Abstract
Objectives:
Adenocarcinoma is known to be associated with ulcerative colitis, but the diagnosis is sometimes challenging, both clinically and pathologically.
Methods and Results:
We present a case of extremely well-differentiated adenocarcinoma associated with ulcerative colitis, in which preoperative diagnosis was not possible. Glands in biopsy specimens showed a serrated appearance that looked like low-grade dysplasia or regenerative mucosa. After an operation due to severe symptoms of stenosis, carcinoma was diagnosed. Tumor cells, especially in invasive glands, tended to show stronger immunoreactivity against anti-CK7, TNF-α and Aurora B antibodies compared to cells of mucosal lesion. Interestingly, CD44v6, one of the adhesion molecules, was less expressed in invasive glands, while those glands exhibited stronger expression of a disintegrin and metalloproteinase 17 (ADAM 17), one of the sheddases that cleaves an extracellular domain of CD44.
Conclusions:
These observations appear interesting to consider the pathogenesis and to diagnose extremely well-differentiated adenocarcinoma in ulcerative colitis, although further investigation is needed.
Keywords: Ulcerative colitis, extremely well-differentiated adenocarcinoma, CK7, TNF-α, CD44v6
Introduction
Various colorectal malignant tumors are known to be associated with inflammatory bowel diseases (IBDs) including ulcerative colitis (UC). Among them, adenocarcinoma is the most common.1 However, adenocarcinoma in IBD might be overlooked by endoscopical examination, because it tends to be poorly circumscribed and multifocal, in contrast to sporadic colorectal adenocarcinoma.2,3 Pathological diagnosis in biopsy specimens is also challenging when distinguishing adenocarcinoma from regenerative atypia or dysplasia, especially when it is accompanied with marked inflammation.
Among carcinoma occurring in IBD, about 11% are reported to be extremely well-differentiated adenocarcinoma (EWDA), which is also called low-grade tubulograndular adenocarcinoma.3 This type of adenocarcinoma is very difficult to diagnose in biopsy specimens due to its minimal cellular and architectural atypia. We have experienced a case of EWDA associated with UC, in which preoperative diagnosis was not possible. Characteristics of the tumor are presented with some intriguing immunohistochemical staining results.
Case report
A 45-year-old man who had been suffering from UC for about 20 years had a total colectomy and ileoanal canal anastomosis performed for rectal adenocarcinoma. About 1 year and 7 months after the operation, redness and erosion were observed around the anastomosis site, and a dysplasia-associated lesion or mass (DALM)-like elevated lesion developed about 4 months later. Regenerative mucosa or low-grade dysplasia was the diagnosis after repeated biopsies. Since symptoms of stenosis were severe, a resection of the ileoanal canal was performed 2 years and 6 months after the first operation.
In three tissues taken in a biopsy about 1 year and 7 months after the first operation, glands were sparsely distributed with background of mild inflammation. Some glands exhibited mild elongation with a decrease in number of goblet cells, but nuclei were uniform and located in the basal area. Regeneration was suspected (Figure 1). In the second and third biopsies, about 2 years and 2 years and 1 month after the first operation, serrated glands were densely distributed. Nuclei were mildly enlarged. Background inflammation was mild. Within five tissues taken in each biopsy, there were no apparent findings that indicated invasion. Low-grade dysplasia was suggested, at least in part (Figure 1). However, three tissues of the subsequent biopsy (2 years and 5 months after the first operation) looked like regenerated mucosa containing a few glands with little nuclear atypia. It was accompanied with mild-to-moderate inflammation (Figure 1).
Figure 1.
Histological features of the biopsy specimens (a-c) 1 year and 7 months, (d-f) 2 years and 1 month and (g-i) 2 years and 7 months after the first operation. Serrated glands are observed: diagnosis was low-grade dysplasia in the specimen of 2 years and 1 month, but regenerative mucosa in the others.
In the operated material, the anastomosis site was severely stenotic (Figure 2). Although there were no apparent elevated masses, the mucosa around the anastomosis was rough and the intestinal wall was thickened hard extending over about 6 cm in length. Histologically, atypical glands proliferated from the mucosa to subserosa: glands tended to show a serrated appearance in the propria mucosa and were tubular below the submucosa (Figure 3). Cellular atypia looked minimal, especially in the superficial area, where cells were uniform with low nuclear cytoplasmic ratio. In invasive glands, nuclei were somewhat irregular and enlarged (Figure 3). In non-tumorous mucosa, there was mild-to-moderate inflammation consistent with UC, accompanying mild basal lymphoplasmacytosis. Glands were shortened and distorted (Figure 3).
Figure 2.

Macroscopic appearance of the resected ileum and colon showing severe stenosis at the anastomosis site.
Figure 3.
Histological features of the operated specimen. (a–c) Glands with minimal atypia tend to show a serrated appearance in the propria mucosa and were tubular below the submucosa. Nuclear atypia is more conspicuous in invasive glands (c). (d-e) In non-tumorous mucosa, mild-to-moderate inflammation with mild basal lymphoplasmacytosis is observed. Glands are shortened and distorted.
sm: submucosa, mp: muscularis propria.
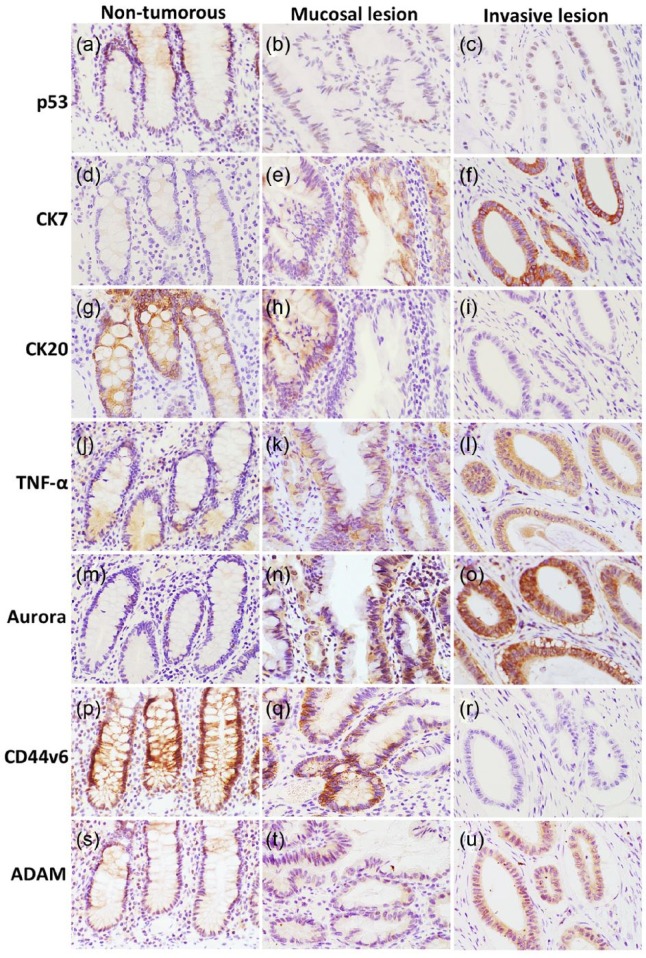
To investigate the characteristics of this tumor, representative sections were immunostained using the EnVision system (Dako, Grostrup, Denmark). The primary antibodies used are summarized in Table 1. On the operated specimen, p53-immunopositive tumor cells were a few and β-catenin was negative. There were many CK7-positive tumorous glands, but CK20 was positive only in some glands in the mucosa (Figure 4). TNF-α and Aurora B were more intensively stained in tumorous glands compared to non-tumorous glands. Invasive glands tended to be stained stronger on immunostaining with CK7, TNF-α and Aurora B (Figure 4). Immunoreactivity against the anti-CD44v6 antibody was focally reduced in tumorous glands in the propria mucosa, and disappeared in invasive glands (Figure 4). By contrast, a disintegrin and metalloproteinase 17 (ADAM 17) were expressed more in invasive glands (Figure 4). There were no apparent differences between tumorous and non-tumorous glands in other immunostains: Aurora A, cycloosygenase-2 (COX-2), and signal transducer and activator of transcription 3 (STAT 3) were positive or weakly positive, and others were negative.
Table 1.
Primary antibodies used in this study.
| Dilution and antigen retrieval | Cat. no. | Source | |
|---|---|---|---|
| p53 | 1:100, mouse, MW* | M7001 | Dako, Glostrup, Denmark |
| β-catenin | 1:1000, mouse, MW* | 610153 | BD Biosciences, San Jose, CA, USA |
| p-c-jun | 1:100, mouse | sc-822-HRP | Santa Cruz Biotechnology, Dallas, TX, USA |
| p16 | 1:1000, mouse, MW | ab54210 | Abcam, Cambridge, MA, USA |
| CK7 | 1:100, mouse, MW | M7018 | Dako |
| CK20 | 1:50, mouse, MW | M7019 | Dako |
| AMACR | 1:300, rabbit, MW* | M3616 | Dako |
| Aurora A | 1:1000, rabbit, MW* | ab1287 | Abcam |
| Aurora B | 1:30, mouse, MW | ab3609 | Abcam |
| TNF-α | 1:20, mouse, MW* | MAB610 | R&D Systems, Minneapolis, MN, USA |
| COX-2 | 1:500, rabbit, MW | 160107 | Cayman, Ann Arbor, MI, USA |
| NFκ-B | 1:500, rabbit | AB1604 | Chemicon, Temecula, CA, USA |
| STAT3 | 1:500, rabbit, MW* | #9132 | Cell Signaling Technology, Danvers, MA, USA |
| CD44v6 | 1:500, mouse, MW* | ab78960 | Abcam |
| ADAM10 | 1:500, goat, MW* | sc-16524 | Santa Cruz Biotech |
| ADAM17 | 1:1000, rabbit, MW* | sc-13973 | Santa Cruz Biotech |
AMACR: α-methylacyl-CoA racemase; TNF: tissue necrosis factor; COX: cyclooxygenase; STAT: signal transducer and activator of transcription; ADAM: a disintegrin and metalloproteinase 17.
MW: microwave antigen retrieval with citrate buffer (pH6.0).
MW*: microwave antigen retrieval with Tris-EDTA (pH9.0).
Figure 4.
Immunohistochemical staining of the operated specimen: (a–c) p53-positive cells are a few. (d–i) Tumor cells show CK7+/CK20− patterns and (j–o) tend to be positive for TNF-α and Aurora B, especially in invasive glands. (p-u) CD44v6 and ADAM17 show staining in a reversed manner.
TNF: tissue necrosis factor; ADAM17: a disintegrin and metalloproteinase 17.
On retrospective examination of biopsy specimens, atypical glands that suggested low-grade dysplasia were positive for CK7 and weakly positive for TNF-α and Aurora B (Figure 5). Some atypical glands exhibited decreased membranous expression of CD44v6 (Figure 5). CK20-positive glands were a few. There was no apparent positive reaction on ADAM17 immunostaining.
Figure 5.
Immunohistochemical staining of the biopsy specimens. Glands of 1 year and 7 months and of 2 years and 5 months are not clearly positive for (a, c) CK7, (d, f) TNF-α or (g, i) Aurora B, but (b, e, h) those of 2 years and 1 month are weakly positive or positive. There were glands showing reduced membranous positivity of CD44v6 (j–l), especially in (k).
Discussion
Pathological diagnosis of dysplasia and adenocarcinoma in UC is sometimes problematic when background inflammation is severe or biopsy materials contain a few atypical glands. Histological appearances of dysplasia in IBD usually resemble a conventional tubular, tubulovillous or villous adenoma, but serrated dysplasia is also known.4 High-grade dysplasia and carcinoma in UC are frequently immunopositive for p53,5 but there are negative cases. In serrated dysplasia, p53 is negative and a serrated pathway of carcinogenesis through the silencing of O(6)-methylguanine-DNA methyltransferase has been proposed.4 EWDA is particularly difficult to distinguish from regenerative atypia or low-grade dysplasia, especially showing a serrated appearance like in our case.
In the resected tumor of the present case, it is uncertain whether mucosal components with minimal atypia are low-grade dysplasia or a part of carcinoma. Since there were no apparent fronts between minimally atypical glands and invasive glands, the latter may be appropriate. However, the mucosa overlying and surrounding EWDA can show indefinite dysplasia, low-grade dysplasia or high-grade dysplasia.3 When superficial serrated lesions are taken in a biopsy, proper diagnosis may be impossible, and the diagnosis might be low-grade dysplasia at most. In the resected tumor of the present case, invasive glands below the muscularis mucosa exhibited more irregular shapes, with more nuclear atypia than superficial serrated glands. Stronger cellular atypia has been reported in deeply invaded glands in EWDA.3 The preoperative biopsy specimens did not contain glands that suggested invasion. Furthermore, the last one was like a regenerative mucosa. Thus, the diagnostic difficulties in biopsy specimens involve spatial and chronological factors. So far, to raise an accuracy of diagnosis, one of the methods to employ could be to take as many specimens from deeper portions of the lesion as possible.
Recent reports describe that CK7 and α-methylacyl-CoA racemase (AMACR)-positive cells tend to be more present in dysplasia and adenocarcinoma in IBD.6–8 In contrast to sporadic colorectal adenocarcinoma commonly showing a CK7−/CK20+ profile, dysplasia/adenocarcinoma in UC tends to contain more CK7-positive cells.6, 7 A CK7+/CK20− pattern in the present case supports that immunostaining with CK7 in addition to p53 may be useful to find dysplasia/adenocarcinoma in UC. If CK7-positive-serrated lesions are observed in biopsy specimens from DALMs, at least low-grade dysplasia can be suggested. However, it should be noted that positive immunoreactivity against CK7 has been observed in inflamed epithelia, hyperplastic polyp and serrated adenoma.7 AMACR is reported to be highly specific to dysplasia and adenocarcinoma in IBD,8 but it was negative in our case.
To investigate more the characteristics of the tumor in the present study, immunohistochemistry against several proteins related to tumorigenesis or tumor progression was used. Among them, TNF-α and Aurora B exhibited increased expression in tumor cells, especially in invasive glands. TNF-α is a proinflammatory cytokine, produced by immune cells, endothelial cells and fibroblasts, and its elevation correlates with the activity of IBD.9 Moreover, TNF-α may be involved in the development9 and invasion10 of IBD-associated carcinoma of the gastrointestinal tract. One of the TNF-α-mediated pathways is through the activation of Src.10 Src activity is increased in malignant and dysplastic epithelia in UC.2,10 Aurora kinases known so far as Auroras A to C are the key regulators of mitosis and are overexpressed in some carcinomas.11,12 Aurora A and Aurora B may be candidates for diagnostic markers in the carcinogenesis in UC patients.13 The result of our case supports that TNF-α and Aurora B are involved in the carcinogenesis of EWDA in UC.
Since carcinoma cells in the present case exhibited remarkable infiltrating potential, the expression of adhesion molecules and related proteins was investigated. UC-associated carcinoma tends to show reduced expression of standard form CD44 compared to sporadic carcinoma.14 Among variants of CD44, we have focused on CD44v6, because increased expression of CD44v6 has been observed in various tumors including colorectal adenocarcinoma, and there are reports describing a correlation with prognosis.15–17 An interesting point of the present case is that invasive glands tended to lack positive immunoreactivity against the anti-CD44v6 antibody, but showed stronger immunoreactivity against the anti-ADAM17 antibody. CD44v6 has a high affinity with extracellular hyaluronan, which entraps tumor cells at the primary site, and its expression tends to be lost at the invasive front and metastatic site.15,18 The ectodomain of CD44 is cleaved by sheddases such as ADAM10 and ADAM17.16,19 Alteration of the receptor site by cleavage may modulate interaction with extracellular hyaluronan, which may give rise to increased invasive ability of the tumor. Similar results have been found between CD44 and matrixmetalloproteinase 9 (MMP-9).20 In addition to MMP-9, cleavage of CD44v6 by ADAM17 may be one of the factors involved in the invasion in the present case.
On biopsy specimens, increased immunoreactivity against anti-CK7, TNF-α and Aurora B antibodies was observed in glands that suggested low-grade dysplasia. Some atypical glands showed decreased expression of CD44v6. Irregular staining pattern of CD44 may be useful for the diagnosis of UC-associated neoplasm.20 Although further investigation is needed, an immunohistochemical panel of CK7, TNF-α, Aurora B and CD44v6 would help a diagnosis of EWDA, especially when there are clinical symptoms like stenosis of our case.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Verbal informed consent was obtained from the patient for their anonymized information to be published in this article.
References
- 1. DeRouche TC, Xiao S-Y, Liu X. Histological evaluation in ulcerative colitis. Gastroenterol Rep 2014; 2: 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kulaylat MN, Dayton MT. Ulcerative colitis and cancer. J Surg Oncol 2010; 101: 706–712. [DOI] [PubMed] [Google Scholar]
- 3. Levi GS, Harpaz N. Intestinal low-grade tubuloglandular adenocarcinoma in inflammatory bowel disease. Am J Surg Pathol 2006; 30: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 4. Srivastava A, Redston M, Farraye FA, et al. Hyperplastic/serrated polyposis in inflammatory bowel disease: a case series of a previously undescribed entity. Am J Surg Pathol 2008; 32: 296–303. [DOI] [PubMed] [Google Scholar]
- 5. Ajioka Y, Watanabe H, Masuda K. Over-expression of p53 protein in neoplastic changes in ulcerative colitis: immunohistochemical study. J Gastroenterol 1995; 30: 33–35. [PubMed] [Google Scholar]
- 6. Stenling R, Lindberg J, Rutegård J, et al. Altered expression of CK7 and CK20 in preneoplastic and neoplastic lesions in ulcerative colitis. APMIS 2007; 11: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 7. Tatsumi N, Kushima R, Vieth M, et al. Cytokeratin 7/20 and mucin core protein expression in ulcerative colitis-associated colorectal neoplasms. Virchows Arch 2006; 448: 756–762. [DOI] [PubMed] [Google Scholar]
- 8. Dorer R, Odze RD. AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett’s esophagus, ulcerative colitis, and Crohn’s disease. Am J Surg Pathol 2006; 30: 871–877. [DOI] [PubMed] [Google Scholar]
- 9. Ślebioda TJ, Kmieć Z. Tumour necrosis factor superfamily members in the pathogenesis of inflammatory bowel disease. Mediat Inflamm 2014; 2014: 325129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawai N, Tsuji S, Tsuji M, et al. Tumor necrosis factor alpha stimulates invasion of Src-activated intestinal cells. Gastroenterology 2002; 122: 331–339. [DOI] [PubMed] [Google Scholar]
- 11. Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res 2006; 12: 6869–6875. [DOI] [PubMed] [Google Scholar]
- 12. Kitzen JJEM, de Jonge MJA, Verweij J. Aurora kinase inhibitors. Crit Rev Oncol Hematol 2010; 73: 99–110. [DOI] [PubMed] [Google Scholar]
- 13. Gerçeker E, Boyacıoglu SO, Kasap E, et al. Never in mitosis gene A-related kinase 6 and aurora kinase A: new gene biomarkers in the conversion from ulcerative colitis to colorectal cancer. Oncol Rep 2015; 34: 1905–1914. [DOI] [PubMed] [Google Scholar]
- 14. Mikami T, Yoshida T, Numata Y, et al. Invasive behavior of ulcerative colitis-associated carcinoma is related to reduced expression of CD44 extracellular domain: comparison with sporadic colon carcinoma. Diagn Pathol 2011; 6: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Afify A, Durbin-Johnson B, Virdi A, et al. The expression of CD44v6 in colon: from normal to malignant. Ann Diagn Pathol 2016; 20: 19–23. [DOI] [PubMed] [Google Scholar]
- 16. Kim YH, Jung J-C. Suppression of tunicamycin-induced CD44v6 ectodomain shedding and apoptosis is correlated with temporal expression patterns of active ADAM10, MMP-9 and MMP-13 proteins in Caki-2 renal carcinoma cells. Oncol Rep 2012; 28: 1869–1874. [DOI] [PubMed] [Google Scholar]
- 17. Saito S, Okabe H, Watanabe M, et al. CD44v6 expression is related to mesenchymal phenotype and poor prognosis in patients with colorectal cancer. Oncol Rep 2013; 29: 1570–1578. [DOI] [PubMed] [Google Scholar]
- 18. Avoranta ST, Korkeila EA, Syrjänen KJ, et al. Lack of CD44variant 6 expression in rectal cancer invasive front associates with early recurrence. World J Gastroenterol 2012; 18: 4549–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamarajan P, Shin JM, Qian X, et al. ADAM17-mediated CD44 cleavage promotes orasphere formation or stemness and tumorigenesis in HNSCC. Cancer Med 2013; 2: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. AbdElazeem MA, El-Sayed M. The pattern of CD44 and matrix metalloproteinase 9 expression is a useful predictor of ulcerative colitis-associated dysplasia and neoplasia. Ann Diagn Pathol 2015; 19: 369–374. [DOI] [PubMed] [Google Scholar]