Abstract
There is considerable evidence that implicates oxidative stress in the pathophysiology of human pregnancy complications. However, the role and the mechanism of maintaining an antioxidant prosurvival uterine environment during normal pregnancy is largely unresolved. Herein we report that the highly active uterine unfolded protein response plays a key role in promoting antioxidant activity in the uterine myocyte across gestation. The unfolded protein response (UPR) senses the accumulation of misfolded proteins in the endoplasmic reticulum (ER) and activates a signaling network that consists of the transmembrane protein kinase eukaryotic translation initiation factor 2 alpha kinase 3/PKR-like-ER kinase (EIF2AK3), which acts to decrease protein translation levels, allowing for a lowered need for protein folding during periods of ER stress. However, independent of its translational regulatory capacity, EIF2AK3-dependent signals elicit the activation of the transcription factor, nuclear factor erythroid 2-like 2 (NFE2L2) in response to oxidative stress. NFE2L2 binds to antioxidant response elements in the promoters of a variety of antioxidant genes that minimize the opportunities for generation of reactive oxygen intermediates. Our analysis demonstrates that in the absence of EIF2AK3, the uterine myocyte experiences increased levels of reactive oxygen species due to decreased NFE2L2 activation. Elevated levels of intracellular reactive oxygen species were observed in the EIF2AK3 null cells, and this was associated with the onset of apoptotic cell death. These findings confirm the prosurvival and antioxidant role of UPR-mediated EIF2AK3 activation in the context of the human uterine myocyte.
Keywords: oxidative stress, pregnancy, unfolded protein response, uterus
INTRODUCTION
During pregnancy, the uterus adapts to many endogenous and exogenous stimuli, including increased exposure to elevated reactive oxygen species (ROS) associated with increasing gestational length [1]. As apoptotic cell death is the consequence of an unresolved oxidative stress (OS), a phenomenon that the pregnant uterus actively avoids [2, 3], we aimed to identify the adaptive mechanisms facilitating the resolution of uterine OS challenges during pregnancy. Recently, our group demonstrated that activation of the uterine endoplasmic reticulum (ER) unfolded protein response (UPR) allows the uterine myocyte to maintain a quiescent phenotype during potential tocolytic challenges [4]. In this study, we reveal that the uterine UPR also acts as a critical mediator in the myometrial antioxidant response.
Several events during pregnancy, such as hypoxia, implantation, angiogenesis, and parturition, generate excessive free radicals and thus lead to OS. An array of uterine antioxidant systems promotes the myocyte's ability to avoid disruption of the redox balance, which affects multiple physiological processes, including pregnancy, normal parturition, and preterm labor [1, 5–8]. Furthermore, nonenzymatic antioxidant levels are decreased in women with pregnancy complications, such as preterm birth, pre-eclampsia, and small-for-gestational-age neonates [9]. Despite the evidence indicating that an effective antioxidant response is very important during pregnancy, little is known about the active mechanism regulating the uterine myocyte antioxidant response. Numerous studies have defined active cross talk between the UPR and OS. Locally, within the ER, the maintenance of redox homeostasis is critical for folding and modification of transmembrane and secreted proteins. The protein-folding process requires an oxidized environment, which is provided by the ER to allow appropriate disulfide bond formation [10]. However, elevated ER stress has been described to give rise to increased intracellular ROS production as a by-product of accelerated oxidative protein folding, further highlighting the association between the maintenance of ER homeostasis and generation of ROS [11–13]. ROS and OS have also been demonstrated to be upstream regulators of the UPR, where it has been demonstrated that increased exposure to extracellular ROS activates the UPR [14]. During the activation of the UPR, the eukaryotic translation initiation factor 2 alpha kinase 3 (EIF2AK3) mediates attenuation of protein translation, reducing the ER protein load and thereby allowing luminal homeostasis to be regained [15, 16]. EIF2AK3 also directly activates the antioxidant response. EIF2AK3, through the site-specific phosphorylation of the transcription factor, nuclear factor erythroid 2-like 2 (NFE2L2/NRF2) [17], promotes its nuclear translocation and its action as the master regulator of the antioxidant response [13]. Phosphorylated NFE2L2 (pNFE2L2) acts as a transcription factor that binds to the antioxidant response elements on the promoters of antioxidant genes, such as NAD(P)H quinone dehydrogenase 1 (NQO1). Consequently, in the absence of EIF2AK3, cells experience constitutive OS and cell death [18, 19].
Briefly, our analysis demonstrates in both the pregnant mouse and the human uterus that there is an elevation in local antioxidant activity associated with an increase in the UPR-mediated EIF2AK3 activation. In vitro analysis of the uterine myocyte hTERT-HM cell line revealed that in the absence of EIF2AK3, activation of NFE2L2 was ablated, as was its downstream antioxidant target NQO1, resulting in increased ROS accumulation and cell death. These data demonstrate that the uterine myocyte utilizes the UPR in an adaptive prosurvival manner to modulate its antioxidant responses in order to avoid ROS accumulation and apoptotic cell death.
MATERIALS AND METHODS
Cell Culture
The human telomerase immortalized uterine myometrial cell line (hTERT-HM) derived from premenopausal nonpregnant uterine tissue was utilized as our in vitro cell culture model system [20]. Cells were cultured in DMEM/F12 (Invitrogen) with 10% (vol/vol) fetal bovine serum (FBS; Invitrogen) supplemented with antibiotic/antimycotic (10 000 U/ml; Invitrogen) at 37°C in a 5% CO2 incubator. All hTERT-HM experiments and analyses represent a minimum of three individual experiments performed on separate occasions (n = 3).
Mice and Uterine Tissue Collection
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University. Timed-pregnant female CD-1 mice (6–8 wk old) were obtained from Charles River and housed according to IACUC guidelines. Uterine tissues (n = 3 for each gestational time point) were harvested between 0800 and 1000 h on E1, E2, E3, E4, E5, E6, E8, E10, E11, E12, E13, E15, E16, E17, E18, and E19 as described previously [4] and flash frozen in liquid nitrogen and stored at −80°C. The flash-frozen pellets were utilized for subsequent mRNA and protein analyses.
Human Subjects and Tissue Acquisition
Myometrial tissues were collected from pregnant women undergoing cesarean hysterectomy (Institutional Review Board [IRB] protocol no. PRO07040011, approval date/14 January 2014). Informed consent was obtained in writing from each woman before surgery using protocols approved by the University of Pittsburgh's IRB. The tissues are acquired through the Department of Obstetrics and Gynecology at the University of Pittsburgh Medical Center.
Myometrial biopsy specimens were collected from two groups of subjects: pregnant nonlaboring women who underwent scheduled cesarean hysterectomy between 32 and 34 wk of gestation due to the presence of placenta accreta with no evidence of infection and pregnant nonlaboring women who underwent a scheduled elective cesarean between 39 and 42 wk of gestation. Myometrial smooth muscle was dissected from each biopsy specimen, flash frozen in liquid nitrogen, and stored at −80°C for subsequent protein analysis.
siRNA Transfection
The hTERT-HM cells were reverse transfected [2] on a six-well plate using Lipofectamine 2000 (Invitrogen). Briefly, 5 nM of scrambled (#4390893) or EIF2AK3 siRNA (CAACAAGAAU AUCCGCAAAtt) from Ambion silencer (#4390824) select (Life Technologies) and 6 μl of Lipofectamine 2000 was used per well of a six-well dish. Control cell groups consisted of the untransfected (U), mock transfected (M), and scrambled siRNA transfected (S).
Tunicamycin Treatment
Tunicamycin (TM) purchased from Calbiochem (catalog no. 654380) was dissolved in 20 μl of 10 M sodium hydroxide. DMEM-F12 medium with 10% FBS was added to obtain a concentration of 1 μg/μl. For the EIF2AK3 knockdown experiments, hTERT-HM cells were treated with 5 μg/ml of TM or vehicle in DMEM-F12 medium with 10% FBS for 1 h after 24 h of transfection with siRNA. The medium was replaced after 1 h of treatment, and cells were harvested after 36 h for Western blotting and quantitative PCR (qPCR) analysis.
Whole Cell Extracts.
Whole cell extracts were made in RIPA buffer (150 mM NaCl, 1% TritonX-100, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA, 50 mM Tris [pH 8.0], and protease and phosphatase inhibitors). Protein estimation was performed using the BCA method, and equal amounts of protein were used for Western blotting.
Nuclear and Cytosol Fractionation from Cells and Tissues
Cytoplasmic and nuclear protein extracts were prepared from frozen mouse and human uterine tissues. Briefly, myometrial tissue was pulverized in liquid nitrogen and homogenized in ice-cold NE1 buffer (10 mM Hepes [pH 7.5], 10 mM MgCl2, 5 mM KCL, and 0.1% TritonX-100) and protease/phosphatase inhibitor cocktail using an IKA homogenizer (IKA Works Inc.). For extraction from hTERT-HM cells, the cells were pelleted at 1000 × g for 3 min, and the cell pellet was suspended in NE1 buffer and passed 15 times through a 23-gauge needle. The homogenate (both cells and tissue) was centrifuged at 2600 × g for 10 min at 4°C, and the supernatant was retained as the cytoplasmic fraction. The nuclear pellet was washed once in NE1 and then resuspended in ice-cold NE2 buffer (25% glycerol, 20 mM HEPES [pH 7.9], 500 mM NaCl, 1.5 mM MgCl2, and 0.2 mM EDTA [pH 8.0]) with protease/phosphatase inhibitor cocktail through vigorous mixing. The resuspended fraction was incubated at 4°C with vigorous shaking for 15 sec at 1400 rpm every 5 min on an Eppendorf thermomixer for 1 h. The nuclear extract was then centrifuged at 10 600 × g for 10 min at 4°C to remove nuclear debris. The supernatant was retained as the nuclear fraction. The endogenous cellular localization of proteins examined in this study dictated the cellular compartment isolated and assessed by immunoblotting.
Nuclear receptor coactivator (NCOA3) and protein disulfide isomerase family a, member 2 (PDIA2), were utilized to ensure equal loading and purity of the isolated fractions. Previous analysis from our laboratory has determined that PDIA2 and NCOA3 expression levels in the cytosolic and nuclear fraction, respectively, in the pregnant mouse and human uterine tissues isolated from multiple gestational time points and the hTERT cell under all conditions examined to date retain stable expression levels relative to subcellular protein concentrations [4, 21].
Immunoblotting and Densitometric Analysis
NuPAGE precast 4%–12% gradient gels (Life Technologies) were used for electrophoresis, and protein was transferred onto Hybond-P polyvinylidene fluoride (PVDF) membranes (Millipore). The PVDF membrane was blocked in 3% nonfat dried milk prepared in Tris-buffered saline with 0.1% Tween-20 for 1 h at room temperature and then incubated with the primary antibodies overnight at 4°C. This was followed by incubation with horseradish peroxidase-conjugated secondary antibodies diluted in 5% nonfat dried milk-TBS-T buffer. Immunoreactive bands were visualized using an ECL detection system (ThermoScientific). The concentrations of primary antibodies and their sources are as follows: 78 kDa glucose regulated protein (GRP78; 1:1000, #CST-9271) growth arrest and DNA damage inducible protein 152 (GADD153; 1:500, #CST-5554), caspase 3 (CASP3; 1:200, #CST-9662), poly ADP ribose polymerase (PARP; 1:200, #CST-5625), EIF2AK3 (1:250, #CST-3192), pEIF2AK3 (1:250, #CST-3179), Cl.CASP3 (1:500, #CST-9664), NFE2L2 (1:250, #CST-12721), NQO1 (1:5000, #CST-3187), NF kappa B p65 subunit (p65; 1:500 #CST-3034), and PDIA2 (1:10000, #3501), were obtained from Cell Signaling Technology. NCOA3 (1:5000, #PA-1845) was obtained from ThermoFisher Scientific, and pNFE2L2 (1:250, ab76026) was obtained from Abcam. The immunoreactive bands were quantified using ImageJ software and normalized to NCOA3 and PDIA2 for nuclear and cytosolic fractions, respectively.
Measurement of ROS Production
2′,7′-Dichloro fluorescein diacetate (DCFDA) (#D6883) purchased from Sigma-Aldrich was used for measuring ROS in the hTERT-HM cells. Briefly, cells were reverse transfected with 5 nM siRNA for 24 h and then treated with TM (5 μg/ml) or vehicle for 1 h in DMEM-F12 medium with 10% FBS. After TM treatment, the cells were left in fresh medium for 24 h. Cells were then treated with DCFDA (5 μM) in Locke solution (154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 2.0 mM CaCl2, 10 mM d-glucose, 5 mM HEPES [pH 7.4]) for 45 min. After treatment, the cells were washed twice with Locke solution and then lysed with ice-cold RIPA buffer in the dark for 20 min. The lysate was spun for 10 min at 10 600 × g to remove cell debris, and the supernatant was used to measure fluorescence of oxidized dichlorofluorescein at an excitation wavelength of 480 nm and an emission wavelength of 535 nm.
RNA Isolation cDNA Synthesis and qPCR
RNA was isolated from uterine tissues and hTERT-HM cells using the RNeasy Mini Kit (Qiagen Inc.) according to the manufacturer's instructions. Total RNA was converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Life Technologies) Primers were designed using MacVector to obtain amplicons from 80 to 150 bp. (See Table 1 for detailed primer information.) Real-time qPCR was performed on the CFX384 Touch Real-Time System (BioRad Laboratories Inc.) using a SYBR Green PCR Master Mix (Life Technologies). For each reaction, 10 ng of cDNA and a final primer concentration of 300 nM were used. Rplp0 was used as a housekeeping gene for mouse tissue and human tissues and cells [2, 21]. Samples were assayed in triplicate (n = 3 for each gestational time point) for mice; for humans, we used n = 5 for term and n = 7 for preterm samples. Expression data were analyzed using the ΔΔCt method.
TABLE 1. .
Human and mouse qPCR primers.
Methylthiazolyldiphenyl-Tetrazolium Assay
A 5-mg/ml stock of methylthiazolyldiphenyl-tetrazolium (MTT) purchased from Sigma-Aldrich (#M5655) was made in pure distilled water. The hTERT-HM cells were untransfected, mock transfected, and reverse transfected with scrambled or EIF2AK3 siRNA for 24 h and treated with TM for 1 h. After 24 and 36 h, 0.5 mg/ml of MTT was added to the cells and incubated in the dark for 3 h. After 3 h, the MTT crystals were dissolved in 0.5 ml of a DMSO/isopropanol (1:1) mixture, and the absorbance was read at 570 nm. The readings were normalized to the untreated control values to get the graph.
TUNEL Staining
The hTERT-HM cells were grown on chamber slides and fixed with 4% paraformaldehyde for 25 min at room temperature and stored in PBS at 4°C. Cells were permeabilized with 0.2% Triton-X in PBS and stained with a fluorometric TUNEL assay as per the manufacturer's instructions (#G3250; Promega).
Statistical Analyses
Values are expressed as mean ± SEM. Student t-test was utilized to ascertain the significance of comparison between two groups on the variables (TM addition) tested, while one-way ANOVA, followed by Bonferroni post hoc analysis, was used to determine the significance between multiple groups. P ≤ 0.05 was considered to indicate statistical significance.
RESULTS
EIF2AK3 Inhibition Suppresses the Human Uterine Myocyte UPR
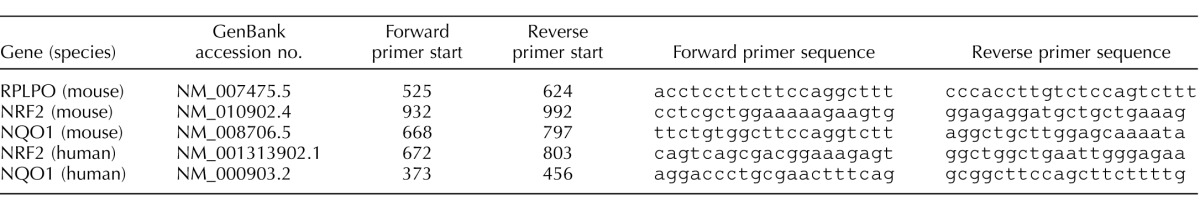
The ability of the uterine myocyte to respond to an ER stress-mediated event in the absence or presence of EIF2AK3 was examined. The siRNA transfection of the telomerase immortalized human uterine myocyte cell line hTERT-HM was utilized to abolish EIF2AK3 signaling. As can be observed in Figure 1A, siRNA mediated EIF2AK3 knockdown was confirmed by Western blot analysis in both the presence and the absence of TM. TM exposure in the U-, M-, and S-transfected hTERT-HM cells hosted the expected UPR with the appearance of an 80-fold elevation in GADD153 and GRP78 levels when compared to those cells not exposed to TM. However, in the absence of EIF2AK3, the UPR was compromised, as indicated by an approximate 50-fold decline in GRP78 and ablation of GADD153 levels in comparison to the TM-treated mock and scrambled siRNA-treated hTERT-HM cells (Fig. 1B).
FIG. 1.
EIF2AK3 knockdown suppresses the uterine myocyte UPR. A) Western blot analysis of the human uterine myocyte hTERT-HM cell line demonstrates successful siRNA-mediated EIF2AK3 ablation (Pi) in comparison to untransfected (U), mock-transfected (M), and scrambled siRNA-transfected (S) cells. In the presence of a TM-induced ER stress, phosphorylated levels of nuclear EIF2AK3 were diminished in the absence of EIF2AK3, as were the UPR mediators GRP78 and GADD153. PDIA2 and NCOA3 act as cytoplasmic and nuclear protein loading controls, respectively. B) Relative optical density (ROD) was analyzed for each protein and normalized to its respective loading control. All experiments were performed in triplicate (n = 3). Student t-test was used for statistical comparison between the S and Pi ± TM samples. *P < 0.05, **P < 0.001.
EIF2AK3 Acts as a Mediator of Prosurvival Signaling in the Human Uterine Myocyte
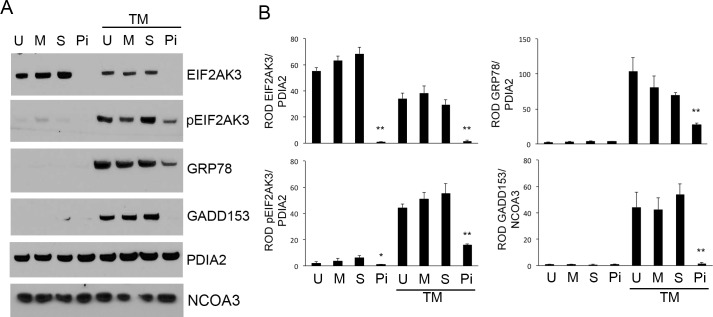
In the presence of TM, hTERT-HM cells devoid of EIF2AK3 displayed increased levels of CASP3 activation, as indicated by the appearance of cleaved active CASP3 proteins observed at 17 and 14 kDa (Fig. 2). TM-activated CASP3 in the absence of EIF2AK3 was also associated with apoptotic consequences, as indicated by the elevated levels of PARP cleavage. U-, M-, S-, and EIF2AK3 siRNA-treated hTERT-HM cells in the absence of TM and U-, S-, and M-transfected hTERT-HM cells in the presence of TM demonstrate no significant increase in CASP3 activation or PARP cleavage. A colorimetric MTT assay for assessing changes in TM-treated hTERT-HM cell metabolic activity, reflecting the number of remaining viable cells, was performed in the presence or absence of EIF2AK3. As can be observed in Figure 3, the TM-treated hTERT-HM cells demonstrated an approximate 18% and 58% decrease in cell viability in the absence of EIF2AK3 24 and 36 h post-TM exposure in comparison to the mock and scrambled siRNA-treated hTERT-HM cells. TUNEL analysis further confirmed increased apoptotic cell death in the TM-treated hTERT-HM cells in the absence of EIF2AK3 (Supplemental Figure S1; Supplemental Data are available online at www.biolreprod.org).
FIG. 2.
EIF2AK3 knockdown promotes ER stress-induced apoptotic CASP3 activation. A) Western blot analysis of the EIF2AK3 null human uterine myocyte hTERT-HM cell line (Pi) demonstrates robust activation of CASP3 and PARP cleavage in the presence of a TM-induced ER stress. In the presence of endogenous EIF2AK3 (U, M, S), minimal CASP3 activation and PARP cleavage was observed. PDIA2 and NCOA3 act as cytoplasmic and nuclear protein loading controls, respectively. B) Relative optical density (ROD) was analyzed for each protein and normalized to its respective loading control. All experiments were performed in triplicate (n = 3). Student t-test was used for statistical comparison between the S and Pi ± TM samples. **P < 0.001.
FIG. 3.
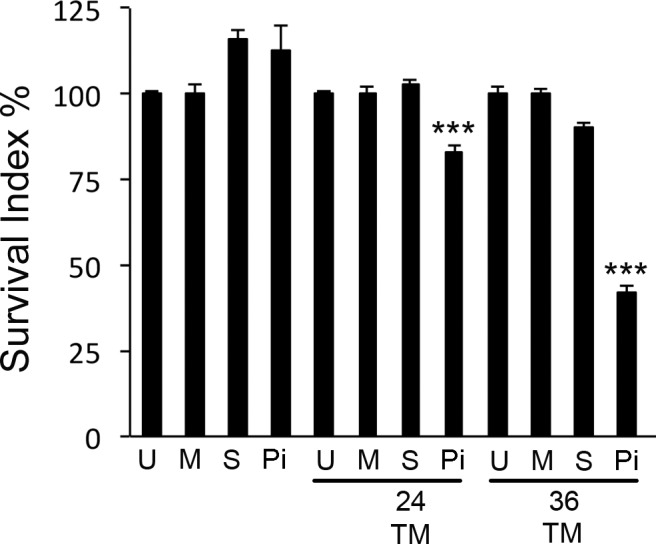
Decreased cell viability is associated with ER stress in the absence of EIF2AK3. Colorimetric MTT assay demonstrated decreased numbers of viable cells in the TM-treated EIF2AK3 null hTERT-HM cell; 24 and 36 h post-TM exposure, cell viability had decreased by approximately 18% and 58%, respectively, in comparison to U-, M-, or S-treated hTERT-HM's and those cells not exposed to TM. All experiments were performed in triplicate (n = 3). Student t-test was used for statistical comparison between the S and Pi ± TM samples. ***P < 0.0001
Decreased Antioxidant Responses in the Human Uterine Myocyte upon EIF2AK3 Knockdown
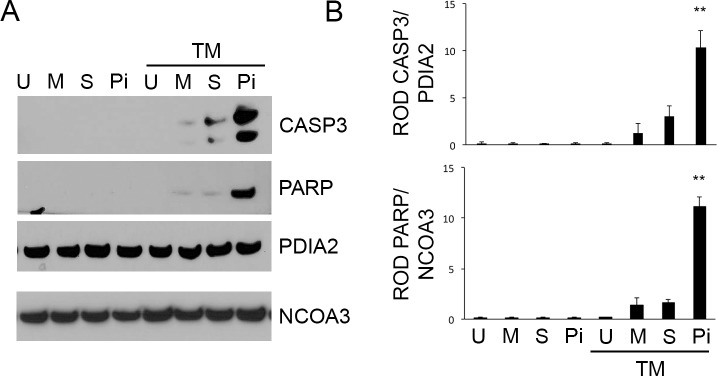
The uterine myocyte ER stress-induced antioxidant response was examined in the hTERT-HM cell in the absence of presence of EIF2AK3. As can be observed in Figure 4, A and B, in the presence of TM, NFE2L2, which acts as a master regulator of antioxidant gene expression, is highly activated, as is indicated by the increased levels of pNFE2L2 in the nuclear fraction of the hTERT-HM cell in comparison to the vehicle-treated hTERT-HM cells. However, in the absence of EIF2AK3, NFE2L2 fails to be activated post-TM exposure. NQO1, a key antioxidant enzyme and a direct downstream target for NFE2L2, is also upregulated on TM exposure; however, in the absence of EIF2AK3, NQO1 remains at basal levels. The mRNA analysis was performed on the TM- and vehicle-treated hTERT-HM cells in the absence and presence of EIF2AK3. As can be observed in Figure 4C, in the absence of EIF2AK3 transcript levels, NFE2L2 and NQO1 were reduced in the absence and presence of TM exposure. Interestingly, basal levels of NF-κB activation were found to be increased in the absence of EIF2AK3, though this was not enhanced by exposure to TM (Supplemental Figure S2).
FIG. 4.
EIF2AK3 knockdown impairs the uterine myocyte antioxidant response. A) Western blot analysis of nuclear levels of pNFE2L2 and cytoplasmic levels of NQO1 were examined in the U-, M-, S-, and Pi-treated hTERT-HM cell line ± TM exposure. On TM exposure, elevated levels of the antioxidant genes pNFE2L2 and NQO1 were observed in the U, M, and S uterine myocytes. In the absence of endogenous EIF2AK3 (Pi) TM exposure, the uterine myocyte failed to host an antioxidant response, as indicated by the basal levels of pNFE2L2 and NQO1. PDIA2 and NCOA3 act as cytoplasmic and nuclear protein loading controls, respectively. B) Relative optical density (ROD) was analyzed for each protein and normalized to its respective loading control. All experiments were performed in triplicate (n = 3). Student t-test was used for statistical comparison between the S and Pi ± TM samples. *P < 0.05. C) qPCR analysis of the mRNA transcripts for NFE2L2 and NQO1 revealed, in the absence of endogenous EIF2AK3, an approximate 50% decline in transcript levels in the absence or presence of TM. RPLPO was utilized as the housekeeping gene, and each experiment was performed in triplicate (n = 3). Student t-test was used for statistical comparison between the S and Pi ± TM samples. **P < 0.001.
Increased OS Associated with EIF2AK3 Ablation in the Human Uterine Myocyte
Utilizing a DCFDA assay, live cells were assayed for hydroxyl, peroxyl, and other ROS activity within the hTERT-HM devoid of EIF2AK3 in the absence or presence of TM-induced ER stress 24 and 36 h post-TM treatment. As can be observed in Figure 5, DCFDA fluorescence demonstrates a 40% and 100% increase in ROS accumulation in the absence of EIF2AK3 at 24 and 36 h, respectively, post-TM exposure, in comparison to U-, M-, and S-, and siRNA-transfected hTERT-HM cells, where an intact antioxidant response observed in Figure 4 allows for appropriate resolution of TM-induced ROS accumulation (Fig. 5).
FIG. 5.
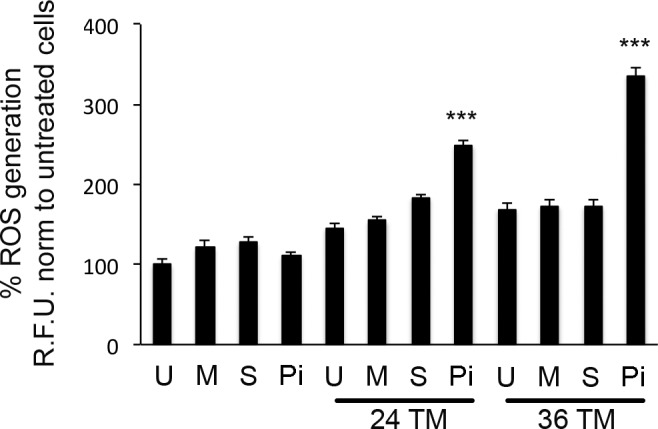
Increased OS associated with ER stress in the absence of EIF2AK3 action. DCFDA fluorescence in the EIF2AK3 null hTERT-HM cells (Pi) demonstrates a 40% and 100% increase in ROS accumulation at 24 and 36 h, respectively, post-TM exposure. The U, M, and S hTERT-HM cells did not demonstrate a significant increase in ROS accumulation in the absence or presence of TM. Relative DCFDA fluorescence was measured at excitation of 480 nm and emission of 535 nm (n = 5 for each condition). Student t-test was used for statistical comparison between S and Pi hTERT-HM cells exposed to TM. ***P < 0.0001.
Uterine pEIF2AK3 and Nuclear NFE2L2 Levels Are Activated Toward Term in the Pregnant Mouse and Human Uterus
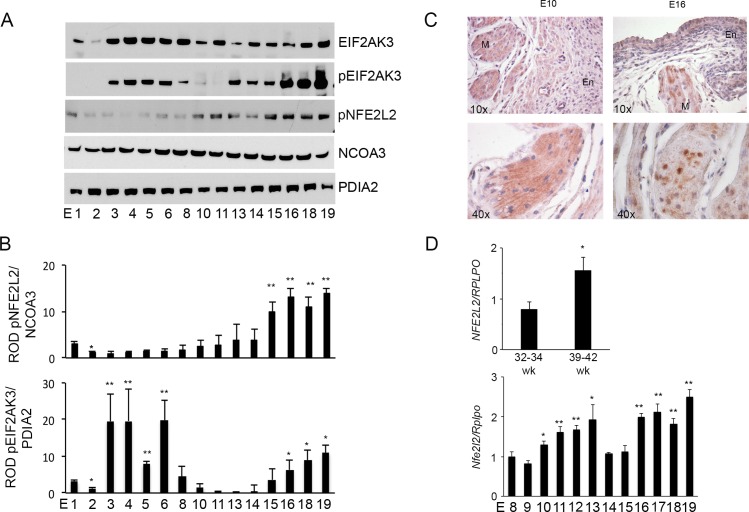
Nuclear and cytosolic proteins isolated from uterine tissues of pregnant mice from Gestation Day (E) 1 to term (E19) were examined for total EIF2AK3 and phosphorylated EIF2AK3 and nuclear NFE2L2 levels. As can be identified in Figure 6, A and B, total uterine EIF2AK3 levels are abundantly expressed across gestation after E2. In contrast, phosphorylated EIF2AK3 indicating activation of the UPR occurs during early gestation from E2 to E8 and from E11, reaching maximal levels at term. Nuclear translocation of NFE2L2 was significantly upregulated from E15 to term.
FIG. 6.
Increased antioxidant signaling in the pregnant mouse and human uterus toward term. A) Western blot analysis was performed on pregnant mouse uterus tissue from E1 to term (E19). Elevated pEIF2AK3 and pNFE2L2 levels were observed as term approached. PDIA2 and NCOA3 acted as cytoplasmic and nuclear protein loading controls, respectively. B) Relative optical density (ROD) was analyzed for each protein and normalized to its respective loading control. Three gestational series were examined (n = 3 for each gestational time point). One-way ANOVA was performed to determine statistical significance, and the Bonferroni post hoc test was used to compare E1 with other gestational age-groups. *P < 0.05, **P < 0.001. C) Immunohistochemical analysis of NFE2L2 at E10 demonstrates abundant cytoplasmic NFE2L2 in both the endometrium (En) and the myometrium (M), which translocated to the nuclear fraction with increasing gestational age, as demonstrated at E16. Nuclear translocation of NFE2L2 is isolated largely to the myometrial compartment and can be observed at E16, where endometrial NFE2L2 remain principally cytoplasmic. D) qPCR analysis of NFE2L2 in human myometrium isolated from pregnant nonlaboring women during their third trimester versus those at term demonstrates a significant increase toward term. RPLPO was utilized as the housekeeping gene (n = 7 myometrium isolated from nonlaboring women during their third trimester and n = 5 from nonlaboring women at term). Student t-test was used for statistical comparison between the 32- to 34-wk and the 39- to 42-wk myometrium. *P < 0.05. The qPCR analysis of Nfe2l2 in pregnant mouse uterus from E6 to term (E19) demonstrates a significant increase toward term. Rplpo was utilized as the housekeeping gene (n = 3 for each gestational time point). One-way ANOVA was performed to determine statistical significance, and the Bonferroni post hoc test was used to compare E8 with other gestational age groups. *P < 0.05, **P < 0.001.
Immunohistochemical analysis of NFE2L2 in the pregnant mouse uterus at E10 and E16 demonstrate its activation and consequent translocation to the nuclear fraction at E16. Nuclear translocation of NFE2L2 is isolated to the myometrial muscle bundles. Endometrial NFE2L2 was detectable throughout gestation, though it remains cytoplasmic and does not translocate to the nuclear compartment, suggesting that the endometrium and myometrium, though adjacent tissues, experience independent tissue-specific OS and antioxidant responses during pregnancy (Fig. 6C).
The mRNA analysis of NFE2L2 levels was examined in myometrial tissue isolated from pregnant nonlaboring women between 32 and 34 wk of gestation and at term between 39 and 42 wk of gestation and pregnant mice from E8 to term. A significant increase in NFE2L2 at the level of transcription in both preterm and term nonlaboring human myometrial samples was observed (Fig. 6D). Similarly, in the pregnant mouse uterus, an increase in NFE2L2 transcripts was observed toward term, suggesting in the pregnant mouse and human uterus a similar gestational profile of the myometrial antioxidant response that increases toward term.
DISCUSSION
We propose that the uterine myocyte across gestation utilizes the resident myometrial UPR to activate appropriate antioxidant responses in the face of ROS generation. Generation of ROS is a vital component and by-product of the aerobic metabolic processes of every normal healthy cell, and multiple lines of investigation have determined during pregnancy that there are elevated levels of ROS produced with increasing gestational length [1]. Generation of ROS, however, can be toxic, resulting in OS-induced cell damage that occurs as a result of increased production of free radicals in the context of an insufficient antioxidant defense. Serial measurements of OS markers in longitudinal studies during pregnancy has helped delineate the etiology of OS-related processes associated with spontaneous preterm birth (sPTB); for example, uterine heme oxygenase 1 activity increases across gestation and peaks at term [22]. In addition, 8-isoprostane, another stable marker of OS, has been examined in the urine and maternal plasma and serum of numerous studies, and its presence at higher levels has been associated with a significant trend for decreased gestational length, pre-eclampsia, preterm premature rupture of membranes, and sPTB [23].
Previous studies have determined that UPR activation is a critical component in the antioxidant response [17]. Our analysis is the first to examine the cross talk between the antioxidant response and the UPR during pregnancy and in the uterine myocyte. Activation of the uterine myocyte UPR signifies the detection of ER stress [24–28]. The ER allows for increased folding and processing of newly synthesized proteins required to accommodate the cellular adaptation to biochemical, hormonal, and mechanical stimuli. ERS signifies the stage when the protein-folding capacity of the ER has been overwhelmed and it can no longer maintain the required protein processing capacity due to prolonged or excessive provocations. The UPR alters transcriptional and translational profiles, allowing for adaptation to the accumulation of unfolded and misfolded proteins that accumulate in the ER due to ER stress, allowing homeostasis to be regained [16, 29–32]. Recently, we have described how activation of the uterine UPR occurs in the pregnant uterus in a gestationally regulated fashion [4]. We demonstrated that appropriate activation of the pregnant uterine UPR is key in determining an appropriate gestational length. In this study, we examined if the activation of the uterine myocyte UPR also regulates the adaptive process to increasing and accumulating levels of OS during pregnancy through regulation of the myometrial antioxidant response.
The UPR is comprised of three distinct though interacting networks that function in concert to deplete unfolded proteins from the lumen of the ER in order to regain homeostasis. With the accumulation of misfolded and unfolded proteins, the chaperone protein GRP78 is released from three transmembrane receptors, inositol-requiring 1 alpha (IRE1α), activating transcription factor 6 (ATF6), and EIF2AK3, to aid in proper protein folding. On release of GRP78, IRE1α, ATF6, and EIF2AK3 are activated, initiating the signal transduction pathways collectively termed the UPR [16, 29–33]. In this context, we have examined the role of EIF2AK3 in regulating uterine myocyte OS, as it has been previously been demonstrated in various other cell types to activate cellular antioxidant responses [17, 18, 34].
In order to determine the effects of the UPR on the myometrial antioxidant response, we utilized an in vitro siRNA approach to knock down uterine myocyte EIF2AK3 (Fig. 1). The hTERT-HM in vitro model afforded us the ability to examine the isolated uterine myocyte responses to in vitro UPR modulation; however, we do recognize that oxidative stress responses are regulated by the modality of the stress being seen by the cell and cell type. While the myometrium is composed primarily of uterine myocytes, there are a number of other cell types, including infiltrating macrophages and neutrophils, endothelial cells of the vasculature, and a variety of additional cell types. Therefore, we recognize that utilizing an in vitro model has its limitations, as any cell-to-cell interaction that may combat or enhance an oxidative stress response is lost.
However, in the context of the hTERT-HM uterine myocyte, EIF2AK3 siRNA was found to significantly reduce the levels of EIF2AK3. On exposure to a TM-induced ER stress, the EIF2KA3 null myocytes demonstrate repressed pEIF2AK3, GRP78, and GADD153 levels, signifying the inability of the uterine myocyte to host an appropriate UPR in the absence of EIF2AK3 (Fig. 1). The consequences of an inappropriate UPR are highlighted in Figure 2, which demonstrates that when the uterine myocyte is exposed to an ER stress in the absence of an intact UPR apoptotic, cell death ensues, as evidenced by increased CASP3 activation, PARP cleavage, and decreased cell viability in the absence of EIF2AK3 (Fig. 3). We speculated that apoptotic cell death in the ER-stressed EIF2AK3 null cell was likely due to a limited antioxidant response, resulting in elevated ROS and an unresolved OS. As can be clearly observed in Figure 4, NFE2L2, the master regulator of antioxidant responses and a direct substrate for pEIF2AK3, failed to accumulate in the nuclear fraction in the absence of EIF2AK3 (Pi), resulting in the incapacitation of the antioxidant response, as indicated by decreased protein and mRNA levels of the downstream target of NFE2L2, the antioxidant enzyme NQO1 (Fig. 4). As we hypothesized, a failure to host an appropriate UPR resulted in the accumulation of ROS, as demonstrated in Figure 5, limited only to the EIF2AK3 (Pi) null myocytes.
Evidence that the UPR-mediated mechanism of antioxidant activity in the uterine myocyte is physiologically relevant with respect to pregnancy is highlighted in Figure 6. In vivo, we observed evidence of a coordinated increase in both the UPR (pEIF2AK3) and the antioxidant response (pNFE2L2) in the pregnant mouse uterus toward term, suggesting that elevated oxidative stress-inducing events are associated with increasing gestational length. As observed in the pregnant uterus, both NFE2L2 protein and mRNA levels increase and become largely nuclear, indicating their activation of the myometrial antioxidant response toward term in the pregnant mouse uterus. In the human myometrium, an increase in NFE2L2 mRNA levels was also observed to term (Fig. 6D). Interestingly, NFE2L2 phosphorylation, activation, and consequent translocation to the nuclear fraction are isolated to the myometrial muscle bundles, suggesting that the uterine myocyte experiences increasing OS toward term in a tissue-specific manner. The endometrium of the pregnant uterus has detectable NFE2L2 throughout gestation, though it remains cytoplasmic and does not translocate to the nuclear compartment, suggesting that the endometrium and myometrium, though adjacent, experience tissue-specific OS and antioxidant responses (Fig. 6C). In previous studies, parturition and infection-mediated preterm labor are linked to increased OS. In the context of the myometrium, elevated levels of ROS have been demonstrated to modify the cellular function with respect to myometrial contractility, where elevated ROS levels are associated with a decreased propensity for contractility. ROS production has been associated with reperfusion-ischemic episodes during labor, which have in part explained during difficult, prolonged labors the depression of contraction characteristic of myometrial dysfunction [35].
It is also important to note that increasing evidence suggests that the signaling network that regulates protein folding, oxidative stress, and ER stress are tightly linked and induce cell death or survival through cross talk directly with the mitochondria. Transfer of calcium from the ER to the mitochondria is important in maintaining control over the prosurvival/proapoptotic signaling pathways [36, 37]. We speculate that understanding the cross talk between the ER and mitochondria with respect to prosurvival signaling will facilitate therapeutic strategies to overcome the problems associated with increased oxidative stress in the context of pregnancy, term, and preterm labor.
In conclusion, this study has demonstrated that EIF2AK3 is likely an important regulator of antioxidant responsiveness in the uterine myocyte. Several publications have highlighted the role of EIF2AK3 in regulating OS in various cell types [18, 34]. The cross talk between the ER stress response and the OS response has previously been shown to be important in regulating various pathological states, such as Alzheimer's [38], myocardial ischemia and reperfusion injury [39], and type 2 diabetes [40]. Elevated OS during pregnancy can be caused by various factors, such as infection, obesity, cigarette smoking, malnutrition, and alcohol consumption, and has been implicated in causing preterm delivery and has been shown to cause DNA damage to the amnion, fetal senescence, enhanced inflammation, and apoptosis in the uterus [41]. Inflammation along with OS has been proposed to be involved in key signaling events that can lead to preterm delivery [42]. LPS-induced preterm delivery has been shown to be caused by increased OS in the myometrium due to macrophage infiltration [42]. Furthermore, antioxidants, such as glutathione and N acetyl cysteine, have been successfully used to partially rescue LPS-induced preterm delivery in mice and rats. Several large randomized controlled clinical trials were performed to assess the use of antioxidant supplements targeting primarily pregnancy outcomes; however, minimal success was achieved [50–53]. As a result, the means by which the local pregnant uterine redox homeostasis is maintained across gestation remain unresolved. Although cross talk between OS and the UPR has been established in other cell types, as outlined above, this study is the first to demonstrate that the local UPR mediated through EIF2AK3 activation is an important regulator of OS management in the uterine myocyte through activation of the antioxidant response, as outlined in Figure 7. We have also demonstrated that a coordinated UPR and antioxidant response occurs in both the pregnant mouse and the human uterus. We speculate that uncovering the UPR as a central mechanism in the regulation of pregnancy-associated ROS management in the uterine myocyte will allow for alternate and novel therapies for the maintenance of gestationally activated uterine myocyte OS. We also speculate that uteri that have a reduced capacity to accommodate an appropriate UPR may be more susceptible to OS-induced pregnancy complications.
FIG. 7.
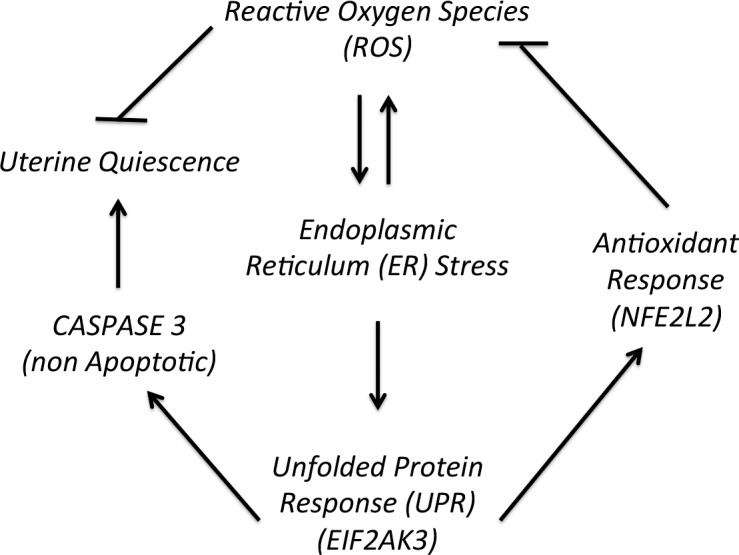
The relationship between ROS, UPR, antioxidant response, and uterine quiescence during pregnancy.
Footnotes
This work was supported by the March of Dimes #21 FY2012-152 and the NICHD 1R01HD065011.
REFERENCES
- Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf) 2002;57:609–613. doi: 10.1046/j.1365-2265.2002.01638.x. [DOI] [PubMed] [Google Scholar]
- Stephenson-Famy A, Marks J, Suresh A, Caritis SN, Simhan H, Jeyasuria P, Condon JC. Antiapoptotic signaling via MCL1 confers resistance to caspase-3-mediated apoptotic cell death in the pregnant human uterine myocyte. Mol Endocrinol. 2012;26:320–330. doi: 10.1210/me.2011-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyasuria P, Wetzel J, Bradley M, Subedi K, Condon JC. Progesterone-regulated caspase 3 action in the mouse may play a role in uterine quiescence during pregnancy through fragmentation of uterine myocyte contractile proteins. Biol Reprod. 2009;80:928–934. doi: 10.1095/biolreprod.108.070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyathanahalli C, Organ K, Moreci RS, Anamthathmakula P, Hassan SS, Caritis SN, Jeyasuria P, Condon JC. Uterine endoplasmic reticulum stress-unfolded protein response regulation of gestational length is caspase-3 and-7-dependent. Proc Natl Acad Sci U S A. 2015;112:14090–14095. doi: 10.1073/pnas.1518309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JM, Gopaul NK, Endresen MJR, Knight M, Linton EA, Dhir S, Anggard EE, Redman CWG. Circulating markers of oxidative stress are raised in normal pregnancy and pre-eclampsia. Br J Obstet Gynaecol. 1998;105:1195–1199. doi: 10.1111/j.1471-0528.1998.tb09974.x. [DOI] [PubMed] [Google Scholar]
- Moretti M, Phillips M, Abouzeid A, Cataneo RN, Greenberg J. Increased breath markers of oxidative stress in normal pregnancy and in preeclampsia. Am J Obstet Gynecol. 2004;190:1184–1190. doi: 10.1016/j.ajog.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Sharma JB, Sharma A, Bahadur A, Vimala N, Satyam A, Mittal S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int J Gynecol Obstet. 2006;94:23–27. doi: 10.1016/j.ijgo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem. 1998;273:7765–7769. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]
- Cohen JM, Beddaoui M, Kramer MS, Platt RW, Basso O, Kahn SR. Maternal antioxidant levels in pregnancy and risk of preeclampsia and small for gestational age birth: a systematic review and meta-analysis. PLoS One. 2015;10:e0135192. doi: 10.1371/journal.pone.0135192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthi S, Jessop CE, Bulleid NJ. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 2006;7:271–275. doi: 10.1038/sj.embor.7400645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Darley-Usmar VM. Role of calcium and superoxide dismutase in sensitizing mitochondria to peroxynitrite-induced permeability transition. Am J Physiol Heart Circ Physiol. 2004;286:H39–H46. doi: 10.1152/ajpheart.00742.2003. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, Takano Y, Shitamura A, Shimada T, Yao J, Kitamura M. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J Biol Chem. 2008;283:4252–4260. doi: 10.1074/jbc.M705951200. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang YH, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase (vol 397, pg 271, 1999) Nature. 1999;398:90–90. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan JY. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Gupta S, Giricz Z, Natoni A, Donnelly N, Deegan S, Szegezdi E, Samali A. NOXA contributes to the sensitivity of PERK-deficient cells to ER stress. FEBS Lett. 2012;586:4023–4030. doi: 10.1016/j.febslet.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67:506–514. doi: 10.1095/biolreprod67.2.506. [DOI] [PubMed] [Google Scholar]
- Suresh A, Subedi K, Kyathanahalli C, Jeyasuria P, Condon JC. Uterine endoplasmic reticulum stress and its unfolded protein response may regulate caspase 3 activation in the pregnant mouse uterus Plos One 2013. 8 e75152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiser D, Kelly DK, Seidman DS, Stevenson DK, Baum M, Dennery PA. Gestational pattern of heme oxygenase expression in the rat. Pediatr Res. 2003;54:172–178. doi: 10.1203/01.PDR.0000072516.83498.07. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen YH, Loch-Caruso R, Mukherjee B, Meeker JD. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth Am J Obstet Gynecol 2015. 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27:315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat. 2004;28:51–65. doi: 10.1016/j.jchemneu.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett. 2004;357:127–130. doi: 10.1016/j.neulet.2003.12.080. [DOI] [PubMed] [Google Scholar]
- Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M. Induction of neuronal death by ER stress in Alzheimer's disease. J Chem Neuroanat. 2004;28:67–78. doi: 10.1016/j.jchemneu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–3629. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi M, Arrowsmith S, Wray S. Hypoxia-induced force increase (HIFI) is a novel mechanism underlying the strengthening of labor contractions, produced by hypoxic stresses. Proc Natl Acad Sci U S A. 2015;112:9763–9768. doi: 10.1073/pnas.1503497112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3:a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota SI, Costa RO, Ferreira IL, Santana I, Caldeira GL, Padovano C, Fonseca AC, Baldeiras I, Cunha C, Letra L, Oliveira CR, Pereira CM, et al. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer's disease. Biochim Biophys Acta. 2015;1852:1428–1441. doi: 10.1016/j.bbadis.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Wang J, Hu X, Jiang H. ERS-PERK signaling pathway-mediated Nrf2/ARE-HO-1 axis: a novel therapeutic target for attenuating myocardial ischemia and reperfusion injury. Int J Cardiol. 2016;203:779–780. doi: 10.1016/j.ijcard.2015.11.033. [DOI] [PubMed] [Google Scholar]
- Mozzini C, Garbin U, Stranieri C, Pasini A, Solani E, Tinelli IA, Cominacini L. Fratta Pasini AM. Endoplasmic reticulum stress and Nrf2 repression in circulating cells of type 2 diabetic patients without the recommended glycemic goals. Free Radic Res. 2015;49:244–252. doi: 10.3109/10715762.2014.997229. [DOI] [PubMed] [Google Scholar]
- Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol. 2014;5:567. doi: 10.3389/fimmu.2014.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi T, Bardou M, Mace G, Sicard P, Wendremaire M, Barrichon M, Richaud S, Demidov O, Sagot P, Garrido C, Lirussi F. Glutathione prevents preterm parturition and fetal death by targeting macrophage-induced reactive oxygen species production in the myometrium. FASEB J. 2015;29:2653–2666. doi: 10.1096/fj.14-266783. [DOI] [PubMed] [Google Scholar]
- Poston L, Briley A, Seed P, Kelly F, Shennan A. Vitamin C and vitamin E in pregnant women at risk of pre-eclampsia—reply. Lancet. 2006;368:199–200. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- Villar J, Purwar M, Merialdi M, Zavaleta N, Ngoc NTN, Anthony J, De Greeff A, Poston L, Shennan A. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG. 2009;116:780–788. doi: 10.1111/j.1471-0528.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM, Mercer BM, Peaceman AM, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HR, Perez-Cuevas R, Xiong X, Reyes H, Julien P, Smith G, Choquette P, Winsor S, Leduc L, Audibert F, Moutquin JM, Wood S, et al. An international trial of vitamins C and E in the prevention of preeclampsia (INTAPP trial) Am J Obstet Gynecol. 2009;201:S2–S3. doi: 10.1016/j.ajog.2010.01.050. [DOI] [PubMed] [Google Scholar]