Abstract
Among the nine classes of ribozymes that have been experimentally validated to date is the metabolite-responsive self-cleaving ribozyme called glmS. This RNA is almost exclusively located in the 5′ untranslated region of bacterial mRNAs that code for the production of GlmS proteins, which catalyze the synthesis of the aminosugar glucosamine-6-phosphate (GlcN6P). Each glmS ribozyme forms a conserved catalytic core that selectively binds GlcN6P and uses this metabolite as a cofactor to promote ribozyme self-cleavage. Metabolite-induced self-cleavage results in down-regulation of glmS gene expression, and thus the ribozyme functions as a key riboswitch component to permit feedback regulation of GlcN6P levels. Representatives of glmS ribozymes also serve as excellent experimental models to elucidate how RNAs fold to recognize small molecule ligands and promote chemical transformations.
Keywords: glucosamine-6-phosphate, metabolite sensing, riboswitch, RNA catalysis, self-cleaving
Regulation of Bacterial Gene Expression by Riboswitches
The typical bacterium encodes for at least 100 different transcription factors, which individually exert regulatory control over the efficiency of transcription initiation. However, each of the stages of gene expression after transcription initiation is also subjected to regulatory control in bacteria. These post-initiation processes include transcription elongation, transcription termination, translation, and mRNA stability. A diverse array of cis- and trans-acting RNA elements has been discovered that can regulate these post-initiation processes. For example, an increasingly large collection of metabolite-sensing, cis-acting regulatory RNAs called riboswitches has been identified (1–2). Riboswitches are mostly located in 5′ leader regions of mRNAs, although some are located within intercistronic portions of polycistronic transcripts. These RNA elements control gene expression in response to direct association with the appropriate metabolite ligand. Over 20 classes have been discovered, each individually responding to a different metabolite ligand.
A variety of metabolite ligands are sensed by riboswitches (1), including cofactors, amino acids, nucleobase-containing metabolites, a second messenger signaling molecule, and an amino sugar precursor of cell wall synthesis. Sensing of the appropriate metabolite is achieved through a ligand-responsive domain (the aptamer), which controls gene expression by altering the configuration of regulatory sequences that interface between the aptamer and the downstream gene (the expression platform). Riboswitch aptamers associate with their target ligands with high affinity and with exquisite specificity. Structural models of ligand-bound riboswitch aptamers (3–5) have revealed that the RNA-bound ligands are stabilized by a combination of hydrogen bonding interactions, electrostatic interactions, and base-stacking energies. Typically, association of the appropriate metabolite ligand is integrated into a feedback inhibition regulatory response, although many riboswitch examples have also been discovered for other types of regulatory circuitry topology, such as substrate-mediated activation of catabolism genes (6).
The majority of bacterial riboswitches couple association of the ligand(s) to gene expression by controlling ribosomal access to a downstream translation initiation site or by controlling formation of a transcription termination site (transcription attenuation). One particular riboswitch class, which responds to thiamin pyrophosphate (TPP), has been demonstrated to affect mRNA processing and stability in plants and lower eukaryotes (7). However, one riboswitch class has been discovered to regulate gene expression through a mechanism other than classical transcription attenuation or ligand-mediated sequestration of the ribosome binding site. Members of this particular riboswitch class, generally called “glmS ribozymes” respond to the cell wall precursor, glucosamine-6-phosphate (GlcN6P) and regulate expression of the glucosamine-6-phosphate synthetase gene (glmS). Instead of transcription attenuation or translational regulation, glmS ribozymes control mRNA stability in bacteria in a ligand-responsive manner, as reviewed herein.
Distribution of glmS ribozymes
The known phylogenetic distribution of glmS ribozymes has greatly increased since the initial identification of this riboswitch class in organisms from the Firmicutes and Fusobacterium phyla (8–9). Representatives have subsequently been identified within the Actinobacteria, Deinococcus-Thermus, Tenericutes, Chloroflexi, Synergistetes, and Dictyoglomi phyla, as well as Thermobaculum terrenum, which has yet to be assigned to a phylum (10–11). Despite this expansion in the phyla containing glmS ribozymes, most examples still reside in Firmicutes.
A total of 463 glmS ribozyme representatives have been identified to date (11). Almost without exception, each organisms carries a single representative that resides immediately upstream of the open reading frame coding for the GlmS protein. There are only two known instances of organisms that carry more than one glmS ribozyme. The Firmicute Dethiobacter alkaliphilus carries one representative located upstream of the glmS ORF and a second representative located upstream of the glmM ORF. GlmM is an enzyme (phosphoglucomutase) that converts GlcN6P into glucosamine-1-phosphate. The Fusobacterium Sebaldella termitidis also carries a glmS ribozyme in the normal location and has a second representative located upstream of the glnA ORF. GlnA is an enzyme (glutamine synthase) that converts glutamate into glutamine (the immediate precursor of GlcN6P). Thus, both additional riboswitches appear to control the expression of genes that are immediately adjacent to GlcN6P in its synthesis and utilization pathways.
It has been suggested (12–13) that glmS ribozymes may be useful targets for the design of novel antibacterial compounds because they control the expression of the glmS gene whose protein product is essential for cell survival (14), Indeed, the glmS ribozyme also is a potentially attractive drug target due its seemingly immutable catalytic core structure, and the abundance of representatives in pathogenic Firmicutes (11, 15). A number of GlcN6P analogs have been prepared and shown to trigger ribozyme function in biochemical assays (12, 16). A structural rationale for function of each analog is evident on examining the atomic-resolution structures of glmS ribozymes (e.g. 17). It appears possible to create additional GlcN6P analogs or perhaps even compounds with completely different chemical scaffolds that could trigger glmS ribozyme action in cells. Such compounds would trick bacteria into repressing glmS gene expression despite the fact that cells are starved for the natural metabolite.
The structural features of glmS ribozymes
Nearly all glmS ribozyme representatives conform to a complex consensus sequence and secondary structure model that can be divided into catalytic core and core support sub-domains. Each ribozyme carries a well-conserved ligand-binding and catalytic core, but with greater variability in sequence and structure outside of this active site. This same pattern of sequence and structural conservation holds true for a mutant glmS ribozyme population that was subjected to in vitro selection (18), suggesting that there are very few if any variants close in sequence space that retain GlcN6P-mediated catalytic activity. Most likely, the strict conservation of the catalytic core is due to the dual necessity to selectively bind GlcN6P and to position this ligand to promote efficient RNA transesterification.
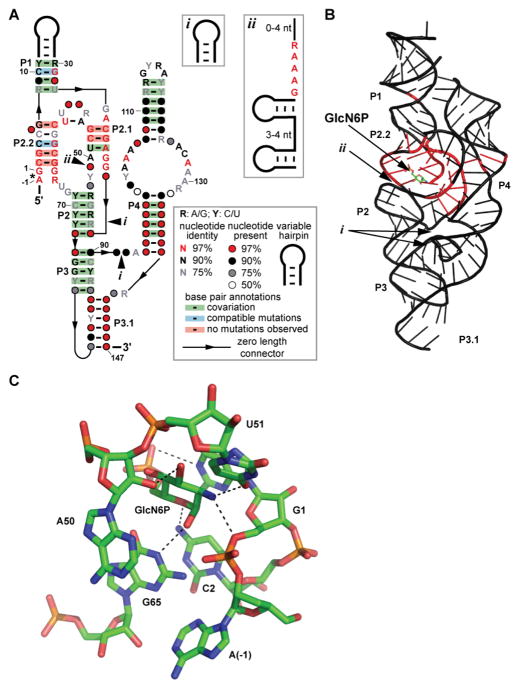
A 3-D structure (Fig. 1) comprised of several roughly coaxial RNA helices and three pseudoknots was identified upon analysis by x-ray crystallography of a glmS ribozyme representative from Thermoanaerobacter tengcongensis (19) and another from Bacillus anthracis (20). Specifically, the P2.1 helix centrally located between the P4 helix on one side and the P1, P2.2, P2, P3, and P3.1 helices. In every glmS ribozyme that possesses a P4 helix, it serves as a support for the catalytic core and binding pocket by employing its GNRA tetraloop as a docking component to form a tertiary interaction with the P1 helix (19–20). In almost every instance when the P4 helix is removed, catalysis is retained (albeit with a substantially reduced rate constant) when high concentrations of cations are present to aid in stable structural formation (21).
FIGURE 1.
Key sequence and structural features of glmS ribozymes., its relation to an atomic-resolution structural model, and RNA contacts to GlcN6P. (A) Consensus sequence and secondary structure model for glmS ribozymes. The asterisk designates the site of self-cleavage. Optional hairpins (i) or optional hairpins and conserved nucleotides (ii) are present in some glmS ribozymes. When provided, numbered nucleotides correspond to positions from a previously reported glmS ribozyme structure (Klein and Ferré-D’Amaré 2006) as depicted in B. (B) Atomic-resolution structural model of a glmS ribozyme from T. tengcongensis (Klein et al. 2007; Protein Databank 3B4C). Nucleotides that are shaded in red are conserved in at least 97% of glmS ribozymes. Arrows depict the locations of optional sub-structures (i) and (ii) when present. (C) Model of the nucleotides forming the coenzyme-binding active site of the T. tengcongensis glmS ribozyme (Klein et al. 2007; Protein Databank 3B4C). Nucleotides are labeled and numbered according to the consensus model in A. Parts A and B are adapted from a previous publication (McCown et al. 2011).
The catalytic core of the ribozyme is largely contained within the P2.1 and P2.2 segments of the ribozyme (Fig. 1; 8, 11, 19, 20). The nucleotides within these regions that have been determined to be responsible for direct binding to GlcN6P are A(-1), G1, C2, A50, U51, and G65 (see numbering of the consensus model in Fig. 1) (19–20). These contacts are located within the most highly-conserved part of the ribozyme, and therefore efforts to design analogs that trigger ribozyme function will likely need to consider this chemical landscape. Also, some parts of the ligand may not be recognized by the ribozyme. For example, there are no direct contacts to the hydroxyl at the 4 position of GlcN6P, which could be exploited in future analog constructions. In contrast, G40 is strictly conserved and is essential for catalysis, but does not participate in ligand binding (17, 19, 20).
Some of the highly-conserved nucleotides make contacts with others to form the rigid structure of the active site, while others establish key contacts between the ribozyme and GlcN6P (19–20). For example, the ribose of nucleotide A(-1) carries the 2′ oxygen nucleophile for the phosphoester transfer reaction that results in RNA cleavage. The 2′-endo ribose pucker of this nucleotide assists in forming a ribose-phosphate backbone conformation that adopts the near in-line geometry necessary for catalysis to occur. The guanine nucleobase of G1, which stacks on top of the sugar ring of GlcN6P, uses its N1 position to form a hydrogen bond to the phosphate portion of the ligand. This may be the source of the ribozyme’s ability to discriminate to some extent against the sulfate analog of GlcN6P (8). Most intriguingly, the amine at the 2 position of the ligand (or hydroxyl of other inactive analogs such as Glc6P) forms a hydrogen bond with the 5′ oxygen of G1. This oxygen is the leaving group during RNA chain scission, and therefore neutralization of developing negative charge on this leaving group oxygen may be a major contributor to the catalytic rate enhancement generated by the ribozyme. Interestingly, the epimeric configuration of the amino group of GlcN6P has little affect on the activity and the structure of the ribozyme (12, 17).
A number of other contacts help bind and position the ligand in the active site. For example, the N4 of C2 forms a hydrogen bond with the oxygen at the 5 position of GlcN6P, and another hydrogen bond is formed between the 2′ oxygen of A50 and the oxygen at the 3 position of the ligand. The contact that C2 makes to the ligand may explain why it is necessary for the ligand to be cyclic for robust activation of the ribozyme (12, 22). However, the contact that A50 makes to GlcN6P does not appear to discriminate between an axial or equatorial position for the hydroxyl group at the 3 position. The O4 of U51 forms a hydrogen bond with the amine of GlcN6P and the N1 of G65 forms a hydrogen bond to the hydroxyl of the anomeric carbon of GlcN6P (20). The N1 of G65 exclusively binds to the α-conformation of anomeric carbon in GlcN6P (12–13).
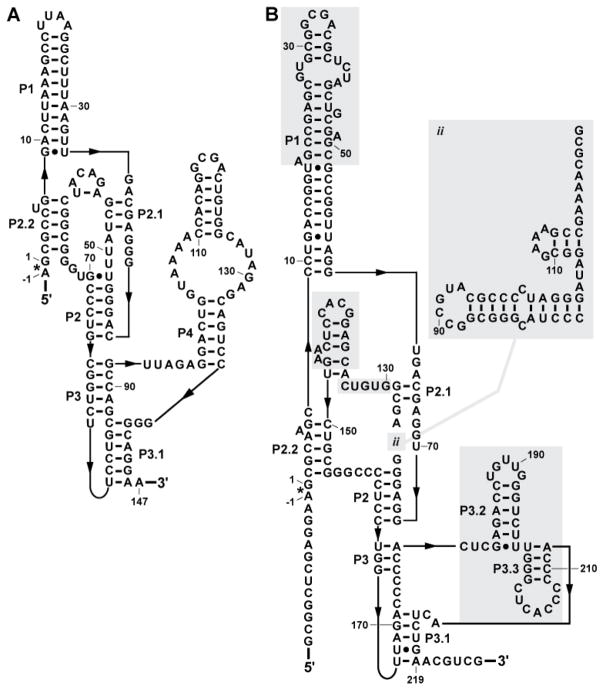
Despite the extensive conservation of the catalytic cores of glmS ribozymes, there are rare exceptions that tolerate mutations. In every Deinococcales species sequenced to date, the nucleotide that corresponds to U51 is a guanosine (11). Within all Thermales species thus far sequenced, as well as within Truepera radiovictrix, there is an additional structural sub-domain that resides just before A50 (Fig. 1). This sub-domain consists of two variable-sequence hairpins separated by a short linker sequence, and a conserved sequence GAAAR that immediately follows the second hairpin. The loop of the second hairpin is almost always a tetraloop and often consists of structured loops such as GNRA or UNCG. Though this module is positioned very close to the binding pocket, it does not appear to affect substrate specificity in biochemical assays (11).
Far more sequence variation is observed outside of the glmS ligand-binding catalytic core, although the vast majority of the variation occurs with retention of the P3 and P4 structures. Some organisms within the Deinococcus-Thermus phylum carry an additional variable-sequence hairpin between the P3 and P4 helices (11). Previously, it was proposed that novel core support structures may take the place of the P4 helical stack (21). Indeed, Truepera radiovictrix carries a glmS ribozyme that appears to dispense with the entire P4 helix (Fig. 2). This ribozyme functions efficiently without the need for high cation concentrations that were required for other glmS ribozymes without the P4 helix (21). Perhaps other glmS ribozymes with similar structural changes that retain robust catalytic activity can be found as more genomic sequences become available.
FIGURE 2.
Comparison of a canonical glmS ribozyme with an extreme variant. (A) The ribozyme from Thermoanaerobacter tengcongensis corresponds well to the consensus glmS ribozyme architecture typical of the vast majority of representatives. The asterisk identifies the site of self-cleavage and arrows denote zero-length connections. (B) A variant glmS ribozyme present in Truepera radiovictrix. Shaded regions identify sequences and structures that are substantially different from the typical glmS ribozyme consensus architecture.
A possible catalytic mechanism for glmS ribozymes
Both biochemical and structural data provide some clues regarding the mechanism of RNA transesterification used by glmS ribozymes. The rate-limiting step for catalysis is the presence of an appropriately formed catalytic core, although the rate of formation of the core is dependent upon specific supporting divalent cations (21, 23–24). However, the activity of the catalytic core was determined to be independent of divalent metal ions, which only serve to assist in structure formation rather than having a direct involvement in bond making or breaking events during RNA cleavage (19–21, 24).
Although GlcN6P is the preferred ligand for glmS ribozymes (8, 12), surprisingly a variety of compounds that carry an amine in a beta position relative to a hydroxyl group (e.g. glucosamine, serinol, ethanolamine, etc.) also can support RNA catalysis, albeit with vastly reduced binding affinities (25). Even Tris buffer at concentrations typically used for biological assays (50 mM) slowly triggers ribozyme self-cleavage (21, 25). In contrast to the activities of compounds remotely similar to GlcN6P, glucose-6-phosphate (Glc6P), which is a very close structural analog of GlcN6P, does not support catalysis (8). Rather, Glc6P functions as a weak competitive inhibitor of ribozyme function (25), which was also shown in an in vivo system (26). Moreover, there are no major structural changes brought about by ligand binding to the RNA (8, 19, 27–28), as would be likely if an allosteric mechanism for ribozyme activation were involved. Finally, both biochemical probing (29) and structural analyses (19–20) reveal that the ligand resides close to the ribozyme cleavage site as described above. These results strongly implicate GlcN6P as a cofactor for ribozyme catalysis, most likely involving the general acid-base properties of the amine functional group (25).
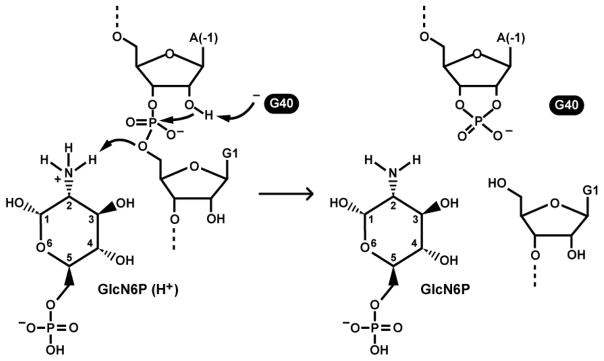
A possible mechanism for RNA cleavage has emerged (Fig. 3) that accounts for many of the structural and functional characteristics observed. A deprotonated N1 of G40 could serve as a general base to deprotonate the 2′ hydroxyl group of A(-1). Recall that this 2′ oxygen is the nucleophile that initiates the cleavage reaction, and is pre-positioned for in-line attack. To further accelerate RNA chain cleavage, a proton from the amine group of GlcN6P is donated to the 5′ oxyanion leaving group of G1. The cleavage mechanism includes features that are similar to aspects of the mechanism employed by RNase A (13, 30).
FIGURE 3.
Possible mechanism of cleavage for glmS ribozymes. Left: the reaction is initiated by deprotonation of the 2′ hydroxyl group of nucleotide A(-1). This creates a strong nucleophile for attack on the adjoining phosphorus center. The reaction is completed by protonation of the 5′ oxygen leaving group by the amine of GlcN6P. Right: Products of cofactor-mediated RNA chain cleavage. Dashed lines indicate continuation of the RNA chain.
Several observations are consistent with this proposed mechanism for glmS ribozymes. For example, changing the G40 nucleotide to any other nucleotide abolishes catalysis despite the fact that there is no expected adverse effect on the active site structure and ligand binding determinants (17, 31). This finding suggests that the properties of the guanosine residue are important only for catalysis, and molecular dynamics models of the catalytic core support a general base role for G40 (32–33). However, some observations remain puzzling and therefore suggest the detailed mechanism for glmS ribozyme function remains incomplete. Particularly notable is the fact that G40 mutation renders the ribozyme completely devoid of self-cleavage activity. This finding is perplexing since all the catalytic machinery within the ribozyme is ready for catalysis, and mutating G40 to adenosine does not drastically alter the binding site of the glmS ribozyme (13) Positioning the cleavage site phosphoester linkage for in-line nucleophilic attack and protonation of the leaving group by GlcN6P should yield measurable rate enhancements even if nucleophile activation does not occur (34–35). Furthermore, the ribozyme appears to favor binding of GlcN6P in its uncharged amino form (20), which yields a bound cofactor that is not well suited for protonation of the 5′ oxygen leaving group. These findings have led to the suggestion that G40 and the GlcN6P cofactor may be chemically coupled wherein each tunes the other’s chemical properties to promote RNA cleavage (30). Regardless, rate constants exhibited by some glmS ribozymes are in excess of 10 min−1 (36), and such rate enhancements require multiple catalytic strategies to be employed in concert (35).
Regulation of mRNA stability by glmS ribozymes
Despite extensive data demonstrating that binding of GlcN6P to the glmS ribozyme stimulates self-cleavage, the basis for intracellular control of gene expression was not immediately obvious upon initial discovery of the ribozyme. The obvious expectation was that self-cleavage is directly coupled to a change in mRNA stability. However, studies on mRNA degradation pathways in Escherichia coli present a conundrum for self-cleaving ribozymes like glmS, where the precursor transcript would be predicted to be similar in stability to the main product generated by ribozyme self-cleavage.
In aggregate, investigations of mRNA degradation in E. coli have demonstrated that molecular features at the 5′ terminus can be critical determinants for mRNA stability (Belasco 2010). Specifically, the degree of phosphorylation at the 5′ terminus can exert an important influence on an initial, rate-limiting step in degradation. For example, mRNA transcripts with a 5′ triphosphate group exhibit a much longer intracellular half-life as compared to transcripts with a 5′ monophosphate, due to lowered affinity for the global RNase enzyme, RNase E. However, transcripts with a 5′ hydroxyl group also exhibit a long intracellular half-life, almost identical to triphosphorylated mRNAs (38–39). Therefore, an mRNA containing a ribozyme at its 5′ terminus, such as glmS, would be predicted to be stable both for the full-length mRNA and after ribozyme self-cleavage, since the corresponding transcripts contain a 5′ terminal triphosphate and hydroxyl, respectively.
A potential solution to this problem was suggested by studies of mRNA degradation by Bacillus subtilis, which differs from E. coli in that it lacks the global RNase E enzyme (39–40). Instead, B. subtilis encodes for at least one different global RNase enzyme, RNase J, which also exhibits 5′-end preferences for a subset of its RNA substrates and has a widespread distribution in bacteria that often contain a glmS ribozyme (40). In B. subtilis, the 3′ cleavage product of the glmS ribozyme self-cleavage reaction, which includes the glmS coding region, is degraded in an RNase J-dependent manner (41). If, however, the same transcript contains a 5′ triphosphate instead of a 5′ hydroxyl group, it is no longer rapidly degraded in an RNase J-dependent manner and the resulting transcripts accumulate within cells.
For B. subtilis, the presence of a 5′ hydroxyl, such as imparted on downstream transcripts by self-cleaving ribozymes, can be a signal for RNase J-mediated degradation. As further proof for this model, heterologous addition of the B. subtilis RNase J enzyme to the E. coli cytosol led to rapid degradation of 5′ hydroxyl-containing transcripts, which otherwise accumulated in the absence of RNase J expression (41). Therefore, ribozyme-mediated control of mRNA stability is likely to be dependent on the appropriate expression of RNase enzymes.
Methods for glmS ribozyme cleavage assays
Various glmS ribozymes can operate under a wide range of conditions in vitro, though there are a few characteristics and limitations that must be considered. A few key considerations are noted herein. For example, representative glmS ribozymes examined in the greatest detail function optimally within a pH range of 6.8 to 8.5 (25). Low millimolar concentrations of divalent magnesium and high millimolar concentrations of monovalent cations support robust activity, although Mg2+ can be substituted for by a variety of other divalent cations (16, 21). The apparent KD for the GlcN6P cofactor is approximately 200 μM, and therefore concentrations above this value will yield optimal cleavage rates (8, 25). Other molecules that mimic the amine and adjacent hydroxyl group arrangement of GlcN6P will trigger ribozyme cleavage, albeit with reduced apparent KD values (25).
Particularly noteworthy is the fact that the commonly-used buffer Tris (2-amino-2-hydroxymethyl-propane-1,3-diol) will also trigger ribozyme activity due to its chemical similarity to the natural cofactor GlcN6P (21, 25). The use of other buffers such as HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) is recommended to prevent unwanted ribozyme action (11, 25). Some glmS ribozymes may require elevated temperatures to function efficiently, such as the Thermus thermophilus glmS ribozyme (11).
When constructing glmS ribozymes, it may be useful to retain a sufficient amount of RNA in the portion 5′ of the cleavage site to differentiate the 3′ fragment from unprocessed RNA. Inclusion of too many flanking nucleotides could interfere with efficient folding of the catalytic domain, particularly if precursor RNAs are purified by denaturing polyacrylamide gel electrophoresis before initiating a cleavage assay. If ribozyme refolding is a problem, adding GlcN6P to a transcription reaction when preparing precursor RNAs can be attempted to conduct ribozyme cleavage assays during transcription.
Materials
1. Preparation of a DNA Template
PCR Buffer (10X). 15 mM MgCl2, 500 mM KCl, 100 mM Tris-HCl (pH 8.3 at 23°C), and 0.1% gelatin in deionized water (dH2O).
Deoxyribonucleoside 5′-triphosphate Mix (10X). 2 mM of each of the following in deionized water: dATP, dGTP, dCTP, and dTTP.
Appropriate primers at 10 μM in dH2O.
Trace amount of the appropriate template DNAs. This template input could come from natural sources such as bacterial genomic DNA, or from synthetic DNA templates.
Thermostable DNA Polymerase (e.g. Taq) (New England BioLabs).
Optional: 100% dimethylsulfoxide (DMSO). Typically used for DNA templates that are above 60% G-C content.
PCR Cleanup Kit (Qiagen).
Gel Extraction Kit (Qiagen).
GPG/Low melt agarose (Applied Biosystems).
Gel electrophoresis rig.
Gel loading buffer (2X). 8 M urea, 20% (w/v) sucrose, 0.1% (w/v) SDS, 0.05% (w/v) bromophenol blue sodium salt, 0.05% (w/v) xylene cyanol, 90 mM Tris-HCl, 90 mM borate, and 10 mM ethylenediaminetetraacetic acid (EDTA) (pH 8.0 at 23°C).
Tris-Borate-EDTA gel-running buffer (1X). 90 mM Tris, 90 mM borate, and 10 mM EDTA (pH 8.0 at 23°C).
2. RNA Transcription of glmS Ribozymes
Transcription buffer (5X). 120 mM MgCl2, 20 mM spermidine, 400 mM HEPES-KOH (pH 7.5 at 23°C), 200 mM dithiothreitol (DTT).
Nucleoside 5′-triphosphate (NTP) mixture (6X). 20 mM in dH2O each of ATP, GTP, CTP, and UTP.
10 μCi of a relevant α-[32P] NTP, usually the most abundant nucleotide in the RNA.
Bacteriophage T7 RNA polymerase.
DNA Template (typically 50 pmol of PCR product).
Reagents and apparatus for polyacrylamide gel electrophoresis (PAGE).
Denaturing 6% polyacrylamide gel (0.75 mm thickness).
Tris-Borate-EDTA gel-running buffer (1X; see above).
Gel-loading buffer (2X; see above).
Hand-held shortwave (254 nm) UV light.
Fluor-coated thin layer chromatography (TLC) plate (Applied Biosystems).
Crush-soak buffer. 200 mM NaCl, 10 mM HEPES-KOH (pH 7.5 at 23°C), and 1 mM EDTA (pH 8.0 at 23°C).
Ethanol (70% and 100%) chilled to −20°C.
3 M sodium acetate (pH 5.0 at 23°C).
X-ray film (BioMax MR; Kodak).
3. RNA Transcription with Ribozyme Cleavage Assay of glmS Ribozymes
Same materials as noted above for typical transcription assays, except that a 2 mM stock solution of GlcN6P (or any other test ligands) is prepared.
PhosphorImager cassette and PhosphorImager with ImageQuant Software (GE Healthcare).
Blotting paper 703 (VWR International).
Gel dryer (e.g. Bio-Rad model 583).
4. glmS Ribozyme Cleavage Assay
PAGE apparatus and materials as noted above.
Radiolabeled glmS ribozyme (no more than 10 nM final concentration).
Ribozyme reaction buffer (2X). 50 mM HEPES-KOH (pH 7.5 at 23°C), 50 mM MgCl2, and 200 mM KCl.
Methods
In general, maintaining clean, RNase-free laboratory settings and materials is essential. Also, safety protocols for handling radioactive samples must be observed.
1. Preparation of a DNA template
Normal PCR protocols can be followed using either genomic DNA or synthetic DNA as the input source. Template product integrity should be confirmed by DNA sequencing.
Design reciprocal primers to generate desired construct for transcription. If performing PCR from genomic DNA, one set of primers suffices. If building a construct from synthetic DNA, performing sequential PCR reactions may be necessary due to the limits on the lengths of synthetic DNAs. The initial 5′ primer of the entire sequence should have the T7 RNA polymerase recognition sequence of 5′-TAATACGACTCACTATA, and the first two transcribed RNA nucleotides should be GG to maximize transcription yields.
-
Combine the following into a microfuge tube to perform a PCR to amplify the region of interest.
10 μL of 10X PCR buffer
10 μL of 10X dNTP mix
5–100 ng of genomic DNA template (not applicable if using only synthetic DNAs)
5 μL of 10 μM 5′ primer
5 μL of 10 μM 3′ primer
Taq DNA polymerase to 0.05 U μL−1 final concentration
5 μL DMSO (required if final construct is above 60% G-C content)
dH2O to 100 μL
Using standard thermocycler conditions, conduct 20–25 cycles with appropriate temperatures for the primers.
Verify PCR product yield by using agarose gel electrophoresis
Use PCR Cleanup Kit if PCR produced a single desired product. If more than one product is generated, use Gel Extraction Kit after extracting the relevant band.
Sequence the resulting PCR products to confirm integrity.
2. RNA Transcription of glmS ribozymes
-
Combine the following in a 1.5 mL microfuge tube:
Approximately 50 pmol DNA template
6 μL of 5X transcription buffer
6 μL of 5X NTPs
10 μCi of α-[32P]NTP whose identity is chosen to maximize radiolabeling. Typically, this will be UTP, though for G-C rich constructs, using GTP is preferred.
25 U μL−1 T7 RNA polymerase final concentration
dH2O to 30 μL
Incubate for 0.5 to 1 hr. at 37°C.
Add 30 μL of 2X loading dye to the reaction to quench transcription and load sample into a 6% denaturing polyacrylamide gel for purification of the desired RNA products.
Run the gel until the product of interest has traversed approximately halfway through the gel, using the bromophenol blue and xylene cyanol markers to estimate the location of the product.
Separate the glass plates of the PAGE rig and place gel into plastic wrap.
Use either UV-shadowing or X-ray film to determine the location of the product in the gel.
Excise the product with a razor blade and transfer gel slice to a fresh microfuge tube using razor blade. Avoid contact between the gel slice with any surfaces that may have contaminating nucleases.
Add 300 μL of crush-soak buffer (no Tris) to the gel and either incubate at room-temperature for 0.5 hr. or at 4°C overnight.
Transfer the crush-soak buffer and RNA product to a new tube and add 30 μL of 3 M sodium acetate (pH 5.0 at 23°C) and 1 mL of cold 100% ethanol. Store at −20°C for at least 20 minutes.
Centrifuge for 20 minutes at 14,000 g at 4°C.
Decant supernatant and add 70% cold ethanol to the tube containing the RNA pellet. Repeat centrifugation step.
Decant supernatant and dry pellet. Resuspend in 50 μL of dH2O and quantify RNA.
3. RNA Transcription with Ribozyme Cleavage Assay of glmS Ribozymes
Make two reactions and follow steps 1–4 from the transcription reaction listed above, except add 3 μL of 2 mM GlcN6P to one reaction.
Place gel onto one piece of plastic wrap on one side and one piece of blotting paper.
Place gel into gel dryer for at least 1 h and place into a PhosphorImager cassette overnight.
Collect image from the PhosphorImager cassette and quantify the data using ImageQuant.
4. glmS Ribozyme Cleavage Assay
-
Add the following into two 1.5 mL microfuge tubes:
10 μL of 2X reaction buffer (no Tris).
Add glmS ribozyme RNA solution, to yield no more than a 10 nM final concentration.
-
Add the following into a 1.5 mL microfuge tube:
2 μL of GlcN6P stock solution.
Fill with dH2O to 20 μL final volume for the combined total in steps 1 and 2.
In another 1.5 mL microfuge tube, only add dH2O to fill a reaction to 20 μL from Step 1. There should be 4 microfuge tubes at this point.
Incubate all four tubes at the desired temperature (37°C is default) for 5 min.
Add the contents from tube #2 into a tube from #1. Add the contents from tube #3 into the other tube from #1.
Incubate at the desired temperature for 5 min, or take aliquots at various time points as desired. Reactions should be terminated by addition to an equal volume of 2X gel loading buffer.
Perform PAGE to separate ribozyme reaction products.
Image the gel using a PhosphorImager and quantify the resulting gel bands.
Conclusions
In total, the discovery and characterization of the glmS ribozyme has provided an important model system for understanding the basis of gene regulation by synthetic and natural, signal-responsive ribozymes. Moreover, the widespread distribution of both the glmS ribozyme and the RNase enzymes that it depends upon provide a mechanistic precedence for how other possible self-cleaving ribozymes could sense and respond to cellular metabolites. Perhaps other novel ribozymes with allosteric- or cofactor-based responses to metabolites still await discovery.
Acknowledgments
We are thankful to the members of the Breaker lab for helpful comments and advice. P.M. is supported by the NIH Training Grant T32GM007499. This work is supported in the Breaker lab by the NIH Grant PO1 GM022778-34 and by the Howard Hughes Medical Institute. R.R.B. is a Howard Hughes Medical Institute Investigator. Research on the glmS ribozyme in the Winkler lab was supported by the University of Texas Southwestern Medical Center Endowed Scholars Fund.
References
- 1.Mandal M, Breaker RR. Gene regulation by riboswitches. Nat Rev Mol Cell Biol. 2004;5:451–463. doi: 10.1038/nrm1403. [DOI] [PubMed] [Google Scholar]
- 2.Breaker RR. Riboswitches and the RNA World. Cold Spring Harb Perspect Biol. 2010 doi: 10.1101/cshperspect.a003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montange RK, Batey RT. Riboswitches: emerging theme in RNA structure and function. Annu Rev Biophys. 2008;37:117–133. doi: 10.1146/annurev.biophys.37.032807.130000. [DOI] [PubMed] [Google Scholar]
- 4.Serganov A, Patel DJ. Amino acid recognition and gene regulation by riboswitches. Biochim Biophys Acta. 2009;1789:592–611. doi: 10.1016/j.bbagrm.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Lau MW, Ferré-D’Amaré AR. Ribozymes and riboswitches: modulation of RNA function by small molecules. Biochemistry. 2010;49:9123–9131. doi: 10.1021/bi1012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dambach MD, Winkler WC. Expanding roles for metabolite-sensing regulatory RNAs. Curr Opin Microbiol. 2009;12:161–169. doi: 10.1016/j.mib.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wachter A. Riboswitch-mediated control of gene expression in eukaryotes. RNA Biol. 2010;7:67–76. doi: 10.4161/rna.7.1.10489. [DOI] [PubMed] [Google Scholar]
- 8.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 9.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, Lee M, Roth A, Sudarsan N, Jona I, et al. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci USA. 2004;101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrick JE, Breaker RR. The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome Biol. 2007;8:R239. doi: 10.1186/gb-2007-8-11-r239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCown PJ, Roth A, Breaker RR. An expanded collection and refined consensus model of glmS ribozymes. RNA. 2011;17:728–736. doi: 10.1261/rna.2590811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim J, Grove BC, Roth A, Breaker RR. Characteristics of ligand recognition by a glmS self-cleaving ribozyme. Angew Chem Int Ed. 2006;45:6689–6693. doi: 10.1002/anie.200602534. [DOI] [PubMed] [Google Scholar]
- 13.Ferré-D’Amaré AR. The glmS ribozyme: use of a small molecule coenzyme by a gene-regulatory RNA. Q Rev Biophys. 2010;43:423–447. doi: 10.1017/S0033583510000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milewski S. Glucosamine-6-phosphate synthase – the multi-facets enzyme. Biochim Biophys Acta. 2002;1597:173–192. doi: 10.1016/s0167-4838(02)00318-7. [DOI] [PubMed] [Google Scholar]
- 15.Blount KF, Breaker RR. Riboswitches as antibacterial drug targets. Nat Biotechnol. 2006;24:1558–1564. doi: 10.1038/nbt1268. [DOI] [PubMed] [Google Scholar]
- 16.Lünse CE, Schmidt MS, Wittmann V, Mayer G. Carba-sugars activate the glmS-riboswitch of Staphylococcus aureus. ACS Chem Biol. 2011 doi: 10.1021/cb200016d. [DOI] [PubMed] [Google Scholar]
- 17.Cochrane JC, Lipchock SV, Smith KD, Strobel SA. Structural and chemical basis for glucosamine 6-phosphate binding and activation of the glmS ribozyme. Biochemistry. 2009;48:3239–3246. doi: 10.1021/bi802069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Link KH, Guo L, Breaker RR. Examination of the structural and functional versatility of glmS ribozymes by using in vitro selection. Nucleic Acids Res. 2006;34:4968–4975. doi: 10.1093/nar/gkl643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein DJ, Ferré-D’Amaré AR. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate activation. Science. 2006;313:1752–1756. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]
- 20.Cochrane JC, Lipchock SV, Strobel SA. Structural investigation of the glmS ribozyme bound to its catalytic cofactor. Chem Biol. 2007;14:97–105. doi: 10.1016/j.chembiol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth A, Nahvi A, Lee M, Jona I, Breaker RR. Characteristics of the glmS ribozyme suggest only structural roles for divalent metal ions. RNA. 2006;12:607–619. doi: 10.1261/rna.2266506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blount KF, Puskarz I, Penchovsky R, Breaker RR. Development and application of a high-throughput assay for glmS riboswitch activators. RNA Biol. 2006;3:77–81. doi: 10.4161/rna.3.2.3102. [DOI] [PubMed] [Google Scholar]
- 23.Brooks KM, Hampel KJ. A rate-limiting conformational step in the catalytic pathway of the glmS ribozyme. Biochemistry. 2009;48:5669–5678. doi: 10.1021/bi900183r. [DOI] [PubMed] [Google Scholar]
- 24.Klawuhn K, Jansen JA, Souchek J, Soukup GA, Soukup JK. Analysis of metal ion dependence in glmS ribozyme self-cleavage and coenzyme binding. Chem Biochem. 2010;11:2567–2571. doi: 10.1002/cbic.201000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy TJ, Plog MA, Floy SA, Jansen JA, Soukup JK, Soukup GA. Ligand requirements for glmS ribozyme self-cleavage. Chem Biol. 2005;12:1221–1226. doi: 10.1016/j.chembiol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Watson PY, Fedor MJ. The glmS riboswitch integrates signals from activating and inhibitory metabolites in vivo. Nat Struct Mol Biol. 2011;18:359–363. doi: 10.1038/nsmb.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hampel KJ, Tinsley MM. Evidence for preorganization of the glmS ribozyme ligand binding pocket. Biochemistry. 2006;45:7861–7871. doi: 10.1021/bi060337z. [DOI] [PubMed] [Google Scholar]
- 28.Klein DJ, Wilkinson SR, Been MD, Ferré-D’Amaré AR. Requirement of helix P2.2 and nucleotide G1 for positioning of the cleavage site and cofactor of the glmS ribozyme. J Mol Biol. 2007;373:178–189. doi: 10.1016/j.jmb.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen JA, McCarthy TJ, Soukup GA, Soukup JK. Backbone and nucleobase contacts to glucosamine-6-phosphate in the glmS ribozyme. Nat Struct Mol Biol. 2006;13:517–523. doi: 10.1038/nsmb1094. [DOI] [PubMed] [Google Scholar]
- 30.Ferré-D’Amaré AR, Scott WG. Small self-cleaving ribozymes. Cold Spring Harbor Perspect Biol. 2010;2:a003574. doi: 10.1101/cshperspect.a003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein DJ, Been MD, Ferré-D’Amaré AR. Essential role of an active-site guanine in glmS ribozyme catalysis. J Am Chem Soc. 2007a;129:14858–14859. doi: 10.1021/ja0768441. [DOI] [PubMed] [Google Scholar]
- 32.Banáš P, Walter NG, Šponer J, Otyepka M. Protonation states of the key active site residues and structural dynamics of glmS riboswitch as revealed by molecular dynamics. J Phys Chem. 2010;114:8701–8712. doi: 10.1021/jp9109699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin Y, Hamelberg D. Deciphering the role of glucosamine-6-phosphate in the riboswitch action of glmS ribozyme. RNA. 2010;16:2455–2463. doi: 10.1261/rna.2334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breaker RR, Emilsson GM, Lazarev D, Nakamura S, Puskarz IJ, Roth A, Sudarsan N. A common speed limit for RNA-cleaving ribozymes and deoxyribozymes. RNA. 2003;9:949–957. doi: 10.1261/rna.5670703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emilsson GM, Nakamura S, Roth A, Breaker RR. Ribozyme speed limits. RNA. 2003;9:907–918. doi: 10.1261/rna.5680603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkinson SR, Been MD. A pseudoknot in the 3′ non-core region of the glmS ribozyme enhances self-cleavage activity. RNA. 2005;11:1788–1794. doi: 10.1261/rna.2203605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belasco JG. All things must pass: contrasts and commonalities in eukaryotic and bacterial mRNA decay. Nat Rev Mol Cell Biol. 2010;11:467–478. doi: 10.1038/nrm2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celesnik H, Deana A, Belasco JG. Initiation of RNA decay in Escherichia coli by 5′ pyrophosphate removal. Mol Cell. 2007;27:79–90. doi: 10.1016/j.molcel.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bechhofer DH. Messenger RNA decay and maturation in Bacillus subtilis. Prog Mol Biol Transl Sci. 2009;85:231–273. doi: 10.1016/S0079-6603(08)00806-4. [DOI] [PubMed] [Google Scholar]
- 40.Condon C, Bechhofer DH. Regulated RNA stability in the Gram-positives. Curr Opin Microbiol. 2011;14:148–154. doi: 10.1016/j.mib.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins JA, Irnov I, Baker S, Winkler WC. Mechanism of mRNA destabilization by the glmS ribozyme. Genes Dev. 2007;21:3356–3368. doi: 10.1101/gad.1605307. [DOI] [PMC free article] [PubMed] [Google Scholar]