Abstract
The measurement of fitness is critical to biological research. Although the determination of fitness for some organisms may be relatively straightforward under controlled conditions, it is often a difficult or nearly impossible task in nature. Plants are no exception. The potential for long-distance pollen dispersal, likelihood of multiple reproductive events per inflorescence, varying degrees of reproductive growth in perennials, and asexual reproduction all confound accurate fitness measurements. For these reasons, biomass is frequently used as a proxy for plant fitness. However, the suitability of indirect fitness measurements such as plant size is rarely evaluated. This review outlines the important associations between plant performance, fecundity, and fitness. We make a case for the reliability of biomass as an estimate of fitness when comparing conspecifics of the same age class. We reviewed 170 studies on plant fitness and discuss the metrics commonly employed for fitness estimations. We find that biomass or growth rate are frequently used and often positively associated with fecundity, which in turn suggests greater overall fitness. Our results support the utility of biomass as an appropriate surrogate for fitness under many circumstances, and suggest that additional fitness measures should be reported along with biomass or growth rate whenever possible.
Keywords: biomass, fecundity, fitness, plant performance, selection
Quantifying the relative fitness of any organism—defined as the reproductive success of a particular genotype compared to all genotypes in a population—is a difficult task (italicized terms are defined in Appendix 1). This is especially true for plants because (1) they are usually hermaphroditic so both male and female reproductive success need to be accounted for (Campbell, 2000); (2) multiple sires may be responsible for fertilizing the seeds of each fruit (Ellstrand, 1984); (3) pollen and seeds can be widely dispersed away from parents, making their success difficult to track (Burczyk et al., 2006); (4) fitness may be accrued through both sexual and asexual reproduction (Silvertown, 2008); and (5) perennial plants can reproduce multiple times over many years (Santos-del-Blanco et al., 2013). Moreover, relative fitness (hereafter: fitness) estimates are confounded because they depend not only on the number of propagules produced (i.e., fecundity), but also on their success, which ultimately determines an individual’s contribution to the next generation (Haldane, 1937).
A complete accounting of all aspects of fitness is rarely conducted in plants because of the large amount of time and effort required. As a result, measurements of plant size or biomass are often used as proxies for fitness. Indeed, the ability to acquire and retain resources generally displays strong positive correlations with plant growth rate, vegetative spread, and reproductive success (Harper and White, 1974; Bazzaz et al., 1987; Herms and Mattson, 1992); however, it is unclear whether size is the most reliable estimate of fitness in natural populations. For example, under intense competition and limiting nutrients—conditions commonly faced in nature—plants may allocate more resources to asexual and sexual reproduction than vegetative growth and, therefore, recruit more offspring (Sugiyama and Bazzaz, 1998; Aarssen et al., 2014; Tracey and Aarssen, 2014). Consequently, the most accurate method to quantify plant fitness should include a determination of the number of sexually and asexually produced progeny recruited into a population from a given individual over its entire life. Because the genetic analysis of every seedling and its potential parents is generally unrealistic, a consensus on whether biomass is an appropriate estimation of fitness is needed. With this in mind, it is important to review the current state of knowledge on the relationship between biomass and fitness so that we can draw conclusions about the accuracy of this relationship for a range of plant life forms, and to outline important exceptions where biomass measures should be supplemented by other fitness estimates (e.g., fecundity). We report the results of our review of published studies that analyze plant fitness, highlight studies that include size or biomass measurements, and note when these measurements show correlations to fruit or seed production. We conclude that size measurements are frequently used as surrogates for fitness in plant biology and often display positive correlations to fecundity metrics. Larger plants generally have more reproductive output, leading to a greater likelihood of leaving viable offspring and higher fitness.
LITERATURE REVIEW OF FITNESS ESTIMATES USED IN PRIMARY RESEARCH
In an effort to assess the frequency of utilizing biomass as a fitness estimate in published research, we reviewed 170 primary research articles that examined plant fitness and documented the metrics that were used for its estimation. We performed this literature review to clarify which metrics are frequently used to estimate fitness and also to determine if biomass measurements correlate with other estimations of fitness. We are not purporting that metrics used in the past are more valuable simply because of their prior use, nor do we include all of the papers published that have examined plant fitness. We used the search term “plant fitness” in Web of Science for all dates and filtered for only research articles. A total of 8548 articles, published between the years 1935 to 2016, matched our search term. Many of these papers did not actually analyze plant fitness (e.g., some examined pathogen or herbivore fitness; some were from outside of the biological sciences altogether). We sorted all of the papers that matched our search term randomly, and then examined the first 170 that analyzed some aspect of plant fitness. For any articles that did not analyze plant fitness (as mentioned above), we highlighted it as unusable and continued our literature review with subsequent papers. No priority was given to publications from specific journals or authors. Additionally, we analyzed any of the 170 studies that used biomass, size, or performance to estimate fitness to determine if any of these studies also found correlations to additional fecundity-related metrics (e.g., seed, fruit, or flower number). All of the studies used in this review and the categories of their fitness estimates are included in Appendix S1 (3.3MB, xls) .
RESULTS
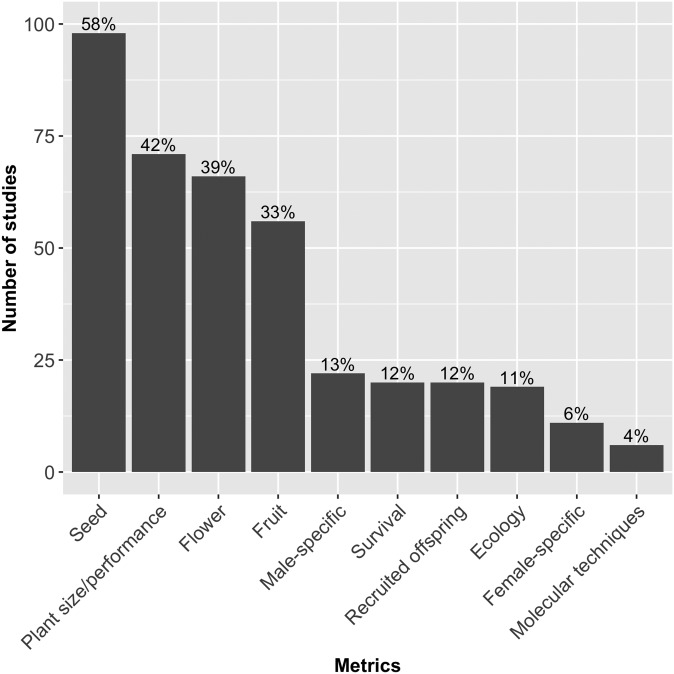
Of the 170 total papers analyzed in our literature review, we report that 42% (n = 71) used biomass, size, or performance as a fitness estimate (Fig. 1). Not surprisingly, most studies used seed-related metrics to estimate fitness (58%, n = 98), while other fecundity-related metrics were also strongly represented (flowers: 39%, n = 66; fruits: 33%, n = 56). It is interesting to note that only 20 studies tracked the number of recruited offspring (or their performance) from parental plants directly (12%) and only six studies traced the offspring recruited through the use of allozymes, microsatellites, or other molecular techniques (4%). This result was unanticipated because determining parental contributions to successive generations is the most direct route to estimating fitness. However, the usage of molecular markers may not be the most feasible for field studies with hundreds of potential parents in a population and low rates of juvenile recruitment in established communities. In addition, because we did not limit our search to a specific date range, many studies preceded the widespread usage of certain molecular methods (e.g., microsatellite markers; 18% or 30 studies were published prior to the year 2000). In addition, the continued development of molecular markers for studies of plant fitness and population genetics will likely result in more researchers embracing these technologies in the near future.
Fig. 1.
Published information on the methods used to estimate plant fitness in literature review. Results depict the number and percentage of times each metric was used in fitness estimations of 170 studies analyzed. Most studies incorporated multiple measurements of fitness, and each measurement is included in this analysis (percentage totals exceed 100). The first 170 studies that analyzed or estimated some aspect of plant fitness were analyzed across all years, sorted randomly. Height of the bars refers to the number of studies, with percentages (out of 170) included at the top of the bars. The ecology category includes studies that used ecological-related measurements to estimate fitness (e.g., rates of herbivory, pollinator visitation, or pathogen infection).
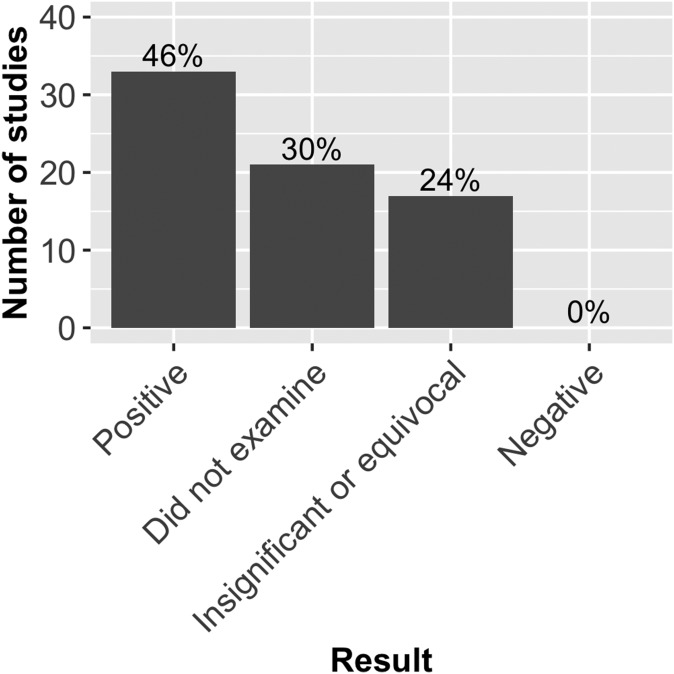
Of the studies that used biomass/size/performance as a fitness estimate, 46% (n = 33) found positive correlations between biomass and various measures of fecundity (fruit, seed, and flower number; Fig. 2). No studies found a negative correlation between biomass and fecundity, 24% (n = 17) found statistically insignificant or equivocal correlations, and 30% (n = 21) of studies that used biomass or size-related metrics did not incorporate any additional measures of fecundity to estimate fitness. When removing studies from the analysis that did not examine both fecundity-related metrics and biomass (n = 21), a significant number of studies found a positive correlation between both measures compared to studies that found insignificant/equivocal results (33 out of 50; exact binomial test; P < 0.05). The fact that no papers found negative correlations between fecundity and biomass was highly significant (0 out of 71; exact binomial test; P < 0.001).
Fig. 2.
Correlations of fecundity-related measurements to biomass estimations of fitness in literature review. Results depict whether a fecundity measurement was positively, negatively, or insignificantly/equivocally correlated to biomass measurements in 71 total studies that estimated fitness with some type of plant size or performance metric. Fecundity included seed-, fruit-, and flower-related measurements. Height of the bars refers to the number of studies, with percentages (out of 71) included at the top of the bars. Twenty-one studies that estimated plant fitness through plant size or performance did not include any additional fecundity-related measurements (30%).
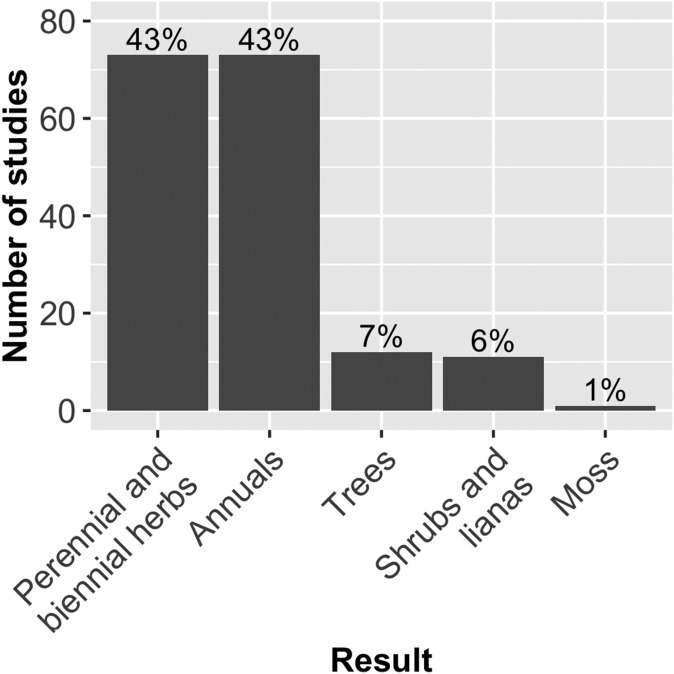
Most of the papers in our literature review used either perennial/biennial herbs (43%, n = 73) or annuals (43%, n = 73) as study systems (Fig. 3). Only 7% (n = 12) of the studies used trees, 6% (n = 11) used shrubs or lianas, and only one study examined a moss. Although a majority of studies employed Arabidopsis thaliana (L.) Heynh. and other members of Brassicaceae as study systems, there were more than 150 plant species examined from a total of 55 different plant families, most of which were angiosperms (Appendix S1 (3.3MB, xls) ).
Fig. 3.
Habits of plants under study in publications analyzed in literature review. Results depict the breakdown of plant habits for study species in the literature review. Height of the bars represents the number of studies that used a plant species of a particular habit, with percentages (out of 170) included at the top of the bars. No studies that examined fern fitness were included in our literature review because of random sorting of the articles.
DISCUSSION
Based on the results of our literature review, biomass estimates of fitness are used frequently in published research owing to its relative ease of measurement and close correlation to fecundity. In contrast, few studies make the effort to determine parental contributions to succeeding generations in natural and controlled conditions. Because few offspring are recruited per capita in established habitats that are free of disturbance (Harper, 1977), this practice requires great effort with low sample sizes in return. Additionally, if more papers with an agricultural focus were included in our review, we would have likely found a greater proportion of studies that tracked parental contributions to offspring, particularly as it relates to desired genes and phenotypes. Although Web of Science includes agricultural studies in its database, only two publications with an agricultural focus were included in our random sorting of articles.
With a large percentage of studies showing positive correlations of biomass to fecundity-related measurements, and a greater likelihood of highly fecund plants leaving successful offspring, we emphasize that biomass measurements are relatively simple, straightforward, and accurate estimations of fitness for plants of the same age. Additionally, we emphasize that measurements of plant size only estimate fitness; researchers should refer primarily to the data that they have measured in their studies. We further highlight several important points regarding the relationship of plant biomass to fitness. First, individual plants that attain a greater size, relative to neighboring competitors, generally have greater fecundity (Harper, 1977). However, both deterministic and stochastic variables influence plant growth and size; therefore, fitness estimates will not always reliably reflect the “quality” of the genotype because an inherent degree of randomness persists in nature. Second, the most important question is not whether bigger plants just produce more seeds, but instead whether bigger individuals leave more successful offspring within a population (Aarssen, 2007). Numerous variables influence the success of progeny (e.g., priority effects, timing of germination, dispersal of offspring), but larger individuals have the potential to leave more offspring due to higher fecundity. Third, using biomass as an estimate for relative fitness should only be used to compare conspecifics. In contrast, making comparisons of fitness between different species based on biomass is problematic, as plant growth forms and life-history strategies can vary considerably. Comparisons of fecundity or biomass between species do not reliably reflect success, as some of the largest and most fecund plant species on Earth are also rare (e.g., giant sequoias and orchids; Parsons, 1994; Nicolè et al., 2005). Fourth, studies of natural, age-structured populations should not use biomass as a fitness estimate because size and growth rate are strongly dependent upon age; the employment of size as a fitness estimate should be restricted to manipulative studies of plants in the same age class. Furthermore, if studies are conducted with natural plants in the same age class, the usage of biomass to estimate fitness may only be reliable for plants within the same population due to numerous uncontrolled variables that likely exist between different habitat types. With these points in mind, we may be able to make the case that the biomass of plants of the same species and age class is generally an accurate estimate of fitness: larger individuals have greater survival and growth, higher reproductive success through male and female function, and a better chance at leaving viable offspring.
Exceptions regarding plant size and fitness
Despite the strong correlations between biomass and fitness frequently found in the literature, exceptions do occur. In most plant species—from annuals to woody perennials—a threshold size must be obtained before allocations to reproduction occur (Wesselingh and de Jong, 1995; Wesselingh and Klinkhamer, 1997; Weiner et al., 2009a; Santos-del-Blanco et al., 2013), and further increases in size beyond this threshold may not result in greater fecundity and siring of ovules on other plants (Klinkhamer et al., 1989; Méndez and Obeso, 1993; de Jong and Klinkhamer, 1994; Pino et al., 2002; Echarte and Andrade, 2003). Furthermore, trees and shrubs possess a high percentage of functionally dead, lignified tissue and experience sizable variations in annual reproductive output (Harper and White, 1974; May and Killingbeck, 1992; Obeso, 2002; Santos-del-Blanco et al., 2013). Because mortality is common for juvenile plants (i.e., viability selection), early stages of growth in these long-lived perennials favor a larger body size, when allocations to reproduction are not necessary (Peet and Christensen, 1987; Mojica and Kelly, 2010). In this instance, the size of juvenile trees and shrubs may be a good predictor of future fitness before a threshold of reproduction is reached. As later declines in growth and productivity occur due to both resource-based and physical factors (e.g., limits on the ability to acquire enough resources or limits on the translocation of water; Weiner and Thomas, 2001), the reliability of plant size as a fitness estimate may track the asymptotic growth rate of these long-lived plants. Regardless of these exceptions, an abundance of evidence demonstrates a positive allometric relationship between plant body size and reproductive output (Harper, 1977; Aarssen and Taylor, 1992; Weiner et al., 2009a). The slope of this relationship will vary for plants depending upon genotype, age, and habitat (Schmid and Weiner, 1993), but larger plant individuals will produce more reproductive structures compared to smaller conspecifics and will have a greater potential to generate viable offspring.
How biomass differentially affects the sexes
Although larger individuals have a greater potential to devote resources to reproduction, it is important to ask whether biomass plays an equal role in male and female success. Sex-specific fitness estimates are traditionally quantified as seed set for females and pollen export for males (Klinkhamer et al., 1997; Campbell, 2000; Goodwillie et al., 2005). When examining the role of size in influencing female fitness, individuals that produce more flowers tend to attract more pollinators and receive more pollen (Harder et al., 1985; de Jong and Klinkhamer, 1994; Wang et al., 2006). However, investigations of hermaphroditic plants including Cynoglossum officinale L., Eichhornia paniculata (Spreng.) Solms, and Mimulus ringens L. have revealed that the number of matings per flower decreases when there are more flowers per plant and the chance of inbreeding increases (Klinkhamer et al., 1989; Harder and Barrett, 1995; Karron and Mitchell, 2012).
With regard to male fitness, plants with more flowers attract more pollinators, thus improving dispersal ability and mating opportunities (de Jong and Klinkhamer, 1994). In an analysis of pollinator visitation rates in natural populations of Echium vulgare L., larger individuals have more flowers and more total visits from pollinators than smaller individuals, but the number of visits per flower decreases on plants with many flowers (Klinkhamer and de Jong, 1990). Also, because larger individuals that are hermaphroditic have an increased likelihood of inbreeding, mating opportunities may be limited through pollen discounting (i.e., because of increased selfing; Holsinger et al., 1984). Additionally, other factors that are not size-related affect paternity rates, including the timing of pollen deposition, donor and recipient genotypes, and pollen competition (Bernasconi, 2003). Furthermore, anemophily (i.e., wind pollination) may have a differential effect on male and female fitness because larger or taller-statured plants may export pollen more easily, while fruit and resultant progeny remain closer to the parent plant when dispersal is not mediated by animals, as demonstrated theoretically by Sakai and Sakai (2003). Therefore, larger wind-pollinated individuals may favor male fitness to a greater degree than female fitness.
It should be noted that a significant body of literature has been devoted to plant sexual allocation theory (Burd, 1994; Wilson et al., 1994; Campbell, 2000), the fitness effects of selfing (Sakai et al., 1997; Barrett, 2003), and strategies to promote outcrossing (Bawa, 1980; Goodwillie et al., 2005). Therefore, these topics will not be reviewed further here. Despite the potential deleterious effects of self-fertilization in larger plants, having more biomass results in a greater pool of resources for reproduction and can lead to more flowers, more mating opportunities, and greater fitness for both male and female function.
Challenges of allocating resources to reproduction
Without a dedicated germline in plants, the formation of reproductive structures depends on the differentiation of vegetative tissue. This generates resource and ecological constraints where plants must balance the costs and benefits of reproduction and vegetative growth (Watson, 2008). Rapidly growing plants can be limited in their ability to produce abundant reproductive structures when pulses of nutrients are acquired late in the growing season (Herms and Mattson, 1992). Moreover, there is significant variation in the allocation of resources to growth and reproduction among plant individuals in the same population (Bazzaz et al., 1987). Studies have demonstrated reproductive plasticity in the annual Senecio vulgaris L. in response to limiting nutrients, but reproductive output still covaries more strongly with biomass in this plant system (Weiner, 2004; Weiner et al., 2009b). Other work has documented the importance of a species’ life-strategy in determining the relative importance of tradeoffs for fecundity at the expense of growth. In an analysis of the relationship between fecundity and plant size in 21 naturally occurring herbaceous species, annuals and biennials demonstrated a greater allocation to reproduction with increasing plant size than did perennials, especially clonal perennials (Aarssen and Taylor, 1992). In the context of the r/K hypothesis, these results emphasize that fecundity is more important for annual and biennial species proximally (r-selected), while performance and survival are more important for perennial species ultimately (K-selected). In a similar manner, fecundity likely plays a greater role in the fitness of colonizing species, while resource acquisition and growth/survival is a more important component of fitness in crowded, established habitats (Grime, 2006).
Clonal plants are particularly useful systems when examining this balance between reproduction and vegetative growth owing to their ability to produce clonal ramets at a lower cost (energetically) than sexual reproduction (Ashmun et al., 1982; Silvertown, 2008; Aarssen, 2014; Herben et al., 2015). For example, in the aquatic clonal plant, Eichhornia crassipes (Mart.) Solms, meristems that develop inflorescences are limited to sexual reproduction only, while meristems that maintain vegetative growth can also produce ramets from axillary buds, thus increasing overall size and future reproductive potential (Geber et al., 1992). Plants that possess more flowering meristems in this system have a reduced ability to produce clonal ramets and less sexual potential in the long term. However, it is important to keep in mind that an estimated 80% of plants are clonal to some degree, yet sexual reproduction remains vital to maintaining genetic diversity in all plant populations (Barrett, 2015). There will be some point within the life of a plant (or its clonal ramets) in which it must generate reproductive tissue, incurring a resource trade-off for the sake of sexual reproduction. Although clonal plants have evolved an ability to counteract this resource constraint, the maintenance of genetic diversity through sexual reproduction is vital to plant populations tracking local and regional environmental changes. As a result, the costs associated with generating reproductive structures will always present a challenge to plants in natural habitats.
Why small plants are plentiful in nature
Despite the positive correlation between biomass and fitness, plant communities still abound with small individuals. A significant body of literature emphasizes the importance of plant reproduction at a smaller body size (Sugiyama and Bazzaz, 1998; Tracey and Aarssen, 2014) because many herbaceous plants will sacrifice growth for an opportunity to produce at least some reproductive structures (Harper, 1977; Aarssen, 2007). The reproductive economy hypothesis posits that most populations of herbaceous plants demonstrate a right-skewed distribution of body size and, therefore, selection favors increased reproduction at the expense of growth. The logic asserts that smaller plant individuals collectively recruit more offspring than larger, less abundant conspecifics, thus allowing smaller plants to persist in the population. Despite this assertion, fitness emphasizes the importance of a single genotype’s genetic contribution relative to others, not the collective contribution of many alleles to successive generations (Fisher, 1930). Still, many small individuals do persist in populations, especially among herbaceous annuals and perennials. This fact can be explained through experimental work over four growing seasons with the annual grass Avena barbata Pott ex Link conducted by Crosby and Latta (2013). Their study clearly demonstrates that larger individuals recruit more offspring than smaller conspecifics per capita, resulting in positive directional selection for body size. Although many small individuals still persist in this population, some form of resource limitation, dominance, or shading is likely forcing smaller plants to eschew additional growth at the expense of reproduction (Ågren, 1985a, b). In addition, it is important to note that if larger individuals have higher fecundity and a greater potential to leave viable offspring, it would seem that plant size in successive generations would continue to increase until physical limits were attained. However, there is little empirical evidence for this occurring in nature, which is likely the result of (1) a low heritability of size-related traits, (2) niche-based constraints on larger offspring in successive generations and beyond (e.g., competition and resource limitation), or (3) an increased likelihood of death from natural disturbances, pathogens, or herbivores. Therefore, the existence of many small plant individuals in natural populations is not the result of having greater fitness when smaller, but instead the result of constraints on reaching a greater size (i.e., a lack of performance).
Downsides to being big
Although larger biomass is likely the result of greater competitive performance or diminished competition from neighbors, additional factors within plant communities may lead to decreased fitness when a larger size is attained. Larger plants may undergo reproduction later in the growing season compared to smaller conspecifics, possibly causing reproduction to occur past the most opportune time in a given habitat, leading to higher mortality of offspring (e.g., during the onset of drought) (Fox, 1990; Latta and Gardner, 2009). An overall greater biomass introduces constraints on nutrient and water transport leading to decreased photosynthesis (Yoder et al., 1994), stomatal conductance (Mencuccini and Grace, 1996), and an increased likelihood of air emboli (Ryan and Yoder, 1997)—although these problems are mostly restricted to trees. Larger plants will also face greater susceptibility to mechanical damage (Everham and Brokaw, 1996) and potentially experience greater damage from herbivores and pathogens due to increased apparency. The apparency hypothesis explains patterns of plant defense in the context of how “apparent” plants are—e.g., early successional or ruderal species are generally smaller and have a shorter life span, experience lower rates of herbivory due to their size and ephemeral nature, and therefore either invest less in chemical defenses or produce qualitative defenses with high toxicity (Feeny, 1976). Larger plants and those with a greater lifespan are antagonized more readily by herbivores and tend to produce quantitative defenses with low toxicity. However, apparent plants may not always be more susceptible to herbivores and pathogens, and patterns of plant defense tend to track resource availability more than apparency (Coley et al., 1985).
In addition, the population can impose a negative level of group selection on larger individuals, relative to smaller conspecifics (Stevens et al., 1995). Natural populations of Silene tatarica (L.) Pers. have shown positive individual selection for the number of vegetative shoots, but negative group selection for the same trait (Aspi et al., 2003). In this system, high levels of herbivory tend to impose a greater degree of group selection on the number of vegetative shoots, regardless of its effects on individual fitness. Although a larger individual plant size may result in greater fecundity and fitness, a high degree of group selection may impose limits on the ability of these individuals to attain a large size. There are likely other types of selection that contrast with a greater plant size that we have failed to mention here. Still, despite potential downsides of being big (e.g., delayed reproduction, greater physical or biological constraints, negative group selection on larger plants, or others), a larger overall size is an indication of greater plant performance that most often results in higher reproductive output and fitness, relative to competitors.
Molecular methods to track offspring and fitness
The development of remarkable dispersal strategies has been a boon to rates of adaptation in plants; however, high dispersal complicates the assignment of parentage to juvenile plants in natural populations. It is relatively straightforward to link fruit or seed set to female fecundity when these structures are still attached to the plant (Meagher, 1986), but if one is considering the viability of offspring or the numbers of juveniles a parental plant is able to recruit into a population, the task becomes difficult. Similarly, assigning paternity to seeds in a single fruit may be a daunting task, as multiple males frequently compete for ovules within the same fruit (Ellstrand, 1984). For these reasons, researchers have embraced the utility of a variety of molecular markers for tracking parentage in natural populations, thereby linking parentage of recruited offspring to fitness (Smouse and Meagher, 1994; Smouse et al., 1999; Garant and Kruuk, 2005; Silvertown, 2008). These markers include allozymes and isozymes, amplified and restriction fragment length polymorphisms (AFLPs and RFLPs), random amplified polymorphic DNAs (RAPDs), and microsatellites (or simple sequence repeats [SSRs]). Currently, researchers almost exclusively track the genetic structure of populations through the use of SSRs and single-nucleotide polymorphisms (SNPs), especially in genome-wide surveys such as restriction-site associated DNA sequencing (RAD-Seq) and genotyping by sequencing (GBS; Elshire et al., 2011). Each molecular marker has advantages and drawbacks that are beyond the scope of this review (for details, please see Parker et al., 1998; Burczyk et al., 2006; and Wheeler et al., 2014). Despite a number of studies employing these techniques to track parentage rates, there is often a limited return on the investment of effort due to low rates of offspring recruitment in established plant populations (Eriksson, 1992) and the requirement of sampling hundreds of potential sires in a given population in paternity exclusion scenarios (Meagher, 1986). Although linking the number of offspring recruited in a population from a given parent is the most accurate method to determine plant fitness, when considering the abovementioned drawbacks, it is not surprising that many studies still employ biomass estimates for fitness (Keller et al., 2000; Sletvold, 2002; Zhang et al., 2011).
Conclusions
Despite its widespread use, no consensus yet exists in the literature regarding the reliability of biomass as a fitness estimate in conspecifics of the same age class. However, agreement does exist on the close association between biomass and fecundity, and because fecundity increases the likelihood of leaving viable offspring (Aarssen and Clauss, 1992; Schmid et al., 1995; Bonser and Aarssen, 2003; Watson, 2008), a strong case can be made for the corollary between biomass and fitness. We acknowledge that there may be circumstances where larger plants have lower fitness, particularly in woody perennials with a large proportion of functionally dead tissue and significant annual fluctuations in reproductive output (May and Killingbeck, 1992; Santos-del-Blanco et al., 2013), as well as in larger plants experiencing higher levels of herbivory (Mauricio et al., 1993; Agrawal et al., 1999). These circumstances represent exceptions to the general rule that larger plants of the same age have higher fecundity. Additionally, quantifying which genotypes recruit the most offspring through molecular markers is the most direct method for determining fitness. Unfortunately, their use is unrealistic in many studies where there may be hundreds of potential sires and low rates of offspring recruitment in established populations (Meagher, 1986; Eriksson, 1992).
With these limitations in mind, we suggest that researchers approach each plant species under study with thoughtful consideration of the factors mentioned above. The best surrogate for fitness is likely to be slightly different for each species and situation examined—even the manner in which we measure plant size will vary based on plant habit and lineage (e.g., basal diameter, leaf area, or above- and belowground biomass). Future research should seek to clarify the relationship between biomass and plant fitness across several lineages and habits to address which specific biomass or performance metrics most accurately track fitness. It would be straightforward to establish an experimental protocol that tests whether the leaf area (for example) of plant individuals is correlated with fecundity and ultimately the number of viable offspring they recruit into the population. The offspring recruited should be tracked through molecular methods to determine parentage, but once these associations become well-defined in the literature, they will serve as a valuable resource to other investigators that work with the same plant system. In conclusion, when analyzing fitness, size is a great place to begin because it is relatively easy to quantify and is closely correlated to fecundity, but we emphasize that multiple measures should be taken whenever feasible.
Supplementary Material
Appendix 1. Glossary of terms.
Allometric scaling: deviations from a proportional increase in the size of certain structures relative to overall body size (contrasted with isometric scaling)
Apparency hypothesis: the division of plants into “apparent” and “non-apparent” groups, in which apparent plants (e.g., shrubs and trees) experience greater herbivory and produce high-molecular-weight organic compounds with low toxicity that act as inhibitors of herbivore digestion (quantitative defenses) and non-apparent plants (e.g., herbs) experience less herbivory and produce defensive compounds in smaller amounts or produce low-molecular-weight organic compounds that are strongly bioactive and highly toxic (qualitative defenses)
Biomass: the amount of organic matter derived from living organisms (also referred to as “size” in this review)
Fecundity: the reproductive output of a plant including the number of seeds, flowers, fruits, and the number of asexual propagules produced
Performance: the ability to acquire resources and survive in the presence of competition or in stressful environments where physiological limits are reached
Relative fitness: the ratio of surviving offspring of a particular genotype to the total surviving offspring for all genotypes in a population
Reproductive economy hypothesis: the ability to produce offspring economically in resource-limited environments, characterized by early sexual maturity at a smaller plant size, smaller seed size, increased rates of self-fertilization, and increased clonal growth
LITERATURE CITED
- Aarssen L. W. 2007. Death without sex—the “problem of the small” and selection for reproductive economy in flowering plants. Evolutionary Ecology 22: 279–298. [Google Scholar]
- Aarssen L. W. 2014. Estimating fitness from offspring counts in clonal seed plants. Ideas in Ecology and Evolution 7: 77–83. [Google Scholar]
- Aarssen L. W., Clauss M. J. 1992. Genotypic variation in fecundity allocation in Arabidopsis thaliana. Journal of Ecology 80: 109–114. [Google Scholar]
- Aarssen L. W., Taylor D. R. 1992. Fecundity allocation in herbaceous plants. Oikos 65: 225–232. [Google Scholar]
- Aarssen L. W., Schamp B. S., Wight S. 2014. Big plants—Do they affect neighbourhood species richness and composition in herbaceous vegetation? Acta Oecologica 55: 36–42. [Google Scholar]
- Agrawal A., Strauss S., Stout M. 1999. Costs of induced responses and tolerance to herbivory in male and female fitness components of wild radish. Evolution 53: 1093–1104. [DOI] [PubMed] [Google Scholar]
- Ågren G. I. 1985a. Theory for growth of plants derived from the nitrogen productivity concept. Physiologia Plantarum 64: 17–28. [Google Scholar]
- Ågren G. I. 1985b. Limits to plant productivity. Journal of Theoretical Biology 113: 89–92. [Google Scholar]
- Ashmun J. W., Thomas R. J., Pitelka L. F. 1982. Translocation of photoassimilates between sister ramets in two rhizomatous forest herbs. Annals of Botany 49: 403–415. [Google Scholar]
- Aspi J., Jäkäläniemi A., Tuomi J., Siikamäki P. 2003. Multilevel phenotypic selection on morphological characters in a metapopulation of Silene tatarica. Evolution 57: 509–517. [DOI] [PubMed] [Google Scholar]
- Barrett S. C. H. 2003. Mating strategies in flowering plants: The outcrossing-selfing paradigm and beyond. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 358: 991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett S. C. H. 2015. Influences of clonality on plant sexual reproduction. Proceedings of the National Academy of Sciences, USA 112: 8859–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa K. 1980. Evolution of dioecy in flowering plants. Annual Review of Ecology and Systematics 11: 15–39. [Google Scholar]
- Bazzaz F., Chiariello N., Coley P., Pitelka L. 1987. Allocating resources to reproduction and defense. Bioscience 37: 58–67. [Google Scholar]
- Bernasconi G. 2003. Seed paternity in flowering plants: An evolutionary perspective. Perspectives in Plant Ecology, Evolution and Systematics 6: 149–158. [Google Scholar]
- Bonser S. P., Aarssen L. W. 2003. Allometry and development in herbaceous plants: Functional responses of meristem allocation to light and nutrient availability. American Journal of Botany 90: 404–412. [DOI] [PubMed] [Google Scholar]
- Burczyk J., Adams W. T., Birkes D. S., Chybicki I. J. 2006. Using genetic markers to directly estimate gene flow and reproductive success parameters in plants on the basis of naturally regenerated seedlings. Genetics 173: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd M. 1994. Bateman’s principle and plant reproduction: The role of pollen limitation in fruit and seed set. Botanical Review 60: 83–139. [Google Scholar]
- Campbell D. R. 2000. Experimental tests of sex-allocation theory in plants. Trends in Ecology & Evolution 15: 227–232. [DOI] [PubMed] [Google Scholar]
- Coley P. D., Bryant J. P., Chapin F. S. 1985. Resource availability and plant antiherbivore defense. Science 230: 895–899. [DOI] [PubMed] [Google Scholar]
- Crosby K., Latta R. G. 2013. A test of the reproductive economy hypothesis in plants: More offspring per capita come from large (not small) parents in Avena barbata. Evolutionary Ecology 27: 193–203. [Google Scholar]
- de Jong T. J., Klinkhamer P. G. L. 1994. Plant size and reproductive success through female and male function. Journal of Ecology 82: 399–402. [Google Scholar]
- Echarte L., Andrade F. H. 2003. Harvest index stability of Argentinean maize hybrids released between 1965 and 1993. Field Crops Research 82: 1–12. [Google Scholar]
- Ellstrand N. 1984. Multiple paternity factors within the fruits of the wild radish, Raphanus sativus. American Naturalist 123: 819–828. [Google Scholar]
- Elshire R. J., Glaubitz J. C., Sun Q., Poland J. A., Kawamoto K., Buckler E. S., Mitchell S. E. 2011. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6: e19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson O. 1992. Evolution of seed dispersal and recruitment in clonal plants. Oikos 63: 439–448. [Google Scholar]
- Everham E. M., Brokaw N. V. L. 1996. Forest damage and recovery from catastrophic wind. Botanical Review 62: 113–185. [Google Scholar]
- Feeny P. 1976. Plant apparency and chemical defense. In J. W. Wallace and R. L. Mansell [eds.], Biochemical interaction between plants and insects, 1–40. Springer, New York, New York, USA. [Google Scholar]
- Fisher R. A. 1930. The genetical theory of natural selection: A complete variorum edition. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Fox G. A. 1990. Drought and the evolution of flowering time in desert annuals. American Journal of Botany 77: 1508–1518. [Google Scholar]
- Garant D., Kruuk L. E. B. 2005. How to use molecular marker data to measure evolutionary parameters in wild populations. Molecular Ecology 14: 1843–1859. [DOI] [PubMed] [Google Scholar]
- Geber M. A., Watson M. A., Furnish R. 1992. Genetic differences in clonal demography in Eichhornia crassipes. Journal of Ecology 80: 329–341. [Google Scholar]
- Goodwillie C., Kalisz S., Eckert C. G. 2005. The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics 36: 47–79. [Google Scholar]
- Grime J. P. 2006. Plant strategies, vegetation processes, and ecosystem properties. John Wiley and Sons, Chichester, United Kingdom. [Google Scholar]
- Haldane J. B. S. 1937. The effect of variation on fitness. American Naturalist 71: 337–349. [Google Scholar]
- Harder L. D., Barrett S. H. 1995. Letter: Mating cost of large floral displays in hermaphrodite plants. Nature 373: 512–515. [Google Scholar]
- Harder L. D., Thomson J. D., Cruzan M. B., Unnasch R. S. 1985. Sexual reproduction and variation in floral morphology in an ephemeral lily, Erythronium americanum. Oecologia 67: 286–291. [DOI] [PubMed] [Google Scholar]
- Harper J. 1977. The population biology of plants. Academic Press, London, United Kingdom. [Google Scholar]
- Harper J., White J. 1974. The demography of plants. Annual Review of Ecology and Systematics 5: 419–463. [Google Scholar]
- Herben T., Šerá B., Klimešová J. 2015. Clonal growth and sexual reproduction: Tradeoffs and environmental constraints. Oikos 124: 469–476. [Google Scholar]
- Herms D., Mattson W. 1992. The dilemma of plants: To grow or defend. Quarterly Review of Biology 67: 283–335. [Google Scholar]
- Holsinger K. E., Feldman M. W., Christiansen F. B. 1984. The evolution of self-fertilization in plants: A population genetic model. American Naturalist 124: 446–453. [Google Scholar]
- Karron J. D., Mitchell R. J. 2012. Effects of floral display size on male and female reproductive success in Mimulus ringens. Annals of Botany 109: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M., Kollmann J., Edwards P. 2000. Genetic introgression from distant provenances reduces fitness in local weed populations. Journal of Applied Ecology 37: 647–659. [Google Scholar]
- Klinkhamer P. G., De Jong T. J. 1990. Effects of plant size, plant density and sex differential nectar reward on pollinator visitation in the protandrous Echium vulgare (Boraginaceae). Oikos 57: 399–405. [Google Scholar]
- Klinkhamer P. G. L., de Jong T. J., de Bruyn G. J. 1989. Plant size and pollinator visitation in Cynoglossum officinale. Oikos 54: 201–204. [Google Scholar]
- Klinkhamer P. G. L., de Jong T. J., Metz H. 1997. Sex and size in cosexual plants. Trends in Ecology & Evolution 12: 260–265. [DOI] [PubMed] [Google Scholar]
- Latta R. G., Gardner K. M. 2009. Natural selection on pleiotropic quantitative trait loci affecting a life-history trade-off in Avena barbata. Evolution 63: 2153–2163. [DOI] [PubMed] [Google Scholar]
- Mauricio R., Bowers M., Bazzaz F. 1993. Pattern of leaf damage affects fitness of the annual plant Raphanus sativus (Brassicaceae). Ecology 74: 2066–2071. [Google Scholar]
- May J., Killingbeck K. 1992. Effects of preventing nutrient resorption on plant fitness and foliar nutrient dynamics. Ecology 73: 1868–1878. [Google Scholar]
- Meagher T. R. 1986. Analysis of paternity within a natural population of Chamaelirium luteum 1. Identification of most-likely male parents. American Naturalist 128: 199–215. [Google Scholar]
- Mencuccini M., Grace J. 1996. Developmental patterns of above-ground hydraulic conductance in a Scots pine (Pinus sylvestris L.) age sequence. Plant, Cell & Environment 19: 939–948. [Google Scholar]
- Méndez M., Obeso J. 1993. Size-dependent reproductive and vegetative allocation in Arum italicum (Araceae). Canadian Journal of Botany 71: 309–314. [Google Scholar]
- Mojica J. P., Kelly J. K. 2010. Viability selection prior to trait expression is an essential component of natural selection. Proceedings of the Royal Society. Series B Biological Sciences 277: 2945–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolè F., Brzosko E., Till-Bottraud I. 2005. Population viability analysis of Cypripedium calceolus in a protected area: Longevity, stability and persistence. Journal of Ecology 93: 716–726. [Google Scholar]
- Obeso J. 2002. The costs of reproduction in plants. New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- Parker P. G., Snow A. A., Schug M. D., Booton G. C., Fuerst P. A. 1998. What molecules can tell us about populations: Choosing and using a molecular marker. Ecology 79: 361–382. [Google Scholar]
- Parsons D. J. 1994. Objects or ecosystems? Giant sequoia management in national parks. In P. S. Aune [technical coordinator], Proceedings of the symposium on the Giant Sequioas: Their place in the ecosystem and society, 109–115. U.S. Forest Service General Technical Report PSW-GTR-151. Pacific Southwest Research Station, Berkeley, California, USA. [Google Scholar]
- Peet R., Christensen N. 1987. Competition and tree death. Bioscience 37: 586–595. [Google Scholar]
- Pino J., Sans F. X., Masalles R. M. 2002. Size-dependent reproductive pattern and short-term reproductive cost in Rumex obtusifolius L. Acta Oecologica 23: 321–328. [Google Scholar]
- Ryan M. G., Yoder B. J. 1997. Hydraulic limits to tree height and tree growth: What keeps trees from growing beyond a certain height? Bioscience 47: 235–242. [Google Scholar]
- Sakai A., Sakai S. 2003. Size-dependent ESS sex allocation in wind-pollinated cosexual plants: Fecundity vs. stature effects. Journal of Theoretical Biology 222: 283–295. [DOI] [PubMed] [Google Scholar]
- Sakai A. K., Weller S. G., Chen M. L., Chou S. Y., Tasanont C. 1997. Evolution of gynodioecy and maintenance of females: The role of inbreeding depression, outcrossing rates, and resource allocation in Schiedea adamantis (Caryophyllaceae). Evolution 51: 724–736. [DOI] [PubMed] [Google Scholar]
- Santos-del-Blanco L., Bonser S. P., Valladares F., Chambel M. R., Climent J. 2013. Plasticity in reproduction and growth among 52 range-wide populations of a Mediterranean conifer: Adaptive responses to environmental stress. Journal of Evolutionary Biology 26: 1912–1924. [DOI] [PubMed] [Google Scholar]
- Schmid B., Weiner J. 1993. Plastic relationships between reproductive and vegetative mass in Solidago altissima. Evolution 47: 61–74. [DOI] [PubMed] [Google Scholar]
- Schmid B., Bazzaz F. A., Weiner J. 1995. Size dependency of sexual reproduction and of clonal growth in two perennial plants. Canadian Journal of Botany 73: 1831–1837. [Google Scholar]
- Silvertown J. 2008. The evolutionary maintenance of sexual reproduction: Evidence from the ecological distribution of asexual reproduction in clonal plants. International Journal of Plant Sciences 169: 157–168. [Google Scholar]
- Sletvold N. 2002. Effects of plant size on reproductive output and offspring performance in the facultative biennial Digitalis purpurea. Journal of Ecology 90: 958–966. [Google Scholar]
- Smouse P. E., Meagher T. R. 1994. Genetic analysis of male reproductive contributions in Chamaelirium luteum (L.) Gray (Liliaceae). Genetics 136: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smouse P. E., Meagher T. R., Kobak C. J. 1999. Parentage analysis in Chamaelirium luteum (L.) Gray (Liliaceae): Why do some males have higher reproductive contributions? Journal of Evolutionary Biology 12: 1069–1077. [Google Scholar]
- Stevens L., Goodnight C. J., Kalisz S. 1995. Multilevel selection in natural populations of Impatiens capensis. American Naturalist 145: 513–526. [Google Scholar]
- Sugiyama S., Bazzaz F. A. 1998. Size dependence of reproductive allocation: The influence of resource availability, competition and genetic identity. Functional Ecology 12: 280–288. [Google Scholar]
- Tracey A. J., Aarssen L. W. 2014. Revising traditional theory on the link between plant body size and fitness under competition: Evidence from old-field vegetation. Ecology and Evolution 4: 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Zhou D., Wang P., Zhang H. 2006. Size-dependent reproductive effort in Amaranthus retroflexus: The influence of planting density and sowing date. Canadian Journal of Botany 84: 485–492. [Google Scholar]
- Watson M. A. 2008. Resource storage and the expression of clonal plant life histories: Forum entry for clone SI. Evolutionary Ecology 22: 471–475. [Google Scholar]
- Weiner J. 2004. Allocation, plasticity and allometry in plants. Perspectives in Plant Ecology, Evolution and Systematics 6: 207–215. [Google Scholar]
- Weiner J., Thomas S. C. 2001. The nature of tree growth and the “age-related decline in forest productivity.” Oikos 94: 374–376. [Google Scholar]
- Weiner J., Campbell L. G., Pino J., Echarte L. 2009a. The allometry of reproduction within plant populations. Journal of Ecology 97: 1220–1233. [Google Scholar]
- Weiner J., Rosenmeier L., Massoni E. S., Vera J. N., Plaza E. H., Sebastià M. T. 2009b. Is reproductive allocation in Senecio vulgaris plastic? Botany 87: 475–481. [Google Scholar]
- Wesselingh R. A., de Jong T. J. 1995. Bidirectional selection on threshold size for flowering in Cynoglossum officinale (hound’s-tongue). Heredity 74: 415–424. [Google Scholar]
- Wesselingh R., Klinkhamer P. 1997. Threshold size for flowering in different habitats: Effects of size-dependent growth and survival. Ecology 78: 2118–2132. [Google Scholar]
- Wheeler G. L., Dorman H. E., Buchanan A., Challagundla L., Wallace L. E. 2014. A review of the prevalence, utility, and caveats of using chloroplast simple sequence repeats for studies of plant biology. Applications in Plant Sciences 2: 1400059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P., Thomson J. D., Stanton M. L., Rigney L. P. 1994. Beyond floral Batemania—gender biases in selection for pollination success. American Naturalist 143: 283–296. [Google Scholar]
- Yoder B. J., Ryan M. G., Waring R. H., Schoettle A. W., Kaufmann M. R. 1994. Evidence of reduced photosynthetic rates in old trees. Forest Science 40: 513–527. [Google Scholar]
- Zhang S., Zhao C., Lamb E. G. 2011. Cotyledon damage affects seed number through final plant size in the annual grassland species Medicago lupulina. Annals of Botany 107: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.