Abstract
The gynecological disease endometriosis is characterized by the deposition and proliferation of endometrial cells outside the uterus and clinically is linked to low body mass index (BMI). Gene expression in the liver of these women has not been reported. We hypothesized that endometriosis may impact hepatic gene expression, promoting a low BMI. To determine the effect of endometriosis on liver gene expression, we induced endometriosis in female mice by suturing donor mouse endometrium into the peritoneal cavity and measuring the weight of these mice. Dual-energy X-ray absorptiometry (DEXA) scanning of these mice showed lower body weight and lower total body fat than controls. Microarray analysis identified 26 genes differentially regulated in the livers of mice with endometriosis. Six of 26 genes were involved in metabolism. Four of six genes were upregulated and were related to weight loss, whereas two genes were downregulated and linked to obesity. Expression levels of Cyp2r1, Fabp4, Mrc1, and Rock2 were increased, whereas Igfbp1 and Mmd2 expression levels were decreased. Lep and Pparg, key metabolic genes in the pathways of the six genes identified from the microarray, were also upregulated. This dysregulation was specific to metabolic pathways. Here we demonstrate that endometriosis causes reduced body weight and body fat and disrupts expression of liver genes. We suggest that altered metabolism mediated by the liver contributes to the clinically observed low BMI that is characteristic of women with endometriosis. These findings reveal the systemic and multiorgan nature of endometriosis.
Keywords: endometriosis, gene expression, liver, metabolism
INTRODUCTION
Endometriosis is one of the most common gynecological disorders among reproductive-aged women [1]. It is characterized by the deposition and proliferation of endometrial cells or tissue outside the uterine cavity [2, 3]. The major symptom of endometriosis is pelvic pain, which affects 50% of patients [4], followed by infertility, which is reported in 40%–50% of patients [5].These symptoms may severely affect a woman's quality of life [6]. Endometriosis is a varied and complex disorder, with patients often reporting diffuse symptoms unrelated to reproduction, and the precise cause and pathophysiology are still not well understood [1, 7]. Women with the disease often complain of weight loss, allergies, fatigue, inflammation, and bowel dysfunction.
The cause of these previously unexplained symptoms is unknown but may originate from dysregulation of multiple molecular pathways in several organ systems outside of the reproductive tract. The existence of lower body mass index (BMI) among women with endometriosis compared to those without the disease is well established [8–15]. No previous studies have investigated the effects of endometriosis on the liver. It is not currently known whether the observed low BMI phenotype in women with endometriosis is directly attributable to the disease and, if so, by what mechanism. Here we sought to determine whether endometriosis could cause metabolic dysregulation.
The liver is a major point of metabolic regulation and a central mediator for the maintenance of energy homeostasis [4, 16–19]. Liver gene expression specifically has been shown to be disrupted in women with obesity [20–22]. Endometriosis creates an altered inflammatory milieu [23–25] which could alter gene expression in remote organs. Indeed, we have previously demonstrated that endometriosis affects uterine gene expression [26] suggesting that endometriosis may lead to altered gene expression in nonreproductive organs as well. Changes in liver gene expression due to endometriosis have not yet been reported; however, hepatic gene dysregulation has been shown to alter BMI in a mouse model [27], and obesity has been clinically associated with altered gene expression in the liver [28, 29]. This makes the liver an interesting candidate for involvement in a possible metabolic component to endometriosis.
Here we compared body weight, body composition, and hepatic gene expression in mice with surgically induced endometriosis with those of control mice that had undergone sham surgery. We identified endometriosis-induced metabolic disruption associated with reduced body weight and fat. These findings may explain the clinically observed low body weight of women with endometriosis.
MATERIALS AND METHODS
All animal experiments were conducted in accordance with an approval from Yale University Animal Care Committee protocol, using a total of 30 mice. Endometriosis was induced in 12-wk-old female C57BL/6 mice (n = 6) by suturing two uterine sections, each consisting of one half a uterine horn from a donor, into the peritoneal cavity according to previously reported techniques [30, 31]. Sham surgeries were performed in control mice (n = 6). Food was consumed ad libitum by all animals, and both groups received the same chow. Mice were weighed weekly on a portable scale (Uline), and body weights were recorded to the nearest 0.1 of a gram, starting 1 wk after the induction surgery. Dual-energy X-ray absorptiometry (DEXA; GE Medical Systems) was performed 7 wk after surgery. In another set of 9-wk-old female C57BL/6 mice, endometriosis was induced according to the same surgical procedures (n = 9 in each group). That set of mice was euthanized by cervical dislocation after CO2 asphyxiation at 21 wk, and livers were collected and stored in RNAlater (Qiagen) at −80°C for RNA and protein isolation. The presence of persistent lesions of endometriosis was confirmed at necropsy.
RNA Isolation
Liver tissue (100 mg) was homogenized in 1 ml of TRIzol reagent (Invitrogen). Homogenates were kept on ice for 5 min, and then 0.2 ml of chloroform was added to each, and samples were vortexed for 15 s, incubated at room temperature for 3 min, and centrifuged at 12 000 rpm at 4°C for 15 min. Then, the aqueous layer was transferred to a fresh tube, and the RNA was precipitated by adding 0.5 ml of isopropyl alcohol, incubated at room temperature for 10 min, and centrifuged at 10 000 rpm for 15 min; then RNA pellets were collected, washed with 75% ethanol, and dissolved in RNase-free water. The total RNA was purified using the RNeasy cleanup kit (Qiagen) and quantified by a NanoDrop spectrophotometer. Purified RNA was immediately used for cDNA synthesis and then subjected to microarray analysis or stored at −80°C until used later.
Mouse Gene Microarray
High-quality total RNA (250 ng) was subjected to WT PLUS reagent kit (Affymetrix) following the manufacturer's instructions. Briefly, total RNA was amplified to create cDNA that was used for in vitro transcription to create complementary RNA (cRNA). The cRNA was cleaned using bead purification and quantitated. The cRNA (15 μg) was used with a random primer to generate a second cycle of first-strand sense direction cDNA. The cDNA was purified using the bead cleanup method and quantitated. The single-stranded cDNA (sscDNA; 5.5 μg) was then enzymatically fragmented using ADP and UDG, using a terminal labeling kit (Affymetrix) and run on a bioanalyzer (Agilent) to ensure proper transcript size. The fragmented material was subsequently labeled using Terminal deoxynucleotidyl transferase, placed into a hybridization cocktail, and hybridized using GeneChip mouse gene 2.0 ST arrays overnight at 45°C. The arrays were washed and stained using the fluidic station model 450 and then scanned using scanner model 3000 7G (both Affymetrix). Affymetrix expression console software was used to generate the raw and normalized data for downstream analysis. MATLAB (MathWorks) was used to analyze the data output.
Real-Time Quantitative PCR Analysis
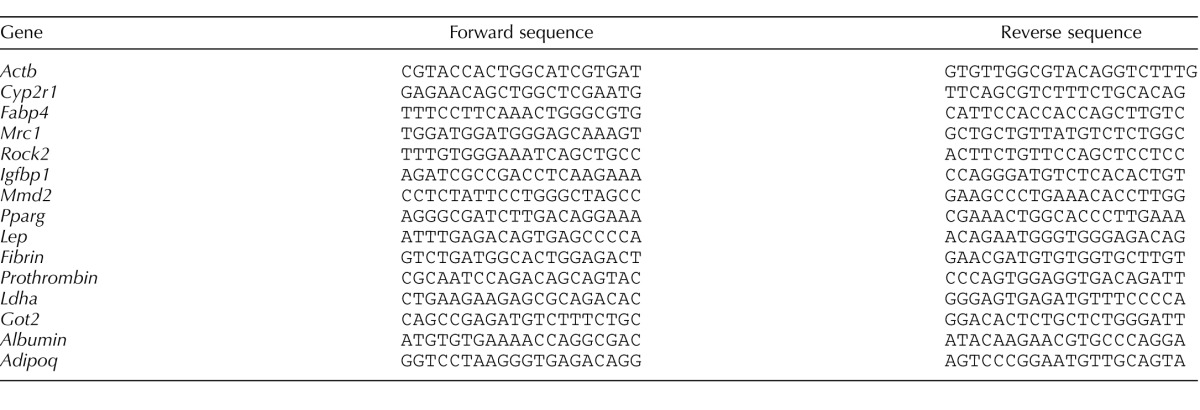
Purified RNA (50 ng) was reverse-transcribed in a 20-μl reaction mixture using iScript cDNA synthesis kit (Bio-Rad Laboratories). Real-time quantitative PCR (real-time qPCR) was performed using SYBR Green (Bio-Rad) and optimized in the MyiQ single-color real-time PCR detection system (Bio-Rad). Primer sequences used for respective genes are listed in Table 1. The specificity of the amplified transcript and absence of primer-dimers was confirmed by a melting curve analysis. Gene expression was normalized to that of β-actin. Relative mRNA expression was calculated using the comparative cycle threshold (Ct) method (2−ΔΔCt) [32, 33]. All experiments were carried out three times and each in triplicate.
TABLE 1.
Primer sequences used for qRT-PCR.
Western Blot Analysis
Liver tissue was homogenized in lysis buffer with protease inhibitor cocktail and phenylmethane sulfonyl fluoride protease inhibitor (Sigma-Aldrich), using tungsten carbide beads in a TissueLyser II (Qiagen). The homogenate was centrifuged at 12 000 rpm for 10 min, the supernatant was collected, and the protein concentration was determined using the Bradford method [34]. Protein samples were prepared in SDS sample buffer while heating at 95°C for 6 min. Protein (25 μg) was subjected to SDS-PAGE, using NuPAGE Novex 4%–12% bis-Tris Midi protein gels (Life Technologies) with 3-(N-morpholino)propanesulfonic acid running buffer. The separated proteins were transferred from the gel onto a polyvinylidene fluoride membrane and blocked with 5% non-fat dry milk. The membranes were then incubated with a specific primary antibody against the desired protein target, followed by a secondary horseradish peroxidase-conjugated antibody. After the membranes were washed, protein bands were visualized by chemiluminescence, using the SuperSignal West Pico and Femto detection kit (Thermo Scientific) according to the manufacturer's protocol. Anti-Cypr1 (sc 48985), anti-Fabp4 (sc 18661), anti-Igfbp1 (sc 6072), anti-Mrc1 (sc 48758), anti-Rock2 (sc 1851), anti-Mmd2 (sc 243496), anti-Lep (sc 9014) and bovine anti-goat secondary antibodies were procured from Santa Cruz Biotechnology Inc. Anti-Pparg (catalog no. 2435) and goat anti-rabbit secondary antibodies were purchased from Cell Signaling Technology.
Dual-Energy X-ray Absorptiometry
Dual-energy X-ray absorptiometry was performed in the mice 7 wk after surgery, using a Lunar PIXImus (GE Medical Systems). Mice were anesthetized using 50 mg/kg ketamine (Fort Dodge Animal Health) and 10 mg/kg xylazine (Lloyd), by intraperitoneal injection.
Statistical Analysis
Body weights and body fat content are shown as box plots. Real-time qPCR results are means ± SEM. Distribution of the variables was investigated using the Kolmogorov-Smirnov test. All statistical analyses were carried out by one-way ANOVA using Prism version 4.00 software (GraphPad), and a P value of 0.05 or less was considered significant. Microarray genes of interest were determined by using fold-change criteria greater than 1.5 and a P value less than 0.05.
RESULTS
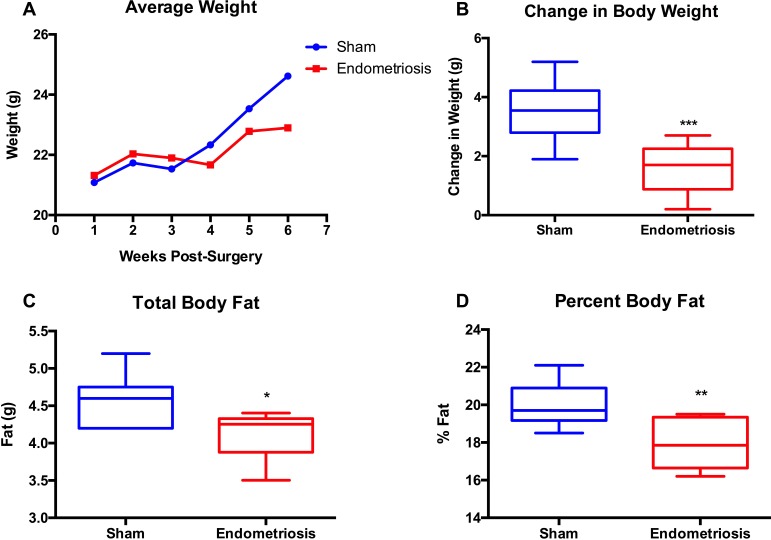
We measured the body weights of endometriosis model mice and sham surgery control mice weekly and compared the net weight changes over time, with the baseline being the mouse's weight 1 wk after surgical induction. All mice gained weight during the postsurgical period, as expected in young mice. However, the weight increase plateaued for the endometriosis group, whereas the controls' body weight continued to increase (Fig. 1A). Beginning at 6 wk post surgery, we found the controls had a significantly greater increase in body weight than the endometriosis mice (P = 0.006) (Fig. 1B).
FIG. 1.
Endometriosis induces a reduced body weight compared to sham controls. The body weights of the mice were measured every week for 6 wk after surgery. A) Average body weights of endometriosis and sham groups (n = 6) from Week 1 to Week 6. B) Changes in body weight for each of the two groups. C and D) Changes in total body fat mass and percentage of body fat, respectively, in the endometriosis and sham groups as determined by DEXA. *P < 0.05; **P < 0.02; ***P < 0.006.
To determine whether these differences in weight gain corresponded to altered body composition in the endometriosis group compared to controls, we performed DEXA in all mice in both the endometriosis and the sham groups (n = 6 per group). The DEXA scans showed that the total body fat content (both the fat mass and the fat percentage) was lower in the endometriosis group than in the sham controls (Fig. 1, C and D).
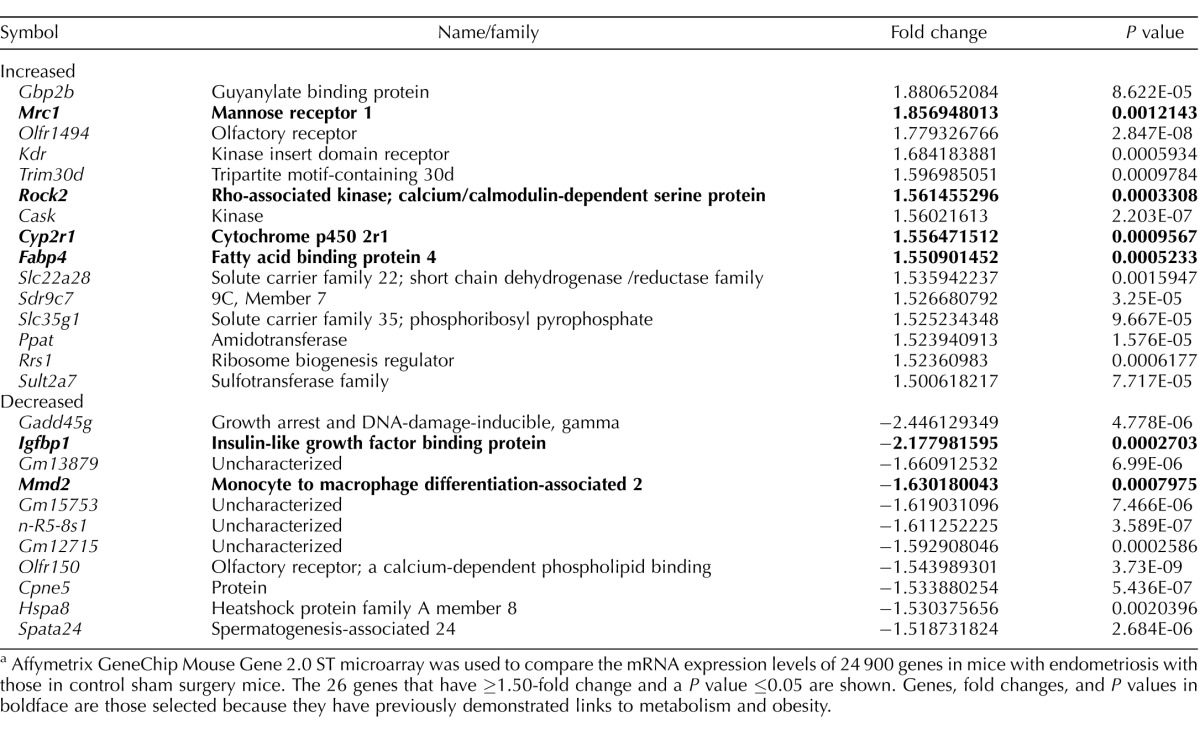
To establish the potential metabolic contribution for this observed low body weight, we compared the relative expression of 24 900 genes in the liver of mice with endometriosis with those in controls by using microarray analysis. Expression of a small number of genes was specifically affected by endometriosis. Of the 26 altered genes, expression of 15 genes was increased, whereas 11 genes showed ≥1.50-fold decreased expression and P values ≤ 0.05 in the endometriosis model compared to sham controls (Table 2). Based on the reported function of the genes identified in our microarray analysis, we noted that one of the genes (Mrc1) with the largest fold upregulation was involved in regulation of metabolism. The Igfbp1 gene, which showed the second largest fold downregulation, also had an essential role in metabolism. Surprisingly, 6 of 26 genes identified in the array had a previously demonstrated role in metabolism. Of those 6 genes linked to metabolism, 4 (Cyp2r1, Fabp4, Mrc1, and Rock2) were upregulated genes tied to weight loss, and 2 (Igfbp1 and Mmd2) were downregulated obesogenic genes in mice with endometriosis compared to controls. Genes subjected to further evaluation were previously related to obesity and metabolic syndrome in the following manner. The cytochrome P450 2R1, or vitamin D 25 hydroxylase (Cyp2r1), gene is involved in liver vitamin D metabolism; disruption of Cyp2r1 has been linked to fatty liver and diabetes risk [35, 36]. Fatty acid binding protein 4 (Fabp4) is expressed primarily in adipose tissue, but low levels of Fabp4 in liver have been shown to cause aberrant lipid storage in the liver [37]. Mannose receptor 1 (Mrc1) is expressed primarily in macrophages, but hepatocyte expression has been demonstrated and characterized [38]. The rho-associated kinase 2 (Rock2) gene mediates fatty acid uptake [39] and appears to have a protective role against weight gain [40–42]. Insulin-like growth factor binding protein 1 (Igfbp1) is involved in glucose metabolism and is expressed primarily in hepatocytes and inversely correlated with liver fat content [43]. Monocyte-to-macrophage differentiation-associated 2 (Mmd2), or progestin and adiponectin receptor 10, is involved in glucose metabolism [44].
TABLE 2.
Gene selection from microarrays.a
Affymetrix GeneChip Mouse Gene 2.0 ST microarray was used to compare the mRNA expression levels of 24 900 genes in mice with endometriosis with those in control sham surgery mice. The 26 genes that have ≥1.50-fold change and a P value ≤0.05 are shown. Genes, fold changes, and P values in boldface are those selected because they have previously demonstrated links to metabolism and obesity.
The microarray results for the 6 genes of interest were confirmed by real-time qPCR (Fig. 2A) and Western blot analysis (Fig. 2B). As shown in Figure 2A, increased expression of several genes was observed, including Cyp2r1 (10.0-fold), Fabp4 (5.4-fold), Mrc1 (4.3-fold), and Rock2 (8.9-fold). Decreased gene expression was noted in Igfbp1 (−333-fold) and Mmd2 (−4.5-fold). We next determined that the altered mRNA levels, as determined by real-time qPCR, resulted in similar changes in protein expression by Western blot analysis as shown in Figure 2B. We decided also to investigate the relative expression of leptin (Lep) and peroxisome proliferator-activated receptor gamma (Pparg), which are essential metabolic genes involved in the same pathways as the genes identified in the microarray. As shown in Figure 2C, gene expression was increased for Lep (16.0-fold) and Pparg (17.6-fold) in mice with endometriosis compared to those in control mice. The increase in mRNA levels for Lep and Pparg was further confirmed by Western blotting as shown in Figure 2D, where the protein levels were increased in mice with endometriosis compared to those in sham controls.
FIG. 2.
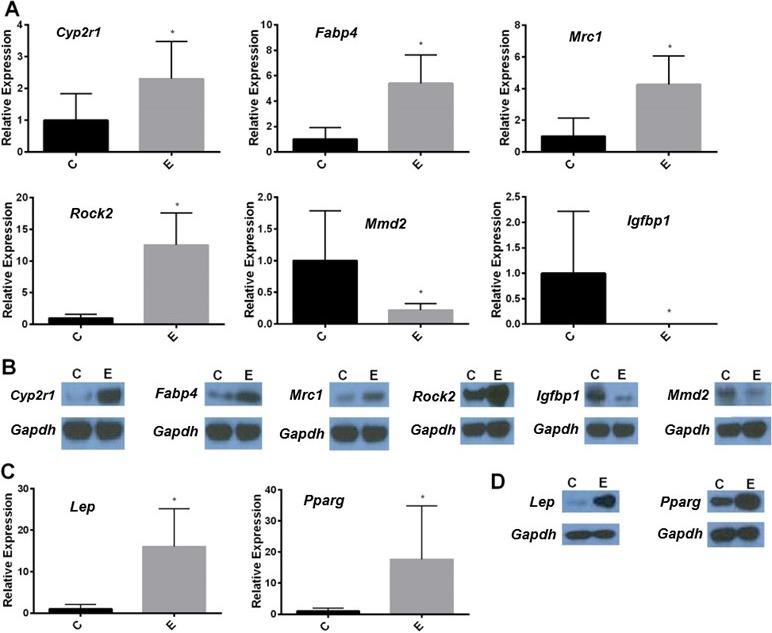
Endometriosis dysregulates liver gene expression. RNA was extracted from liver tissue collected from sham surgery mice or mice in which endometriosis was induced. A) Real-time qPCR results shows the altered liver gene expression as identified in the array. The levels of Cyp2r1, Fabp4, Mrc1, and Rock2 expression were increased, whereas those of Mmd2 and Igfbp1 expression were decreased. Data are relative fold-change expression in mice with endometriosis (E) compared to sham surgery controls (C). The expression levels of all genes were normalized to those of β-actin. Bars in each graph are mean ± SEM of two individual experiments, each performed in triplicate (n = 16 mice per group). *Statistical significance (P < 0.05). B) Western blot analysis shows the protein levels. GADPH was used as a protein loading control. Liver protein (25 μg) was subjected to 4%–12% SDS-PAGE and immunoblotting. The protein product levels of Cyp2r1, Fabp4, Mrc1, and Rock2 genes were increased, whereas those of Igfbp1, Mmd2 were decreased. In addition to those genes identified in the array, we showed the increased expression of mRNA levels of the Lep and Pparg genes in real-time qPCR (C) and increased protein levels by Western blot analysis (D) in endometriosis (E), compared to those in controls (C).
Dysregulation of the gene expression was highly specific. Most genes (24 874) analyzed in the array were not differentially expressed between the diseased and control mice. We examined the mRNA expression of multiple liver genes: albumin, clotting factors fibrin and prothrombin, and the enzymes aspartate aminotransferase mitochondrial (Got2) and lactate dehydrogenase A (Ldha) genes. We found no differences in mRNA expression among any of these genes by real-time qPCR, as shown in Figure 3. These results show that liver function, outside of energy homeostasis, was not disrupted. We also compared mRNA levels for adiponectin (another major metabolic hormone) to demonstrate the specificity of the anorexigenic metabolic disruption. We found adiponectin was not differentially expressed between endometriosis and sham control groups as shown in Figure 3. These results support the metabolic specificity of the observed altered gene expression caused by endometriosis.
FIG. 3.
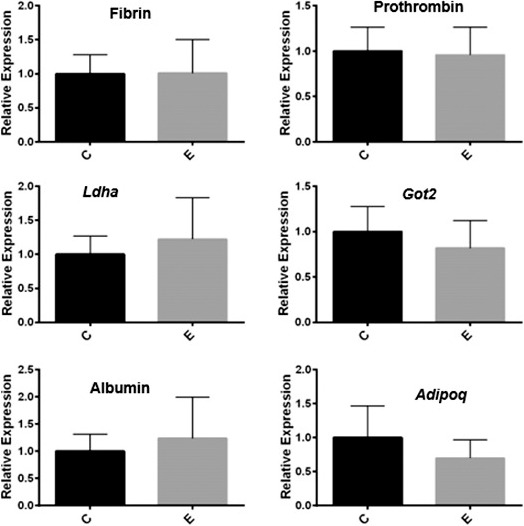
Disruption of hepatic gene expression is specific. RNA was extracted from liver tissue collected from sham surgery mice and mice in which endometriosis was induced. Real-time qPCR results from liver mRNA show comparable gene expression levels of fibrin, prothrombin, Ldha, Got2, albumin, and Adipoq (adiponectin) between the endometriosis (E) and control (C) groups. Data are relative fold-change expression compared to that in sham surgery controls, and the expression levels of all genes were normalized to those of β-actin. The bars in each graph are mean ± SEM of two individual experiments, each performed in triplicate (n = 6 mice per group). None of the differences was statistically significant.
DISCUSSION
In this study, we demonstrate, in an animal model, that endometriosis resulted in the same low BMI phenotype as that clinically observed in women with endometriosis. The decrease in body weight in the endometriosis group was further supported by DEXA scan, which revealed that total body fat content was significantly lower in the endometriosis group than in the sham control group. This finding affirms the fact that the previously observed clinical correlation is actually causally related to endometriosis.
We also exhibited the first evidence of a molecular mechanism explaining the low BMI seen in women with endometriosis. These findings have implications for both the understanding and treatment of endometriosis. The hepatic gene alterations were specific to a defined set of metabolic pathways. Although these dysregulated genes were identified using a mouse model, the metabolic pathways identified are highly conserved between mice and humans. The fact that our mice also exhibited lower body weight than that of controls supports the clinical relevance of this molecular mechanism.
Previous studies suggest that the four genes (Cyp2r1, Fabp4, Mrc1, and Rock2) that we identified as having increased expression in mice with endometriosis have an anorexigenic role. Cyp2r1 has a protective role against obesity and diabetes [35]. An association between Cyp2r1 single-nucleotide polymorphisms and BMI or weight has been previously reported [45]. Similarly, levels of Cyp2r1 expression are lower in obese mice than in the normal weight controls [46]. Rock2 expression has been inversely associated with obesity [40, 42]. Fabp4 has been associated with insulin sensitivity, lipid metabolism, and inflammation [47]. Expression of the Fabp4 gene is higher in visceral adipose tissue of lean controls than in obese patients and is thought to be associated with regulation of BMI [48]. Pparg expression is decreased in obese individuals [49] but upregulated here in mice with endometriosis. Lagou et al.[50] reported that expression of functional Pparg was decreased in obese children due to Pparg gene polymorphism (the Pparg Pro12Ala and C1432T polymorphisms), while Li et al. [51] showed that overweight children have lower Pparg concentrations in omental adipose tissue than in control children between 2 and 14 yr of age; results that are in agreement with our data. We observed that levels of transcription factor Pparg increased in our murine endometriosis model where total body fat, weight, and percentage of body fat content were decreased. The increased expression of Pparg in endometriosis may contribute to prevention of obesity in young animals but be reversed after the obesity is established in adulthood. Mrc1 is associated with insulin sensitivity [52] and has been shown to have reduced expression in obese mice [53]. Increased expression of these genes promotes low BMI. Additionally, decreased expression of genes associated with obesity and metabolic disease was demonstrated. Igfbp1 is a biomarker of obesity [54], and liver expression levels are inversely correlated with insulin sensitivity [43], whereas Mmd2 is an adiponectin receptor, and expression has been shown to be disrupted in type 2 diabetes [55]. Our results suggest that endometriosis disrupts metabolism in a way that could combat obesity and metabolic syndrome and lead to low BMI.
We also sought to determine whether endometriosis affected expression of other metabolic genes in the liver that may not have been detected by the array. We investigated relative expression of a few essential metabolic genes that function in the same pathways as the genes identified. Leptin has a demonstrated role in establishing satiety and modulating food consumption and appetite [56, 57], and Pparg has been shown to regulate fatty acid and glucose metabolism [58]. Both Lep and Pparg were significantly increased in our endometriosis murine model compared to controls. Previous studies have reported increased leptin levels in women with endometriosis and hypothesized that leptin might promote establishment and proliferation of endometrial lesions [59]. It has been also shown that rAAV-leptin-treated rats maintained a lower body weight than untreated rats [60]. However, our results suggest that endometriosis increases leptin expression rather than leptin leading to endometriosis; endometriosis contributes to physiological changes that promote low body weight.
Liver function, outside of energy homeostasis, was normal. The dysregulation of gene expression was shown to be specific to those metabolic genes. There was no affect on the majority of liver genes examined including albumin, clotting factors fibrin and prothrombin, and enzymes Got2 and Ldha. We also compared mRNA levels for adiponectin, another major metabolic hormone, to demonstrate the specificity of the anorexigenic metabolic disruption. There were no differences between the expression of adiponectin in the endometriosis model and that in the sham control group, suggesting that there is a precise mechanism of metabolic disruption caused by endometriosis, involving leptin and not adiponectin.
Although mice do not spontaneously develop endometriosis, and animal models involving surgical attachment of uterine horns to the peritoneal wall do not fully recapitulate the pathogenesis of endometriosis, the model used here mimics many aspects of the disease that are observed in humans. Increased inflammation due to endometriosis in a mouse model was demonstrated by several investigators by measuring the elevated levels of proinflammatory markers including cytokines/chemokines [61–63]. However, the mechanism(s) by which endometriosis affects the liver is not known. Although endometriosis is seen commonly in pelvic organs and peritoneum, endometriosis lesions have been reported in other remote organs of the body [64]. Endometriosis may be transported to the liver directly through the peritoneal cavity or through lymphatic and blood vessels [65]. We failed to identify any visible endometriosis in the livers of the experimental animals; however, small implants of the disease were possibly present and able to alter liver metabolism. Previously, we demonstrated that endometrial stem cells could migrate from endometriosis lesions to the uterus [66]; perhaps these cells may migrate to other organs that include the liver and affect local gene expression. Alternatively, the effect may not be mediated by direct endometrial cell infiltration of the liver. Inflammation of the peritoneal cavity associated with endometriosis could affect gene expression [67, 68], possibly involving the actions of circulating microRNAs that we have shown to be differentially expressed in the sera of women with endometriosis [69]. A better understanding of the mechanism by which endometriosis affects liver gene expression and metabolism may lead to identification of novel anti-obesity treatments.
In summary, we demonstrate that endometriosis leads to a decreased body weight and the disruption of hepatic gene expression. The effect selectively targets a limited number of genes associated with metabolism. These alterations to liver metabolism likely contribute to the low BMI observed in women with endometriosis, demonstrating a previously unknown metabolic component to this disease. Here we provide evidence that endometriosis is a metabolic, systemic, and multi-organ disease.
Footnotes
Supported by National Institutes of Sciences grant NIH RO1 HD076422 to T.H.S. Presented in part at the 63rd Annual Meeting of the Society for Reproductive Investigation, March 16–19, 2016, Montreal, Quebec, Canada.
REFERENCES
- Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39:535–549. doi: 10.1016/j.ogc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC. Clinical Practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008;1127:92–100. doi: 10.1196/annals.1434.007. [DOI] [PubMed] [Google Scholar]
- Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D. Impact of endometriosis on women's lives: a qualitative study. BMC Womens Health. 2014;14:1–12. doi: 10.1186/1472-6874-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznaurova YB, Zhumataev MB, Roberts TK, Aliper AM, Zhavoronkov AA. Molecular aspects of development and regulation of endometriosis. Reprod Biol Endocrinol. 2014;12:50. doi: 10.1186/1477-7827-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah DK, Correia KF, Vitonis AF, Missmer SA. Body size and endometriosis: results from 20 years of follow-up within the Nurses' Health Study II prospective cohort. Hum Reprod. 2013;28:1783–1792. doi: 10.1093/humrep/det120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann SE, Freudenheim JL, Darrow SL, Batt RE, Zielezny MA. Endometriosis and body fat distribution. Obstet Gynecol. 1993;82:545–549. [PubMed] [Google Scholar]
- Vitonis AF, Baer HJ, Hankinson SE, Laufer MR, Missmer SA. A prospective study of body size during childhood and early adulthood and the incidence of endometriosis. Hum Reprod. 2010;25:1325–1334. doi: 10.1093/humrep/deq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay Pillet M-C, Schneider A, Borghese B, Santulli P, Souza C, Streuli I, de Ziegler D, Chapron C. Deep infiltrating endometriosis is associated with markedly lower body mass index: a 476 case–control study. Hum Reprod. 2012;27:265–272. doi: 10.1093/humrep/der346. [DOI] [PubMed] [Google Scholar]
- Shahbazi S, Shahrabi-Farahani M. Evaluation of the correlation between body mass index and endometriosis among Iranian fertile women. Gynecol Endocrinol. 2016;32:157–160. doi: 10.3109/09513590.2015.1101439. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;121:94–98. doi: 10.1016/j.ejogrb.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril. 2005;84:1366–1374. doi: 10.1016/j.fertnstert.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KW, Shin JH, Park MS, Kim T, Kim SH, Hur JY. Association of body mass index with severity of endometriosis in Korean women. Int J Gynaecol Obstet. 2009;105:39–42. doi: 10.1016/j.ijgo.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Della Torre S, Rando G, Meda C, Stell A, Chambon P, Krust A, Ibarra C, Magni P, Ciana P, Maggi A. Amino acid-dependent activation of liver estrogen receptor alpha integrates metabolic and reproductive functions via IGF-1. Cell Metab. 2011;13:205–214. doi: 10.1016/j.cmet.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Koliaki C, Roden M. Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol Cell Endocrinol. 2013;379:35–42. doi: 10.1016/j.mce.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Owen OE, Reichard GA, Jr., Patel MS, Boden G. Energy metabolism in feasting and fasting. Adv Exp Med Biol. 1979;111:169–188. doi: 10.1007/978-1-4757-0734-2_8. [DOI] [PubMed] [Google Scholar]
- van den Berghe G. The role of the liver in metabolic homeostasis: implications for inborn errors of metabolism. J Inherit Metab Dis. 1991;14:407–420. doi: 10.1007/BF01797914. [DOI] [PubMed] [Google Scholar]
- Elam MB, Cowan GS, Jr, Rooney RJ, Hiler ML, Yellaturu CR, Deng X, Howell GE, Park EA, Gerling IC, Patel D, Corton JC, Cagen LM, et al. Hepatic gene expression in morbidly obese women: implications for disease susceptibility. Obesity (Silver Spring) 2009;17:1563–1573. doi: 10.1038/oby.2009.49. [DOI] [PubMed] [Google Scholar]
- Elam MB, Yellaturu C, Howell GE, Deng X, Cowan GS, Kumar P, Park EA, Hiler ML, Wilcox HG, Hughes TA, Cook GA, Raghow R. Dysregulation of sterol regulatory element binding protein-1c in livers of morbidly obese women is associated with altered suppressor of cytokine signaling-3 and signal transducer and activator of transcription-1 signaling. Metabolism. 2010;59:587–598. doi: 10.1016/j.metabol.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlberg D, Rudling M, Angelin B, Bjorkhem I, Forsell P, Nilsell K, Einarsson K. Hepatic cholesterol metabolism in human obesity. Hepatology. 1997;25:1447–1450. doi: 10.1002/hep.510250623. [DOI] [PubMed] [Google Scholar]
- Lousse JC, Van Langendonckt A, Defrere S, Ramos RG, Colette S, Donnez J. Peritoneal endometriosis is an inflammatory disease. Front Biosci. 2012;4:23–40. doi: 10.2741/e358. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Kane MA, Sidell N. Pathogenesis of Endometriosis: roles of retinoids and inflammatory pathways. Semin Reprod Med. 2015;33:246–256. doi: 10.1055/s-0035-1554920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli GM, Abrao MS, Mechsner S. Can chemokines be used as biomarkers for endometriosis? A systematic review. Hum Reprod. 2014;29:253–266. doi: 10.1093/humrep/det401. [DOI] [PubMed] [Google Scholar]
- Naqvi H, Mamillapalli R, Krikun G, Taylor HS. Endometriosis located proximal to or remote from the uterus differentially affects uterine gene expression. Reprod Sci. 2016;23:186–191. doi: 10.1177/1933719115613449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N, Farrelly D, Hagan D, Hillyer D, Arbeeny C, Sabrah T, Treloar A, Brown K, Kalinowski S, Mookhtiar K. Expression of human hepatic glucokinase in transgenic mice liver results in decreased glucose levels and reduced body weight. Diabetes. 1997;46:11–16. doi: 10.2337/diab.46.1.11. [DOI] [PubMed] [Google Scholar]
- Baudrand R, Carvajal CA, Riquelme A, Morales M, Solis N, Pizarro M, Escalona A, Boza C, Perez G, Dominguez A, Arrese M, Fardella CE. Overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in hepatic and visceral adipose tissue is associated with metabolic disorders in morbidly obese patients. Obes Surg. 2010;20:77–83. doi: 10.1007/s11695-009-9937-0. [DOI] [PubMed] [Google Scholar]
- Torrecilla E, Fernandez-Vazquez G, Vicent D, Sanchez-Franco F, Barabash A, Cabrerizo L, Sanchez-Pernaute A, Torres AJ, Rubio MA. Liver upregulation of genes involved in cortisol production and action is associated with metabolic syndrome in morbidly obese patients. Obes Surg. 2012;22:478–486. doi: 10.1007/s11695-011-0524-9. [DOI] [PubMed] [Google Scholar]
- Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod. 2009;80:79–85. doi: 10.1095/biolreprod.108.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Barr A, Manning DG. Proteins Techniques of Analysis Boca Raton, FL: CRC Press, Inc; 1999. 227 245 [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:298–306. doi: 10.1016/S2213-8587(13)70200-6. [DOI] [PubMed] [Google Scholar]
- Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, Silecchia G, Leonetti F, Fraioli A, Picardi A, Morini S, Cavallo MG. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180–2187. doi: 10.1002/hep.25930. [DOI] [PubMed] [Google Scholar]
- Syamsunarno MR, Iso T, Hanaoka H, Yamaguchi A, Obokata M, Koitabashi N, Goto K, Hishiki T, Nagahata Y, Matsui H, Sano M, Kobayashi M, et al. A critical role of fatty acid binding protein 4 and 5 (FABP4/5) in the systemic response to fasting. PLoS One. 2013;8:e79386. doi: 10.1371/journal.pone.0079386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XS, Brondyk W, Lydon JT, Thurberg BL, Piepenhagen PA. Biotherapeutic target or sink: analysis of the macrophage mannose receptor tissue distribution in murine models of lysosomal storage diseases. J Inherit Metab Dis. 2011;34:795–809. doi: 10.1007/s10545-011-9285-9. [DOI] [PubMed] [Google Scholar]
- Park CJ, Karrar E, Chen P, Liao JK. Abstract 19339: ROCK2 mediates the uptake of fatty acids through the transcriptional regulation of CD36. Circulation. 2014;130:A19339. [Google Scholar]
- Chen P, Park C, Karrar E, Wang C, Liao J. Abstract 19727: ROCK2 mediates browning of white fat and protects against obesity. Circulation. 2014;130:A19727. [Google Scholar]
- Wang C, Liao JK. Abstract 2268: altered basal metabolism and increased obesity in mice with targeted deletion of rho-associated coiled-coil forming kinase (ROCK)-2. Circulation. 2009;120:S613. [Google Scholar]
- Wang C, Liao JK. Abstract 1426: decrease basal metabolism and energy expenditure and increase obesity in mice with targeted deletion of ROCK2. Circulation. 2008;118:S323. [Google Scholar]
- Kotronen A, Lewitt M, Hall K, Brismar K, Yki-Jarvinen H. Insulin-like growth factor binding protein 1 as a novel specific marker of hepatic insulin sensitivity. J Clin Endocrinol Metab. 2008;93:4867–4872. doi: 10.1210/jc.2008-1245. [DOI] [PubMed] [Google Scholar]
- Jin T, Xu D, Ding Q, Zhang Y, Mao C, Pan Y, Wang Z, Chen Y. Identification of the topology and functional domains of PAQR10. Biochem J. 2012;443:643–653. doi: 10.1042/BJ20112105. [DOI] [PubMed] [Google Scholar]
- Dorjgochoo T, Shi J, Gao YT, Long J, Delahanty R, Xiang YB, Cai Q, Shu XO. Genetic variants in vitamin D metabolism-related genes and body mass index: analysis of genome-wide scan data of approximately 7000 Chinese women. Int J Obes (Lond) 2012;36:1252–1255. doi: 10.1038/ijo.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Park CY, Han SN. High fat diet-Induced obesity alters vitamin D metabolizing enzyme expression in mice. Biofactors. 2015;41:175–182. doi: 10.1002/biof.1211. [DOI] [PubMed] [Google Scholar]
- Makowski L, Hotamisligil GS. Fatty acid binding proteins–the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004;134:2464S–2468S. doi: 10.1093/jn/134.9.2464S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Postigo M, Queipo-Ortuno MI, Fernandez-Garcia D, Gomez-Huelgas R, Tinahones FJ, Cardona F. Adipose tissue gene expression of factors related to lipid processing in obesity. PLoS One. 2011;6:e24783. doi: 10.1371/journal.pone.0024783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akyurek N, Aycan Z, Cetinkaya S, Akyurek O. Yilmaz Agladioglu S, Ertan U. Peroxisome proliferator activated receptor (PPAR)-gamma concentrations in childhood obesity. Scand J Clin Lab Invest. 2013;73:355–360. doi: 10.3109/00365513.2013.786121. [DOI] [PubMed] [Google Scholar]
- Lagou V, Scott RA, Manios Y, Chen TL, Wang G, Grammatikaki E, Kortsalioudaki C, Liarigkovinos T, Moschonis G, Roma-Giannikou E, Pitsiladis YP. Impact of peroxisome proliferator-activated receptors gamma and delta on adiposity in toddlers and preschoolers in the GENESIS Study. Obesity (Silver Spring) 2008;16:913–918. doi: 10.1038/oby.2008.1. [DOI] [PubMed] [Google Scholar]
- Li X, Lindquist S, Angsten G, Yi J, Olsson T, Hernell O. Adiponectin and peroxisome proliferator-activated receptor gamma expression in subcutaneous and omental adipose tissue in children. Acta Paediatr. 2008;97:630–635. doi: 10.1111/j.1651-2227.2008.00715.x. [DOI] [PubMed] [Google Scholar]
- Boon MR, Bakker LE, Haks MC, Quinten E, Schaart G, Van Beek L, Wang Y, Van Schinkel L, Van Harmelen V, Meinders AE, Ottenhoff TH, Van Dijk KW, et al. Short-term high-fat diet increases macrophage markers in skeletal muscle accompanied by impaired insulin signalling in healthy male subjects. Clin Sci (Lond) 2015;128:143–151. doi: 10.1042/CS20140179. [DOI] [PubMed] [Google Scholar]
- Pan X, Wang P, Luo J, Wang Z, Song Y, Ye J, Hou X. Adipogenic changes of hepatocytes in a high-fat diet-induced fatty liver mice model and non-alcoholic fatty liver disease patients. Endocrine. 2015;48:834–847. doi: 10.1007/s12020-014-0384-x. [DOI] [PubMed] [Google Scholar]
- Lim U, Turner SD, Franke AA, Cooney RV, Wilkens LR, Ernst T, Albright CL, Novotny R, Chang L, Kolonel LN, Murphy SP, Le Marchand L. Predicting total, abdominal, visceral and hepatic adiposity with circulating biomarkers in Caucasian and Japanese American women. PLoS One. 2012;7:e43502. doi: 10.1371/journal.pone.0043502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Liu B, Zhang N, Liu Z, Liang D, Li F, Cao Y, Feng X, Zhang X, Yang Z. Magnolol inhibits lipopolysaccharide-induced inflammatory response by interfering with TLR4 mediated NF-kappaB and MAPKs signaling pathways. J Ethnopharmacol. 2013;145:193–199. doi: 10.1016/j.jep.2012.10.051. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Goodson S, Halford JC. Regulation of appetite: role of leptin in signalling systems for drive and satiety. Int J Obes Relat Metab Disord. 2001;25(suppl 1):S29–34. doi: 10.1038/sj.ijo.0801693. [DOI] [PubMed] [Google Scholar]
- DePaoli AM. 20 years of leptin: leptin in common obesity and associated disorders of metabolism. J Endocrinol. 2014;223:T71–81. doi: 10.1530/JOE-14-0258. [DOI] [PubMed] [Google Scholar]
- Janani C. Ranjitha Kumari BD. PPAR gamma gene–a review. Diabetes Metab Syndr. 2015;9:46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Nacul AP, Lecke SB, Edelweiss MI, Morsch DM, Spritzer PM. Gene expression of leptin and long leptin receptor isoform in endometriosis: a case-control study. Obstet Gynecol Int. 2013;2013:879618. doi: 10.1155/2013/879618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT, Dube M, Branscum AJ, Wong CP, Olson DA, Zhong X, Kweh MF, Larkin IV, Wronski TJ, Rosen CJ, Kalra SP, Iwaniec UT. Hypothalamic leptin gene therapy reduces body weight without accelerating age-related bone loss. J Endocrinol. 2015;227:129–141. doi: 10.1530/JOE-15-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chen Y, Kuang Y, Bagchi MK, Taylor RN, Katzenellenbogen JA, Katzenellenbogen BS. Multiple beneficial roles of repressor of estrogen receptor activity (REA) in suppressing the progression of endometriosis. Endocrinology. 2016;157:900–912. doi: 10.1210/en.2015-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Nie J, Liu X, Zheng Y, Guo SW, Trichostatin A. a histone deacetylase inhibitor, reduces lesion growth and hyperalgesia in experimentally induced endometriosis in mice. Hum Reprod. 2010;25:1014–1025. doi: 10.1093/humrep/dep472. [DOI] [PubMed] [Google Scholar]
- Chen QH, Zhou WD, Su ZY, Huang QS, Jiang JN, Chen QX. Change of proinflammatory cytokines follows certain patterns after induction of endometriosis in a mouse model. Fertil Steril. 2010;93:1448–1454. doi: 10.1016/j.fertnstert.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Markham SM. Extrapelvic endometriosis. In: EJ Thomas, Rock JA., editors. Modern Approaches to Endometriosis. Dordrecht: Springer Netherlands; 1991. pp. 151–182. In. (eds.) [Google Scholar]
- Liu K, Zhang W, Liu S, Dong B, Liu Y. Hepatic endometriosis: a rare case and review of the literature. Eur J Med Res. 2015;20:48. doi: 10.1186/s40001-015-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr S, Naqvi H, Komm B, Taylor HS. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology. 2014;155:1489–1497. doi: 10.1210/en.2013-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli P, Marcellin L, Tosti C, Chouzenoux S, Cerles O, Borghese B, Batteux F, Chapron C. MAP kinases and the inflammatory signaling cascade as targets for the treatment of endometriosis? Expert Opin Ther Targets. 2015;19:1465–1483. doi: 10.1517/14728222.2015.1090974. [DOI] [PubMed] [Google Scholar]
- Ural UM, Tekin YB, Cure M, Sahin FK. Serum YKL-40 levels as a novel marker of inflammation in patients with endometriosis. Clin Exp Obstet Gynecol. 2015;42:495–497. [PubMed] [Google Scholar]
- Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril. 2015;103:1252–1260. doi: 10.1016/j.fertnstert.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]