Abstract
Spontaneous preterm labor occurs in two subsets of patients with sterile intra-amniotic inflammation, a process induced by alarmins such as high-mobility group box-1 (HMGB1). Inflammasomes are implicated in the process of spontaneous preterm labor. Therefore, we investigated whether HMGB1 initiates an inflammasome-associated inflammatory response in the chorioamniotic membranes. Incubation of the chorioamniotic membranes with HMGB1 1) induced the release of mature IL-1beta and IL-6; 2) upregulated the mRNA expression of the pro-inflammatory mediators NFKB1, IL6, TNF, IL1A, IFNG, and HMGB1 receptors RAGE and TLR2; 3) upregulated the mRNA expression of the inflammasome components NLRP3 and AIM2 as well as NOD proteins (NOD1 and NOD2); 4) increased the protein concentrations of NLRP3 and NOD2; 5) increased the concentration of caspase-1 and the quantity of its active form (p20); and 6) upregulated the mRNA expression and active form of MMP-9. In addition, HMGB1 concentrations in chorioamniotic membrane extracts from women who underwent spontaneous preterm labor were greater than in those from women who had undergone spontaneous labor at term. Collectively, these results show that HMGB1 can induce an inflammatory response in the chorioamniotic membranes, which is partially mediated by the inflammasome. These results provide insight into the mechanisms whereby HMGB1 induces preterm labor and birth in mice and explain why the concentration of this alarmin is increased in women who undergo spontaneous preterm labor.
Keywords: AIM2, alarmins, caspase-1, DAMPs, interleukin-1β, interleukin-6, MMP-9, NLRP3, NOD2, parturition, pregnancy, preterm birth, preterm labor, RAGE, sterile inflammation, sterile intra-amniotic inflammation, TLR-2
INTRODUCTION
Preterm birth (PTB), delivery prior to the 37th wk of gestation, is the leading cause of perinatal morbidity and mortality worldwide [1]. In 2015, 9.63% of all births in the United States were classified as preterm [2]. Preterm neonates are at an increased risk for short- and long-term morbidity, and prematurity places a substantial burden on the healthcare system and society [3–6]. Two-thirds of PTBs occur after spontaneous preterm labor [7]. Spontaneous preterm labor is a syndrome associated with multiple pathological processes [8], such as intra-amniotic infection/inflammation, which has been causally linked to PTB [9–12].
Intra-amniotic inflammation can be due to microorganisms (bacteria or viruses) or endogenous danger signals derived from necrosis or cellular stress [13, 14], termed damage-associated molecular pattern molecules [15, 16] or alarmins [17]. The pro-inflammatory process induced by alarmins is termed sterile inflammation [18]. We have used the term sterile intra-amniotic inflammation to describe the inflammatory process (interleukin-6 [IL-6] ≥2.6 ng/ml), in which microorganisms cannot be detected by cultivation and molecular microbiology techniques [19–29]. Sterile intra-amniotic inflammation appears to be more common than microbial-associated intra-amniotic inflammation in patients with preterm labor and intact chorioamniotic membranes [20]. This process is also frequently observed in patients with a sonographic short cervix [21] and in those with preterm prelabor rupture of the membranes (PPROM) with clinical chorioamnionitis at term [22]. Because the concentrations of several alarmins, including IL-1α [30], S100 calcium-binding protein B [31], heat-shock protein 70 [32], and high-mobility group box-1 (HMGB1) [33, 34], are increased in the amniotic fluid of women with intra-amniotic inflammation, we have previously proposed that these danger signals are responsible for sterile inflammation [32–34].
The median amniotic fluid concentration of HMGB1 is significantly higher in patients with sterile intra-amniotic inflammation than in those without intra-amniotic inflammation [20]. Indeed, patients with sterile intra-amniotic inflammation who delivered within 7 days after amniocentesis had higher amniotic fluid concentrations of HMGB1 than those who delivered after 7 days [20]. Amniotic fluid concentrations of HMGB1 are also higher in women who underwent spontaneous preterm labor with intra-amniotic infection/inflammation than in those without this clinical condition [33]. Recently, we provided in vivo evidence demonstrating that the intra-amniotic administration of HMGB1 induces preterm labor and birth in mice [35].
HMGB1 is an evolutionarily conserved protein that stabilizes nucleosome formation and facilitates gene transcription while localized in the nucleus, yet acts as an alarmin when released extracellularly [36, 37]. This alarmin activates innate immune cells via pattern recognition receptors such as the receptor for advanced glycation end products (RAGE) [38], toll-like receptor (TLR)-2, and TLR-4 [39] in order to initiate inflammatory responses. In vitro studies of monocytes [40], neutrophils [41, 42], dendritic cells [43], and endothelial cells [44] have demonstrated that HMGB1 promotes the activation of pro-inflammatory transcription factor NF-κB that, in turn, induces the production of pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, IL-8, IL-6, and IL-1β. However, the mechanisms whereby HMGB1 induces a sterile inflammatory response in the amniotic cavity are poorly understood.
Previously, we proposed that the mechanisms responsible for physiological (i.e., sterile) inflammation involve inflammasomes [45–47], which are cytoplasmic high-molecular-weight multisubunit protein complexes capable of inducing an inflammatory response through the production of IL-1β and IL-18 [48–66]. Their basic structure consists of 1) an inflammasome sensor molecule, 2) the adaptor protein ASC (an apoptosis-associated speck-like protein), and 3) pro-caspase-1 (pro-CASP-1) [48–66]. Following activation, the inflammasome complex induces the autocatalytic cleavage of pro-CASP-1 into its active form that, in turn, can cleave pro-IL-1β and pro-IL-18 into their mature and released forms [67–75]. In line with our hypothesis, the chorioamniotic membranes express inflammasome components (e.g., NLR family pyrin domain containing 3 [NLRP3]), nucleotide-binding oligomerization domain-containing (NOD) proteins, and active forms of CASP-1, as well as release mature IL-1β in spontaneous labor at term [47], suggesting a role for the inflammasome in the sterile inflammatory process of parturition. Yet, whether HMGB1 could induce the inflammasome-mediated release of IL-1β in the chorioamniotic membranes is unknown.
The aims of this study were to determine whether 1) HMGB1 induces the release of mature IL-1β and IL-6 by the chorioamniotic membranes as well as the upregulation of pro-inflammatory mediators; 2) HMGB1-induced release of mature IL-1β is associated with the upregulation of inflammasome components, NOD proteins, and the activation of CASP-1; 3) these changes are linked to an increased mRNA abundance and active form of matrix metalloproteinase 9 (MMP-9) in the chorioamniotic membranes; and 4) the chorioamniotic membranes from patients who underwent spontaneous preterm labor have an increased concentration of HMGB1 compared to those who had undergone spontaneous labor at term.
MATERIALS AND METHODS
Human Subjects
Chorioamniotic membrane samples were obtained from the Bank of Biological Specimens of the Detroit Medical Center, Wayne State University (WSU), and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS, Detroit, MI). The Institutional Review Boards (IRBs) of WSU and NICHD approved the collection and use of biological materials for research purposes (WSU IRB No. 110605MP4F, WSU IRB No. 082403MP2F, and NICHD No. OH95-CH-N040). All participating women provided written consent, and samples were collected 30 min after delivery.
To evaluate the in vitro effects of HMGB1 in the chorioamniotic membranes, samples were collected from women who delivered at term without labor and underwent an elective cesarean section (n = 23). Demographic and clinical characteristics of the study population are shown in Table 1. Patients with multiple births or with neonates having congenital or chromosomal abnormalities were excluded. Labor was defined by the presence of regular uterine contractions at a frequency of at least two contractions every 10 min with cervical changes resulting in delivery [76]. In each case, tissue sections of the chorioamniotic membranes were evaluated for acute histologic chorioamnionitis according to published criteria [77, 78] by pathologists who were blinded to the clinical outcome. Samples collected from women with labor or acute histologic chorioamnionitis were excluded from this study.
TABLE 1.
Demographic and clinical characteristics of term non-labor cases utilized for in vitro treatment with HMGB1.

IQR, interquartile range.
To determine the concentration of HMGB1 in the chorioamniotic membranes, samples were collected from four study groups (term delivery without labor, TNL; term delivery with labor, TIL; preterm delivery without labor, PTNL; and preterm delivery with labor, PTL; n = 8–12 each) and processed on the same day. Demographic and clinical characteristics of the study groups are shown in Table 2. Preterm labor was diagnosed by the presence of regular uterine contractions (at least three in 30 min) and documented cervical changes in patients with gestational ages between 20 and 36-6/7 wk. Preterm delivery was defined as birth prior to the 37th wk of gestation.
TABLE 2.
Demographic and clinical characteristics of cases used to determine HMGB1 concentrations.a
IQR, interquartile range; TNL, term delivery without labor; TIL, term delivery with labor; PTNL, preterm delivery without labor; PTL, preterm delivery with labor; NS, not significant.
Kruskal-Wallis test.
Chi-squared test.
In Vitro Incubation of the Chorioamniotic Membranes with HMGB1
Chorioamniotic membrane samples were spread out flat onto a sterile cutting board. A dermatological biopsy punch (12 mm Acu-Punch; Acuderm Inc.) was used to obtain tissue explants (three to six explants per sample) from the chorioamniotic membranes. Tissue explants were placed into a Falcon 24-well plate (Corning) in 500 μl of 1× Dulbecco modified Eagle medium (Corning) containing 10% fetal bovine serum (Gibco, Life Technologies Corporation) and 1% penicillin/streptomycin (Gibco) with or without 10 ng/ml–50 μg/ml of ultrapure HMGB1 (RE-HM050; IBL International Corporation). Tissue explants were incubated with 100 ng/ml of lipopolysaccharide (Escherichia coli 0111:B4; Sigma Aldrich) as a positive control. The tissue explants were incubated for 24h in a humidified 5% CO2 incubator. Following incubation, tissue supernatants were collected and stored at −80°C until use. In addition, tissue explants were homogenized in either their conditioned medium to prepare tissue extracts or in 1× sterile PBS (Gibco) containing protease inhibitor cocktail (Roche) to prepare tissue lysates. Tissue explants were also placed into Ambion RNAlater Solution (Thermo-Fisher Scientific, Inc.) for quantitative RT-PCR (qRT-PCR) analysis or into 10% formalin (Thermo-Fisher Scientific) for histology.
Enzyme-Linked Immunosorbent Assays
The concentrations of NLRP3, AIM2, NOD1, NOD2 in tissue lysates, CASP-1 in tissue extracts, and IL-6 and mature IL-1β in tissue supernatants were measured using specific and sensitive enzyme-linked immunosorbent assays (ELISA) (kits for NLRP3, NOD1, and NOD2 from Cusabio; kits for AIM2 and CASP-1 from Cloud Clone; kit for IL-6 and IL-1β from R&D Systems), following the manufacturers' instructions. Briefly, recombinant human standards and the samples were incubated in duplicate wells of the 96-well microplates precoated with monoclonal antibodies specific for target analytes. After washing the unbound substances, enzyme-conjugated antibodies bound to the target analytes were added to the wells. After the incubation, assay plates were washed to remove the unbound antibodies, followed by the addition of a substrate solution that developed color proportional to the amount of target protein bound in the initial step. Finally, the color development was stopped by the addition of a sulfuric acid solution, and the microplates were read using a programmable spectrophotometer (SpectraMax M5 Multi-Mode Microplate Reader; Molecular Devices). The sensitivities of the assays were <0.039 ng/ml for NLRP3, <0.056 ng/ml for AIM2, <3.9 pg/ml for NOD1, <6.25 pg/ml for NOD2, <0.112 ng/ml for CASP-1, <0.70 pg/ml for IL-6, and <1 pg/ml for mature IL-1β. The IL-1β ELISA kit measures approximately 6.1% of the pro-IL-1β. The immunoassays for NLRP1 and NLRC4 did not meet our criteria for validation; therefore, immunoblotting was performed.
RNA Isolation, cDNA Generation, and qRT-PCR Analysis
TRIzol (Invitrogen, Life Technologies Corporation) and a Qiagen RNeasy kit (Qiagen) were used to extract total RNA from the chorioamniotic membrane tissues (n = 6–7 each). RNA purity and concentration were assessed with the NanoDrop 1000 spectrophotometer (Thermo-Fisher Scientific, Inc.), and RNA integrity was evaluated with the Bioanalyzer 2100 (Agilent Technologies). The Super-Script III First-Strand Synthesis System (Invitrogen) and oligo (dT)20 primers (Invitrogen) were utilized to generate cDNA. Gene expression profiling was performed on the BioMark System for high-throughput qRT-PCR (Fluidigm) and on the ABI 7500 FAST Real-Time PCR System (Applied Biosystems, Life Technologies Corporation) with TaqMan gene expression assays (Applied Biosystems) listed in Supplemental Table S1 (Supplemental Data are available online at www.biolreprod.org).
Immunoblotting
Chorioamniotic membrane tissue lysates (n = 7 each for NLRP1 and NLRC4) or tissue extracts (n = 9 each for CASP-1) were subjected to 4%–12% SDS-polyacrylamide gel electrophoresis (Invitrogen). After electrophoresis, separated proteins were transferred onto nitrocellulose membranes (Bio-Rad), and the membranes were blocked with StartingBlock T20 Block Buffer (Thermo-Fisher Scientific, Inc.) and probed overnight at 4°C with the following primary antibodies: anti-NLRP1 (1:500, ALX-210-904; Enzo Life Sciences), anti-NLRC4 (1:1000, 659702; BioLegend), and anti-CASP-1 (1:500, MAB6215; R&D Systems). Next, either of the following horseradish peroxidase-conjugated secondary antibodies was added: anti-rabbit IgG (7074; Cell Signaling) or anti-mouse IgG (7076; Cell Signaling). Signals were detected by chemiluminescence with ChemiGlow West reagents (ProteinSimple). Images were acquired using the FUJIFILM LAS-4000 Imaging System (Fujifilm North America Corporation), and semiquantification was performed by the ImageJ 1.44p software (NIH). Finally, nitrocellulose membranes were stripped with Restore Plus Western Blot Stripping Buffer (Pierce Biotechnology, Thermo-Fisher Scientific, Inc.) for 15 min, washed with 19 mM Tris and 137 mM NaCl containing 0.1% Tween-20 (1706531; Bio-Rad), blocked, and reprobed for 1 h at room temperature with either mouse anti-GAPDH (Santa Cruz Biotechnology) or anti-ACTB (A5316; Sigma-Aldrich).
Zymography
To determine the active form of MMP-9, chorioamniotic membrane tissue extracts (n = 8 each) were subjected to 10% Zymogram (gelatin) gel electrophoresis (Novex, Life Technologies Corporation). After electrophoresis, the gels were incubated for 30 min in Zymograph Renaturing Buffer (Novex, Life Technologies Corporation) followed by an additional 30-min incubation in Zymograph Developing Buffer (Novex, Life Technologies Corporation), both with gentle agitation. Gels were then placed in a freshly made developing buffer and incubated overnight at 37°C. Following incubation, gels were washed in double-distilled H2O, stained for 4 h with SimplyBlue SafeStain (Invitrogen), and washed with double-distilled H2O again. Images were taken using Alpha Innotech FluorChem SP (ProteinSimple) and semiquantified using ImageJ 1.44p software.
Determination of HMGB1 Concentration in the Chorioamniotic Membranes
Chorioamniotic membrane tissue extracts from the four study groups (TNL, TIL, PTNL, and PTL; n = 8–12 each) were prepared as follows: 10–12 tissue explants were obtained from each membrane using a dermatological punch (12 mm Acu-Punch; Acuderm Inc.). Tissue explants were placed at 37°C in a humidified 5% CO2 incubator for 24 h in 500 μl of Dulbecco modified Eagle medium (Gibco, Life Technologies) in a 24-well plate. Following incubation, tissue explants were homogenized in their conditioned medium using a mechanical tissue homogenizer (T-25 Ultra-Turrax; IKA Works, Inc.). Tissue extracts were centrifuged at 14 000 × g for 3–5 min at 4°C, and the supernatant was collected and filtered using a syringe filter (0.22 μm Millex-GV Syringe Filter Unit, 33mm polyvinylidene fluoride, gamma-sterilized; EMD Millipore). The concentration of HMGB1 in these tissue extracts was determined using an ELISA kit (IBL International) following the manufacturers' instructions. The sensitivity of the HMGB1 ELISA kit was <0.2 ng/ml.
Statistical Analyses
Statistical analyses were performed using SPSS, version 21.0 (IBM Corporation). For gene expression data, Ct values over technical replicates were averaged and gene expression relative to references (ACTB, GAPDH, RPLPO) were quantified by subtracting target gene Ct values from mean reference gene Ct values within the same sample. A Shapiro-Wilk test was performed to determine whether the data were normally distributed. Normally distributed data were analyzed by a paired t-test. Nonnormally distributed data were analyzed by either a Wilcoxon signed-rank test for paired samples or a Mann-Whitney U-test for unpaired samples. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
Incubation with HMGB1 Induces the Release of Mature IL-1β and IL-6 by the Chorioamniotic Membranes
Because HMGB1 concentrations in cytoplasmic fractions of the chorioamniotic membranes range between 400 and 500 ng/ml [79], we incubated chorioamniotic membrane explants with either 1 μg/ml or 10 μg/ml of this alarmin. Incubation of the chorioamniotic membranes with either 1 μg/ml or 10 μg/ml of HMGB1 did not increase the release of mature IL-1β or IL-6 (Supplemental Fig. S1 and data not shown). Yet, incubation of the chorioamniotic membranes with lipopolysaccharide increased the release of mature IL-1β and IL-6 (positive control, Supplemental Fig. S1). Next, we increased the concentrations of HMGB1 to 25 or 50 μg/ml. Incubation of the chorioamniotic membranes with 25 or 50 μg/ml of HMGB1 increased the release of mature IL-1β (Fig. 1A) and IL-6 (Fig. 1B) when compared to negative controls. We used 50 μg/ml of HMGB1 for subsequent experiments.
FIG. 1.
Incubation with HMGB1 induces the release of mature IL-1β and IL-6 by the chorioamniotic membranes. A) Protein concentrations of mature IL-1β in the chorioamniotic membranes upon incubation with either media alone (negative control) or media containing HMGB1 (25 and 50 μg/ml, n = 13 each). B) Protein concentrations of IL-6 in the chorioamniotic membranes upon incubation with either media alone or media containing HMGB1 (25 and 50 μg/ml, n = 11–12 each).
Incubation with HMGB1 Increases the mRNA Abundance of Pro-Inflammatory Mediators in the Chorioamniotic Membranes
Previous studies demonstrated that HMGB1 activates the NF-κB pathway, which consequently upregulates the expression of pro-inflammatory cytokines [40–44]. Therefore, we investigated whether this alarmin would cause the upregulation of NF-κB and several pro-inflammatory cytokines in the chorioamniotic membranes. Incubation of the chorioamniotic membranes with HMGB1 increased the mRNA abundance of NFKB1, IL6, TNF, IL1A, and IFNG compared to negative controls (Fig. 2, A and C–F). Incubation of the chorioamniotic membranes with HMGB1 tended to increase the mRNA abundance of IL1B compared to negative controls, yet this increase did not reach statistical significance (Fig. 2B; P = 0.053). However, the mRNA abundance of IL18 was unaltered upon HMGB1 incubation (Fig. 2G). These data demonstrate that HMGB1 can induce a pro-inflammatory response in the chorioamniotic membranes.
FIG. 2.
Incubation with HMGB1 increases the mRNA abundance of pro-inflammatory mediators by the chorioamniotic membranes. Messenger RNA abundance of NFKB1 (A), IL1B (B), IL6 (C), TNF (D), IL1A (E), IFNG (F), IL18 (G), RAGE (H), and TLR2 (I) in the chorioamniotic membranes upon incubation with either media alone or media containing HMGB1 (n = 7 each). Relative gene expressions are presented as −ΔCt values.
HMGB1 interacts with RAGE, TLR-2, and TLR-4 [38, 39] and can increase the expression of these receptors in a positive feedback mechanism, which contributes to disease progression in several pathological conditions [80]. Therefore, we investigated whether incubation with HMGB1 would increase the expression of RAGE, TLR2, and TLR4 in the chorioamniotic membranes. Incubation with HMGB1 increased the mRNA abundance of RAGE and TLR2 (Fig. 2, H and I), but not TLR4 (data not shown), in the chorioamniotic membranes compared to negative controls. These data demonstrate that HMGB1 increases the mRNA abundance of RAGE and TLR2 in the chorioamniotic membranes.
Incubation with HMGB1 Increases the mRNA Abundance and Protein Concentration of NLRP3 and NOD2 in the Chorioamniotic Membranes
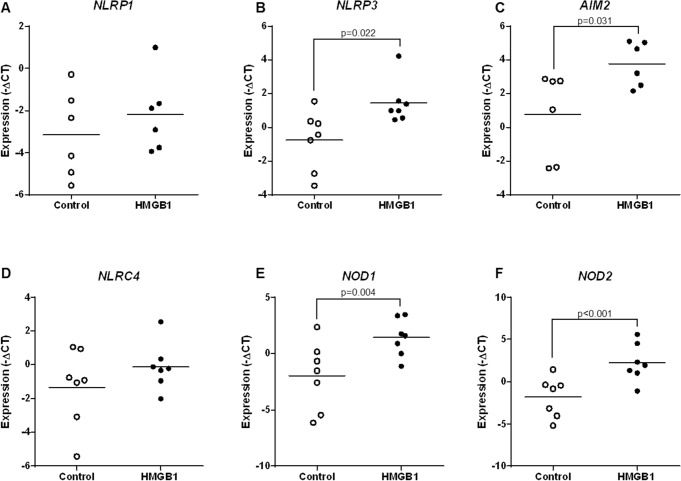
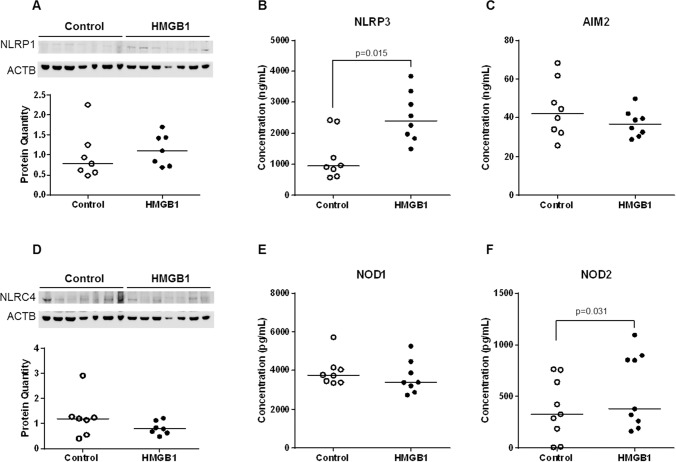
Next, we investigated whether HMGB1 upregulates the mRNA and protein expressions of major inflammasome components (NLRP1, NLRP3, NLRC4, and AIM2) and NOD proteins (NOD1 and NOD2) in the chorioamniotic membranes. Incubation with HMGB1 increased the mRNA abundance of NLRP3, AIM2, NOD1, and NOD2 compared to negative controls (Fig. 3, B, C, E, and F). However, the mRNA abundance of NLRP1 and NLRC4 was unchanged upon HMGB1 incubation (Fig. 3, A and D). HMGB1 increased the protein concentrations of NLRP3 and NOD2 in the chorioamniotic membranes compared to negative controls (Fig. 4, B and F). In contrast, the protein concentrations of NLRP1, AIM2, NLRC4, and NOD1 were unaltered upon HMGB1 incubation (Fig. 4, A and C–E). These data demonstrate that HMGB1 simultaneously upregulates the mRNA and protein expression of NLRP3 and NOD2 in the chorioamniotic membranes.
FIG. 3.
Incubation with HMGB1 increases the mRNA abundance of inflammasome components and NOD proteins in the chorioamniotic membranes. Messenger RNA abundance of NLRP1 (A), NLRP3 (B), AIM2 (C), NLRC4 (D), NOD1 (E), and NOD2 (F) in the chorioamniotic membranes upon incubation with either media alone or media containing HMGB1 (n = 6–7 each). Relative gene expressions are presented as −ΔCt values.
FIG. 4.
Incubation with HMGB1 increases the protein concentration of NLRP3 and NOD2 in the chorioamniotic membranes. Protein concentrations of NLRP1 (A, immunoblotting), NLRP3 (B, ELISA), AIM2 (C, ELISA), NLRC4 (D, immunoblotting), NOD1 (E, ELISA), and NOD2 (F, ELISA) in the chorioamniotic membranes upon incubation with either media alone or media containing HMGB1 (n = 7–9 each). ACTB was used as a loading control.
Incubation with HMGB1 Increases the Protein Concentration and Activation of Caspase-1
We then investigated whether elevated concentrations of NLRP3 and NOD2 were associated with the activation of CASP-1 in the chorioamniotic membranes. Activation of CASP-1 results in the generation of two subunits: p10 and p20 [69]. Incubation of the chorioamniotic membranes with HMGB1 tended to increase the mRNA abundance of CASP1 compared to negative controls, yet this increase did not reach statistical significance (Fig. 5A). Incubation with HMGB1 increased the total protein concentration of CASP-1 in the chorioamniotic membranes compared to negative controls (Fig. 5B). Moreover, the pro- and active forms of CASP-1 (p20) were increased in the chorioamniotic membranes upon incubation with HMGB1 (Fig. 5C). There was a 2.8-fold increase in the active form of CASP-1 (p10) upon incubation of the chorioamniotic membranes with HMGB1, yet this increase did not reach statistical significance (Fig. 5C). Because an increase in the p20 subunit alone is a sufficient marker for the activation of this inflammatory caspase [81, 82], these data demonstrate that HMGB1 induces the activation of CASP-1 in the chorioamniotic membranes.
FIG. 5.
Incubation with HMGB1 increases the protein concentration and activation of caspase-1. A) Messenger RNA abundance of CASP1 in the chorioamniotic membranes upon incubation with either media alone or media containing HMGB1 (n = 7 each). Relative gene expressions are presented as −ΔCt values. B) Protein concentrations of CASP-1 in the chorioamniotic membranes upon incubation with either media alone or media containing HMGB1 (n = 9 each). C) Immunoblotting of CASP-1 in the chorioamniotic membranes upon incubation with either media alone or media containing HMGB1 and its quantification (n = 9 each). GAPDH was used as a loading control.
Incubation with HMGB1 Increases the mRNA Abundance and Active Form of MMP-9 in the Chorioamniotic Membranes
The median concentration of HMGB1 is higher in amniotic fluid samples from women with PPROM than in those from women who undergo spontaneous preterm labor with intact membranes [33]. In addition, amniotic fluid MMP-9 concentrations are increased among women with PPROM compared to those who undergo spontaneous preterm labor with intact membranes [83], and a polymorphism in the MMP-9 promotor is associated with an increased risk of PPROM [84]. Therefore, we investigated whether incubation with HMGB1 would increase the mRNA abundance and active form of MMP-9 in the chorioamniotic membranes. Incubation with HMGB1 increased the mRNA abundance (Fig. 6A) and active form (Fig. 6B) of MMP-9 in the chorioamniotic membranes compared to negative controls. An increase in the mRNA abundance of MMP-9 and its active form suggests that HMGB1 may be implicated in the mechanisms of rupture of the membranes.
FIG. 6.
Incubation with HMGB1 increases the mRNA abundance and active form of MMP-9 in the chorioamniotic membranes. A) Messenger RNA abundance of MMP9 in the chorioamniotic membranes upon incubation with either media alone or media containing HMGB1 (n = 7 each). Relative gene expressions are presented as −ΔCt values. B) Zymography of the active form of MMP-9 in the chorioamniotic membranes upon incubation with either media alone or media containing HMGB1 and its quantification (n = 8 each).
HMGB1 Concentration Is Increased in the Chorioamniotic Membranes from Women Who Underwent Spontaneous Preterm Labor
HMGB1 concentration in the cytoplasmic fraction of the chorioamniotic membranes is not different between women who underwent spontaneous labor at term and those who delivered at term without labor [79]. In this study, we determined whether the concentration of HMGB1 was increased in the chorioamniotic membrane extracts from women who underwent spontaneous preterm labor. The chorioamniotic membrane extracts from women who underwent spontaneous preterm labor had greater concentrations of HMGB1 than those from women who had undergone spontaneous labor at term (Fig. 7). These findings demonstrate that the chorioamniotic membranes from women undergoing spontaneous preterm labor have an elevated concentration of the alarmin HMGB1.
FIG. 7.

HMGB1 concentration is increased in the chorioamniotic membranes from women who underwent spontaneous preterm labor. Protein concentrations of HMGB1 in the chorioamniotic membranes from women who delivered at term with labor (TIL, n = 9) or without labor (TNL, n = 12) or those who delivered preterm with labor (PTL, n = 11) or without labor (PTNL, n = 8).
DISCUSSION
Principal Findings of the Study
Incubation of the chorioamniotic membranes with HMGB1 1) induced the release of mature IL-1β and IL-6; 2) upregulated the mRNA expression of the pro-inflammatory mediators NFKB1, IL6, TNF, IL1A, and IFNG, as well as HMGB1 receptors RAGE and TLR2; 3) upregulated the mRNA expression of NLRP3, AIM2, NOD1, and NOD2; 4) increased the protein concentrations of NLRP3 and NOD2; 5) increased the protein concentration of CASP-1 and the quantity of its active form (p20); and 6) upregulated the mRNA abundance and active form of MMP-9. In addition, HMGB1 concentrations in the chorioamniotic membrane extracts from women who underwent spontaneous preterm labor were greater than in those from women who had undergone spontaneous labor at term. Collectively, these data suggest that HMGB1 induces an inflammatory response in the chorioamniotic membranes, which is partially mediated by the inflammasome.
Incubation of the Chorioamniotic Membranes with HMGB1 Induces the Release of Mature IL-1β and IL-6 as well as the Upregulation of Pro-inflammatory Mediators
HMGB1 induces the activation of NF-κB in innate immune cells [40–43]. The study herein demonstrated that HMGB1 also upregulated the mRNA expression of NF-κB in the chorioamniotic membranes. This was accompanied by the upregulation of downstream targets such as IL6, TNF, IL-1A, and IFNG. Importantly, incubation of the chorioamniotic membranes with HMGB1 also increased the concentrations of mature IL-1β and IL-6. These cytokines participate in the pathophysiology of spontaneous preterm labor [23]. In particular, IL-1β is a central mediator of pathological inflammation because its administration induces preterm labor and birth in mice [85, 86] and monkeys [10–12]. This effect is ameliorated by the administration of its antagonist IL-1RA [86, 87]. IL-1β is synthesized as a zymogen, which is processed into its mature form by the inflammasome [48, 88]. In addition, neutrophil- and macrophage-derived serine proteases (e.g., proteinase 3, elastase, cathepsin-G, chymase, and chymotrypsin) and metalloproteases (e.g., meprin α and meprin β) can cleave pro-IL-1β into its mature form [75, 89–92]. Recently, we provided evidence demonstrating that the inflammasome is implicated in the processing of mature IL-1β in the chorioamniotic membranes in the setting of physiological inflammation during term parturition [47]. Our recently published data also support a role for the inflammasome in the processing of mature IL-1β in pathological inflammation (i.e., acute histologic chorioamnionitis) in term [93] and preterm [94] gestations. These findings are concordant with previously reported observations demonstrating that 1) amniotic fluid IL-1β concentrations are greater in women who had undergone spontaneous term labor compared to those who delivered at term without labor [95] and 2) amniotic fluid IL-1β concentrations are elevated in women who had undergone spontaneous preterm labor with intra-amniotic infection compared to those without this clinical complication [30]. IL-1β actively participates in the process of labor by inducing 1) the biosynthesis of prostaglandin E2 by the human amnion [96] and myometrial cells [97, 98], 2) the expression of cyclooxygenase-2 in human myometrial cells [99], and 3) the expression of matrix-metabolizing enzymes (MMP-1, MMP-3, MMP-9, and cathepsin S) in human cervical smooth muscle cells [100]. Collectively, these data show that HMGB1 induces the release of mature IL-1β in the chorioamniotic membranes, which we suggest is partially mediated by the inflammasome. The fact that incubation of the chorioamniotic membranes with HMGB1 also induces the release of IL-6, an inflammasome-independent cytokine, suggests that this alarmin activates inflammasome and non-inflammasome pathways in order to promote preterm labor and birth [35].
We also found that incubation of the chorioamniotic membranes with HMGB1 upregulated the mRNA expression of RAGE and TLR2. RAGE and TLR2 are putative receptors for HMGB1 [38, 39] that are regulated by NF-κB. Because we demonstrated that HMGB1 increased the expression of NFKB1, it is not surprising that it also upregulated the expression of RAGE and TLR2. This positive-feedback mechanism is thought to participate in the amplification of pathological inflammation [80], and in our model, it may contribute to the pro-inflammatory milieu that accompanies spontaneous preterm labor.
Incubation of the Chorioamniotic Membranes with HMGB1 Increases the mRNA Abundance and Protein Concentration of NLRP3 and NOD2
Physiological inflammation during spontaneous labor at term includes the participation of inflammasomes [47]. Specifically, we demonstrated that the mRNA abundance and protein expression of NLRP3 and NOD2 are increased in the chorioamniotic membranes of women who underwent spontaneous labor at term compared to those who delivered at term without labor [47]. Herein, we demonstrated that incubation of the chorioamniotic membranes with HMGB1 increased the mRNA abundance and protein expression of NLRP3 and NOD2. Together, these data suggest that the HMGB1-induced inflammatory response in the chorioamniotic membranes resembles the physiological inflammation in the in vivo scenario.
The NLRP3 inflammasome includes the NLRP3 protein (also known as cryopyrin), the adaptor molecule ASC containing two death-fold domains (one pyrin domain and one CARD), and pro-caspase-1 [101–103]. Activation of the NLRP3 inflammasome can be induced by chemically and structurally different stimuli, including crystalline material [104, 105], extracellular ATP released from dying cells [106], peptide aggregates such as vaccine adjuvants [107–111], phospholipid cardiolipin and mitochondrial DNA [112–114], and bacterial toxins [106, 115, 116]. NLRP3 inflammasome activation requires two steps: priming and assembly of the inflammasome complex [117, 118]. The priming step is initiated by pattern recognition receptors, cytokine receptors, or any other factor able to induce the activation of NF-κB, which results in the upregulation of NLRP3 to a functional level and pro-IL-1β expression [117–119]. The second step is posttranscriptional and allows the assembly of the NLRP3 inflammasome complex [117, 118]. The fact that incubation of the chorioamniotic membranes with HMGB1 upregulated the mRNA and protein expression of NLRP3 suggests that this alarmin can initiate the activation of the inflammasome. Yet, whether the assembly of the NLRP3 inflammasome complex occurs in the chorioamniotic membranes upon HMGB1 incubation requires further investigation.
The NOD2 protein is an intracellular receptor that recognizes bacterial peptidoglycan segments but does not recruit inflammasome components [120–129]. NOD2 can also mediate sterile inflammatory processes such as those related to the endoplasmic reticulum stress response [130]. In dendritic cells, NOD2 can act synergistically with the NLRP3 inflammasome in response to bacterial muramyl dipeptide and uric acid [131]. A previous study showed that the mRNA expression of NOD2 is greater in the chorioamniotic membranes from women who underwent spontaneous preterm labor than in those who delivered preterm without labor [132]. Herein, we demonstrated that the incubation of the chorioamniotic membranes with HMGB1 upregulated the mRNA and protein expression of NOD2. Together, these data suggest that NOD2 and the NLRP3 inflammasome may be implicated in the HMGB1-induced preterm labor process [35].
Incubation of the Chorioamniotic Membranes with HMGB1 Induces the Activation of Caspase-1
Oligomerization of the inflammasome leads to the recruitment of ASC, which binds and activates pro-CASP-1 via its CARD domain [64, 133]. Active forms of CASP-1 are able to convert inactive pro-IL-1β into its bioactive and secreted form [68–70, 88, 134]. In the current study, incubation of the chorioamniotic membranes with HMGB1 induced the activation of CASP-1, which coincided with the release of the mature form of IL-1β. In concordance with this finding, the active forms of CASP-1 and the mature form of IL-1β are increased in the chorioamniotic membranes in spontaneous labor at term [47]. In addition, the mRNA abundance and active form of CASP-1 (p10) are increased in the rupture zone of the chorioamniotic membranes and myometrium from women who undergo spontaneous labor at term when compared to those from women who deliver at term without labor [135]. Taken together, these data demonstrate that HMGB1 is implicated in the activation of CASP-1 in the chorioamniotic membranes, which may participate in the maturation of IL-1β during the process of labor.
Incubation of the Chorioamniotic Membranes with HMGB1 Induces the Expression and Activation of MMP-9
Inflammasome activation [136] and IL-1β expression [100] are associated with an enhanced production of extracellular matrix remodeling enzymes, such as MMP-9. Because incubation of the chorioamniotic membranes with HMGB1 is characterized by the upregulation of inflammasome components and maturation of IL-1β, we hypothesized that this alarmin would induce the expression and activation of MMP-9. Consistent with our hypothesis, HMGB1 increased the mRNA expression and active form of MMP-9 in the chorioamniotic membranes. MMPs are a superfamily of zinc enzymes that participate in the degradation of the extracellular matrix [137–141]. Particularly, MMP-9 (also known as gelatinase B) [142] is expressed by resident cells and infiltrating leukocytes at the maternal-fetal interface and is associated with the processes of term and preterm parturition [143–154]. Collectively, these data suggest that HMGB1 promotes the activation of MMP-9, which may participate in the process of preterm parturition induced by this alarmin [35].
Increased Concentration of HMGB1 in the Chorioamniotic Membranes of Patients Who Undergo Spontaneous Preterm Labor
Patients with sterile intra-amniotic inflammation and elevated amniotic fluid concentrations of HMGB1 delivered earlier than those with low concentrations of this alarmin [20]. In addition, intra-amniotic administration of HMGB1 induces preterm labor and birth in mice [35]. Herein, we showed that the chorioamniotic membrane extracts from women who underwent spontaneous preterm labor had greater HMGB1 concentrations than those who had undergone spontaneous labor at term. These data demonstrate that the chorioamniotic membranes from women who underwent spontaneous preterm labor contain high concentrations of HMGB1, which may contribute to the quantity of this alarmin in the amniotic fluid of women with sterile intra-amniotic inflammation.
A limitation of the current in vitro study is that the effects of HMGB1 were observed at high concentrations. Incubation of the chorioamniotic membranes with low concentrations of HMGB1 did not increase the release of mature IL-1β or IL-6 (Supplemental Fig. S1). It is likely that physiological concentrations of HMGB1 induce PTB in mice [35] by triggering the release of other alarmins which, in turn, will amplify the inflammatory response in the amniotic cavity and promote labor. However, the findings herein provide insight into the mechanisms whereby HMGB1 induces preterm labor and birth, and further in vivo studies are needed in order to extend our observations.
The study herein demonstrates that HMGB1 induces a pro-inflammatory response in the chorioamniotic membranes, which may be partially mediated by the inflammasome. This response was characterized by the upregulation of pro-inflammatory mediators, increased expression and activation of MMP-9, and the processing of mature IL-1β, which is likely mediated by inflammasome-derived CASP-1 activation. Collectively, these findings provide insight into the mechanisms whereby HMGB1 induces preterm labor and birth in mice [35] and explain why the concentration of this alarmin is increased in women who undergo spontaneous preterm labor [20, 33].
ACKNOWLEDGMENT
We gratefully acknowledge Yaozhu Leng, Lorri McLuckie, Rona Wang, and Yang Jiang for their contributions to the execution of this study. We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit for their help in collecting human samples. We also thank staff members of the PRB Clinical Laboratory and the PRB Histology/Pathology Unit for the processing and examination of the pathological sections.
Footnotes
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No. HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. Presented, in part, at the 63rd Annual Scientific Meeting of the Society for Reproductive Investigation, March 16–19, 2016, Montreal, QC, Canada.
REFERENCES
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- Martin JA, Martin MPH, Hamilton BE, Osterman MJK. Births in the United States, 2015. NCHS Data Brief. 2016 No. 258, http://www.cdc.gov/nchs/data/databriefs/db258.pdf. Accessed September 2016. [PubMed] [Google Scholar]
- Behrman RE, , Butler AS , editors. Preterm Birth: Causes, Consequences, and Prevention. Washington DC: National Academic Press; 2007. Societal costs of preterm birth. In. (eds.) [PubMed] [Google Scholar]
- Lubow JM, How HY, Habli M, Maxwell R, Sibai BM. Indications for delivery and short-term neonatal outcomes in late preterm as compared with term births. Am J Obstet Gynecol. 2009;200:e30–e33. doi: 10.1016/j.ajog.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol. 2014;210:426. doi: 10.1016/j.ajog.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3:121–126. doi: 10.1177/107155769600300304. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA, Job AH, Chougnet CA, Kallapur SG. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod. 2015;92:56. doi: 10.1095/biolreprod.114.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson B. Intra-amniotic infection and inflammation in preterm birth–is bacteria always the connection? Commentary on the article by Miralles et al. on page 570. Pediatr Res. 2005;57:473–474. doi: 10.1203/01.PDR.0000156475.50488.B0. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Deisseroth A, Rubartelli A. Damage associated molecular pattern molecules. Clin Immunol. 2007;124:1–4. doi: 10.1016/j.clim.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–358. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–474. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014;28:1–17. doi: 10.3109/14767058.2014.954243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394–1409. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, Margolis L. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213:836. doi: 10.1016/j.ajog.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, Gotsch F, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43:19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Dong Z, Docheva N, Martinez-Varea A, Yoon BH, Hassan SS, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016;44:5–22. doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaemsaithong P, Korzeniewski SJ, Kusanovic JP, Docheva N, Martinez-Varea A, Ahmed AI, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med. 2016;44:23–32. doi: 10.1515/jpm-2015-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Dong Z, Chaiyasit N, Ahmed AI, Yoon BH, et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med. 2016;44:77–98. doi: 10.1515/jpm-2015-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Chaiyasit N, Dong Z, Yoon BH, Hassan SS, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med. 2016;44:53–76. doi: 10.1515/jpm-2015-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Kusanovic JP, Yoon BH, Kim JS, Chaiyasit N, Ahmed AI, Qureshi F, Jacques SM, Kim CJ, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med. 2016;44:33–51. doi: 10.1515/jpm-2015-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, Kusanovic JP, Tolosa JE, Hassan SS, Espinoza J. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35:385–393. doi: 10.1515/JPM.2007.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, Edwin S, Gomez R, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21:449–461. doi: 10.1080/14767050802054550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T. Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–567. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol. 2016;75:3–7. doi: 10.1111/aji.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, Morser J, Stern D, Schmidt AM. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–C879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- Pineles BL, Romero R, Montenegro D, Tarca AL, Than NG, Hassan S, Gotsch F, Draghici S, Espinoza J, Kim CJ. “The inflammasome” in human parturition. Reprod Sci. 2007;14:59A. [Google Scholar]
- Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, Tarca AL, Abrahams VM, et al. A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol. doi: 10.1111/aji.12440. (in press) Published online ahead of print 8 March 2016 as DOI: 10.1111/aji.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Petrilli V, Papin S, Tschopp J. The inflammasome. Curr Biol. 2005;15:R581. doi: 10.1016/j.cub.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol. 2009;183:7623–7629. doi: 10.4049/jimmunol.0902425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G. Inflammasomes as microbial sensors. Eur J Immunol. 2010;40:611–615. doi: 10.1002/eji.200940180. [DOI] [PubMed] [Google Scholar]
- Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187:597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- Horvath GL, Schrum JE, De Nardo CM, Latz E. Intracellular sensing of microbes and danger signals by the inflammasomes. Immunol Rev. 2011;243:119–135. doi: 10.1111/j.1600-065X.2011.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Nunez G. Immunology. Orchestrating inflammasomes. Science. 2012;337:1299–1300. doi: 10.1126/science.1229010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Strowig T, Flavell RA. Inflammasomes: far beyond inflammation. Nat Immunol. 2012;13:321–324. doi: 10.1038/ni.2257. [DOI] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RA, Kronheim SR, Merriam JE, March CJ, Hopp TP. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem. 1989;264:5323–5326. [PubMed] [Google Scholar]
- Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989;86:5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, Elliston KO, Ayala JM, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, Huebner K, Black RA. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- American College of Obstetrics, Gynecology Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin Number 49, December 2003: dystocia and augmentation of labor Obstet Gynecol 2003. 102 1445 1454 [DOI] [PubMed] [Google Scholar]
- Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta. 2008;29(Suppl A):S86–S91. doi: 10.1016/j.placenta.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213:S29–S52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Behnia F, Polettini J, Saade GR, Campisi J. Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging. 2016;8:216–230. doi: 10.18632/aging.100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- Guey B, Bodnar M, Manie SN, Tardivel A, Petrilli V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc Natl Acad Sci U S A. 2014;111:17254–17259. doi: 10.1073/pnas.1415756111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zeng MY, Yang D, Motro B, Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, Mazor M, Maymon E, Berry S. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–1130. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- Ferrand PE, Parry S, Sammel M, Macones GA, Kuivaniemi H, Romero R, Strauss JF., III. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod. 2002;8:494–501. doi: 10.1093/molehr/8.5.494. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- Romero R, Sepulveda W, Mazor M, Brandt F, Cotton DB, Dinarello CA, Mitchell MD. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol. 1992;167:863–872. doi: 10.1016/s0002-9378(12)80003-2. [DOI] [PubMed] [Google Scholar]
- Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci U S A. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A, Hanzawa K, Kumagai K, Okamura H, Takada H. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 2001;167:6568–6575. doi: 10.4049/jimmunol.167.11.6568. [DOI] [PubMed] [Google Scholar]
- Herzog C, Haun RS, Kaushal V, Mayeux PR, Shah SV, Kaushal GP., Meprin A. and meprin alpha generate biologically functional IL-1beta from pro-IL-1beta. Biochem Biophys Res Commun. 2009;379:904–908. doi: 10.1016/j.bbrc.2008.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Than NG, Chaemsaithong P, Chaiworapongsa T, Dong Z, Tarca AL, Abrahams VM, Yeo L, et al. A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci. doi: 10.1177/1933719116675058. (in press). Published online ahead of print 16 November 2016 as DOI: 10.1177/1933719116675058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, Than NG, Chaiworapongsa T, Panaitescu B, Dong Z, Tarca AL, Abrahams VM, et al. A role for the inflammasome in spontaneous preterm labor with acute histologic chorioamnionitis. Reprod Sci. doi: 10.1177/1933719116687656. (in press). DOI: 10.1177/1933719116687656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, Athanassiadis AP, Mitchell MD. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–238. [PubMed] [Google Scholar]
- Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Romero R, Molnar M, Todd H, Baldassare JJ. Cytokine-initiated signal transduction in human myometrial cells. Am J Reprod Immunol. 1993;30:49–57. doi: 10.1111/j.1600-0897.1993.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Rastogi P, Molnar M, Romero R. Interleukin-1beta-induced prostaglandin E2 production in human myometrial cells: role of a pertussis toxin-sensitive component. Am J Reprod Immunol. 2001;45:142–147. doi: 10.1111/j.8755-8920.2001.450304.x. [DOI] [PubMed] [Google Scholar]
- Belt AR, Baldassare JJ, Molnar M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999;181:359–366. doi: 10.1016/s0002-9378(99)70562-4. [DOI] [PubMed] [Google Scholar]
- Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF., III. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol. 1999;154:1755–1762. doi: 10.1016/S0002-9440(10)65431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Zamboni DS, Roy CR, Flavell RA. NALP3: a key player in caspase-1 activation. J Endotoxin Res. 2006;12:251–256. doi: 10.1177/09680519060120040701. [DOI] [PubMed] [Google Scholar]
- Chae JJ, Cho YH, Lee GS, Cheng J, Liu PP, Feigenbaum L, Katz SI, Kastner DL. Gain-of-function pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. 2011;34:755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ. Mark Saltzman W, Mellman I, Ledizet M, Fikrig E, Flavell RA, Fahmy TM. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA. Cardiolipin and the Nlrp3 inflammasome. Cell Metab. 2013;18:610–612. doi: 10.1016/j.cmet.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Costello MJ, Joyce SK, Abrahams VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57:67–80. doi: 10.1111/j.1600-0897.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- King AE, Horne AW, Hombach-Klonisch S, Mason JI, Critchley HO. Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: a potential role in innate immune protection and menstruation. Mol Hum Reprod. 2009;15:311–319. doi: 10.1093/molehr/gap020. [DOI] [PubMed] [Google Scholar]
- Mulla MJ, Yu AG, Cardenas I, Guller S, Panda B, Abrahams VM. Regulation of Nod1 and Nod2 in first trimester trophoblast cells. Am J Reprod Immunol. 2009;61:294–302. doi: 10.1111/j.1600-0897.2009.00694.x. [DOI] [PubMed] [Google Scholar]
- Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas I, Mulla MJ, Myrtolli K, Sfakianaki AK, Norwitz ER, Tadesse S, Guller S, Abrahams VM. Nod1 activation by bacterial iE-DAP induces maternal-fetal inflammation and preterm labor. J Immunol. 2011;187:980–986. doi: 10.4049/jimmunol.1100578. [DOI] [PubMed] [Google Scholar]
- Kersse K, Bertrand MJ, Lamkanfi M, Vandenabeele P. NOD-like receptors and the innate immune system: coping with danger, damage and death. Cytokine Growth Factor Rev. 2011;22:257–276. doi: 10.1016/j.cytogfr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Shah A, Hirsch E. TRIF is an essential adaptor protein of TLR3 and NOD2 synergy in macrophages–a role for viral priming in inflammation-induced parturition. Am J Obstet Gynecol. 2014;210:S231–S232. [Google Scholar]
- Hoang M, Potter JA, Gysler SM, Han CS, Guller S, Norwitz ER, Abrahams VM. Human fetal membranes generate distinct cytokine profiles in response to bacterial Toll-like receptor and nod-like receptor agonists. Biol Reprod. 2014;90:39. doi: 10.1095/biolreprod.113.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra-Gounder AM, Byndloss MX, Seyffert N, Young BM, Chavez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham OH, Tiffany CR, de Jong MF, Kerrinnes T, et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature. 2016;532:394–397. doi: 10.1038/nature17631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti-Andreoni C, Beretta O, Licandro G, Qian HL, Urbano M, Vitulli F, Ricciardi-Castagnoli P, Mortellaro A. Synergism of NOD2 and NLRP3 activators promotes a unique transcriptional profile in murine dendritic cells. J Leukoc Biol. 2010;88:1207–1216. doi: 10.1189/jlb.1009652. [DOI] [PubMed] [Google Scholar]
- Lappas M. NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol Reprod. 2013;89:14. doi: 10.1095/biolreprod.113.110056. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Lappas M. Caspase-1 activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1beta secretion. Am J Reprod Immunol. 2014;71:189–201. doi: 10.1111/aji.12174. [DOI] [PubMed] [Google Scholar]
- Wu D, Choi JC, Coselli J, Shen YH, LeMaire SA. NLRP3 inflammasome activates matrix metalloproteinase-9: potential role in smooth muscle cell dysfunction in thoracic aortic disease. J Surg Res. 2013;179:204. [Google Scholar]
- Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner JF., Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Light A, Hammes SR. LH-Induced steroidogenesis in the mouse ovary, but not testis, requires matrix metalloproteinase 2- and 9-mediated cleavage of upregulated EGF receptor ligands. Biol Reprod. 2015;93:65. doi: 10.1095/biolreprod.115.130971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Shynlova O, Lye SJ. Matrix metalloproteinase expression in the rat myometrium during pregnancy, term labor, and postpartum. Biol Reprod. doi: 10.1095/biolreprod.115.138248. 2016; 95:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopata I, Wize J. A latent gelatin specific proteinase of human leucocytes and its activation. Biochim Biophys Acta. 1979;571:305–312. doi: 10.1016/0005-2744(79)90100-1. [DOI] [PubMed] [Google Scholar]
- Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, Menon R. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol. 1998;179:1248–1253. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- Athayde N, Edwin SS, Pacora P, Romero R. Human decidual cells respond to lipopolysaccharide, proinflammatory cytokines (interleukin 1-beta and tumor necrosis factor-alpha) and interleukin-8 with release of matrix metalloproteinase-9 In The 45th Annual Meeting of the Society of Gynecologic Investigation, Atlanta, GA 1998. 5 62A 63A [Google Scholar]
- Athayde N, Romero R, Gomez R, Maymon E, Pacora P, Mazor M, Yoon BH, Fortunato S, Menon R, Ghezzi F, Edwin SS. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med. 1999;8:213–219. doi: 10.1002/(SICI)1520-6661(199909/10)8:5<213::AID-MFM3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol. 2000;183:887–894. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- Sorokin Y, Romero R, Mele L, Wapner RJ, Iams JD, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Harper M, Caritis SN, Miodovnik M, et al. Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth <32 weeks and adverse neonatal outcomes. Am J Perinatol. 2010;27:631–640. doi: 10.1055/s-0030-1249366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- Vadillo-Ortega F, Gonzalez-Avila G, Furth EE, Lei H, Muschel RJ, Stetler-Stevenson WG, Strauss JF., III. 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol. 1995;146:148–156. [PMC free article] [PubMed] [Google Scholar]
- Vadillo-Ortega F, Hernandez A, Gonzalez-Avila G, Bermejo L, Iwata K, Strauss JF., III. Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinases-1 levels in amniotic fluids from pregnancies complicated by premature rupture of membranes. Am J Obstet Gynecol. 1996;174:1371–1376. doi: 10.1016/s0002-9378(96)70687-7. [DOI] [PubMed] [Google Scholar]
- Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab. 2002;87:1353–1361. doi: 10.1210/jcem.87.3.8320. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205:235. doi: 10.1016/j.ajog.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69:212–230. doi: 10.1111/aji.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Castrejon M, Meraz-Cruz N, Gomez-Lopez N, Flores-Pliego A, Beltran-Montoya J, Viveros-Alcaraz M, Vadillo-Ortega F. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor. Am J Reprod Immunol. 2014;71:86–93. doi: 10.1111/aji.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]