Graphical abstract
Keywords: Basil, Seed, Mucilage, Extraction, Variables, Optimization
Abstract
Aqueous extraction of basil seed mucilage was optimized using response surface methodology. A Central Composite Rotatable Design (CCRD) for modeling of three independent variables: temperature (40–91 °C); extraction time (1.6–3.3 h) and water/seed ratio (18:1–77:1) was used to study the response for yield. Experimental values for extraction yield ranged from 7.86 to 20.5 g/100 g. Extraction yield was significantly (P < 0.05) affected by all the variables. Temperature and water/seed ratio were found to have pronounced effect while the extraction time was found to have minor possible effects. Graphical optimization determined the optimal conditions for the extraction of mucilage. The optimal condition predicted an extraction yield of 20.49 g/100 g at 56.7 °C, 1.6 h, and a water/seed ratio of 66.84:1. Optimal conditions were determined to obtain highest extraction yield. Results indicated that water/seed ratio was the most significant parameter, followed by temperature and time.
Introduction
Basil (Ocimum basilicum L.) is an annual herb that belongs to the family Lamiaceae. The aromatic herb is about 20–60 cm long with white/purple flowers, ovate/lanceolate leaves, and a hairy-petiole [1]. The plant is native to India and Iran, and grows throughout the temperate, tropical and subtropical regions of the world [2]. In India, it is indigenous toward lower hills of Punjab and Himalayas, and is cultivated over 3000 ha of land throughout the tropical and peninsular regions [3]. About 350 tons of essential oil (from basil leaves) is annually produced in India, against world’s production of 500 tons [4], [5].
Basil seed is a tiny black, ellipsoid seed. These seeds are popularly used in traditional desserts (such as sherbet and faloodeh) and also considered important in traditional medicine (to treat colic ulcer, dyspepsia, and diarrhea) [6]. They have a remarkable feature of considerable hydration capacity that is attributed to its adhered seed mucilage. Mucilage produced is reported to be deposited in testa cells during seed development. It reportedly acts as a reservoir for loosely bound water at high water potential during seed germination and early seedling development. On soaking in water, the seed’s outer pericarp swells into a gelatinous mass called hydrogel [7]. During soaking, columnar structures arise unfolded from the pericarp and hold the mucilage tightly to the surface of seed core. The porous layer of exudated mucilage remains tightly adhered and clinged to the core throughout the process of water imbibitions [8], [9].
In recent years, many reports have explored mucilage from various plant seeds of Salvia hispanica, Alyssum homolocarpum, and Descurainia sophia [10], [11], [12]. A major emphasis in all these studies has been channelled toward investigating mucilage extraction from novel sources, and the effect of various parameters, such as temperature, time, water/seed ratio, pH and stirring modes for the release of hydrosoluble compounds. Various such reports indicated varied levels of yields usually dependent on extraction methods and parameters employed [13], [14]. To analyse the effect of extraction conditions on the extraction yield obtained, modeling by response surface methodology (RSM) is a widely accepted method [15].
The present work was carried out to systematically investigate the extraction optimization of mucilage using response surface methodology (RSM), from Ocimum basilicum L. accession found in Kashmir, India. A great variability exists amongst the chemotypes of genus Ocimum, cultivated around the world. Therefore, a variation in the quantities of extracted gum is expected, depending upon its origin.
Material and methods
Materials
Sample collection and preparation
Seeds of Ocimum basilicum L. were procured from local farmers of a high altitude Kashmir region of India. The seeds were cleaned and stored in air tight containers until further use.
Reagents
Sodium hydroxide and hydrochloric acid were procured from Merck Laboratories, Mumbai, India. The reagents used were of analytical grade.
Methods
Proximate analysis
Moisture (925.10), protein (920.87), fat (920.85) and ash (923.03) contents of basil seed were determined according to the standard methods of AOAC [16]. Carbohydrate content was determined by difference. The units for the proximate analysis were g/100 g.
Experimental design
Response surface methodology was employed to study the effect of independent variables X1 (extraction temperature), X2 (extraction time), and X3 (water/seed ratio) on the extraction yield (Y). The levels incorporated for independent variables were based on the results of preliminary analysis. A rotatable centred central composite design (CCRD) was selected to propose the model for the response Y. Apart from linear and quadratic interactions, cubic interactions were also observed in the evaluation of model. Therefore, the experimental data were fit into a second order polynomial equation with extended cubic interactions.
The model proposed for response (Y) was
| (1) |
where Y is the extraction yield (dependent variable) and coefficients represent the intercept (), the main (), quadratic (), interactions effects (, ), and Ei the error term.
Mucilage extraction
Extraction of mucilage was performed using sieving as a mechanical technique. An experimental design of 20 runs at different levels of independent variables (temperature 40–91 °C, time 1.6–3.3 h and water/seed ratio 18:1–77:1) was used. All the experiments were performed in triplicate. An optimal alkaline pH 8 was applied to all the experimental runs.
Mucilage was extracted using distilled water. The pH of water was adjusted to 8, using 0.2 M NaOH or HCl solutions. Seeds were added to a specific proportion of water at a desired temperature. Slurry was maintained at a constant temperature and continuously stirred using a magnetic stirrer under reflux conditions for the entire extraction period. Later, mucilage was separated from seeds using a rubber spatula on a mesh screen. Slurry obtained was passed through a screen of mesh size 10. Separated mucilage and a seed suspension were obtained, which was dried at 50 °C for 10 h in a conventional hot air oven. Also, the adhered mucilage from the dried seeds was separated by rubbing them over a 40 mesh screen. Finally, the weight of whole dried extract of mucilage was recorded.
Extraction yield
Extraction yield for each experimental run was obtained in triplicates. The mucilage obtained from various experimental runs was weighed and yield obtained by the following equation:
| (2) |
Statistical analysis
Experimental data were analysed using a statistical package. Design-Expert version 9.0.6.2 (Stat-Ease Inc., Minneapolis, USA) was employed to predict the response surface methodology for the experimental data. Central composite rotatable design (CCRD) included 20 experimental runs with three replicates of each. The data obtained were fit in the model Eq. (1) where Y is the extraction yield.
Validation of response surface models
In order to determine the adequacy of the model, the predicted and experimental responses were compared. Validity for each experimental run was obtained and adequacy of model was evaluated by analysis of variance (ANOVA). Values for coefficient of determination (R2), adjusted-R2 and predicted-R2 were determined and analysed.
Results and discussion
Proximate analysis
The proximate composition for basil seed is presented in Table 1. A moisture content of 9.4 g/100 g was obtained, which was in range with earlier reports for Salvia hispanica seeds [10]. Ash content of seeds was 5.6 g/100 g. However, seeds showed high content of lipids (33 g/100 g), low protein (10 g/100 g), and a reasonable amount of carbohydrates (43 g/100 g). This variation may be due to the high altitude of ecosystem in which the basil seed sample was grown. Also, various studies on different agricultural plant seeds have reported tendency of higher lipid and lower protein content with an increase in altitude [17].
Table 1.
Proximate composition of basil seeds (n = 3).
| Parameters (g/100 g) | Seed |
|---|---|
| Moisture | 9.4 ± 0.32 |
| Proteina | 10.0 ± 0.46 |
| Fata | 33.0 ± 0.61 |
| Asha | 5.6 ± 0.22 |
| Carbohydratea (by difference) | 43.9 ± 0.22 |
On a dry weight basis.
Model fitting
For model fitting of variation in extraction yield, the sequential sum of squares was analysed. The analysis showed that adding cubic terms significantly improved the model. Therefore, the second-order polynomial equation with extended cubic interactions was employed. Adding cubic interactions significantly improved the model. The model can be referred to as a reduced cubic model. Regression equation obtained for the mucilage yield is represented as follows:
| (3) |
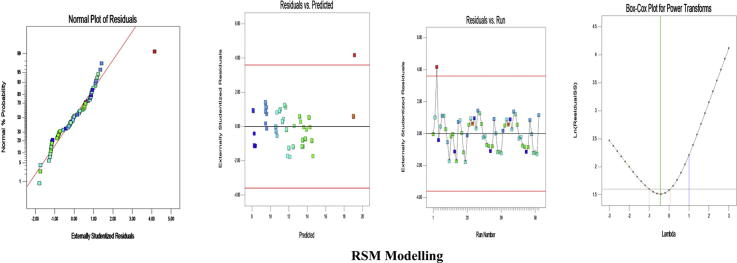
The empirical model was tested by various confirmatory experimental runs. A triplicate of each experimental run was performed (Table 2). Studentized residuals versus predicted values were checked for constant error. Influential values were observed from externally studentized residuals. Predicted values for yield were determined from the design model and compared with the experimental values obtained (Fig. 1). On comparing, the validity for each experimental run was determined. Box-Cox plot was also observed for power transformations. A standard deviation of 2.5 was observed for the model. Model adequacy was evaluated by determination of R2, adjusted R2, and predicted R2; values of 97.41%, 96.57%, and 94.8% were obtained for each respectively. Predicted R2 (94.89%) and adjusted R2 (96.57%) show reasonable agreement with a difference of less than 2%. ANOVA determined a mean value of 11.94, C.V. of 3.99%, and a PRESS value of 19.27. An insignificant lack of fit and a standard error of 0.48 further validate the model. Adequate precision of 43.277 indicates an adequate signal. Thus, it is implied that the model can be used to design space and also applied successfully (Table 3).
Table 2.
Central composite arrangement for variables X1 (temperature), X2 (time), X3 (water ratio), and their response (mucilage yield, %).
| Run | Variables |
Mucilage yield (g/100 g) |
|||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Water/seed ratio (w/v) | Experimental |
Predicted | |||
| X1 | X2 | X3 | Y1 | Y2 | Y3 | Y | |
| 1 | −0.596 (50) | −0.529 (2) | −0.608 (30) | 14.3 | 14.4 | 14.4 | 14.21 |
| 2 | 0.589 (80) | −0.529 (2) | −0.608 (30) | 11.5 | 11.35 | 11.35 | 11.54 |
| 3 | −0.596 (50) | 0.647 (3) | −0.608 (30) | 20.5 | 19.25 | 19.25 | 18.54 |
| 4 | 0.589 (80) | 0.647 (3) | −0.608 (30) | 8.1 | 8.49 | 8.49 | 8.04 |
| 5 | −0.596 (50) | −0.529 (2) | 0.605 (65) | 11.59 | 11.42 | 11.42 | 11.57 |
| 6 | 0.589 (80) | −0.529 (2) | 0.605 (65) | 10.01 | 10 | 10 | 8.96 |
| 7 | −0.596 (50) | 0.647 (3) | 0.605 (65) | 12.10 | 12.07 | 12.07 | 12.03 |
| 8 | 0.589 (80) | 0.647 (3) | 0.605 (65) | 13.40 | 13.41 | 13.40 | 13.68 |
| 9 | −0.991 (40) | 0.059 (2.5) | −0.001 (47.5) | 10.52 | 10.51 | 10.51 | 10.65 |
| 10 | 1.024 (91) | 0.059 (2.5) | −0.001 (47.5) | 11.21 | 11.68 | 11.68 | 11.51 |
| 11 | −0.003 (65) | −1.000 (1.6) | −0.001 (47.5) | 13.86 | 13.54 | 13.54 | 13.67 |
| 12 | −0.003 (65) | 1.000 (3.3) | 0.001 (47.5) | 13.6 | 13.20 | 13.21 | 13.93 |
| 13 | −0.003 (65) | 0.059 (2.5) | −1.024 (18) | 7.97 | 7.86 | 7.86 | 8.95 |
| 14 | −0.003 (65) | 0.059 (2.5) | 1.021 (77) | 13.96 | 14.20 | 14.20 | 14.20 |
| 15 | −0.003 (65) | 0.059 (2.5) | −0.001 (47.5) | 9.91 | 9.86 | 9.86 | 9.54 |
| 16 | 0.589 (80) | −0.529 (2) | −0.261 (40) | 11.04 | 10.59 | 10.59 | 10.73 |
| 17 | −0.596 (50) | 0.647 (3) | 0.085 (50) | 12.61 | 11.99 | 11.99 | 12.51 |
| 18 | −0.596 (50) | 0.647 (3) | −0.261 (40) | 13.10 | 12.99 | 12.99 | 13.21 |
| 19 | 0.589 (80) | −0.529 (2) | 0.085 (50) | 11.40 | 11.49 | 11.49 | 11.83 |
| 20 | −0.003 (65) | 0.059 (2.5) | −0.001 (47.5) | 9.56 | 9.56 | 9.98 | 9.54 |
Y1, Y2, Y3 are the experimental yields of mucilage.
w/v means, weight/volume.
Actual values for X1, X2, X3 are enclosed within brackets.
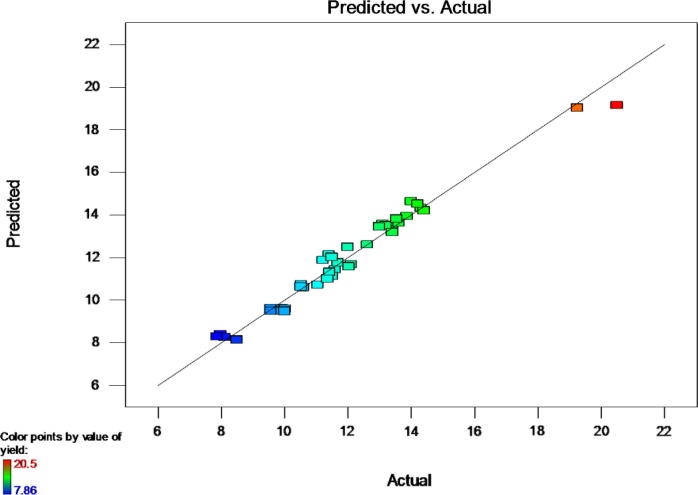
Fig. 1.
Comparison of actual and predicted yields for extraction of basil seed mucilage.
Table 3.
Evaluation of polynomial model (Central Composite Rotatable Design).
| Source | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| Model | 14 | 367.71 | 26.26 | 115.51 | <0.0001 |
| Residuals | 5 | 9.78 | 0.23 | ||
| Lack of fit | 4 | 9.66 | 0.24 | 6.39 | 0.0751 |
| Pure error | 1 | 0.11 | 0.038 | ||
| Corr total | 19 | 377.64 |
DF, degrees of freedom; SS, sum of squares; MS, mean square; F value; P value.
Interpretation of response surface plots for extraction yield
Experimental values for mucilage yield varied from 7.86 to 20.5 g/100 g in 20 different extraction conditions (Table 2). Maximum basil seed mucilage yield is higher than that of cress seed [14], flaxseed [18], and chia seeds [19], which have an extraction yield in the range of 6.46 g/100 g, 7.9 g/100 g and 6.97 g/100 g respectively. The difference in yield occurs due to the variability amongst chemotypes of various genuses across the world [20]. And it can be predicted from the results that the basil seed from the Kashmir region of India produces reasonable amounts of mucilage.
Analysis of variance of variables and their interactions are presented in Table 4. The magnitude of each coefficient measures its importance. Significance for each coefficient was analysed by the P-value obtained in ANOVA. Values of P (P < 0.05) indicate the significance of terms. Lesser values for P indicate more coefficient significance. Results from ANOVA show that the yield was significantly influenced by temperature and water/seed ratio. Extraction time had a lesser significance. Various interaction effects were observed in the model. All the interactions had a significant effect on extraction yield. Regression Eq. (3) can be used to make predictions about the response (Table 5). The coefficients are scaled to accommodate the units of each factor. However, to determine the relative impact of each factor and gain a better understanding of obtained results, 2D Contour plots and 3D response surface were plotted. They illustrate the interaction between variables and facilitate the location of optimal extraction conditions.
Table 4.
ANOVA for Response Surface Reduced Cubic Model.
| Source | Sum of squares | Mean square | F value | P-value | ||
|---|---|---|---|---|---|---|
| DF | Prob > F | |||||
| Block | 0.17 | 2 | 0.087 | |||
| Model | 368.42 | 14 | 26.32 | 124.38 | <0.0001 | Significant |
| A-temp | 3.71 | 1 | 3.71 | 17.52 | 0.0001 | |
| B-time | 2.52 | 1 | 2.52 | 11.91 | 0.0013 | |
| C-water | 11.26 | 1 | 11.26 | 53.20 | <0.0001 | |
| AB | 8.31 | 1 | 8.31 | 39.28 | <0.0001 | |
| AC | 53.43 | 1 | 53.43 | 252.55 | <0.0001 | |
| BC | 1.66 | 1 | 1.66 | 7.86 | 0.0075 | |
| A2 | 6.65 | 1 | 6.65 | 31.41 | <0.0001 | |
| B2 | 60.38 | 1 | 60.38 | 285.39 | <0.0001 | |
| C2 | 12.29 | 1 | 12.29 | 58.10 | <0.0001 | |
| ABC | 46.49 | 1 | 46.49 | 219.73 | <0.0001 | |
| A2B | 7.67 | 1 | 7.67 | 36.26 | <0.0001 | |
| A2C | 15.62 | 1 | 15.62 | 73.84 | <0.0001 | |
| AC2 | 52.28 | 1 | 52.28 | 247.12 | <0.0001 | |
| C3 | 7.89 | 1 | 7.89 | 37.31 | <0.0001 | |
| Residual | 9.10 | 43 | 0.21 | |||
| Lack of fit | 8.98 | 40 | 0.22 | 5.94 | 0.0829 | Non significant |
| Pure error | 0.11 | 3 | 0.038 | |||
| Cor total | 377.69 | 59 |
Table 5.
Regression results for the Response Surface Cubic Model.
| Source | Results |
|---|---|
| Std. Dev. | 0.46 |
| Mean | 11.94 |
| C.V.% | 3.85 |
| PRESS | 17.64 |
| R-squared | 0.9759 |
| Adj R-squared | 0.9681 |
| Pred R-squared | 0.9533 |
| Adeq precision | 44.942 |
Effect of temperature and time
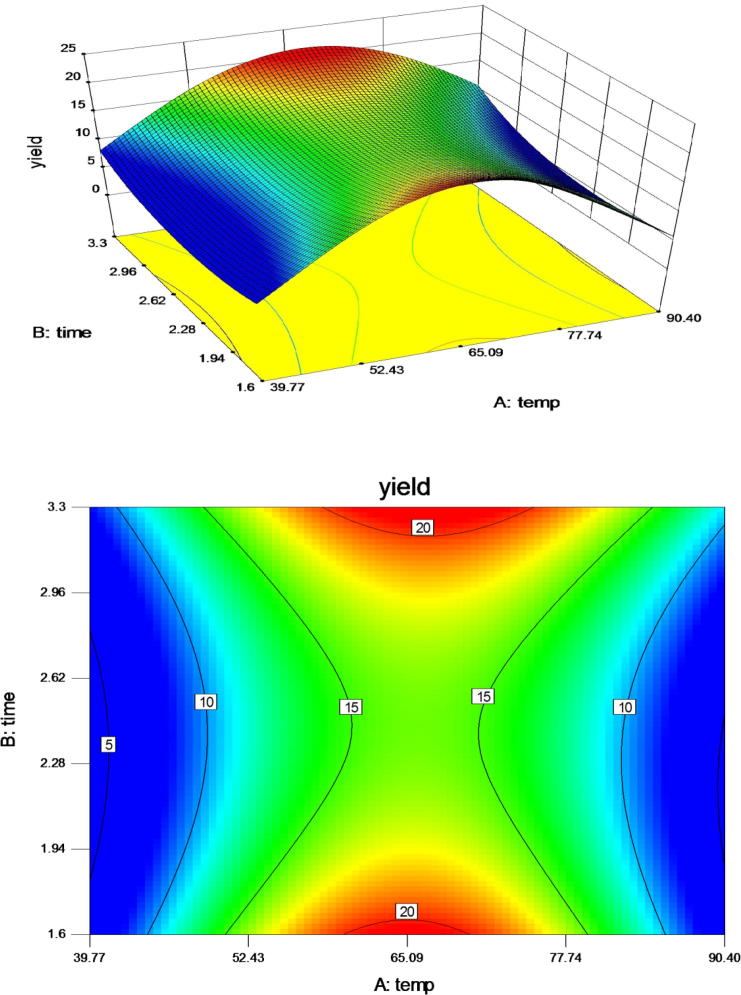
The effect of temperature and time, presented in Fig. 2 shows a strong interaction between temperature and time. An extraction yield of 10.52 g/100 g was obtained at a relatively low temperature (40 °C). Extraction yield considerably increased with increase in temperature from 50 °C to 65 °C. It can be inferred from Fig. 2 that yield is higher at 50–65 °C. Response surface shows that extraction yield increased to a maximum point and then decreased. Maximum yield of 20.5 g/100 g was obtained at 50 °C and started to decrease at and above 80 °C. Temperature allows better penetration of water into solid matrix to solubilize the compounds. As a result, the mucilage was easily released and the extraction yield increased [19]. At higher temperatures (≤50 °C and ≥80 °C), seeds become less sticky and mucilage release occurs [21]. However, at and above 80 °C, degradation of polysaccharides leads to decrease in the mucilage yield [14]. Also, increasing the time of extraction led to an increase in the extraction yield. Extraction time influences the efficiency of extraction and increases the yield. Liquid penetrates, dissolves and subsequently diffuses out the mucilage from seed pericarp. Trends in extraction yield showed an increasing tendency from 2 to 3 h and a decreasing trend is observed above 3 h. This might be due to the exposure of the seed to aqueous medium [22]. The combined effect of temperature and time can be best explained by mass transfer effect. Mass transfer effect causes the mucilage to diffuse at a higher rate, showing a strong interaction between temperature and time [23], [24], [25]. The effect of time was more pronounced at higher temperatures (50–65 °C) but prolonged extraction time might have caused changes in the polysaccharides structure and decreased the yield. A combined effect of increase in temperature and extraction time led to an increase in the yield of mucilage. Highest extraction yield (20.5 g/100 g) of seed mucilage was obtained at a high temperature and short extraction time of 2 h. However, on increasing the temperature beyond a certain point of time (3 h) led to a decrease in the yield. This indicated that about 2 h is a sufficient time for mucilage extraction. Decrease in yield, after 2 h time occurs due to the hydrolysis of polysaccharides at higher temperature [14]. Various other studies pertaining to response surface methodology, reported a decrease in yield of bioactive compounds with an increase in temperature. The decreasing yield was a result of thermal degradation of bioactive compounds at high temperatures [26]. Results correspond with those obtained for Alyssum homolocarpum seed [11]. Similar results were demonstrated for the extraction of mucilage from chia seeds [19].
Fig. 2.
Response surface and contour plot illustration for the effect of temperature and time on extraction yield at water ratio 1:58.
Effect of water/seed ratio and time
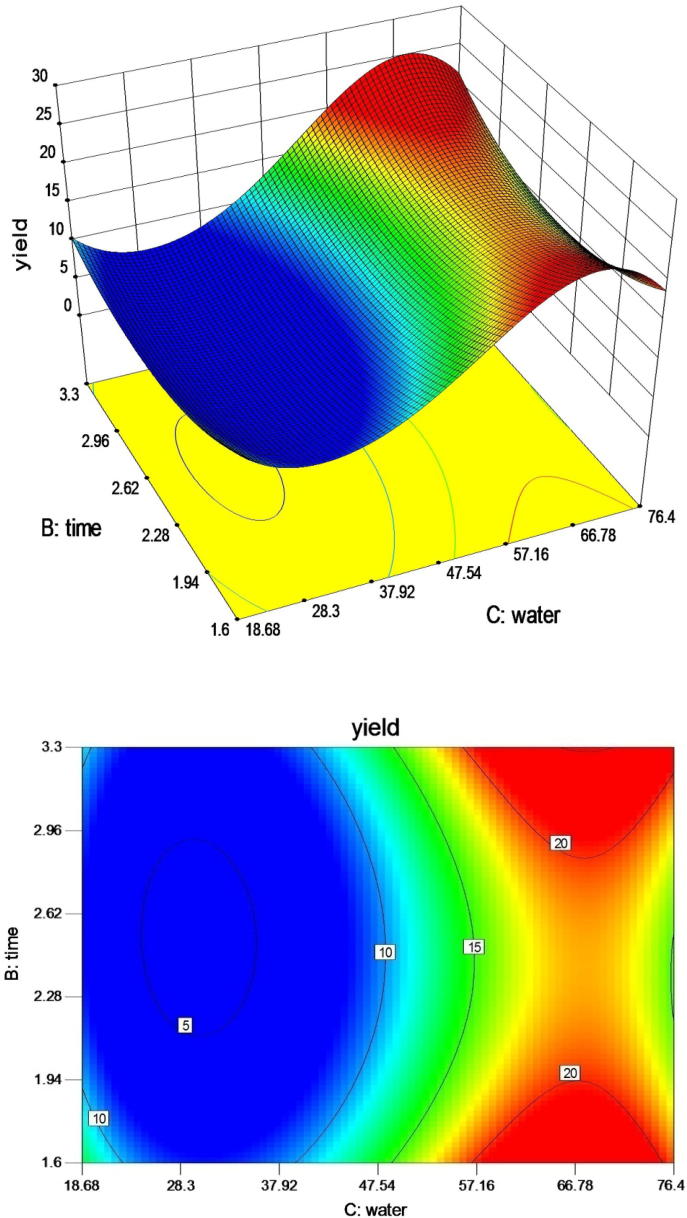
The effect of water/seed ratio and time is shown in Fig. 3. Lowest extraction yield of 7.86 g/100 g was obtained at water/seed ratio of 18:1. Increase in water/seed ratio up to 30:1 increased the yield to a certain maximum value of 20.5 g/100 g. Temperature and time held constant, and higher water/seed ratios (18:1, 30:1, 40:1, 47.5:1, 50:1, 65:1, 77:1) showed an increase in mucilage yield. Increase in time also showed increased extraction yield. Extraction time leads to an increased exposure of seeds to aqueous medium [22]. Fig. 3 shows increase in the yield. The response surface shows the effect of time is more pronounced at higher water/seed ratios. Extraction time influences the extraction efficiency and selectivity of the fluid. A significantly long extraction time has a positive effect on the yield of polysaccharides [27]. Response surface shows somewhat linear interaction between water/seed ratio and time. Combined effect of increase in extraction time and water/seed ratio increased the yield. However, the graph predicted that yield increases steadily and slowly rather than a sharp increase. Similar results were also obtained where a longer extraction time favoured the polysaccharide production from Malva sylvestris [28]. Also, cress seeds showed similar results for extraction yield [14]. Yield of mucilage increased with increasing the water/seed ratio. This may be due to the availability of more liquid that acts as a driving force to exude mucilage out of the seeds as the volume of water/seed ratio was increased [11]. A greater mucilage yield was also reported from Alyssum homolocarpum, wild sage seed gum, and Opuntia spp. seeds as function of water ratio [22].
Fig. 3.
Response surface and contour plot illustration for the effect of water ratio and time on extraction yield at a temperature of 65 °C.
Effect of temperature and water/seed ratio
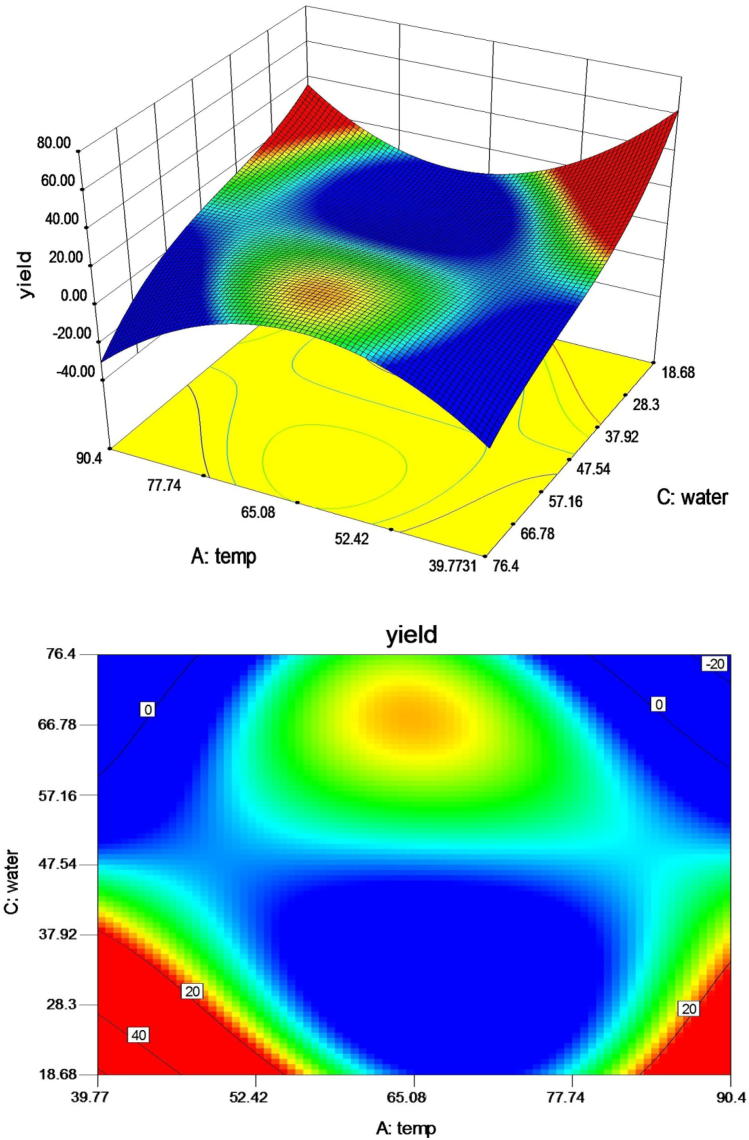
Effect of temperature and water seed ratio is presented in Fig. 4. Results obtained showed slight co-relation of lesser significance. Response surface showed a steady increase to equilibrium and later an abrupt increase in yield. At water/seed ratios 30:1, 40:1, 50:1 and 65:1, an increase in temperature led to a decrease in yield. However, at a water/seed ratio 47.5:1 and an increase in temperature from 40 to 65 °C showed an increasing trend. It can be revealed from Fig. 4, decreased water/seed ratios at higher temperatures, increase the yield significantly. Water acts a driving force and temperature allows better penetration of aqueous medium to increase yield [19], [22].
Fig. 4.
Response surface and contour plot illustration for the effect of water ratio and temperature on extraction yield at 2.42 h.
Single factor results
Effect of extraction time on yields
An extraction time of about 1.6–3.3 h was adopted. Table 2 shows that keeping the temperature at 50 °C and water/seed ratio of 1:30 constant, a higher yield is obtained on increasing extraction time from 2 to 3 h. Therefore, it is concluded that increase in the extraction time resulted in higher extraction yield. Also, the yield reached the highest when an extraction time was 3 h. Similar, results have been reported for extraction of polysaccharides from Dioscorea nipponica Makino [29].
Effect of extraction temperature on yields
The effect of extraction temperature showed that increasing the temperature leads to a significant increase in extraction yield. However, on increasing the temperature beyond 65 °C, decrease in mucilage yield was observed. Table 2 shows a significant increase in yield when temperature was elevated from 40 to 50 °C. The highest yield was also obtained at a temperature of 50 °C. A similar interaction was observed for Tricholoma matsutake [30].
Effect of water/seed ratio on yields
Temperature and time held constant, and elevated water/seed ratios (18:1, 30:1, 40:1, 47.5:1, 50:1, 65:1, 77:1) resulted in increased extraction yield. Gum extraction from Dioscorea nipponica Makino reported similar results [29].
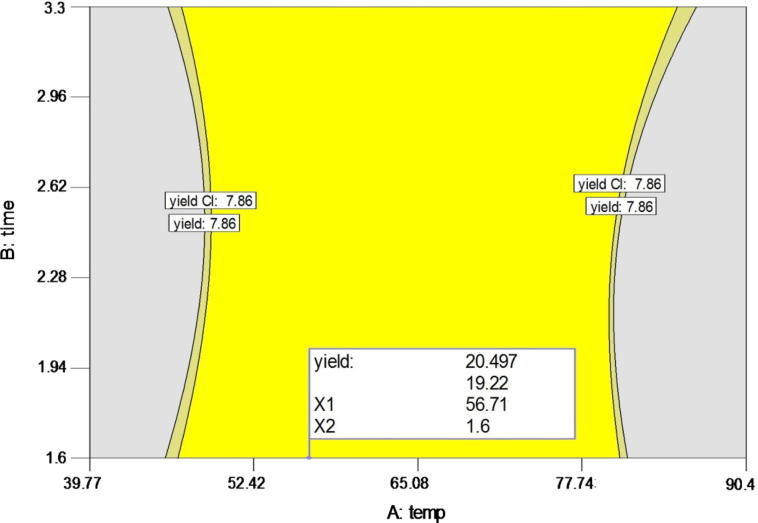
Mucilage optimization
Numerical and graphical optimizations were used to determine the optimal conditions. Optimum condition was based on the highest extraction yield. An optimal condition of 56.71 °C, 1.6 h, and water/seed ratio of 66.84:1 was predicted by Design-Expert, with an extraction yield of 20.49 g/100 g. A graphical representation shown in Fig. 5 illustrates the optimal yield. The graphical plot was obtained by superimposing the contour plots of all the analysed results for various experimental runs. The plot illustrates the best extraction conditions to obtain the highest extraction yield of basil seed mucilage. Validation of the developed model was obtained by performing various experimental runs. Model adequacy to predict optimal conditions was tested by comparing the experimental levels with optimization levels. Results showed that the experimental and predicted yield values were not significantly different (Fig. 1). The experimental value for yield obtained was 20.5 g/100 g which is in line with that of predicted value.
Fig. 5.
Graphical illustration showing optimal conditions for the extraction of mucilage.
Conclusions
Response surface modeling for extraction provides a way to realize the interdependence of extraction conditions on the yield of basil seed mucilage. Results show that the effect of water/seed ratio has statistical significance in the extraction of mucilage. Second order polynomial model with extended cubic interactions was obtained to predict the extraction yield of mucilage. Response analysis demonstrated a significant reduced cubic regression. Model exhibited R2 value of 97.41% with an insignificant lack of fit. The optimum conditions were obtained by the software as 56.71 °C, 1.6 h, and water/seed ratio of 66.84:1. And an optimal extraction yield of 20.5 g/100 g was obtained by graphical optimization of results.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Klimankovaa E., Holadová K., Hajšlová J., Čajka T., Poustka J., Koudela M. Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008;107:464–472. [Google Scholar]
- 2.Lal S., Mistry K.N., Thaker R., Shah S.D., Vaidya P.B. Genetic diversity assessment in six medicinally important species of Ocimum from central Gujarat (India) utilizing RAPD, ISSR and SSR markers. Int J Adv Biol Res. 2012;2(2):279–288. [Google Scholar]
- 3.Bhasin M. Ocimum-taxonomy, medicinal potentialities and economic value of essential oil. J Biosphere. 2012;1:48–50. [Google Scholar]
- 4.Kadam P.V., Yadav K.N., Jagdale S.K., Shivatare R.S., Bhilwade S.K., Patil M.J. Evaluation of Ocimum sanctum and Ocimum basillicum Mucilage – as a pharmaceutical excipient. J Chem Pharm Res. 2012;4(4):1950–1955. [Google Scholar]
- 5.Sarfaraz Z., Anjum F.M., Khan M.I., Arshad M.S., Nadeem M. Characterization of basil (Ocimum basilicum L.) parts for antioxidant potential. Afr J Food Sci Technol. 2011;2(9) (204-203) [Google Scholar]
- 6.Simon J.E., Morales M.R., Phippen W.B., Vieira R.F., Hao Z. Perspectives on new crops and uses. In: Janick J., editor. Source of aroma compounds and a popular culinary and ornamental herb. ASHS Press; Alexandria, VA: 1999. pp. 499–505. [Google Scholar]
- 7.Azoma J., Sakamoto M. Cellulosic hydrocolloid system present in seed of plants. Trends Glycosci Glyc. 2003;15:1–14. [Google Scholar]
- 8.Zhou D., Ponder M., Barney J., Welbaum G. The production and function of mucilage by sweet basil (Ocimum basilicum L.) seeds. Virginia Tech. 2012;515 [Google Scholar]
- 9.Salgado-Cruza M.P., Calderon-Domingueza G., Chanona-Pereza J., Farrera-Rebolloa R.R., Mendez-Mendezb J.V., Diaz-Ramireza M. Chia (Salvia hispanica L.) seed mucilage release characterization. A microstructural and image analysis study. Ind Crops Prod. 2013;51:453–462. [Google Scholar]
- 10.Muñoz L.A., Cobos A., Diaz O., Aguilera J.M. Chia seeds: microstructure, mucilage extraction and hydration. J Food Eng. 2012;108:216–224. [Google Scholar]
- 11.Koocheki A., Mortazavi S.A., Shahidi F., Razavi S.M.A., Kadkhodaee R., Milani J. Rheological properties of mucilage extracted from Alyssum homolocarpum seed as a new source of thickening agent. J Food Process Eng. 2010;33:861–882. [Google Scholar]
- 12.Golalikhani M., Khodaiyan F., Khosravi A. Response surface optimization of mucilage aqueous extraction from flixweed (Descurainia sophia) seeds. Int J Biol Macromol. 2014;70:444–449. doi: 10.1016/j.ijbiomac.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Koocheki A., Taherian A.R., Razavi S.M.A., Bostan A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocoll. 2009;23:2369–2379. [Google Scholar]
- 14.Karazhiyan H., Razavi S.M.A., Phillips G.O. Extraction optimization of a hydrocolloid extract from cress seed. Food Hydrocoll. 2011;25:915–920. [Google Scholar]
- 15.Wani A.A., Kaur D., Wani I.A., Sogi D.S. Extraction optimization of watermelon seed protein using response surface methodology. LWT-Food Sci Technol. 2008;41:1514–1520. [Google Scholar]
- 16.AOAC. Official methods of analysis of the association of official analytical chemists. Virginia, USA: AOAC Inc.; 1990.
- 17.Ayerza R., Coates W. Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.) Ind Crops Prod. 2011;34:1366–1371. [Google Scholar]
- 18.Cui S.W., Mazza G., Oomah B.D., Biliaderism C.G. Optimization of an aqueous extraction process for flaxseed gum by response surface methodology. LWT-Food Sci Technol. 1994;27:363–369. [Google Scholar]
- 19.Campos B.E., Ruivo T.D., Scapim M.R.S., Madrona G.S., Bergamasco R.C. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT-Food Sci Technol. 2016;65:874–883. [Google Scholar]
- 20.Kumar V., Rani A., Solanki S., Hussain S.M. Influence of growing environment on the biochemical composition and physical characteristics of soybean seed. J Food Comp Anal. 2006;19:188–195. [Google Scholar]
- 21.Lonein M., Merson R.L. Academic Press Inc.; New York: 1979. Food engineering. Principles and selected applications; p. 494. [Google Scholar]
- 22.Jouki M., Mortazavi S.A., Yazdi F.T., Koocheki A. Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. Int J Biol Macromol. 2014;66:113–124. doi: 10.1016/j.ijbiomac.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Braga M.E.M., Moreschi S.R.M., Meireles M.A.A. Effects of supercritical fluid extraction on Curcuma longa L. and Zingiber officinale R. starches. Carbohydr Polym. 2006;63:601–608. [Google Scholar]
- 24.Ye C., Jiang C.J. Optimization of extraction process of crude polysaccharides from Plantago asiatica L. by response surface methodology. Carbohydr Polym. 2011;84:495–502. [Google Scholar]
- 25.Balke D.T., Diosady L.L. Rapid aqueous extraction of mucilage from whole white mustard seed. Food Res Int. 2000;33:347–356. [Google Scholar]
- 26.Singh B., Oberoi D.P.S., Wani I.A., Sogi D.S. Effect of temperature, salt concentration, pH and time on thermal degradation of pumpkin (Cucurbita pepo) puree. Adv Food Sci. 2009;31(2):96–101. [Google Scholar]
- 27.El Batal H., Hasib A. Optimization of extraction process of carob bean gum purified from carob seeds by response surface methodology. Chem Process Eng Res. 2013;12:1–8. [Google Scholar]
- 28.Samavati V., Manoochehrizade A. Polysaccharide extraction from Malva sylvestris and its anti-oxidant activity. Int J Biol Macromol. 2013;60:427–436. doi: 10.1016/j.ijbiomac.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 29.Luo D. Optimization of total polysaccharide extraction from Dioscorea nipponica Makino using response surface methodology and uniform design. Carbhydr Polym. 2012;90:284–288. doi: 10.1016/j.carbpol.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 30.Yin X., You Q., Jiang Z. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr Polym. 2011;86:1358–1364. doi: 10.1016/j.carbpol.2013.11.072. [DOI] [PubMed] [Google Scholar]