one branch per tree was dip-inoculated with autoclaved ddH2O as experimental controls. Plants were incubated in an enclosed incubation chamber in 12 hours of light and 12 hours of dark that temperatures ranged from 30°C to 25°C respectively. Plant leaves or needles were harvested three weeks post-infection, homogenized in 1 mL autoclaved ddH2O and plated on YPD, YPD + NAT (100 mg/L), YPD + NEO (100 mg/L), and YPD + NAT (100 mg/L) + NEO (100 mg/L).
Growth and mating on plant materials and agar
Freshly grown Cryptococcus cells were harvested from YPD agar plates and suspended in 1 mL autoclaved ddH2O and OD600 was measured. Cells were diluted to OD600 = 1.0. Then, 10 μL of each strain were spotted individually or in combination with the opposite mating partner on agar or directly on autoclaved plant materials placed on top of 2% water agar (20 g Difco Bacto® agar/L) in petri-plates. Plant-based agars contained 30 g Difco Bacto® agar + 20 g ground plant materials (fresh A. thaliana plants, Black cherry (Prunus serotina) wood chips, Almond, Coco, Long leaf pine (Pinus palustris) needles, pine wood chips, Sugar maple (Acer saccharum) wood chips, Western red cedar (Thuja plicata), Douglas fir (Pseudotsuga menziesii), Western hemlock (Tsuga heterophylla) wood chips, or Mopane wood chips + 1 mL 20% glucose per L and pH was not adjusted. Plant materials were ground uniformly with a coffee bean grinder that was cleaned thoroughly with water and ethanol between each preparation. Black cherry and sugar maple wood chips (obtained from Dr. Paul Manion, Cazenovia, New York); A. thaliana plants (Dong lab, Duke University); Almonds (Whole, dry, and non-salted, Kroger brand); pine shavings (Petco, bedding); Douglas fir, Western red cedar, and Hemlock chips (obtained from Jamie, Murry, GEM Shavings, Auburn, Washington) were homogenized in a spice grinder. Coca agar was composed of 20 g Hershey’s powder cocoa + 20 g Difco Bacto® agar and pH was not adjusted. V8 agar pH 5, V8 agar pH 7, MS agar, Niger seed agar, and Filament agar were made following standard methodology. V8 fusion agar was made substituting V8 Strawberry Banana fusion juice (50 mL) for standard V8 juice + 0.5 g KH2PO4 + 30 g Difco Bacto® agar, and pH was adjusted to pH 5 or pH 7. Fusion agar consisted of 100 mL V8 Strawberry Banana fusion juice + 30 g Difco Bacto® agar and pH was not adjusted.
Tree needles (Hemlock, Long leaf pine, Eastern red cedar), leaves (Sugar maple), wood chips (Black cherry), Pine button plug (General Unfinished Flat Head no.315038 pine, Home Depot), or Oak button plugs (General Unfinished Flat Head no.313038 oak, Home Depot) were autoclaved, allowed to cool overnight, and aseptically placed in petri plates on top of 2% water agar before being inoculated with 15 μL of H99α, KN99a, or H99α + KN99a mating mixture; JEC21α, JEC20a, or JEC21α + JEC20a mating mixture; or NIH444α, NIH194a, or NIH444α + NIH194a mating mixture. Plates were incubated in a dark drawer at room temperature and observed weekly for the production of hyphae or basidia.
In vivo murine model
Six-week-old female A/JCr mice (Cat. No. 01A24, NCI-Frederick) or male BALB/c mice (Cat. No. 01B05, NCI-Frederick) were used. All animal studies were conducted in the Division of Laboratory Animal Resources (DLAR) facilities at Duke University Medical Center (DUMC) and animals were handled according to the guidelines defined by the United States Animal Welfare Act and in full compliance with the DUMC Institutional Animal Care Use Committee (IACUC). Animal models were reviewed and approved by DUMC IACUC under IACUC protocol # A217-11-08. Mice were acclimated in the facility for one week prior to infection and were housed in cages at 21°C and 50% humidity with a 12 hrs. light/12 hrs. dark cycle and given ample food and water daily. Cryptococcus cells were grown on Arabidopsis agar (20 g/L A. thaliana plants + 20 g/L agar) at 30°C for six days. Cells were harvested by scraping and washing from the agarose plates with autoclaved ddH2O and resuspended in 10 mL of autoclaved ddH2O. Cell were counted with a hemocytometer and resuspended to 2 x 106 cells/mL. All mice were sedated with Nembutal (sodium pentobarbital) prior to intranasal inoculation with 106 Cryptococcus cells in 40 μl. We expect the mice will become ill from inoculation with Cryptococcus which may exhibit as social isolation, lack of grooming, weight loss, loss of balance, inability to feed, loss of sternal recumbency, limb paralysis, seizures, convulsion, and coma. Animals were monitored daily by the primary author and Duke University DLAR staff for signs of disease development, distress, or suffering as described previously and were euthanized utilizing CO2 when weight loss ≥ 15% of original body weight or they exhibited neurological symptoms or cranial swelling. Kaplan-Meier survival curves were constructed by GraphPad Prism version 6.03 (Windows, GraphPad Software, La Jolla California USA, www.graphpad.com).
Electron microscopy
To examine mating reactions for morphological features associated with mating, scanning electron microscopy studies were conducted. First, one centimeter square blocks of agar, whole needles, or sectioned leaf tissues were collected and fixed in 2% glutaraldehyde (Electron Microscopy Sciences, EMS, Hatfield, PA, USA) with 0.05% malachite green oxalate (EMS) in 0.1 M sodium cacodylate buffer and incubated at 4°C until further processing. The fixation buffer was removed, and blocks were dehydrated by ethanol series, critical point dried (Pelco CPD2, Ted Pella, Inc., Redding, California, USA), sputter coated, and imaged with the FEI XL30 SEM-FEG (FEI Company, Hillsboro, Oregon, USA) at the electron microscopy facility at North Carolina State University.
Results
Cryptococcus colonizes live Arabidopsis thaliana plants
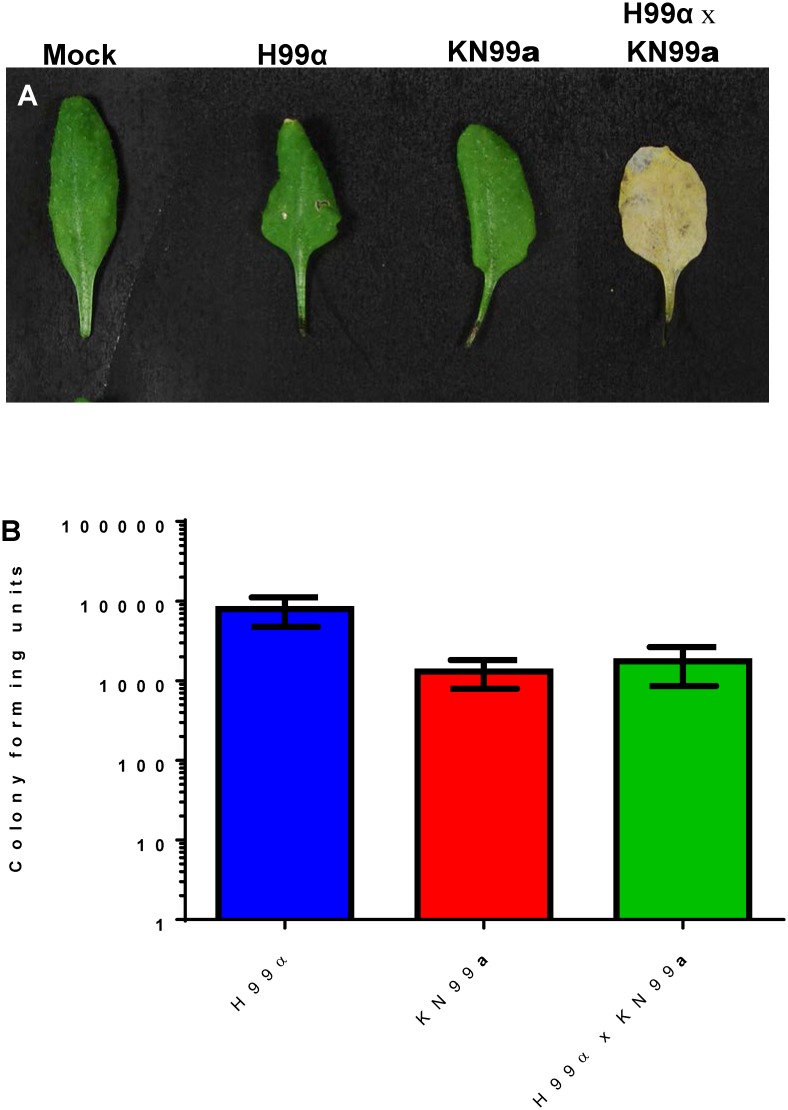
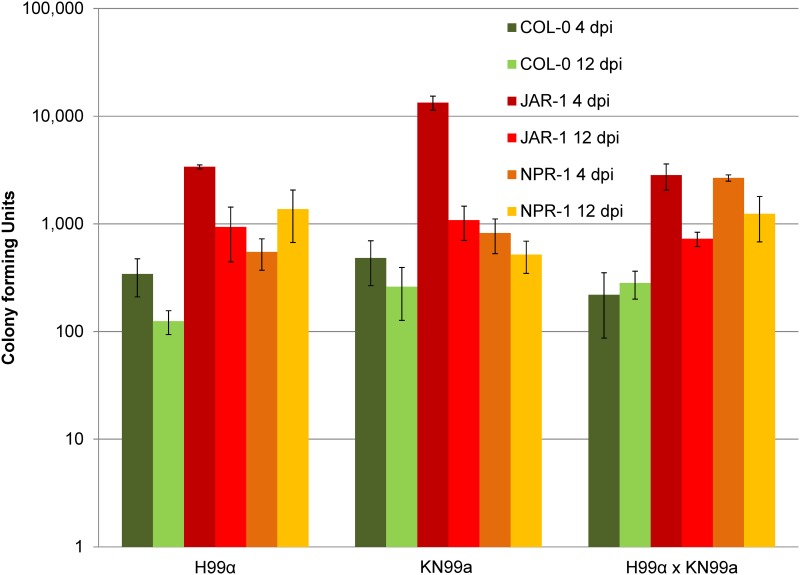
Cryptococcus can colonize a broad variety of live plants. We demonstrate that C. neoformans (VNIV, Fig 1) C. deuterogattii (VGII), and C. gattii (VGI, S1 Fig) can colonize mature soil grown A. thaliana plants as previously described [30, 39]. Mature soil grown A. thaliana plants were colonized more readily by H99α in comparison to KN99a (p < 0.0001). Survival and colonization of A. thaliana by Cryptococcus is strain-dependent (S1 Fig). Colonization of A. thaliana by C. neoformans is not dependent on laccase, capsule, or calcineurin (p > 0.100). However, we observed reduced colonization of C. deuterogattii (VGII) calcineurin (cna1Δ) mutant strains on A. thaliana plants (p < 0.001) S1 Fig). Chlorotic symptoms were only observed in leaves inoculated with mated pairs (MATa x MATα) and were not correlated with increased fungal colonization (Fig 1, KN99a versus H99α x KN99a, p > 0.100). A. thaliana mutants jar1-1 (p < 0.001) and npr1-1 (12 dpi and H99α x KN99a p < 0.001) were more permissible to C. neoformans colonization in comparison to Wild-type (Col-0) (Fig 2). Scanning electron microscopy indicates that C. neoformans colonizes live soil-grown wild type and mutant lines of A. thaliana (S2 Fig). Wild-type (Col-0) and jar1-1 A. thaliana seedlings inoculated with mated mixtures of C. neoformans (H99α x KN99a) displayed reduced growth and increased pigmentation (likely anthocyanin) in comparison to seedlings inoculated with individual strains (S3 Fig).
Fig 1. Cryptococcus neoformans (VNIV) can colonize mature soil grown Arabidopsis thaliana.
(A) Mating mixtures can induce chlorosis, (B) Colony forming units (CFUs + SEM) indicate that both individual and mating strains of Cryptococcus can colonize A. thaliana plants. Chlorosis was only associated with mated mixtures of C. neoformans (VNI).
Fig 2. Arabidopsis thaliana mutants display increased susceptibility to Cryptococcus colonization.
A. thaliana jar1-1 mutants display increased colonization by C. neoformans (VNI) at four and twelve days post infection (dpi). A. thaliana npr1-1 mutants display increased colonization to mixed C. neoformans infection at 4 and 12 days post-infection. Error bars represent CFUs + SEM.
Cryptococcus colonizes live Douglas fir, Western hemlock, Mopane, and Eucalyptus seedlings
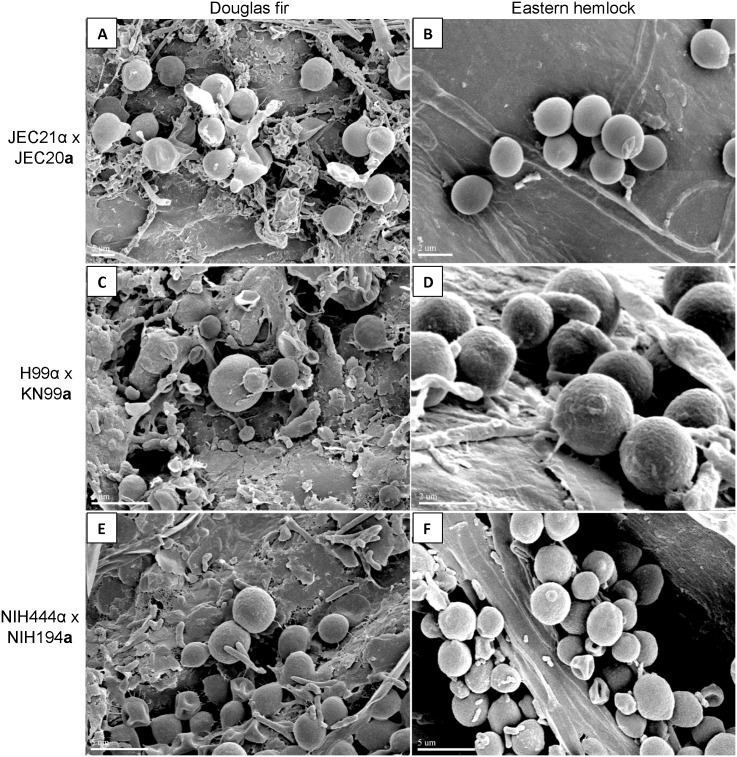
C. deneoformans, C. neoformans, C. deuterogattii, C. bacillisporus, and C. gattii can colonize Douglas fir and Western hemlock seedlings. Electron microscopy demonstrates that C. deneoformans (VNIV), C. neoformans (VNI), C. deuterogattii (VGII), and C. gattii (VGI) can colonize live Douglas fir and Western hemlock needles in mixed communities with other naturally acquired microorganisms (Fig 3). Douglas fir seedlings appeared healthy at one week post inoculation (Fig 4), but developed browning needles and progressive disease symptoms on young buds by three weeks (Fig 5). Disease symptoms progressively developed with prolonged incubation. Obvious disease symptoms were not observed on Western hemlock seedlings even after prolonged incubation (Fig 5). Douglas fir seedlings inoculated with C. neoformans, C. bacillisporus (VGIII), and C. gattii (VGI) displayed more severe needle browning and bud symptoms than those inoculated with C. deneoformans. Disease symptoms were associated with both mated mixtures and individually inoculated strains. Disease symptoms were not observed on Eucalyptus or Mopane seedlings inoculated with C. deneoformans, C. neoformans, C. bacillisporus, or C. gattii (S4 Fig).
Fig 3. Cryptococcus can colonize live Douglas fir and Western hemlock trees.
Scanning electron micrographs are shown of mixed mating strains of (A) Cryptococcus deneoformans (VNIV) producing filaments on Douglas fir trees, and (B) colonizing Eastern hemlock trees. Colonization of Cryptococcus neoformans (VNIV) on Douglas fir (C) or Western hemlock (D), and of C. deuterogattii (VGII) x C. gattii (VGI) on Douglas fir (E) or Hemlock (F) are shown. Scale bar = 5 μm.
Fig 4. Douglas fir and Eastern hemlock infection model at one week post infection.
(A) Top row displays Douglas fir inoculated with (A) C. deneoformans JEC21α, JEC20a, and JEC21α + JEC20a mixed; Middle row shows, C. neoformans H99α, KN99a, and H99α + KN99a mixture; Bottom row shows, C. deuterogattii (NIH312α), C. gattii (NIH194a), and NIH312α + NIH194a mixture. (B) Inoculated buds and needles predominantly appear green and healthy but some browning or loss of needles are observed for both individually inoculated strains and mated mixtures. (B) Left column shows, C. deneoformans JEC21α, JEC20a, and JEC21α + JEC20a mixed; Middle column under labeled control shows, C. neoformans H99α, KN99a, and H99α + KN99a mixture; and right column shows, C. deuterogattii (NIH312α), C. gattii (NIH194a), and NIH312α + NIH194a mixture.
Fig 5. Cryptococcus can infect conifers and shows progressive disease associated symptoms.
Conifer infection model at four weeks post-infection displays exacerbated needle browning, bud drooping, and needle dropping consistent with infection. Douglas fir (top row) and Eastern hemlock (bottom row) four weeks post inoculation with, (left column) C. deneoformans JEC21α, JEC20a, and JEC21α + JEC20a mixed; (middle column) C. neoformans H99α, KN99a, and H99α + KN99a mixture; (right column) C. bacillisporus (NIH312α), C. gattii (NIH194a), and NIH312α + NIH194a mixture. Douglas fir displays more plant infection symptoms in contrast to Eastern hemlock.
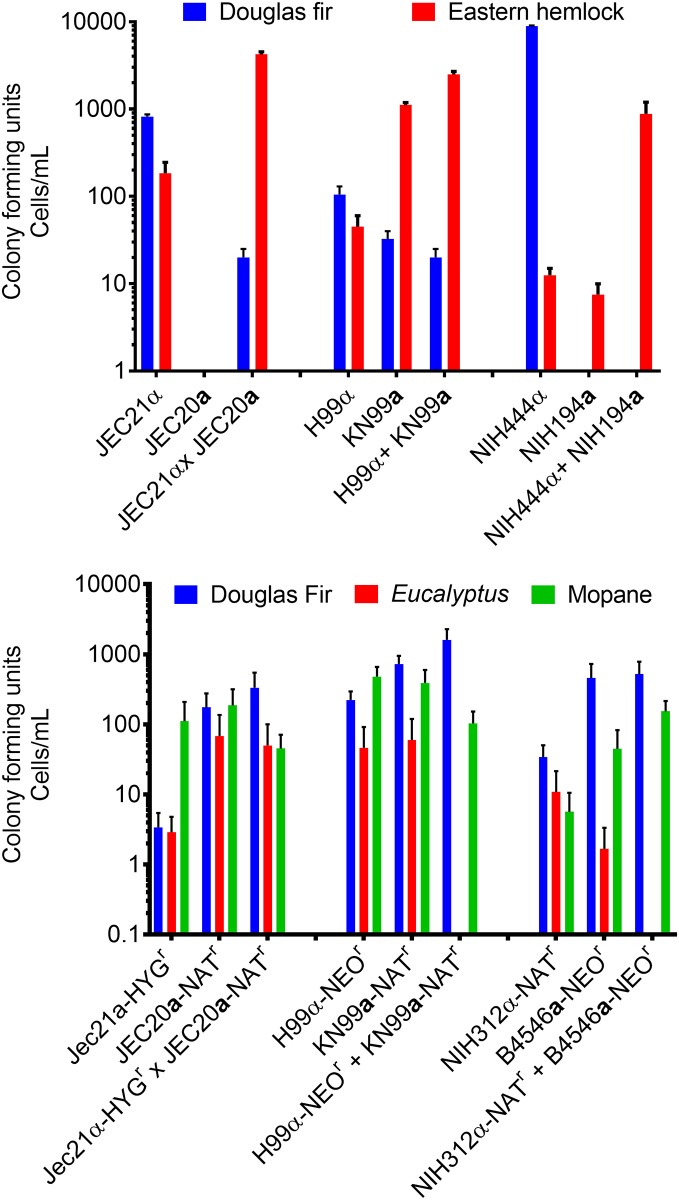
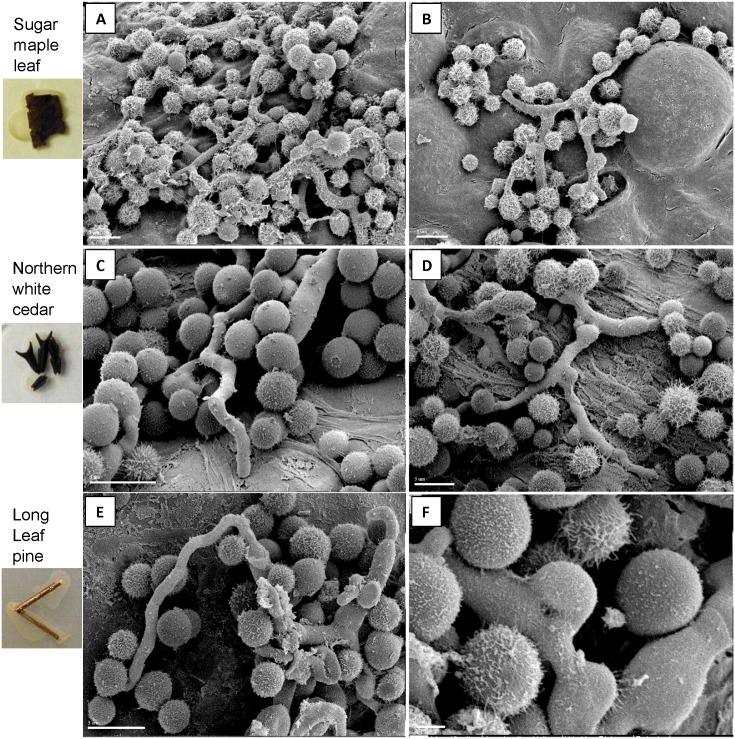
Cryptococcus recovery from infected tree seedlings appears to be plant- and strain-dependent, but C. deneoformans, C. neoformans, C. deuterogattii C. bacillisporus, and C. gattii were able to colonize tree seedlings (Fig 6). C. neoformans were more consistently recovered from inoculated Douglas fir and Western hemlock seedlings compared with C. deneoformans and C. deuterogattii C. bacillisporus, or C. gattii (Fig 6a). Cryptococcus cell recovery from Eucalyptus was inconsistent between strains (Fig 6b). Cryptococcus recovery was enhanced by utilizing Cryptococcus strains containing a drug-resistance marker (Fig 6b). No double drug resistant colonies were isolated from trees inoculated with mixed mating strains. Furthermore, we observed by light microscopy (S5 Fig) and scanning electron microscopy (Fig 7) that Cryptococcus can grow saprobically, filament (Oak, Hemlock, Maple, and Long leaf pine), and mate on dead plant materials including Sugar maple leaves and needles of Cedar and Long leaf pine (Fig 7).
Fig 6. Conifer trees are susceptible to colonization by Cryptococcus.
Colony forming units (CFUs + SEM) at three weeks post-inoculation are shown. (A) Cryptococcus cells were recovered from Douglas fir and Eastern hemlock seedlings. C. neoformans was recovered from both Douglas fir and Eastern hemlock; however, recovery of C. deneoformans, C. gattii, C. bacillisporus, C. deuterogattii strains was inconsistent. In a plant infection trial, Douglas fir, Eucalyptus, and Mopane infection models were compared utilizing engineered strains containing various drug-resistant cassettes. Cryptococcus cells were recovered from Eucalyptus, Mopane, and Douglas fir infected with individual and mated strains. Error bars represent +SEM.
Fig 7. Cryptococcus can grow saprobically and mate on dead plant materials.
Scanning electron micrographs depict C. neoformans (VNI) displaying robust filamentation on sugar maple leaves (top row), northern white cedar needles (middle row) and long leaf pine needles (bottom row). Fused clamp connections are indicative of productive matings (arrow).
Cryptococcus can mate on agar containing plant materials
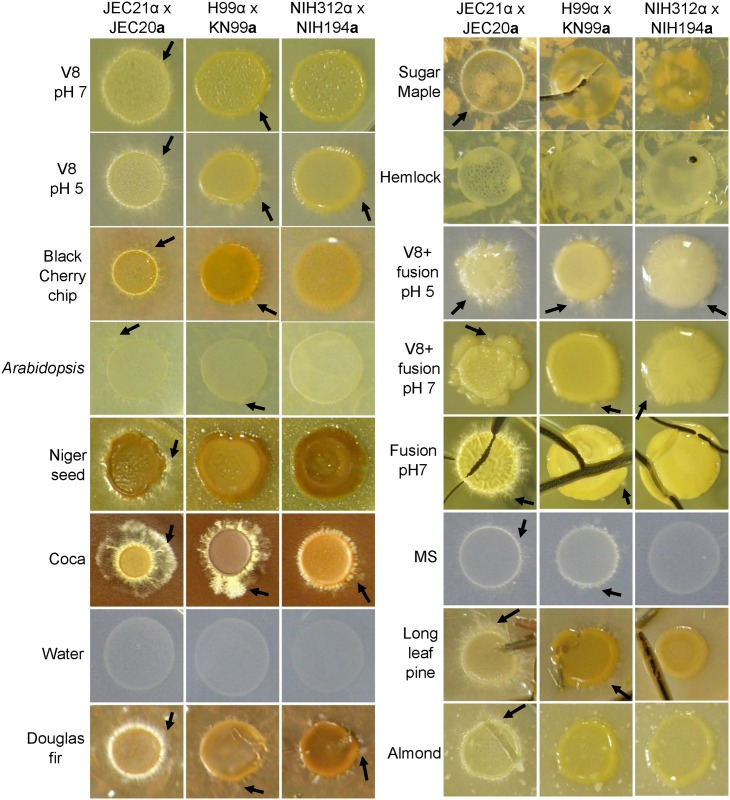
C. neoformans is highly fertile and prolifically mates on plant agars. Mating of C. deneoformans and C. neoformans was observed on Black cherry chip, Arabidopsis, Niger seed, Coca, Douglas fir, Sugar maple, Longleaf pine, and Almond agars, as well as on classic V8 and MS agar and newly concocted V8-fusion and Fusion agars (Fig 8 and S6 Fig). Strains of C. bacillisporus (VGIII) x C. gattii (VGI) had fewer observed matings and were slower to mate on many of the tested media (Fig 8 and S6 Fig). Mating of C. bacillisporus (VGIII) x C. gattii (VGI) was observed on Douglas fir, Cocoa, V8-fusion, Fusion, and classic V8 (pH 5) agar (Fig 8). Filamentation and basidia observed by direct microscopic analysis confirmed the mating of C. deneoformans, C. neoformans, and C. bacillisporus (VGIII) x C. gattii (VGI). S7 Fig depicts colony morphology and S8 Fig displays photomicrographs of the production of hyphae and basidia on standard mating media in contrast to mating on newly described plant-based agars displayed in S8 Fig (colony morphology) and S10 Fig (filamentation and basidia).
Fig 8. Enhanced mating of Cryptococcus is observed on plant material-based agars.
C. deneoformans and C. neoformans display filamentation (arrows) around the periphery of the colony on many types of plant-based agars at four weeks post inoculation. Mating and the production of basidia were confirmed (S7, S8, S9 and S10 Figs). Mating of C. bacillisporus x C. gattii was less prolific and was confirmed based on microscopic observation of hyphae, basidia, and spores. (S7, S8, S9 and S10 Figs). For C. bacillisporus x C. gattii arrows highlight detectable areas of filamentation with confirmation by light microscopy.
Cryptococcus gattii can grow in extract broth
C. deuterogattii and C. bacillisporus, can grow and proliferate in A. thaliana and Pigeon guano extract broth. The OD of C. deuterogattii and C. bacillisporus cells followed normal growth dynamics over 72 hours, logarithmically increasing over 24 hours before reaching stationary phase (S11a Fig). Viable cells were obtained at the conclusion of the growth curve (S11b Fig). A. thaliana and pigeon guano extract broth have no additional nutrients, and growth was similar to YPD broth over 8 hours but then rapidly reached stationary phase around 12 hours; viable cells were recovered at the termination of the growth curve at 72 hours.
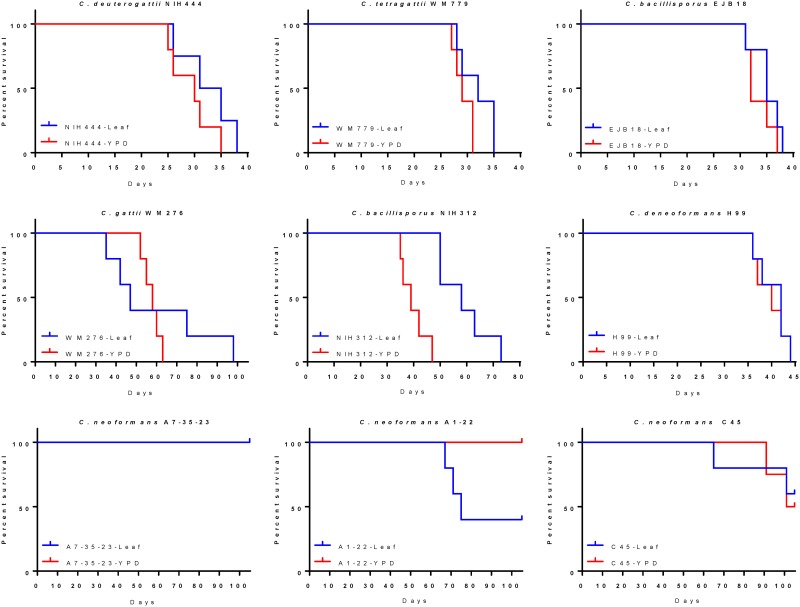
The impact on virulence due to growth on Arabidopsis agar is strain dependent
C. deneoformans, C. neoformans and C. gattii, C. deuterogattii, C. bacillisporus, and C. tetragattii strains were grown on Arabidopsis agar for one week, harvested from plates, and tested for virulence in the intranasal murine model (Fig 9). Hypervirulence as a result of growth on plant materials was only observed for Cryptococcus neoformans isolate A1-22 (Fig 9). Hypovirulence as a result of growth on Arabidopsis agar was observed for C. bacillisporus VGIII isolate NIH312 (Fig 9). No other statistically significant differences (P > 0.05) were observed for growth on YPD agar or Arabidopsis agar were observed for NIH444, WM779, EJB18, WM276, H99, A7-35-33 or C45 (Fig 9).
Fig 9. Strain dependent changes in the virulence of Cryptococcus as a result of passage on plant-based agars are depicted.
Kaplan-Meier survival curves of nine Cryptococcus strains grown on Arabidopsis agar are shown. C. bacillisporus NIH312 (E) demonstrated reduced virulence and C. neoformans A1-22 (H) displayed increased virulence following one week of growth on Arabidopsis plant agar. No significant differences were observed between YPD agar and Arabidopsis agar for any other strain.
Discussion
The human pathogen, Cryptococcus, can utilize plant hosts as reservoir for mating and dispersal. It is presently understood that C. deneoformans, C. neoformans, and VGI, VGII, and VGIII crosses can colonize and mate on A. thaliana in the laboratory setting, but little is known regarding the ability of Cryptococcus to colonize trees that it is known to associate with in nature other than limited reports of infection of almond and Eucalyptus seedlings. In this study, we demonstrated that Cryptococcus can colonize live and dead plant tissues from a number of natural plant hosts and that these plant materials can trigger the transition of yeast cells to a filamentous stage supporting the important role of these hosts in the environmental lifecycle of Cryptococcus. Prolific filamentation of Cryptococcus was observed on dead sterilized plant materials and on water agar containing plant matter suggesting that Cryptococcus deneoformans, C. neoformans, C. gattii, C. deuterogattii, C. bacillisporus, and C. tetragattii may interact with plants at various stages of their lifecycle including seedlings, mature plants, to necrotic and dead plant matter by adapting different life strategies as a biotroph, saprobe, or as pathogen. The ability of Cryptococcus to transition to alternative life strategies in association with host may be impacted by the presence of both mating types because plant pathogenicity and the filamentation, mating, and the generation of double drug resistant progeny by Cryptococcus was only observed under those circumstances [30]. However, we observed filamentation, production of clamp connections, and basidia by Cryptococcus in saprobic association with sterile plant matter and agar supplemented with plant matter indicating the sexual lifecycle can also be completed in saprobic association with plant matter. It is possible that the pathogenic Cryptococcus species (VN and VG inclusive) could form epiphytic and endophytic associations with plants because C. laurentii and C. albidus, C. flavus, C. podzolicus, C. hungaricus, and other Cryptococcus sp. have been reported to form non-pathogenic associations with a variety of host plants [43–46].
Consistent with previous reports we observed A. thaliana is readily colonized by C. deneoformans, C. neoformans, C. gattii, C. deuterogattii, and C. bacillisporus. Evidence has been previously reported that Cryptococcus mating triggers a jasmonic acid (JA)-dependent defense response in Arabidopsis and that Salicylic acid (SA) signaling pathway involving NPR1 is suppressed [30]. Here, we show that both JA and SA signaling pathways reduce the colonization of Cryptococcus in Arabidopsis as the JA and SA signaling mutants, jar1-1 and npr1-1, both show increased fungal colonization. This is consistent with previous reports that Arabidopsis SA signaling or synthesis mutants eds1 (enhanced disease susceptibility 1; lipase/signal transducer/triacylglycerol lipase), nahG (transgenic line degrading salicylic acid; SA), sid2 (SA-induction deficient), and npr1 (nonexpressor of PR genes 1; pathogenesis-related 1) are all more susceptible to Cryptococcus infection [39, 41]. Other mutant A. thaliana ecotypes rpm1 (resistance to Pseudomonas syringae pv maculicola 1), pad4 (phytoalexin deficient 4), and Atprn1 (PRN1; one of four members of an iron-containing subgroup of the cupin superfamily, accumulates flavonoids) that are components of SA-mediated pathways, were also observed to be more susceptible to Cryptococcus infection [39, 41]. The data suggest that both the SA and JA pathways are required to hinder the colonization and growth of Cryptococcus in Arabidopsis and are not necessarily antagonistic to each other consistent with resistance to the pathogenic fungus Fusarium graminearum [47].
Furthermore, in laboratory trials, Cryptococcus mutants (ste12α, cap59, lac1) show reduced ability to colonize A. thaliana plants and could be differentially regulated between species and molecular types as in mating and virulence [41, 48]. Laccase was not essential for the colonization of A. thaliana by C. deuterogattii [42] but was important for virulence in C. neoformans, suggesting that different Cryptococcus species may have adapted different strategies to cope with the evolutionary pressures encountered in the plant ecological niche [41].
The interactions between Cryptococcus and plants are likely dependent on both the environmental conditions, the genetics of Cryptococcus, and the genetics of the host, A. thaliana. Conserved factors that are utilized for both plant colonization and virulence within animal host could be maintained in the natural ecological niche providing for the natural reservoirs of infectious genotypes. Furthermore, both yeast and mated co-cultures were observed to colonize live plants and plant tissues that were associated with the appearance of disease symptoms [30, 39, 42].
In this study, we demonstrate the ability of C. deneoformans, C. neoformans and C. gattii, C. bacillisporus, C. deuterogattii, and C. tetragattii to colonize live non-sterile Douglas fir and Hemlock trees and to undergo saprobic filamentation, mating, and the production of spores on dead plant material, implicating the potential for long-term association of Cryptococcus with plants. Therefore, plants may serve an important role in maintaining, optimizing, and enhancing virulence factors during the environmental lifecycle of the opportunistically acquired human pathogen, Cryptococcus.
Supporting information
LAC1 and CNA1 are not required for A. thaliana colonization.
(DOCX)
Scanning electron microscopy indicates active colonization of mutant (jar1-1, npr1-1) and wildtype (Col-0) A. thaliana plants.
(DOCX)
(A) Mating mixtures can alter growth of immature A. thaliana seedlings. (B) jar1-1 seedlings display increased deleterious symptoms associated with Cryptococcus neoformans (VNI) colonization.
(DOCX)
Leaves from two individual Eucalyptus or Mopane seedlings were harvested at three weeks post inoculation.
(TIF)
Robust filamentation of C. deneoformans is observed in association with black cherry chips and oak but limited filamentation is also observed in association with Sugar maple leaf, Hemlock needle, and long leaf pine needle. Robust filamentation of C. neoformans was observed in association with oak and limited filamentation with Sugar maple and Long leaf pine.
(DOCX)
Mating of C. neoformans is observed in as little as five to seven days post-inoculation. Detectable mating of C. bacillisporus (VGIII) x C. gattii (VGI) took longer, no filamentation or mating was observed one week post-inoculation. Red boxes denote hyphal growth and mating by light microscopic observation of basidia.
(DOCX)
C. deneoformans and C. neoformans produces filaments more prolifically than C. bacillisporus (VGIII) x C. gattii (VGI) on most media. Newly created fusion media and mixed V8-fusion media induce robust filamentation and mating of C. bacillisporus (VGIII) x C. gattii (VGI).
(DOCX)
C. deuterogattii (VGII) x C. gattii (VGI), C. deneoformans, and C. neoformans robustly form basidia and spores on newly synthesized fusion and V8-fusion blend media.
(TIF)
C. bacillisporus (VGIII) x C. gattii (VGI) only sparsely filamented on Arabidopsis, black cherry, Coca, Long leaf pine, Sugar maple, and hemlock agars (S7 Fig).
(DOCX)
C. bacillisporus (VGIII) x C. gattii (VGI) also produced basidia on Arabidopsis, black cherry, Coca, Sugar maple, and hemlock agars.
(DOCX)
(A) Reduced growth in Arabidopsis or Pigeon guano extract broth. (B) Colony forming units vs absorbance at OD600 confirms a higher level of viable cells in YPD broth vs Arabidopsis of pigeon guano extract broth.
(DOCX)
Acknowledgments
We thank Valerie Knowlton, Center for Electron Microscopy, NC State University for assistance with scanning electron microscopy, Paul Manion for supplying wood identified to species, and Jamie, Murry, GEM Shavings for wood shavings used in this study. In addition, we thank Cecelia Wall, Anna Floyd-Averette, Blake Billmyre, Sheng Sun, and Joanna Kingsbury for facilitating experiments and reviewing the manuscript at various stages. These studies were supported by NIH/NIAID R37 Merit Award AI39115-18.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
These studies were supported by NIH/NIAID R37 Merit Award AI39115-18.
References
- 1.Pfaller M.A. and Diekema D.J., Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol, 2004. 42(10): p. 4419–31. 10.1128/JCM.42.10.4419-4431.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durden F.M. and Elewski B., Fungal infections in HIV-infected patients. Semin Cutan Med Surg, 1997. 16(3): p. 200–12. [DOI] [PubMed] [Google Scholar]
- 3.Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16–48. 10.1016/j.fgb.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 4.Johnson L.B., Bradley S.F., and Kauffman C.A., Fungaemia due to Cryptococcus laurentii and a review of non-neoformans cryptococcaemia. Mycoses, 1998. 41(7–8): p. 277–80. [DOI] [PubMed] [Google Scholar]
- 5.Khawcharoenporn T., Apisarnthanarak A., and Mundy L.M., Non-neoformans cryptococcal infections: a systematic review. Infection, 2007. 35(2): p. 51–8. 10.1007/s15010-007-6142-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinde S.M., Vanarse K.S., and Pandit A.N., Systemic humicolus cryptococcosis. Indian Pediatr, 2004. 41(11): p. 1162–4. [PubMed] [Google Scholar]
- 7.Powel MS, Alizadeh AA, Budvytiene I, Schaenman JM, Banaei N. First isolation of Cryptococcus uzbekistanensis from an immunocompromised patient with lymphoma. Journal of Clinical Microbiology. 2012;50(3):1125–7. 10.1128/JCM.05678-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrnes EJ 3rd, Li W, Ren P, Lewit Y, Voelz K, Fraser JA, et al. A diverse population of Cryptococcus gattii molecular type VGIII in Southern Californian HIV/AIDS patients. PLOS Pathogens. 2011;7(9):e1002205 10.1371/journal.ppat.1002205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Springer DJ, Billmyre RB, Filler EE, Voelz K, Pursall R, Mieczkowski PA, et al. Cryptococcus gattii VGIII isolates causing infections in HIV/AIDS patients in Southern California: Identification of the local environmental source as arboreal. PLOS Pathog. 2014;10(8):e1004285 10.1371/journal.ppat.1004285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith RM, Mba-Jonas A, Tourdjman M, Schimek T, DeBess E, Marsden-Haug N, et al. Treatment and outcomes among patients with Cryptococcus gattii infections in the United States Pacific Northwest. PLOS One. 2014;9(2):e88875 10.1371/journal.pone.0088875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira-Paim K, Ferreira TB, Andrade-Silva L, Mora DJ, Fonseca FM, Springer DJ, et al. Phylogenetic analysis of Cryptococcus laurentii isolates reveals a high frequency of sibling species. PLOS ONE. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebecker B, Naglik JR, Hube B, Jacobsen ID. Pathogenicity mechanisms and host response during oral Candida albicans infections. Expert Rev Anti Infect Ther. 2014;12(7):867–79. 10.1586/14787210.2014.916210 [DOI] [PubMed] [Google Scholar]
- 13.Kwon-Chung K.J. and Sugui J.A., Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLOS Pathog, 2013. 9(12): p. e1003743 10.1371/journal.ppat.1003743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Gorman C.M., Airborne Aspergillus fumigatus conidia: a risk factor for aspergillosis. Fungal Biology Reviews, 2011. 25(3): p. 151–157. [Google Scholar]
- 15.Bennett J.W., Aspergillus: a primer for the novice. Med Mycol, 2009. 47 Suppl 1: p. S5–12. [DOI] [PubMed] [Google Scholar]
- 16.Fisher FS, Bultman MW, Johnson SM, Pappagianis D, Zaborsky E. Coccidioides niches and habitat parameters in the southwestern United States: a matter of scale. Ann N Y Acad Sci. 2007;1111:47–72. 10.1196/annals.1406.031 [DOI] [PubMed] [Google Scholar]
- 17.Pappagianis D., Epidemiology of coccidioidomycosis. Curr Top Med Mycol, 1988. 2: p. 199–238. [DOI] [PubMed] [Google Scholar]
- 18.Howard D.H., The epidemiology and ecology of blastomycosis, coccidioidomycosis and histoplasmosis. Zentralbl Bakteriol Mikrobiol Hyg A, 1984. 257(2): p. 219–27. [PubMed] [Google Scholar]
- 19.Barker BM, Jewell KA, Kroken S, Orbach MJ. The population biology of coccidioides: epidemiologic implications for disease outbreaks. Ann N Y Acad Sci. 2007;1111:147–63. 10.1196/annals.1406.040 [DOI] [PubMed] [Google Scholar]
- 20.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367(23):2214–25. 10.1056/NEJMoa1204781 [DOI] [PubMed] [Google Scholar]
- 21.Chamany S, Mirza SA, Fleming JW, Howell JF, Lenhart SW, Mortimer VD, et al. A large histoplasmosis outbreak among high school students in Indiana, 2001. Pediatr Infect Dis J. 2004;23(10):909–14. [DOI] [PubMed] [Google Scholar]
- 22.Cairns L, Blythe D, Kao A, Pappagianis D, Kaufman L, Kobayashi J, et al. Outbreak of coccidioidomycosis in Washington state residents returning from Mexico. Clin Infect Dis. 2000;30(1):61–4. 10.1086/313602 [DOI] [PubMed] [Google Scholar]
- 23.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science translational medicine. 2012;4(165):165rv13 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 24.Kidd SE, Bach PJ, Hingston AO, Mak S, Chow Y, MacDougall L, et al. Cryptococcus gattii dispersal mechanisms, British Columbia, Canada. Emerg Infect Dis. 2007;13(1):51–7. 10.3201/eid1301.060823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coles FB, Schuchat A, Hibbs JR, Kondracki SF, Salkin IF, Dixon DM, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136(4):475–87. [DOI] [PubMed] [Google Scholar]
- 26.Klein BS, Vergeront JM, Weeks RJ, Kumar UN, Mathai G, Varkey B, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314(9):529–34. 10.1056/NEJM198602273140901 [DOI] [PubMed] [Google Scholar]
- 27.Kwon-Chung K.J., A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia, 1975. 67(6): p. 1197–200. [PubMed] [Google Scholar]
- 28.Erke K.H., Light microscopy of basidia, basidiospores, and nuclei in spores and hyphae of Filobasidiella neoformans (Cryptococcus neoformans). J Bacteriol, 1976. 128(1): p. 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen K., De Obaldia A.L., and Heitman J., Cryptococcus neoformans mates on pigeon guano: Implications for the realized ecological niche and globalization. Eukaryotic Cell, 2007. 6(6): p. 949–959. 10.1128/EC.00097-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue C, Tada Y, Dong X, Heitman J. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host and Microbe. 2007;1(4):263–73. 10.1016/j.chom.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 31.Botes A, Boekhout T, Hagen F, Vismer H, Swart J, Botha A. Growth and mating of Cryptococcus neoformans var. grubii on woody debris. Microbial Ecology. 2009;57(4):757–65. 10.1007/s00248-008-9452-1 [DOI] [PubMed] [Google Scholar]
- 32.Kwon-Chung K.J., A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia, 1976. 68(4): p. 943–6. [PubMed] [Google Scholar]
- 33.Kwon-Chung K.J., Bennett J.E., and Theodore T.S., Cryptococcus bacillisporus sp. nov.: serotype BC of Cryptococcus neoformans. International Journal of Systematic and Evolutionary Microbiology, 1978. 28(4): p. 616. [Google Scholar]
- 34.Lin X, Litvintseva AP, Nielsen K, Patel S, Floyd A, Mitchell TG, et al. alpha AD alpha hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLOS Genet. 2007;3(10):1975–90. 10.1371/journal.pgen.0030186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahid M, Han S, Yoell H, Xu J. Fitness distribution and transgressive segregation across 40 environments in a hybrid progeny population of the human-pathogenic yeast Cryptococcus neoformans. Genome. 2008;51(4):272–81. 10.1139/G08-004 [DOI] [PubMed] [Google Scholar]
- 36.Li W, Averette AF, Desnos-Ollivier M, Ni M, Dromer F, Heitman J. Genetic Diversity and Genomic Plasticity of Cryptococcus neoformans AD Hybrid Strains. G3 (Bethesda). 2012;2(1):83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phadke S.S., Feretzaki M., and Heitman J., Unisexual reproduction enhances fungal competitiveness by promoting habitat exploration via hyphal growth and sporulation. Eukaryot Cell, 2013. 12(8): p. 1155–9. 10.1128/EC.00147-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saul N., Krockenberger M., and Carter D., Evidence of recombination in mixed-mating-type and alpha-only populations of Cryptococcus gattii sourced from single Eucalyptus tree hollows. Eukaryot Cell, 2008. 7(4): p. 727–34. 10.1128/EC.00020-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Springer DJ, Ren P, Raina R, Dong Y, Behr MJ, McEwen BF, et al. Extracellular fibrils of pathogenic yeast Cryptococcus gattii are important for ecological niche, murine virulence and human neutrophil interactions. PLOS ONE. 2010;5(6):e10978 10.1371/journal.pone.0010978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huerfano S., Castaneda A., and Castaneda E., Experimental infection of almond trees seedlings (Terminalia catappa) with an environmental isolate of Cryptococcus neoformans var. gattii, serotype C. Rev Iberoam Micol, 2001. 18(3): p. 131–2. [PubMed] [Google Scholar]
- 41.Warpeha K.M., Park Y.D., and Williamson P.R., Susceptibility of intact germinating Arabidopsis thaliana to human fungal pathogens Cryptococcus neoformans and C. gattii. Appl Environ Microbiol, 2013. 79(9): p. 2979–88. 10.1128/AEM.03697-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Springer, D.J., The unique impact of environment on virulence of the pathogenic yeast Cryptococcus gattii, in Biomendical Sciences. 2009, State University of New York at Albany. p. 317.
- 43.Rhoden SA, Garcia A, Azevedo JL, Pamphile JA. In silico analysis of diverse endophytic fungi by using ITS1-5,8S-ITS2 sequences with isolates from various plant families in Brazil. Genet Mol Res. 2013;12(2):935–50. 10.4238/2013.April.2.10 [DOI] [PubMed] [Google Scholar]
- 44.Vieira ML, Hughes AF, Gil VB, Vaz AB, Alves TM, Zani CL, et al. Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell. (Solanaceae). Can J Microbiol. 2012;58(1):54–66. 10.1139/W11-105 [DOI] [PubMed] [Google Scholar]
- 45.Zachow C, Berg C, Muller H, Meincke R, Komon-Zelazowska M, Druzhinina IS, et al. Fungal diversity in the rhizosphere of endemic plant species of Tenerife (Canary Islands): relationship to vegetation zones and environmental factors. ISME J. 2009;3(1):79–92. 10.1038/ismej.2008.87 [DOI] [PubMed] [Google Scholar]
- 46.Gomes NC, Fagbola O, Costa R, Rumjanek NG, Buchner A, Mendona-Hagler L, et al. Dynamics of fungal communities in bulk and maize rhizosphere soil in the tropics. Appl Environ Microbiol. 2003;69(7):3758–66. Epub 2003/07/04. 10.1128/AEM.69.7.3758-3766.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makandar R, Nalam V, Chaturvedi R, Jeannotte R, Sparks AA, Shah J. Involvement of salicylate and jasmonate signaling pathways in Arabidopsis interaction with Fusarium graminearum. Mol Plant Microbe Interact. 2010;23(7):861–70. 10.1094/MPMI-23-7-0861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren P, Springer DJ, Behr MJ, Samsonoff WA, Chaturvedi S, Chaturvedi V. Transcription factor STE12alpha has distinct roles in morphogenesis, virulence, and ecological fitness of the primary pathogenic yeast Cryptococcus gattii. Eukaryot Cell. 2006;5(7):1065–80. Epub 2006/07/13. 10.1128/EC.00009-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LAC1 and CNA1 are not required for A. thaliana colonization.
(DOCX)
Scanning electron microscopy indicates active colonization of mutant (jar1-1, npr1-1) and wildtype (Col-0) A. thaliana plants.
(DOCX)
(A) Mating mixtures can alter growth of immature A. thaliana seedlings. (B) jar1-1 seedlings display increased deleterious symptoms associated with Cryptococcus neoformans (VNI) colonization.
(DOCX)
Leaves from two individual Eucalyptus or Mopane seedlings were harvested at three weeks post inoculation.
(TIF)
Robust filamentation of C. deneoformans is observed in association with black cherry chips and oak but limited filamentation is also observed in association with Sugar maple leaf, Hemlock needle, and long leaf pine needle. Robust filamentation of C. neoformans was observed in association with oak and limited filamentation with Sugar maple and Long leaf pine.
(DOCX)
Mating of C. neoformans is observed in as little as five to seven days post-inoculation. Detectable mating of C. bacillisporus (VGIII) x C. gattii (VGI) took longer, no filamentation or mating was observed one week post-inoculation. Red boxes denote hyphal growth and mating by light microscopic observation of basidia.
(DOCX)
C. deneoformans and C. neoformans produces filaments more prolifically than C. bacillisporus (VGIII) x C. gattii (VGI) on most media. Newly created fusion media and mixed V8-fusion media induce robust filamentation and mating of C. bacillisporus (VGIII) x C. gattii (VGI).
(DOCX)
C. deuterogattii (VGII) x C. gattii (VGI), C. deneoformans, and C. neoformans robustly form basidia and spores on newly synthesized fusion and V8-fusion blend media.
(TIF)
C. bacillisporus (VGIII) x C. gattii (VGI) only sparsely filamented on Arabidopsis, black cherry, Coca, Long leaf pine, Sugar maple, and hemlock agars (S7 Fig).
(DOCX)
C. bacillisporus (VGIII) x C. gattii (VGI) also produced basidia on Arabidopsis, black cherry, Coca, Sugar maple, and hemlock agars.
(DOCX)
(A) Reduced growth in Arabidopsis or Pigeon guano extract broth. (B) Colony forming units vs absorbance at OD600 confirms a higher level of viable cells in YPD broth vs Arabidopsis of pigeon guano extract broth.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.