Abstract
Background
Cholinergic systems regulate the synaptic transmission resulting in the contribution of the nociceptive behaviors. Anterior cingulate cortex is a key cortical area to play roles in nociception and chronic pain. However, the effect of the activation of cholinergic system for nociception is still unknown in the cortical area. Here, we tested whether the activation of cholinergic receptors can regulate nociceptive behaviors in adult rat anterior cingulate cortex by integrative methods including behavior, immunohistochemical, and electrophysiological methods.
Results
We found that muscarinic M1 receptors were clearly expressed in the anterior cingulate cortex. Using behavioral tests, we identified that microinjection of a selective muscarinic M1 receptors agonist McN-A-343 into the anterior cingulate cortex dose dependently increased the mechanical threshold. In contrast, the local injection of McN-A-343 into the anterior cingulate cortex showed normal motor function. The microinjection of a selective M1 receptors antagonist pirenzepine blocked the McN-A-343-induced antinociceptive effect. Pirenzepine alone into the anterior cingulate cortex decreased the mechanical thresholds. The local injection of the GABAA receptors antagonist bicuculline into the anterior cingulate cortex also inhibited the McN-A-343-induced antinociceptive effect and decreased the mechanical threshold. Finally, we further tested whether the activation of M1 receptors could regulate GABAergic transmission using whole-cell patch-clamp recordings. The activation of M1 receptors enhanced the frequency of spontaneous and miniature inhibitory postsynaptic currents as well as the amplitude of spontaneous inhibitory postsynaptic currents in the anterior cingulate cortex.
Conclusions
These results suggest that the activation of muscarinic M1 receptors in part increased the mechanical threshold by increasing GABAergic transmitter release and facilitating GABAergic transmission in the anterior cingulate cortex.
Keywords: Muscarinic M1 receptor, McN-A-343, antinociception, GABA receptors, anterior cingulate cortex, mechanical stimulation
Background
The anterior cingulate cortex (ACC) is a major brain area which is involved in the processing of both sensory nociceptive information and the anticipation of painful stimuli.1,2 Human neuroimaging studies have proved that the ACC can be activated by different kinds of pain.3–5 In animal studies, in vivo electrophysiological measurements showed that peripheral nociceptive stimulation increases neural activity in the ACC of mice and rats.6–8 Furthermore, chronic pain models activate glutamatergic transmission and produce synaptic plasticity in the ACC.1,9–11 In addition to the activation of glutamatergic transmission, γ-aminobutyric acid (GABA)ergic transmission also plays an important role for nociception and is involved in nerve injury models.12
The cholinergic system has many projections to the central nervous system,13 and muscarinic receptors are expressed in many brain areas.14 Muscarinic receptors have been classified into five subtypes, M1, M2, M3, M4, and M5,15 and the existence of all receptor subtypes in the brain has been reported.16 Cholinergic projections play important roles in nociceptive behaviors, and the critical involvement of the cholinergic system in pain inhibitory pathways has long been known. For example, Sullivan et al.17 reported that the systemic administration of a muscarinic receptor agonist WAY-132984 produced antihyperalgesic and antiallodynic effects in animal models of neuropathic pain. At the spinal cord level, the activation of muscarinic receptors has been shown to produce antinociceptive effects.18–22 Furthermore, muscarinic receptors are involved in formalin-induced nociception,23 and the muscarinic M1 receptor is involved in the antinociceptive effect caused by the intrathecal injection of clonidine in mice.24 We also reported that the intrathecal injection of the muscarinic receptor agonist McN-A-343 produces antinociceptive effects during thermal and mechanical stimulation.25,26 These results suggest that muscarinic receptors are critical for nociception. These findings suggest that cholinergic neurons and muscarinic receptors have important roles in pain signaling at central nervous system levels. However, it is still unclear whether the cholinergic system contributes to anitinociception at the supraspinal level.
Here, to understand the role of muscarinic receptors of the ACC in the processing of sensory nociception, we characterized the properties of antinociceptive effects following muscarinic receptor activation in the ACC. We found that the activation of muscarinic M1 receptors in the ACC can produce antinociceptive effects in rats without affecting locomotor functions. Blocking GABAA receptors in the ACC regulates the nociceptive behaviors. Finally, to explore a possible mechanism, we performed whole-cell patch-clamp recordings and revealed that the activation of muscarinic M1 receptors facilitates GABAergic transmission in the ACC.
Materials and methods
Animals
Male Wistar rats (Kyudo, Kumamoto, Japan or CLEA, Hirosaki, Japan), weighing 280–300 g, were used throughout the experiment. Rats were housed at 23 ± 2℃ with a 12/12 h light/dark cycle (light on at 07:00 h) and were given free access to commercial food and tap water. Experimental procedures were based on the Guidelines of the Committee for Animal Care and Use of Fukuoka University and Hirosaki University.
Microinjection surgery into the ACC of rat
Microinjection of drugs was undertaken via a guide cannula (23 gauge) placed into the right ACC (A: 1.9; L: 0.6; V: 3.2 mm to bregma) according to the atlas of Paxinos and Watson.27 The guide cannula was fixed to the skull with dental cement. After surgery, rats were housed individually and allowed to recover for at least three days before experiments. For ACC microinjections of drugs, a 29-gauge injection needle was attached to a polyethylene tube fitted to a 5-µl Hamilton syringe. Then, the rat was restrained by hand, the stylet was withdrawn, and the injection needle was inserted into the guide cannula. Solutions were injected in a volume of 200 nl, and the injections were delivered slowly over a period of 2 min. For additional confirmation of the placement of the cannula in the ACC of the brain, at the end of experiments, rats were injected in ACC with 200 nl of 0.5% Evans blue and were then deeply anesthetized with pentobarbital (60 mg/kg, intraperitoneal, i.p.) and decapitated. The brains were then removed, sliced, and cannula tip placement and distribution of the dye in the ACC were confirmed. Data from rats with an incorrect placement of the cannula were excluded from the analysis.
Mechanical pressure stimulation
To assess for the changes in the nociceptive mechanical threshold, paw withdrawal thresholds to pressure stimulation was assessed using the Dynamic Plantar Aesthesiometer (model 37450; Ugo Basile, Italy). Rats were placed individually in a plastic cage (wide× length × height: 9.7 × 16 × 14 cm) with a wire mesh bottom. After at least 30-min acclimation period, the unit raises a straight metal filament (0.5 mm diameter) until it touches the medial plantar surface of the hind paw and begins to exert an upwards force until the paw is withdrawn or the preset cut-off is reached (50 g). The force required to elicit a withdrawal response is measured in grams. The mean value of three trials with at least 5-min intervals between trials was taken as the withdrawal threshold. All behavioral tests were performed during the light portion of the circadian cycle (9:00 a.m. to 5:00 p.m.). After the final behavioral measurement, all rats were deeply anesthetized with pentobarbital (60 mg/kg, i.p.) and processed for tissue preparation as described below.
Rota-rod test
Each rat was subjected to the rota-rod treadmill test once a day for a total period of three days (diameter, 7.6 cm; Muromachi Kikai, Tokyo, Japan). The treadmill was set to a rotating speed of 15 rpm. Rats that stayed on the treadmill rotating at 15 rpm for 120 s were considered complete responders; their latencies were recorded as 120 s. The results were presented as drop latency(s) measured before and after the ACC injection. The value was determined individually as the mean of three trials.
Immunohistochemistry
Rats were deeply anesthetized by pentobarbital (50 mg/kg, i.p.) and perfused transcardially with phosphate-buffered saline (PBS, composition in mM: NaCl 137, KCl 2.7, KH2PO4 1.5, NaH2PO4 8.1; pH 7.4) followed by 4% paraformaldehyde in PBS. Brains were removed and placed in fixative for two days and then stored in 25% sucrose solution for two days at 4℃, until frozen sections were cut for immunohistochemical staining. Brain sections were cut at 50 µm with a ROM-380 microtome (Yamato Kohki, Saitama, Japan). For immunohistochemical staining, the brain sections were incubated for 30 min at room temperature in a blocking solution (1% normal goat serum) and then were incubated for seven days at 4℃, in the primary antibody for muscarinic receptor (rabbit polyclonal anti-muscarinic M1 receptor, 1:1,000; Millipore, Billerica, MA). After incubation, the brain sections were washed and incubated for 2 h at room temperature in the secondary antibody solution (Alexa Fluor 488 donkey anti-sheep IgG and Alexa Fluor 546 goat anti-rabbit IgG, 1:1,000; Invitrogen Corp., Carlsbad, CA). The brain sections were analyzed using an LSM510 Imaging System (Carl Zeiss GmbH, Jena, Germany).
In vitro whole-cell patch-clamp recordings
Coronal brain slices (400 µm) at the level of the ACC were prepared using standard methods.10,28 Brain slices were transferred to a submerged recovery chamber with oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid containing (in mM) 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2.0 MgSO4, 2.0 CaCl2, 26.0 NaHCO3, and 20.0 glucose at room temperature for at least 1 h. Experiments were performed in a recording chamber on the stage of a BX50WI microscope (Olympus, Tokyo, Japan) with infrared differential interference contrast optics for visualization. For recording in the ACC, GABAA receptors-mediated inhibitory postsynaptic currents (IPSCs) were recorded from layer II/III neurons with a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Whole-cell recordings were made using voltage-clamp mode (Vh = –60 mV) in the presence of an a-amino-3-hydroxy-5-methyl-4isoxazolyle propionic acid/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 µM), a N-methyl-D-aspartate receptor antagonist D(–)-2-amino-5-phosphonopentanoic acid (D-AP5, 50 µM), and a GABAB receptor antagonist CGP55845 (3 µM). The recording pipettes (3-5 MΩ) were filled with a solution containing (in mM) 130 CsCl2, 2 MgCl2, 0.5 ethyleneglycol-bis(2-aminoethylether)-N,N,N9,N9-tetra acetic acid, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 3 Mg(adenosine triphosphate)2, and 0.4 guanosine triphosphate (pH 7.3 with CsOH). To record miniature IPSCs (mIPSCs), tetrodotoxin (TTX, 1 µM) was also applied in the bath solution. For analyzing GABAA receptors-mediated tonic currents, bicuculline methiodide (BMI, 10 µM) was used.28,29 The initial access resistance was 15–30 MΩ and was monitored throughout the experiment. Data were discarded if the access resistance changed >15% during experiment. Data were filtered at 2 kHz and digitized at 10 kHz.
Drugs
McN-A-343 (4-3-chlorophenyl-carbamoyloxy-2-butynyltrimethylammonium) was purchased from Research Biochemicals (RBI, Natick, MA). Pirenzepine hydrochloride, BMI, CNQX, CGP55845, TTX, and D-AP5 were purchased from Tocris Bioscience. All drugs were dissolved in sterile saline just before the experiment.
Statistical analysis
Data are expressed as means ± SEM. Statistical analyses were done with StatView (Abacus Concepts, Berkely, CA). Results of time course experiments were analyzed for significance with repeated measures analysis of variance (ANOVA) with post hoc tests, Dunnett’s test (comparisons to pre-injection 0 min). The differences among the groups were analyzed by two-way repeated measures ANOVA, followed by the Tukey–Kramer test for multiple comparisons or Student’s t test for two comparisons. A value of P < 0.05 was taken to indicate a statistically significant difference.
Results
Microinjection of McN-A-343 into the ACC produced antinociceptive effects in response to mechanical stimulation
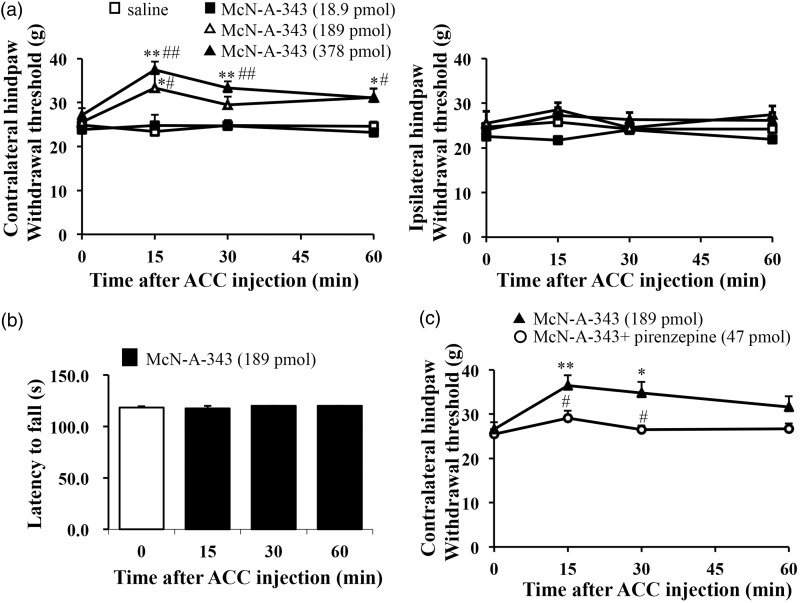
We tested whether the activation of muscarinic receptors at the supraspinal level can show antinociceptive effects. A physiological study by Davies et al.30 showed that McN-A-343 has selectivity for the muscarinic M1 receptor. We performed microinjection surgery in right side of the ACC in adult rats and allowed at least three days of recovery postsurgery. Nociceptive thresholds (in grams) were assessed by measuring hindpaw withdrawal responses to mechanical pressure with a sharp tip by dynamic plantar aesthesiometer. We evaluated the mechanical threshold, using the electric von Fey test, in injured rats microinjected with McN-A-343 into the ACC. The microinjection of McN-A-343 (189 pmol and 378 pmol) produced antinociceptive effects at the peak of 15 min and lasted at least for 60 min (F3, 12 = 3.576, P = 0.047 for 189 pmol, n = 5; F3,21 = 12.588, P = 0.0001 for 378 pmol, n = 8) to the hindpaw contralateral to microinjected site, while microinjection of saline did not produce any effect (F3, 18 = 0.222, P = 0.879, n = 7) (Figure 1(a)). Moreover, the withdrawal thresholds to the hindpaw ipsilateral to microinjected site were not affected by the microinjection of McN-A-343 (Figure 1(a)).
Figure 1.
Activation of M1 receptors in the ACC dose-dependently increased mechanical pressure threshold. (a) A microinjection of selective muscarinic M1 agonist, McN-A-343 (18.9-389 pmol) dose-dependently enhanced mechanical threshold to the hindpaw contralateral to microinjected site (saline, n = 7; 18.9 pmol, n = 5; 189 pmol, n = 5; 389 pmol, n = 8). (b) McN-A-343 (0.189 pmol) into the ACC showed normal locomotor activity. The latency to fall was measured for up to 2 min at rotation speed of 10 rpm in the rota-rod test (n = 8). (c) The effects of a selective M1 antagonist, pirenzepine (47 pmol) on McN-A-343(189 pmol)-induced antinociceptive effect (McN-A-343, n = 8; McN-A-343 + prirenzepine, n = 6). All values are means ± S.E.M. *P < 0.05 vs. 0 min, **P < 0.01 vs. 0 min, #P < 0.05 vs. saline (a) or McN-A-343 (c), ##P < 0.01 vs. saline (a) or McN-A-343 (c). ACC: anterior cingulate cortex.
We confirmed whether the pharmacological drug into the ACC could affect motor function or sedation. A dose of 189-pmol McN-A-343 into the ACC was used, which caused the antinociceptive effect (Figure 1(a)). The local injection of McN-A-343 into the ACC did not induce significant changes in the motor activity according to a rota-rod treadmill test in rats (Figure 1(b)). The result indicated that ACC injection of McN-A-343 at dose of antinociceptive effect does not produce motor dysfunction or sedation.
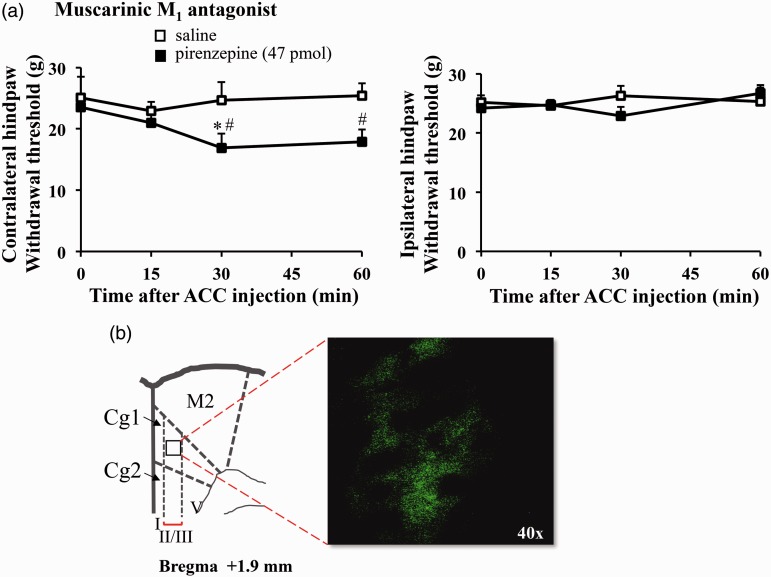
Next, we studied whether blocking M1 receptors in the ACC can affect the mechanical threshold (Figure 1(c)). In addition to McN-A-343, we also used a selective M1 antagonist pirenzepine at a previously reported dose.31 The antinociceptive effect of microinjected McN-A-343 in the ACC did not show the presence of pirenzepine (47 pmol) (F3, 15 = 1.670, P = 0.210, n = 6) (Figure 1(c)). Moreover, pirenzepine alone decreased paw withdrawal thresholds (F3, 12 = 3.756, P = 0.041, n = 5) and induced hypersensitivity on the mechanical threshold compared to a control group (Figure 2(a)). We further identified whether M1 receptors express in the ACC (Figure 2(b)) by using immunohistochemistry and observed a clear expression of M1 receptors in the ACC. Taken together, these results suggest that muscarinic M1 receptors in the ACC play critical roles in nociception, without affecting motor function.
Figure 2.
Inhibiting M1 receptors in the ACC decreased the mechanical threshold. (a) A selective M1 antagonist, pirenzepine (47 pmol) into the ACC reduced mechanical thresholds (saline, n = 3; pirenzepine, n = 5). All values are means ± S.E.M. *P < 0.05 vs. 0 min, #P < 0.05 vs. saline. (b) Immunohistochemical method showed that muscarinic M1 receptors expressed in the ACC. The scheme was modified from the atlas of Paxinos and Watson (1986) for rats. Coronal sections show with 40-fold (×40) under microscope.
ACC: anterior cingulate cortex; Cg1: cingulate cortex area 1; Cg2: cingulate cortex area 2; M2: secondary motor cortex.
Involvement of the GABAergic system in the McN-A-343-induced antinociceptive effect
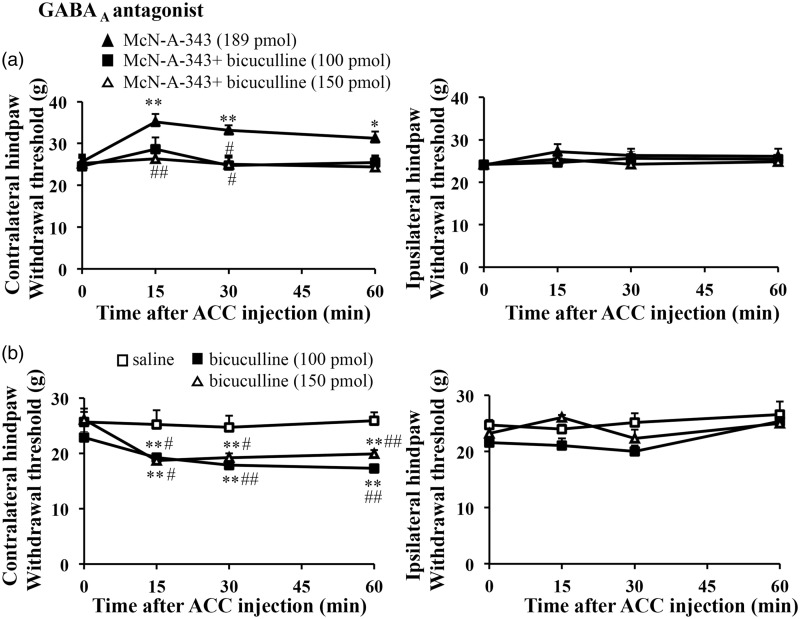
GABAergic transmission in the ACC can modulate excitatory transmission resulting in altered behaviors.32 We tested whether GABAergic transmission contributes to nociceptive behaviors in the ACC. To determine whether GABAergic systems are involved in the McN-A-343-induced antinociceptive effect, rats were treated with the GABAA receptors antagonist bicuculline. Bicuculline blocked McN-A-343-induced increases in the mechanical threshold (F3, 15 = 0.547, P = 0.657 for 150 pmol, n = 6) (Figure 3(a)). On the other hand, microinjection of bicuculline (100 pmol, 150 pmol) alone significantly produced hypersensitivity on the mechanical threshold (F3,12 = 20.060, P = 0.001 for 100 pmol, n = 5; F3,18 = 13.480, P = 0.001 for 150 pmol, n = 7) (Figure 3(b)).
Figure 3.
Blocking of GABAA receptors in the ACC reduced mechanical threshold. (a) The effects of the GABAA antagonist, bicuculline on McN-A-343(189 pmol)-induced antinociceptive effect (McN-A-343, n = 7; McN-A-343 + bicuculline100 pmol, n = 5; McN-A-343 + bicuculline 150 pmol, n = 6). (b) The local injection of the GABAA receptors antagonist, bicuculline (100 or 150 pmol) into the ACC decreased mechanical thresholds (saline, n = 4; bicuculline 100 pmol, n = 5; 150 pmol, n = 7). All values are means ± S.E.M. *P < 0.05 vs. 0 min, **P < 0.01 vs. 0 min, #P < 0.05 vs. McN-A-343 (a) or saline (c), ##i < 0.01 vs. McN-A-343 (a) or saline (c). ACC: anterior cingulate cortex
Activation of M1 receptors regulate GABAergic transmissions in the ACC
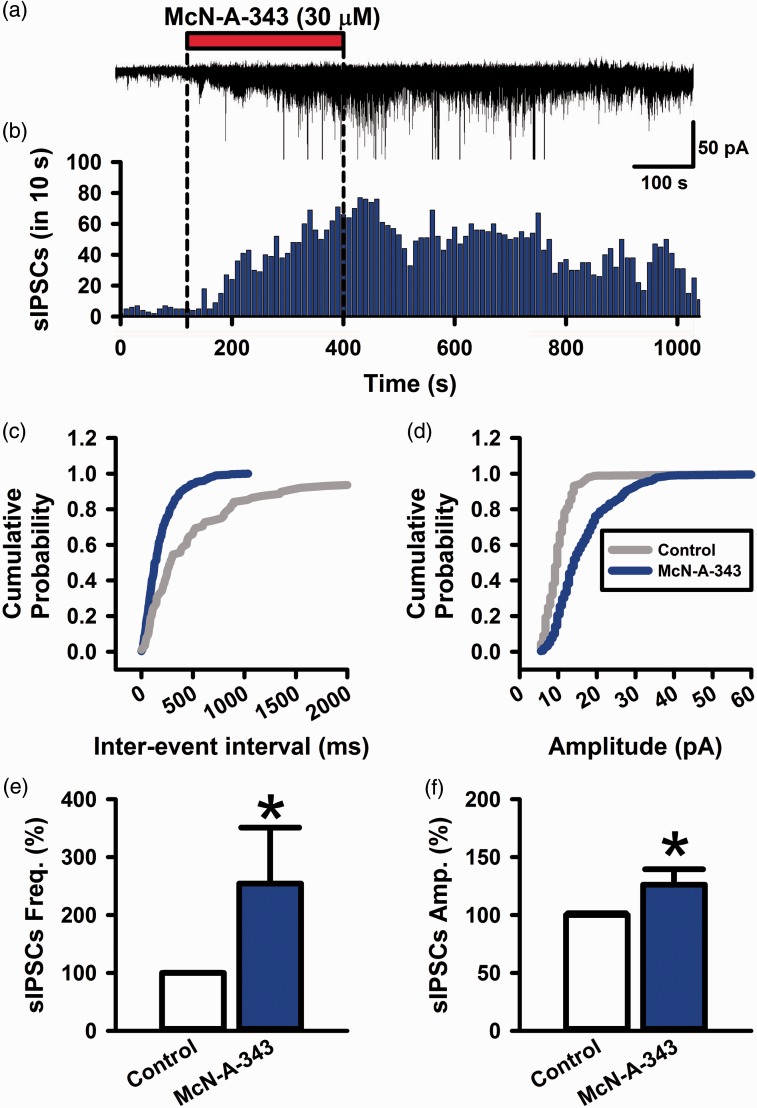
Muscarinic M1 receptors are G-protein-coupled receptors of class Gq.33 It is possible that the activation of M1 receptors can facilitate GABAergic transmissions in the ACC (Figures 4 to 6). To test this possibility, we performed in vitro whole-cell patch-clamp recordings from adult rat brain slices. First, we recorded spontaneous inhibitory postsynaptic currents (sIPSCs) from layer II/III pyramidal neurons with a holding membrane potential at −60 mV (n = 7 from five rats). The sIPSCs were analyzed while blocking glutamatergic receptors by CNQX (10 µM) and AP-5 (50 µM) and GABAB receptors by CGP55845 (3 µM) in the bath solution (Figure 4(a) and (b)). The sIPSCs were inhibited by GABAA receptors antagonist bicuculline; therefore, the sIPSCs were GABAA receptors-mediated currents. McN-A-343 (30 µM) in bath solution significantly produced enhancement of the frequency of sIPSCs (304 ± 47% of control, n = 7, P < 0.05) (Figure 4(c) and (e)). McN-A-343 enhanced the amplitude of sIPSCs (146 ± 19% of control, n = 7, P < 0.05) (Figure 4(d) and (f)). These results suggest that muscarinic M1 receptors activate spontaneous GABAergic transmission via GABAA receptors in the ACC.
Figure 4.
McN-A-343 facilitates GABAA receptors mediated sIPSCs in the ACC. (a) Bath application of McN-A-343 (30 µM) increased the frequency of IPSCs in a neuron. (b) Histogram of a recording from (a). (c and d) Cumulative curves of inter-event interval and amplitude of sIPSCs before and after the application of McN-A-343 (30 µM). (e and f) The averaged graphs of the frequency and amplitude of sIPSCs (n = 7 from 5 rats). All values are means ± S.E.M. *P < 0.05 vs. Control (E&F).
sIPSCs: spontaneous inhibitory postsynaptic currents.
Figure 5.
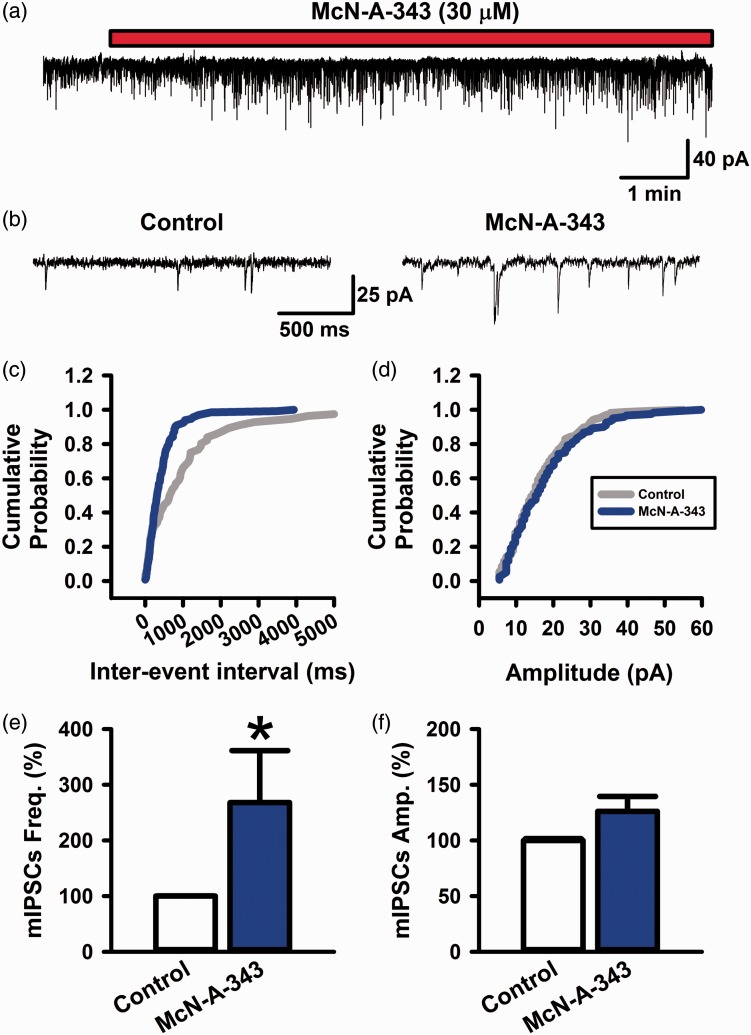
Activation of muscarinic M1 receptors enhanced the frequency of mIPSCs in the ACC. (a and b) Bath application of McN-A-343 (30 µM) increased the frequency of mIPSCs in a neuron. (c and d) Cumulative curves of inter-event interval and amplitude of mIPSCs before and after the application of McN-A-343 (30 µM). (e and f) The averaged graphs of the frequency and amplitude of mIPSCs (n = 8 from 5 rats). All values are means ± S.E.M. *P < 0.05 vs. Control (e).
Figure 6.
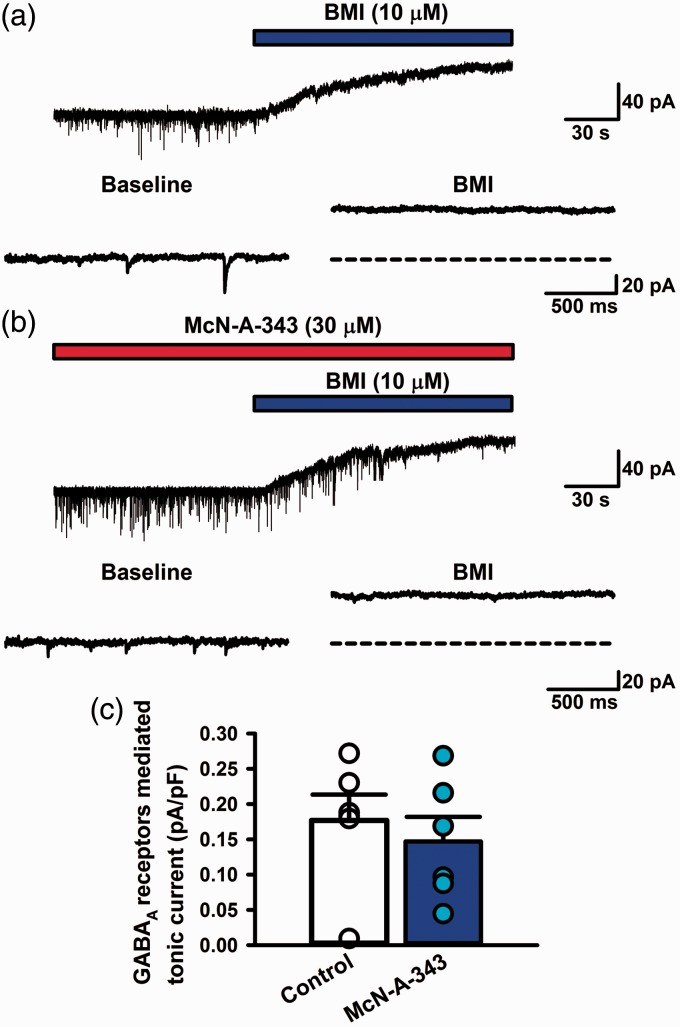
Activation of muscarinic M1 receptors did not affect GABAA receptors mediated currents in the ACC. (a) In a control group, BMI (10 µM) abolished mIPSCs and induced an outward shift of the holding current (Ihold). The dashed line depicts the Ihold in the absence of BMI. (b) In a McN-A-343-treated group, BMI (10 µM) abolished mIPSCs and induced an outward shift of the Ihold. The dashed line depicts the Ihold in the absence of BMI. (c) The averaged graph of tonic GABAA receptors mediated currents (pA/pF) in control and McN-A-343-treated group (n = 6 from 5 rats each group, P > 0.05). All values are means ± S.E.M.
BMI: bicuculline methiodide; GABA: γ-aminobutyric acid.
We tested whether the activation of muscarinic M1 receptors could facilitate miniature inhibitory postsynaptic currents (mIPSCs) (Figure 5). To block sodium channels, we applied TTX (1 µM) in the bath solution. We gave McN-A-343 (30 µM) on mIPSCs in the bath solution (Figure 5(a) and (b)). McN-A-343 (30 µM) in bath solution significantly produced enhancement of the frequency of mIPSCs (268 ± 94% of control, n = 8, P < 0.05) (Figure 5(c) and (e)). On the other hand, McN-A-343 did not enhance the amplitude of sIPSCs (126 ± 13% of control, n = 8, P > 0.05) (Figure 5(d) and (f)). These results suggest that muscarinic M1 receptors activate miniature GABAergic transmitter release in the ACC.
Activation of M1 receptors did not affect GABAA receptors-mediated tonic currents
GABA acting on GABAA receptors produces not only phasic but also tonic inhibitions by persistent activation of extrasynaptic receptors. Tonic GABAA receptors-mediated currents have been observed in the cortical area.28 We examined whether the activation of muscarinic M1 receptors could regulate tonic GABAA receptors-mediated currents (Figure 6). Blocking glutamatergic transmission, GABAB receptors, and sodium channels, we applied BMI (10 µM) in bath solution (Figure 6). Tonic GABAA receptors-mediated currents were observed in a control group (0.18 ± 0.04 pA/pF, n = 6: Figure 6(c)). In the presence of McN-A-343 (30 µM) at least for 10 min, BMI was given in the bath solution (Figure 6(b)). BMI inhibited tonic GABAA receptors-mediated currents (0.15 ± 0.03 pA/pF, n = 6: Figure 6(c)). GABAA receptors-mediated tonic currents did not change among control and McN-A-343-treated groups (P > 0.05: Figure 6(c)). BMI significantly reduced the frequency of mIPSCs in a McN-A-343-treated group (4 ± 1% of McN-A-343 group, n = 8, P < 0.05). These results suggest that the activation of muscarinic M1 receptors may not change extrasynaptic GABAA receptors-mediated currents in the acute-treated condition.
Discussion
In the present study, we combined immunohistochemical, behavioral, and electrophysiological methods and found that the activation of muscarinic M1 receptors in the ACC produces antinociceptive effects on the mechanical pressure threshold in rats without affecting locomotor activity or motor function. We further showed that GABAergic transmission in the ACC was involved in muscarinic M1-mediated behaviors. Finally, the activation of muscarinic M1 receptors increased GABAA receptor-mediated synaptic transmission in the ACC.
Activation of muscarinic M1 receptors in the ACC produces antinociceptive behaviors
Although the effects of muscarine have been studied by systemic injections or manipulations at the spinal cord level, several studies have reported that supraspinal cholinergic antinociception is mediated by M1 receptors.19,34–37 In this study, we found that in the ACC, a key brain area for nociception, the activation of muscarinic M1 receptors produced antinociceptive effects on mechanical thresholds. Anatomical studies have reported that cholinergic neurons project to all layers of the ACC.38–40 ACC injections of the muscarinic M1 receptor selective agonist McN-A-343 produced antinociceptive behaviors in a dose-dependent manner (Figure 1(a)), and the antagonist pirenzepine inhibited McN-A-343-induced antinociceptive effect (Figure 1(c)). In addition, the inhibition of M1 receptors in ACC caused the nociceptive behaviors (Figure 2(a)). In accordance, human41 and rat42 autoradiographic studies have shown the existence of muscarinic M1 receptors in the ACC. Indeed, we confirmed that muscarinic M1 receptors were expressed in the rat ACC. Taken together, our present results suggest that muscarinic M1 receptors in the ACC are involved in the antinociceptive effects on responses to noxious mechanical stimuli.
GABAergic transmission in the ACC involved in nociceptive behavior
We further explored the antinociceptive effects of M1 receptors in the ACC. It has been reported that acetylcholine and muscarine activate GABA receptors in the spinal dorsal horn via release of the inhibitory neurotransmitter, GABA.43,44 Importantly, inhibitory interneurons, such as GABAergic neurons, are involved in antinociceptive processing in the ACC sites.45,46 In this study, we found that in vivo inhibition of GABAA receptors in the ACC reduces mechanical thresholds. On the other hand, the McN-A-343-induced antinociceptive effect was attenuated by co-injection of the GABAA antagonist bicuculline. The muscarinic M1 receptor is coupled to Gq proteins and activates phospholipase C leading to an increase in intracellular calcium, which is able to cause the release of GABA from GABAergic neurons.47 Our behavioral data also show that the inhibition of GABAA receptors in the ACC caused the nociceptive behaviors. These results suggest that the antinociceptive behaviors by the activation of muscarinic M1 receptors may require the facilitation of GABAergic transmission in the ACC.
Facilitation of muscarinic M1 receptors in the ACC amplifier GABAergic transmission
By using whole-cell patch-clamp recordings, we examined the functional roles of muscarinic M1 receptors on GABAergic transmission in the layer II/III from the ACC (Figures 4 to 6). The activation of muscarinic M1 receptors amplifies GABAergic transmission (Figures 4 and 5). We found that the enhancement in GABAergic transmission by activating muscarinic M1 receptors is the result of an increased presynaptic probability of GABA neurotransmitter release in ACC synapses, as demonstrated by the increased sIPSCs and mIPSCs frequency. For the postsynaptic mechanisms of muscarinic M1 receptors, the activation of muscarinic M1 receptors increased the amplitude of sIPSCs; however, the amplitude of mIPSCs did not change by stimulating muscarinic M1 receptors. Although postsynaptic M1 receptors may have the modulatory mechanisms on postsynaptic GABAA receptors, the different mechanisms are still unknown in this study. Further studies are needed to find how postsynaptic muscarinic M1 receptors modulate GABAA receptors by the activity-dependent GABA transmitter releases. We further tested whether the activation of muscarinic M1 receptors can regulate extrasynaptic GABAA receptors-mediated currents (Figure 6). As a result, McN-A-343 treatment for 10–30 min did affect the amplitude of tonic GABAA receptors-mediated currents. Therefore, at least acute treatment of the muscarinic M1 receptors agonist may not affect extrasynaptic GABAA receptors-mediated functions. Together, these results suggest that the enhanced GABAergic transmission by the activation of muscarinic M1 receptors results from the increased probability of presynaptic GABA neurotransmitter release and a possible postsynaptic modification of functional transient GABAA receptors.
These findings show that cholinergic system in ACC produces an antinociceptive effect on responses to mechanical nociceptive stimulation via muscarinic M1 receptors and, at least in part, through neuronal pathways involving in both increased GABA transmitter release and modulating postsynaptic GABAA receptors in the ACC.
Conclusion
We found that the activation of M1 receptors in the ACC produces antinociceptive effects to the mechanical stimuli. Furthermore, the facilitation of M1 receptors in the ACC stimulates GABAergic transmission via GABAA receptors.
Acknowledgments
The authors thank Dr. Descalzi G for critical reading and editing of the manuscript.
Author Contributions
KK and KH designed the experiments and wrote the draft of the manuscript. KK performed electrophysiological analysis. YM, KH, FE, and KM performed behavioral analysis. KI, KM, and SU participated in experimental conception and design and edited the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a Grant-in-Aid from The Ministry of Education, Culture, Sports, Science and Technology of Japan (#23590731 to KH), a grant from the Advanced Materials Institute and the Central Research Institute of Fukuoka University (#066006 to KH), Hirosaki University Grant for Exploratory Research by Young Scientists and Newly-appointed Scientists, The Ichiro Kanehara Foundation, The Nakatomi Foundation, Kato Memorial Bioscience Foundation, and The Karoji Memorial Fund for Medical Research in Hirosaki University (to KK).
References
- 1.Bliss TV, Collingridge GL, Kaang BK, et al. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 2016; 17: 485–496. [DOI] [PubMed] [Google Scholar]
- 2.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005; 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shackman AJ, Salomons TV, Slagter HA, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 2011; 12: 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apkarian AV, Bushnell MC, Treede RD, et al. Human brain mechanisms of pain perception and regulation in health and disease. Euro J Pain 2005; 9: 463–484. [DOI] [PubMed] [Google Scholar]
- 5.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga K, Li X, Chen T, et al. In vivo whole-cell patch-clamp recording of sensory synaptic responses of cingulate pyramidal neurons to noxious mechanical stimuli in adult mice. Mol Pain 2010; 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol 2001; 532: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu MF, Pang ZP, Zhuo M, et al. Prolonged membrane potential depolarization in cingulate pyramidal cells after digit amputation in adult rats. Mol Pain 2005; 1: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuo M. Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci 2016; 39: 136–145. [DOI] [PubMed] [Google Scholar]
- 10.Koga K, Descalzi G, Chen T, et al. Co-existence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015; 85: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XY, Ko HG, Chen T, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010; 330: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis MF, Boyce-Rustay JM. Neuropathic pain: models and mechanisms. Curr Pharm Des 2009; 15: 1711–1716. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong DM, Saper CB, Levey AI, et al. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol 1983; 216: 53–68. [DOI] [PubMed] [Google Scholar]
- 14.Levey AI, Kitt CA, Simonds WF, et al. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 1991; 11: 3218–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eglen RM, Watson N. Selective muscarinic receptor agonists and antagonists. Pharmacol Toxicol 1996; 78: 59–68. [DOI] [PubMed] [Google Scholar]
- 16.Wei J, Walton EA, Milici A, et al. m1-m5 muscarinic receptor distribution in rat CNS by RT-PCR and HPLC. J Neurochem 1994; 63: 815–821. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan NR, Leventhal L, Harrison J, et al. Pharmacological characterization of the muscarinic agonist (3 R,4 R)-3 -(3-hexylsulfanyl-pyrazin-2-yloxy)-1-aza-bicyclo[2.2.1]heptane (WAY-132983) in in vitro and in vivo models of chronic pain. J Pharmacol Exp Ther 2007; 322: 1294–1304. [DOI] [PubMed] [Google Scholar]
- 18.Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology 1995; 82: 331–343. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto ET, Marion L. Characterization of the antinociception produced by intrathecally administered muscarinic agonists in rats. J Pharmacol Exp Ther 1993; 266: 329–338. [PubMed] [Google Scholar]
- 20.Yaksh TL, Dirksen R, Harty GJ. Antinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and cat. Eur J Pharmacol 1985; 117: 81–88. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe M, Takasu K, Kasuya N, et al. Role of descending noradrenergic system and spinal alpha2-adrenergic receptors in the effects of gabapentin on thermal and mechanical nociception after partial nerve injury in the mouse. Br J Pharmacol 2005; 144: 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schechtmann G, Song Z, Ultenius C, et al. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain 2008; 139: 136–145. [DOI] [PubMed] [Google Scholar]
- 23.Honda K, Harada A, Takano Y, et al. Involvement of M3 muscarinic receptors of the spinal cord in formalin-induced nociception in mice. Brain Res 2000; 859: 38–44. [DOI] [PubMed] [Google Scholar]
- 24.Honda K, Koga K, Moriyama T, et al. Intrathecal alpha2 adrenoceptor agonist clonidine inhibits mechanical transmission in mouse spinal cord via activation of muscarinic M1 receptors. Neurosci Lett 2002; 322: 161–164. [DOI] [PubMed] [Google Scholar]
- 25.Koga K, Honda K, Ando S, et al. Intrathecal clonidine inhibits mechanical allodynia via activation of the spinal muscarinic M1 receptor in streptozotocin-induced diabetic mice. Eur J Pharmacol 2004; 505: 75–82. [DOI] [PubMed] [Google Scholar]
- 26.Honda K, Ando S, Koga K, et al. The spinal muscarinic receptor subtypes contribute to the morphine-induced antinociceptive effects in thermal stimulation in mice. Neurosci Lett 2004; 371: 235–238. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 5th ed San Diego, CA: Academic Press, 2005. [Google Scholar]
- 28.Yamada J, Furukawa T, Ueno S, et al. Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cereb Cortex 2007; 17: 1782–1787. [DOI] [PubMed] [Google Scholar]
- 29.Migita K, Tomiyama M, Yamada J, et al. Phenotypes of pain behavior in phospholipase C-related but catalytically inactive protein type 1 knockout mice. Mol Pain 2011; 7: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies RH, Scholes HE, Virdi S, et al. Inhibition of field stimulation-induced contractions of rabbit vas deferens by muscarinic receptor agonists: selectivity of McN-A-343 for M1 receptors. J. Pharm Pharmacol 2001; 53: 487–496. [DOI] [PubMed] [Google Scholar]
- 31.Abe K, Taguchi K, Kato M, et al. Characterization of muscarinic receptor subtypes in the rostral ventrolateral medulla and effects on morphine-induced antinociception in rats. Eur J Pharmacol 2003; 465: 237–249. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Ren WH, Zhang YQ, et al. GABAergic disinhibition facilitates polysynaptic excitatory transmission in rat anterior cingulate cortex. Biochem Biophys Res Commun 2005; 338: 1634–1639. [DOI] [PubMed] [Google Scholar]
- 33.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 1998; 50: 279–290. [PubMed] [Google Scholar]
- 34.Bartolini A, Ghelardini C, Fantetti L, et al. Role of muscarinic receptor subtypes in central antinociception. Br J Pharmacol 1992; 105: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghelardini C, Galeotti N, Figini M, et al. The central cholinergic system has a role in the antinociception induced in rodents and guinea pigs by the antimigraine drug sumatriptan. J Pharmacol Exp Ther 1996; 279: 884–890. [PubMed] [Google Scholar]
- 36.Ghelardini C, Galeotti N, Bartolini A. Loss of muscarinic antinociception by antisense inhibition of M(1) receptors. Br J Pharmacol 2000; 129: 1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naguib M, Yaksh TL. Characterization of muscarinic receptor subtypes that mediate antinociception in the rat spinal cord. Anesth Analg 1997; 85: 847–853. [DOI] [PubMed] [Google Scholar]
- 38.Mesulam MM, Rosen AD, Mufson EJ. Regional variations in cortical cholinergic innervation: chemoarchitectonics of acetylcholinesterase-containing fibers in the macaque brain. Brain Res 1984; 311: 245–258. [DOI] [PubMed] [Google Scholar]
- 39.Lewis DA. Distribution of choline acetyltransferase-immunoreactive axons in monkey frontal cortex. Neuroscience 1991; 40: 363–374. [DOI] [PubMed] [Google Scholar]
- 40.Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience 2001; 103: 593–614. [DOI] [PubMed] [Google Scholar]
- 41.Villiger JW, Faull RL. Muscarinic cholinergic receptors in the human spinal cord: differential localization of [3H]pirenzepine and [3H]quinuclidinylbenzilate binding sites. Brain Res 1985; 345: 196–199. [DOI] [PubMed] [Google Scholar]
- 42.Wamsley JK, Gehlert DR, Roeske WR, et al. Muscarinic antagonist binding site heterogeneity as evidenced by autoradiography after direct labeling with [3H]-QNB and [3H]-pirenzepine. Life Sci 1984; 34: 1395–1402. [DOI] [PubMed] [Google Scholar]
- 43.Baba H, Kohno T, Okamoto M, et al. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J Physiol 1998; 508(Pt 1): 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li DP, Chen SR, Pan YZ, et al. Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol 2002; 543: 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Millan MJ. Descending control of pain. Prog Neurobiol 2002; 66: 355–474. [DOI] [PubMed] [Google Scholar]
- 46.Jasmin L, Wu MV, Ohara PT. GABA puts a stop to pain. Curr Drug Targets CNS Neurol Disord 2004; 3: 487–505. [DOI] [PubMed] [Google Scholar]
- 47.Harsing LG, Jr, Zigmond MJ. Postsynaptic integration of cholinergic and dopaminergic signals on medium-sized GABAergic projection neurons in the neostriatum. Brain Res Bull 1998; 45: 607–613. [DOI] [PubMed] [Google Scholar]