Abstract
Objective
Medical management (MM) with antiplatelet (AP) and statin therapy is recommended for most patients undergoing vascular surgery and has been advocated by the Vascular Quality Initiative (VQI). We analyzed the effect of VQI participation on perioperative (preoperative and postoperative) MM use over time and the effect of discharge MM on patient survival.
Methods
We studied VQI patients treated with MM preoperatively and at discharge from 2005 to 2014, including all elective carotid endarterectomy/carotid stenting (n = 28,092), suprainguinal/infrainguinal bypass (n = 11,362), peripheral vascular interventions (n = 24,476), open/endovascular abdominal aortic aneurysm repair (n = 13,503), and thoracic endovascular aneurysm repair (n = 702). We examined trends of MM use over time, as well as the effect of duration of VQI participation on MM use. Multivariable logistic regression analysis was performed to identify factors associated with MM use. In addition, the Cox proportional hazards model was used to identify factors associated with 5-year survival.
Results
MM with AP and statin preoperatively and postoperatively across VQI centers improved from 55% in 2005 to 68% in 2009, with a subsequent overall decline to 62% by 2014, coincident with many new centers with lower MM rates joining VQI in 2010. Longer center participation in VQI was associated with improved perioperative MM overall. This was also noted across all procedure types, with MM increasing from 47% to 82% for aneurysm repairs and 69% to 83% for carotid procedures from 1 to 12 years of participation in VQI. After multivariable adjustment, centers in VQI ≥3 years were 30% more likely to have patients on MM (odds ratio, 1.3, 95% confidence interval [CI], 1.3–1.4). Importantly, discharge on AP and statin therapy was associated with improved 5-year survival, compared with discharge on neither medication (82% [95% CI, 81%–83%] vs 67% [95% CI, 62%–72%]), and an adjusted hazard ratio for death of 0.6 (95% CI, 0.5–0.7; P < .001). Discharge on a single medication was associated with intermediate survival at 5 years (AP only: 77% [95% CI, 75%–79%]; statin only: 73% [95% CI, 68%–77%]).
Conclusions
These data demonstrate that MM is associated with improved survival after a number of vascular procedures. Importantly, VQI participation improves the use of MM, demonstrating that involvement in an organized quality effort can affect patient outcomes.
Patients undergoing vascular surgical procedures often present with multiple cardiovascular morbidities. Up to 75% of patients with peripheral arterial disease (PAD) will ultimately die of cardiovascular causes.1 Secondary treatment for cardiovascular disease in patients with PAD is based on medical management (MM). Multiple intersocietal consensus guidelines recommend treatment of patients with coronary artery disease (CAD) and symptomatic PAD with antiplatelet (AP) and 3-hydroxy-3 methyl-glutaryl-coenzyme A (statin) medications in addition to smoking cessation and blood pressure control.1–6
Despite these guidelines, only one-third of Americans with PAD are taking an AP or statin medication, or both, based on a recent National Health and Nutrition Examination Study. Lack of AP and statin therapy in these patients was associated with higher long-term mortality.7 Further, there is wide variation in the use of AP and statin medications at the time of intervention for PAD, which has also been associated with 5-year survival.7 Moreover, despite improvement over time, there is variation by procedure and among centers in perioperative AP and statin usage.8
In 2011, the Society for Vascular Surgery launched the Vascular Quality Initiative (VQI) to improve the care and outcomes for patients with vascular disease.9 Although variation exists in the use of MM with AP and statins, factors that may improve medication use, such as participation in the VQI, are not well described. The purpose of this study was to describe the utilization of AP and statin medications perioperatively and to understand factors associated with improved perioperative medication use across centers participating in VQI as well as the effect of MM on overall survival.
METHODS
Database
This is a retrospective analysis of data collected prospectively by the VQI, a nationwide quality improvement initiative developed originally in 2002 in New England10 to improve outcomes of vascular procedures.9 Registry data are compared with hospital claims in annual audits, and missing cases are retrieved to track all procedures.10 Mortality data are supplemented by semi-annual matching of registry data with the Social Security Death Index (SSDI).
Construction of analytic cohort
Because statin use at discharge was tracked beginning in 2005, all patients undergoing their first-time intervention in the VQI data set from 2005 to 2014 for carotid endarterectomy, carotid artery stenting, infrainguinal or suprainguinal arterial bypass, peripheral vascular interventions (PVIs), open or endovascular abdominal aortic aneurysm (AAA) repair (EVAR), and thoracic endovascular aortic repair (TEVAR) for aneurysmal disease (other TEVAR indications, such as trauma or dissection, were excluded) were identified. This yielded our initial cohort of 94,961 patients undergoing first-time procedures. These patients were selected because patients with these conditions meet the criteria outlined in multiple guidelines to support AP and statin use for patients with cerebrovascular disease and symptomatic PAD.1,3–5,11
Although aneurysmal disease carries no specified societal recommendations regarding medication treatment with AP and statin medications, most of these patients have indications for known coronary risk factors that support their use.2 These factors include a history of CAD, hypertension, positive stress test result, prior coronary revascularization, a prior arterial bypass or peripheral intervention, or prior carotid revascularization. All TEVAR patients and 98.9% of AAA patients had at least one of these cardiovascular risk factors to recommend AP and statin use. Only 1.1% of AAA patients (n = 584) had none of these risk factors. As noted in our prior study, patients with none of these cardiac risk factors had similar survival and outcomes to AAA patients with cardiac comorbidities, and thus, they were included in the final study cohort.8
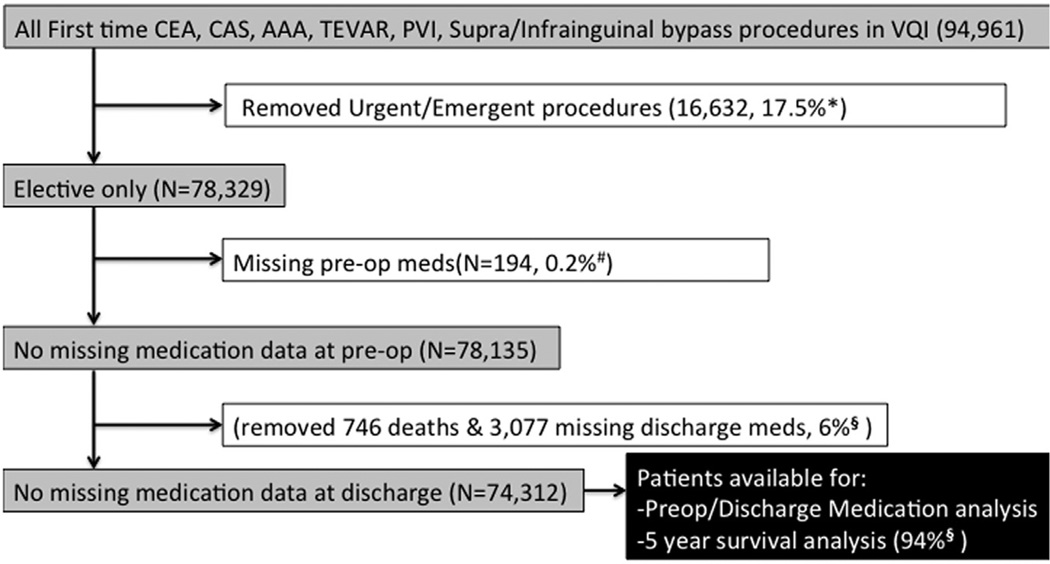
All cases were elective; urgent or emergency cases were excluded (3096 emergency, 12,843 urgent, and 693 missing urgency data). These exclusion criteria were designed to provide a cohort of patients with the potential to be prescribed AP and statin medications before elective surgery. Finally, 194 patients (0.25%) were removed from analysis for missing preoperative medication data. This resulted in 78,135 patients with preoperative data available (Fig 1). To analyze the use of AP and statins preoperatively and postoperatively, our final patient cohort excluded 746 (0.95%) who were not eligible for discharge medications because they died in-hospital and 3077 (3.9%) who were missing discharge medication data. This resulted in 74,312 patients with preoperative and postoperative medications for review (Fig 1).
Fig 1.
Identification of cases for analysis. AAA, Abdominal aortic aneurysm; CAS, carotid artery stenting; CEA, carotid endarterectomy; PVI, peripheral vascular intervention; TEVAR, thoracic endovascular aortic repair; VQI, Vascular Quality Initiative. *Proportion represents total urgent/emergency of all first time procedures. #Denominator is all eligible patients for analysis (elective first-time procedures). §Denominator is all patients available for perioperative analysis.
Definitions of exposures and outcomes
Our primary outcome measure was treatment with AP and statin preoperatively and at discharge. Statin use was defined as being on any type of statin medication at any dose. Patients were considered on AP medication if they were taking aspirin (any dose) or any P2Y12a antagonist (commonly clopidogrel). Preoperative medication use was defined as taking the medication ≤36 hours of surgery. Eligible patients classified as being intolerant to AP (254 [0.3%]) and statin (1397 [1.8%]) were considered as not taking these medications. Patients were reassessed for AP and statin use at the 1-year follow-up but not routinely outside this encounter in follow-up. No serologic tests were done on drug efficacy, lipid levels, or other biochemical markers. Exposure variables for MM included each patient’s demographics and preoperative factors.
Our secondary outcome was 5-year survival. The effect of medication use at discharge on survival was evaluated. We hypothesized that survival might be affected by medications prescribed at discharge that could reduce long-term cardiovascular events. Patients were excluded from the 5-year survival analysis if they died in-hospital or ≤30 days postoperatively (746 [0.95%]) or had missing discharge medication data (3077 [3.9%]). This left 74,312 patients for 5-year survival analysis (Fig 1). Patients who died ≤30 days were excluded because these likely represented events related to their operation. All survival analysis was started on postoperative day 30 to better gauge the long-term effect of MM.
Long-term survival was determined from the VQI database 1-year follow-up reports and by matching patient information with the SSDI. There is no information on any patient’s cause of death, only the time from the procedure until their death. Patients not in the SSDI or who did not have a hospital record indicating they had died were considered alive and censored at the time of data analysis (March 13, 2014). Definitions of medical comorbidities in the VQI cohort have been previously published.12
Physicians, nurses, or clinical data abstractors entered data prospectively on clinical and demographic variables. Research analysts were blinded to patient, surgeon, and hospital identity. The Committee for the Protection of Human Subjects at Dartmouth Medical School has approved the use of deidentified data from VQI for research purposes. Patient consent was not obtained. The VQI is a federally listed Patient Safety Organization authorized under the Patient Safety and Quality Improvement Act of 2005 to allow collection of patient data without consent.
Statistical analysis
Our primary outcome measure was the use of MM in the perioperative period, defined as AP and statin used preoperatively and prescribed at discharge. Our secondary outcome was 5-year survival with patients stratified by their medication use (none, AP only, statin only, or both) to associate the effect of MM on survival. Independent variables for analysis included patient and procedural characteristics. In addition, for each patient procedure, the centers were categorized by the duration of their participation within the VQI at the time of the operation to assess the associated effect of participation in VQI on MM. Comparison of the effects of independent variables on MM was done using the t-test for continuous data and χ2 test for categoric data. To adjust for patient cofactors, logistic regression analysis was used. Univariate comparisons for survival analyses were made with log-rank for categoric variables or Cox proportional hazards for continuous variables, starting at 30 days to exclude events ≤30 days.
For multivariable logistic regression and survival analysis with Cox proportional hazards, variables of clinical significance and those with a P value of <.1 by univariate analysis were included in a backwards stepwise multivariable model. Variables were removed from the model using the likelihood ratio test.
Test of trend for MM over time was assessed by logistic regression. Continuous variables with nonlinear risk were categorized for analysis. Age was categorized by quartiles. Probability values of <.05 were considered significant. Analyses were done using Stata 13 software (StataCorp LP, College Station, Tex).
RESULTS
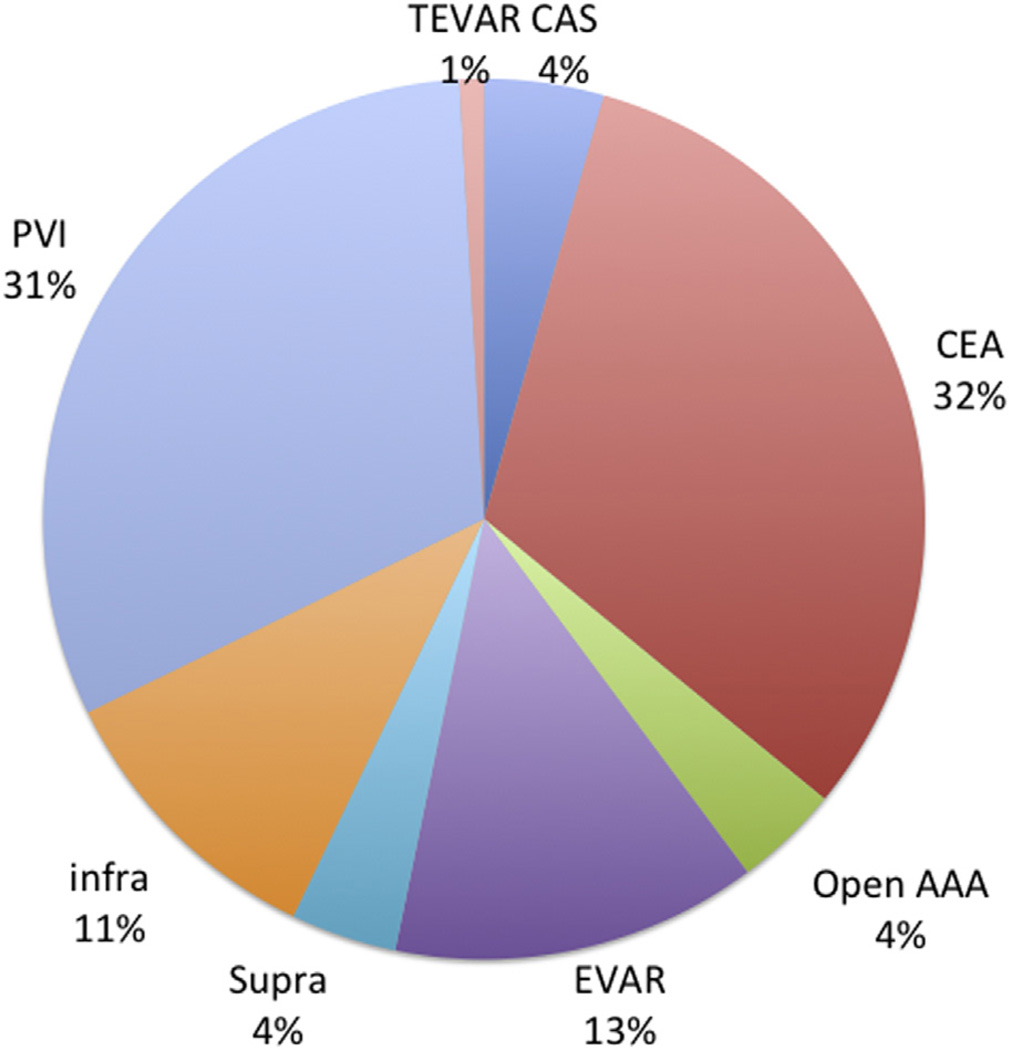
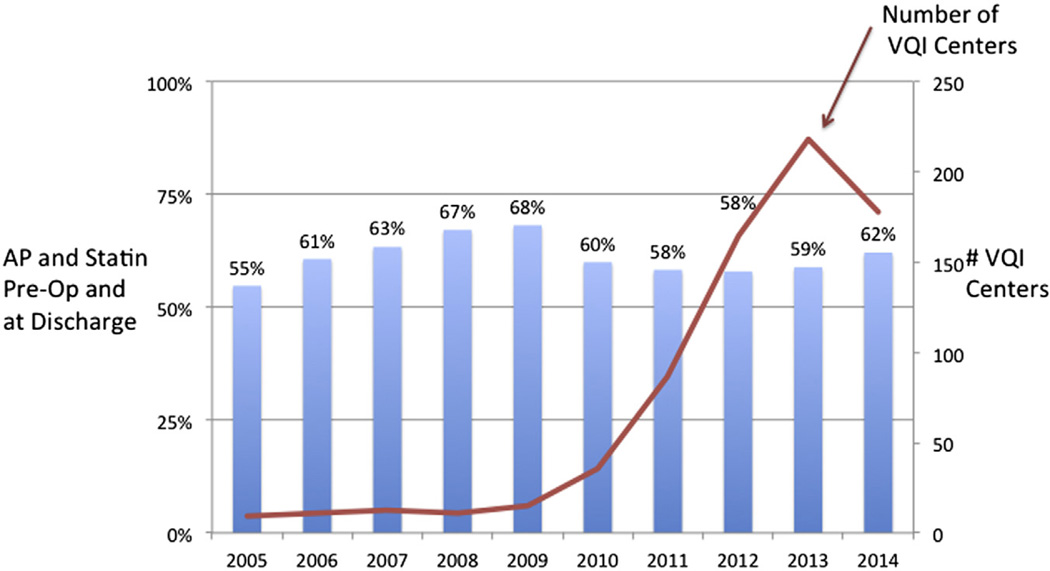
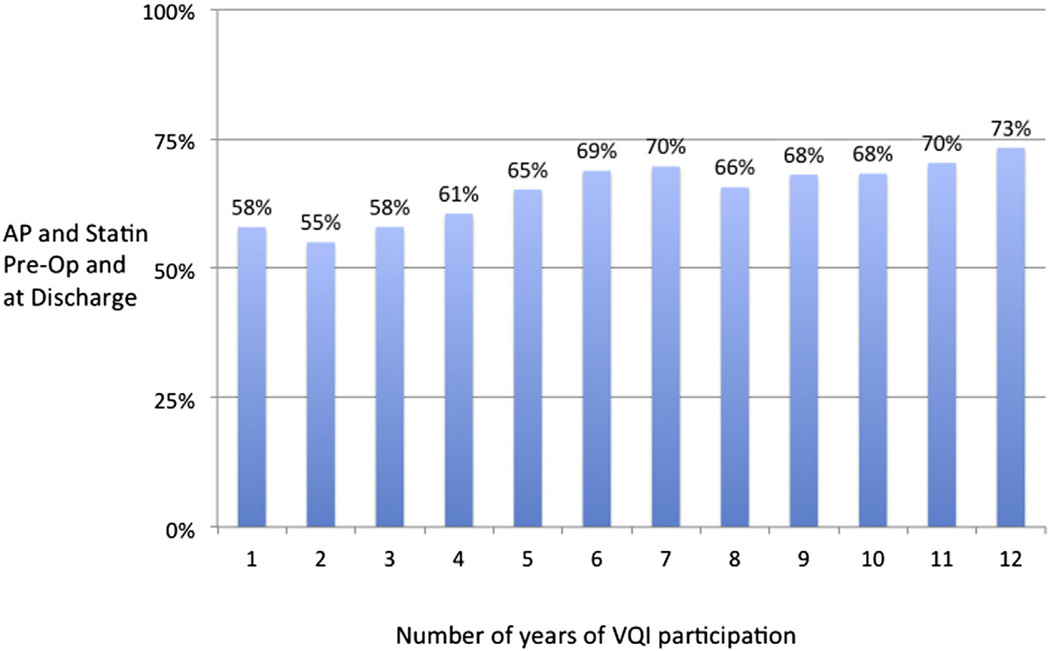
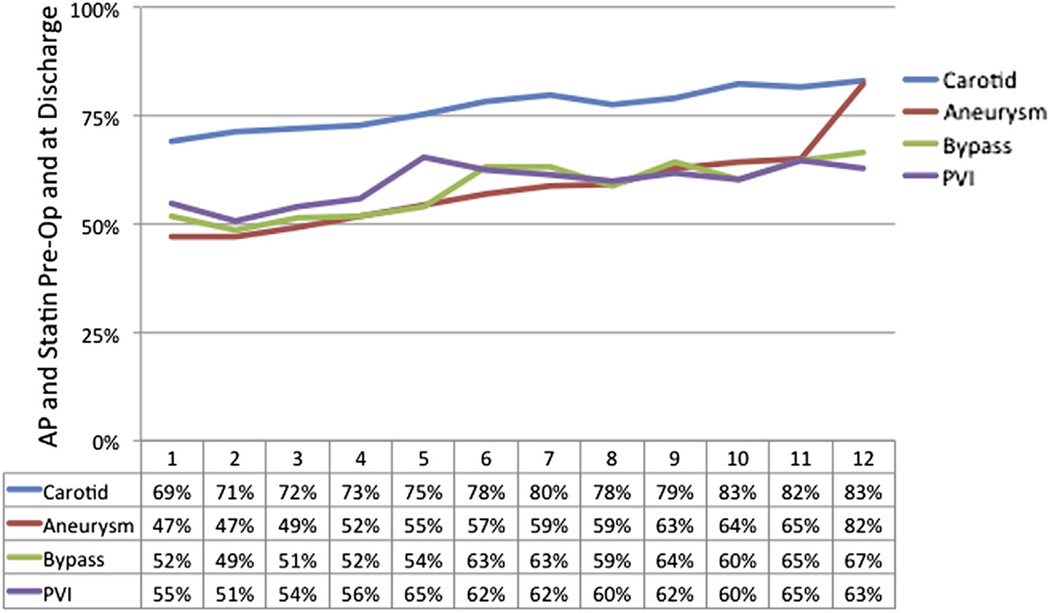
From 2005 to 2013, 78,135 individual patients underwent first-time elective procedures within VQI (3430 carotid artery stenting, 24,662 carotid endarterectomy, 3007 open AAAs, 10,496 EVARs, 3039 suprainguinal bypasses, 8323 infrainguinal bypasses, 24,476 PVIs, and 702 TEVARs; Fig 2). From 2005 to 2009, MM using both AP and statin both preoperatively and postoperatively improved from 55% to 68% across the early centers participating in VQI. Beginning in 2010, the number of centers participating and entering data in VQI increased from 15 to >200, and at the same time, MM decreased to 58% in 2011 and 2012, improving to 62% in 2014 (Fig 3). When evaluated by a center’s duration of VQI participation, MM improved from 58% in year 1 to 69% by year 6, with stabilization after 6 years (P < .001; Fig 4). When evaluated by procedure, the increase in MM was seen across all procedure types over time in VQI (P < .001 for each procedure; Fig 5).
Fig 2.
Distribution of procedures for patients undergoing vascular surgical procedures in Vascular Quality Initiative (VQI) 2005–2012. CAS, Carotid artery stenting; CEA, carotid endarterectomy; EVAR, endovascular abdominal aortic aneurysm repair; infra, infrainguinal bypass; open AAA, open abdominal aortic aneurysm repair; PVI, peripheral vascular intervention; supra, suprainguinal bypass; TEVAR, thoracic endovascular aortic repair.
Fig 3.
Proportion of patients in Vascular Quality Initiative (VQI) on antiplatelet (AP) and statin medications preoperatively and VQI expansion of centers, 2005–2014.
Fig 4.
Proportion of patients in Vascular Quality Initiative (VQI) on antiplatelet (AP) and statin medications preoperatively by center’s duration of participation in VQI.
Fig 5.
Proportion of patients in Vascular Quality Initiative (VQI) on antiplatelet (AP) and statin medications preoperatively by center’s duration of participation in VQI by procedure type. Aneurysm, Open or endovascular abdominal aortic aneurysm (AAA) or thoracic endovascular aortic repair (TEVAR); Bypass, suprainguinal or infrainguinal arterial bypass; Carotid, carotid endarterectomy or carotid artery stent; PVI, peripheral vascular intervention.
Factors associated with MM
When patient characteristics of those receiving MM were compared with those receiving less optimal MM, there was no significant difference in age or history of chronic obstructive pulmonary disease (COPD). Rates of any congestive heart failure (CHF) severity and American Society of Anesthesiologists Physical Status Classification were similar (Table I). However, patients on less optimal MM were more likely to be African American, Hispanic, current smokers, and on dialysis. Patients more likely to be on MM had concomitant hypertension, diabetes, CAD, prior coronary revascularization, an abnormal stress test result, and were on a β-blocker (Table I).
Table I.
Patient characteristics
| Variables | Total preoperative cohort (N = 74,835) |
Less optimal MMa (n = 30,428) |
Optimal MMb (n = 44,407) |
P |
|---|---|---|---|---|
| Age, mean (SD), years | 69.3 (10.4) | 69.3 (11.3) | 69.2 (9.8) | .24 |
| Male, % | 64 | 62 | 65 | <.001 |
| Race, % | <.001 | |||
| Hispanic | 4 | 4 | 3 | <.001 |
| White | 89 | 87 | 90 | |
| American Indian | 0.2 | 0.3 | 0.2 | |
| Asian | 0.6 | 1 | 1 | |
| Black | 8 | 9 | 7 | |
| Native Hawaiian | 0.1 | 0.1 | 0.1 | |
| >1 race | 0.1 | 0.1 | 0.1 | |
| Unknown | 2 | 3 | 2 | |
| Any smoking history, % | 81 | 80 | 81 | <.001 |
| Smoking, % | <.001 | |||
| Never | 19 | 20 | 19 | |
| Prior (>12 months ago) | 46 | 42 | 48 | |
| Current | 35 | 38 | 33 | |
| Hypertension, % | 87 | 82 | 90 | <.001 |
| Diabetes mellitus, % | <.001 | |||
| None | 63 | 67 | 61 | |
| Diet | 4 | 4 | 4 | |
| Oral medication | 17 | 14 | 18 | |
| Insulin | 15 | 15 | 16 | |
| CAD, % | <.001 | |||
| None | 71 | 78 | 65 | |
| History of MI | 19 | 14 | 22 | |
| Stable angina | 9 | 6 | 10 | |
| Unstable angina or MI | 2 | 1 | 3 | |
| Prior coronary revascularization, % | 34 | 24 | 42 | <.001 |
| Any CHF, % | 12 | 11 | 12 | <.001 |
| Any COPD, % | 24 | 24 | 24 | .308 |
| Dialysis, % | <.001 | |||
| No | 97 | 96 | 97 | |
| Transplant | 0 | 0.5 | 0.5 | |
| On dialysis | 3 | 4 | 2 | |
| Creatinine >1.8 mg/dL | 6 | 6 | 6 | .677 |
| All stress test, % | <.001 | |||
| Not done | 67 | 70 | 65 | |
| Normal | 24 | 23 | 25 | |
| Abnormal | 9 | 6 | 10 | |
| Living at home, % | 98 | 97 | 98 | <.001 |
| ASA, % | <.001 | |||
| 1 | 1 | 2 | 1 | |
| 2 | 16 | 17 | 15 | |
| 3 | 69 | 68 | 70 | |
| 4 | 13 | 14 | 13 | |
| 5 | 1 | 1 | 0 | |
| History of | ||||
| Bypass, % | 10 | 10 | 11 | <.001 |
| CEA, % | 9 | 7 | 11 | <.001 |
| AAA repair, % | 3 | 3 | 3 | .832 |
| PTA/stent, % | 18 | 16 | 19 | <.001 |
| Major amputation, % | 2 | 2 | 2 | <.001 |
| Preoperative ACEi, % | 49 | 40 | 55 | <.001 |
| Intolerant/noncompliant | 2 | 2 | 2 | |
| Chronic anticoagulation, % | 10 | 13 | 9 | <.001 |
| β-Blockers, % | <.001 | |||
| None | 37 | 47 | 31 | |
| Perioperative | 6 | 6 | 7 | |
| Chronic | 53 | 44 | 59 | |
| Intolerant/noncompliant | 1 | 1 | 1 | |
| Operative day only | 2 | 2 | 2 |
AAA, Abdominal aortic aneurysm; ASA, American Society of Anesthesiologists; ACEi, angiotensin-converting enzyme inhibitor; CAD, coronary artery disease; CEA, carotid endarterectomy; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; MM, medical management; PTA, percutaneous transluminal angioplasty; SD, standard deviation.
Less optimal MM: failure to be on both medication preoperatively and at discharge.
Optimal MM: antiplatelet and statin medication preoperatively and at discharge.
After multivariable analysis, patients less likely to be on MM included those aged >78 years, African American and Hispanic patients, those transferred from another hospital, patients with CHF, COPD, on dialysis, living in a nursing home preoperatively, having a prior major amputation, and all noncarotid interventions (Table II).
Table II.
Multivariable factors associated with less medical management (MM)
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age ≥78 years (Ref <63) | 0.8 | 0.7–0.8 | <.001 |
| Ethnicity | |||
| Not Hispanic or Latino | 1.0 (Ref) | ||
| Hispanic or Latino | 0.8 | 0.8–0.9 | <.001 |
| Transfer | |||
| None | 1.0 (Ref) | ||
| Hospital | 0.8 | 0.7–0.9 | <.001 |
| Rehabilitation | 1 | 0.8–1.3 | .679 |
| CHF | 0.9 | 0.8–0.9 | <.001 |
| Prior major amputation | 0.9 | 0.8–0.9 | .001 |
| Race | |||
| White | 1.0 (Ref) | ||
| American Indian/Alaskan | 0.8 | 0.5–1.1 | .108 |
| Asian | 1.1 | 0.9–1.3 | .589 |
| African American | 0.9 | 0.58–1.9 | .002 |
| Native Hawaiian/Pacific Islander | 1.2 | 0.7–2.0 | .602 |
| >1 race | 1.3 | 0.7–2.6 | .443 |
| Unknown/other | 1 | 0.9–1.1 | .897 |
| COPD | |||
| None | 1.0 (Ref) | ||
| Not treated | 0.9 | 0.8–0.9 | .019 |
| On medication | 1.0 | 0.9–1.0 | .252 |
| On oxygen | 0.9 | 0.8–0.9 | .005 |
| Dialysis | |||
| None | 1.0 (Ref) | ||
| Working transplant | 0.8 | 0.7–1.1 | .157 |
| On dialysis | 0.6 | 0.5–0.7 | <.001 |
| Living status | |||
| Home | 1.0 (Ref) | ||
| Nursing home | 0.7 | 0.6–0.8 | <.001 |
| Homeless | 1.0 | 0.6–1.8 | .844 |
| β-Blocker noncompliant Procedure |
0.3 | 0.2–0.5 | <.001 |
| CAS | 1.0 (Ref) | ||
| CEA | 1.0 | 0.9–1.1 | .839 |
| oAAA | 0.38 | 0.3–0.4 | <.001 |
| EVAR | 0.37 | 0.3–0.4 | <.001 |
| Suprainguinal bypass | 0.39 | 0.3–0.4 | <.001 |
| Infrainguinal bypass | 0.39 | 0.3–0.4 | <.001 |
| PVI | 0.45 | 0.4–0.5 | <.001 |
| TEVAR | 0.37 | 0.3–0.4 | <.001 |
CAS, Carotid artery stenting; CEA, carotid endarterectomy; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; EVAR, endovascular abdominal aortic aneurysm repair; oAAA, open abdominal aortic aneurysm repair; OR, odds ratio; PVI, peripheral vascular intervention; TEVAR, thoracic endovascular aortic repair.
By multivariable analysis, patients more likely to be on MM were male, former smokers, and had higher rates of cardiovascular risk factors such as hypertension, diabetes, CAD, prior coronary revascularization, and a positive stress test result. In addition, patients were more likely to be on MM after a prior bypass, PVI, carotid intervention, and currently on a β-blocker. Finally, participation in VQI ≥3 years was independently associated with an increased odds of MM use (odds ratio, 1.3, 95% confidence interval [CI], 1.3–1.4; P < .001), even when adjusted for year of surgery (Table III).
Table III.
Multivariable factors associated with more medical management (MM)
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age, years | |||
| <63 | 1.0 (Ref) | ||
| 63–70 | 1.1 | 1.0–1.2 | <.001 |
| 71–77 | 1 | 1.0–1.1 | .744 |
| Male | 1.1 | 1.0–1.1 | <.001 |
| Smoking | |||
| Never | 1.0 (Ref) | ||
| Former | 1.2 | 1.1–1.2 | <.001 |
| Current | 1 | 1.0–1.1 | .22 |
| Hypertension | 1.4 | 1.3–1.4 | <.001 |
| Diabetes | |||
| None | 1.0 (Ref) | ||
| Diet | 1 | 0.9–1.1 | .89 |
| Oral medication | 1.3 | 1.2–1.3 | <.001 |
| Insulin | 1.1 | 1.1–1.2 | <.001 |
| CAD | |||
| None | 1.0 (Ref) | ||
| Prior MI | 1.2 | 1.2–1.3 | <.001 |
| Stable angina | 1.3 | 1.2–1.4 | <.001 |
| Unstable angina/recent MI | 1.5 | 1.3–1.7 | <.001 |
| Prior coronary revascularization | 1.7 | 1.7–1.8 | <.001 |
| Stress test | |||
| Not done | 1.0 (Ref) | ||
| Normal | 1.1 | 1.1–1.2 | <.001 |
| Ischemia | 1.4 | 1.3–1.5 | <.001 |
| MI | 1.1 | 1.0–1.3 | .053 |
| Both | 1.3 | 1.1–1.6 | .002 |
| Prior bypass | 1.1 | 1.1–1.2 | .003 |
| Prior carotid intervention | 1.3 | 1.2–1.3 | <.001 |
| Prior PVI | 1.3 | 1.2–1.4 | <.001 |
| β-blockers | |||
| None | 1.0 (Ref) | ||
| Preoperative only | 1.7 | 1.5–1.8 | <.001 |
| Chronic | 1.5 | 1.5–1.6 | <.001 |
| Intolerant | 1.5 | 1.3–1.8 | <.001 |
| Operative day only | 1.1 | 1.0–1.3 | .025 |
| VQI | |||
| <3 years | 1.0 (Ref) | ||
| ≥3 years | 1.3 | 1.3–1.4 | <.001 |
| Year | |||
| 2005 | 1.0 (Ref) | ||
| 2006 | 1.4 | 1.1–1.7 | .002 |
| 2007 | 1.5 | 1.2–1.8 | <.001 |
| 2008 | 2 | 1.6–2.4 | <.001 |
| 2009 | 2.1 | 1.78–2.6 | <.001 |
| 2010 | 2 | 1.7–2.4 | <.001 |
| 2011 | 2.1 | 1.7–2.5 | <.001 |
| 2012 | 1.9 | 1.6–2.3 | <.001 |
| 2013 | 1.9 | 1.6–2.3 | <.001 |
| 2014 | 2 | 1.7–2.5 | <.001 |
AAA, Abdominal aortic aneurysm; CAD, coronary artery disease; CI, confidence interval; MI, myocardial infarction; OR, odds ratio; PVI, peripheral vascular intervention; VQI, Vascular Quality Initiative.
Five-year survival
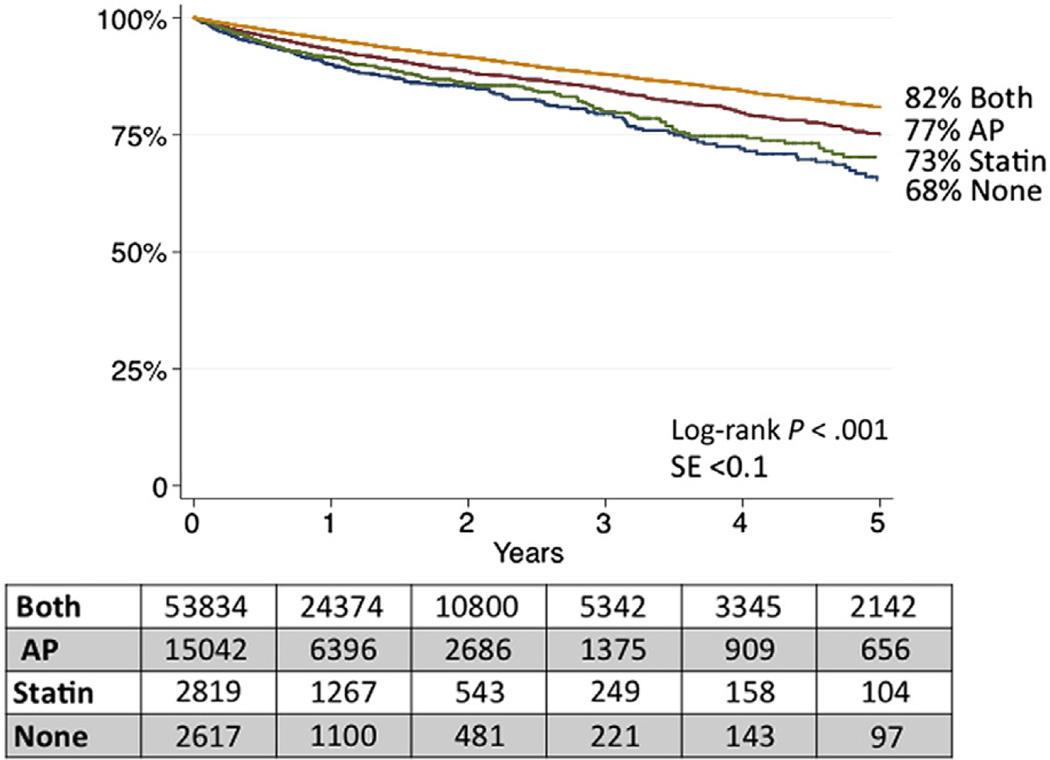
Postdischarge 5-year survival for the entire cohort was 80% (95% CI, 79%–81%). However, when stratified by discharge medication status, survival was highest for those prescribed both AP and statin medication at discharge, at 82% (95% CI, 81%–83%), and lowest for those discharged on neither medication, at 67% (95% CI, 62%–73%). Survival after discharge on a single agent was intermediate (AP only: 77% [95% CI, 75%–79%]; statin only: 73% [95% CI, 68%–77%]; Fig 6). The survival difference of these groups was statistically significant (log-rank P < .001).
Fig 6.
Five-year survival by discharge medication status. AP, Antiplatelet; SE, standard error.
By multivariable Cox proportional hazards analysis, male patients, current smokers at the time of surgery, and those of increasing age had higher 5-year mortality. In addition, increasing severity of diabetes, CAD, CHF, COPD, and advanced renal function were all associated with worse 5-year survival. Need for prior arterial bypass, prior major amputation, and nonindependent living status were also associated with worse survival (Table IV).
Table IV.
Multivariable predictors of 5-year survival
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age, years | |||
| <63 | 1.0 (Ref) | ||
| 63–70 | 1.3 | 1.2–1.5 | <.001 |
| 71–77 | 1.9 | 1.7–2.1 | <.001 |
| ≥78 | 3.5 | 3.2–3.9 | <.001 |
| Male | 1.1 | 1.0–1.2 | <.001 |
| Smoking | |||
| Never | 1.0 (Ref) | ||
| Former | 1 | 0.9–1.1 | .303 |
| Current | 1.2 | 1.1–1.3 | <.001 |
| Diabetes | |||
| None | 1.0 (Ref) | ||
| Diet | 1.3 | 1.1–1.5 | <.001 |
| Oral medication | 1.2 | 1.1–1.3 | <.001 |
| Insulin | 1.4 | 1.3–1.6 | <.001 |
| CAD | |||
| None | 1.0 (Ref) | ||
| Prior MI | 1.2 | 1.1–1.3 | <.001 |
| Stable angina | 1.1 | 0.9–1.2 | .096 |
| Unstable angina/recent MI | 1.1 | 0.9–1.4 | .306 |
| CHF | |||
| None | 1.0 (Ref) | ||
| Asymptomatic | 1.7 | 1.5–1.9 | <.001 |
| Mild/moderate | 1.9 | 1.7–2.1 | <.001 |
| Severe | 2.4 | 1.9–2.9 | <.001 |
| COPD | |||
| None | 1.0 (Ref) | ||
| Not treated | 1.2 | 1.1–1.4 | .011 |
| On medication | 1.5 | 1.4–1.6 | <.001 |
| On oxygen | 2.2 | 1.9–2.5 | <.001 |
| GFR, mL/min/1.73 m2 | |||
| >60 | 1.0 (Ref) | ||
| 40–59 | 1.2 | 1.2–1.3 | <.001 |
| 30–39 | 1.6 | 1.5–1.8 | <.001 |
| <30 | 2.7 | 2.4–3.0 | <.001 |
| Missing | 2.9 | 2.6–3.3 | <.001 |
| Living status | 2.1 | 1.8–2.4 | <.001 |
| Prior bypass surgery | 1.2 | 1.1–1.3 | <.001 |
| Prior major amputation | 1.3 | 1.2–1.4 | <.001 |
| Procedure | |||
| CAS | 1.0 (Ref) | ||
| CEA | 0.7 | 0.6–0.8 | <.001 |
| oAAA | 0.8 | 0.6–0.9 | .017 |
| EVAR | 0.9 | 0.7–1.0 | .06 |
| Suprainguinal bypass | 1.2 | 1.0–1.5 | .057 |
| Infrainguinal bypass | 1.2 | 1.0–1.4 | .03 |
| PVI | 1.1 | 1.0–1.3 | .128 |
| TEVAR | 1.3 | 0.9–2.0 | .124 |
| Medication | |||
| None | 1.0 (Ref) | ||
| Aspirin | 0.8 | 0.7–0.9 | <.001 |
| Statin | 0.8 | 0.7–0.9 | .011 |
| Both | 0.6 | 0.5–0.7 | <.001 |
| VQI | |||
| <3 years | 1.0 (Ref) | ||
| ≥3 years | 1 | 0.9–1.1 | .684 |
CAS, Carotid artery stenting; CEA, carotid endarterectomy; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; EVAR, endovascular abdominal aortic aneurysm repair; GFR, glomerular filtration rate; HR, hazard ratio; MI, myocardial infarction; oAAA, open abdominal aortic aneurysm repair; PVI, peripheral vascular intervention; TEVAR, thoracic endovascular aortic repair; VQI, Vascular Quality Initiative.
MM was the only preoperative nonprocedural factor associated with improved survival. Discharge on only AP or statin medication was associated with improved survival (hazard ratio [HR], 0.8; 95% CI, 0.7–0.9) compared with discharge on neither medication. Discharge on both agents appeared to have a synergistic effect, with the most improved adjusted survival (HR, 0.6; 95% CI, 0.5–0.8) after adjustment for other patient factors (Table IV). Although longer center participation (≥3 years) in VQI at the time of the procedure was associated with improved MM, it was not associated with improved subsequent survival (HR, 1.0; 95% CI, 0.9–1.1; P = .68).
DISCUSSION
In this analysis of the VQI registry, we examined the use of AP and statin therapy surrounding vascular procedures and the association of these medications with long-term survival. Our study has three primary findings. First, within a nationwide vascular surgical registry and quality improvement initiative, there continues to be significant variability in MM with AP and statin medications, before and after vascular procedures. Patients undergoing noncarotid interventions, African Americans, those of Hispanic or Latino descent, older patients, and those with severe life-limiting comorbid conditions are the least likely to be on these medications.
Secondly, younger patients with known cardiovascular risk factors are more likely to be on these medications.
Thirdly, and perhaps most importantly, participation within a quality improvement registry, such as VQI, was strongly associated with improved MM after several years of participation, and this improvement in MM was associated with better long-term survival for patients with vascular disease. We hypothesize that this is due to sites receiving feedback about their MM and thus an incentive to improve. The discussions at regional quality group meetings provide benchmark comparisons with other sites and additional incentive to improve over time. This is important, because patients discharged on AP and statins were associated with the highest overall and adjusted survival.
Undertreatment of PAD remains a gap in secondary prevention of cardiovascular care. The National Health and Nutrition and Examination Survey data demonstrate that nearly a third of all Americans with PAD are not on a statin or aspirin.7 Yet, treatment with these medications is associated with reduction in overall mortality of nearly 65%.7 This effect is larger than our findings and may be due to the heterogeneity of patients in the National Health and Nutrition and Examination Survey study with PAD. The Dutch Randomised Endovascular Aneurysm Management trial investigators demonstrated a similar survival benefit of patients with AAA with statin therapy.13 Our current findings across VQI extend our prior findings, which demonstrated a similar reduction in long-term mortality with AP and statin treatment in the Vascular Study Group of New England (18% overall benefit vs 14% in current analysis). We also saw similar improvements in MM over time and across procedures in New England.8
Identifying patient groups that are more likely to be medically undertreated is an important goal for physicians who care for patients with vascular disease, because these populations are often the most likely to benefit from these treatments. For example, Hispanic or Latino patients and African Americans were less likely to be on MM. Disparities for these groups have been described in many areas of cardiovascular care and outcomes.14 In some cases, participation in quality improvement initiatives has been associated with elimination of racial and ethnic disparities in cardiovascular care. Gaps in guideline treatment of acute myocardial infarction were eliminated in an analysis of patients treated within the American Heart Association’s Get With The Guidelines-Coronary Artery Disease observational registry and quality improvement initiative from 2000 to 2007.15 This supports the concept that participation in quality improvement initiatives can result in overall improvement in process measures such as medication utilization.
In an effort to improve MM throughout the VQI, the Arterial Quality Committee in May 2014 adopted a practice recommendation that all eligible patients undergoing arterial procedures be discharged on AP and statin medications.16 Eligible patients for AP medications include those not on oral anticoagulants or documented intolerance to AP medications. Eligible patients for statin use include those not on dialysis and those without documented intolerance or allergy. A goal of 90% adherence to this goal was described in this practice recommendation. Although ambitious, this represents a goal that has been achieved by many centers participating in VQI. As our data demonstrate, this practice is associated with an unadjusted 14% survival advantage and an adjusted 40% reduction in 5-year mortality compared with being on neither medication. Given that many arterial procedures are performed to improve survival or quality of life, many would agree that vascular practitioners should also optimize the MM of these patients. Prescribing such medications at the time of discharge is certainly an opportunity to improve patient outcomes, especially if patients have been poorly managed in this regard before their operation.
Although longer participation in the VQI was associated with improved MM, it was not associated with improved survival in our multivariable analysis after adjustment for other patient factors. Our initial analysis did suggest VQI participation was associated with improved survival, but when additional procedures and centers were added in the final analysis, this association did not persist. One explanation may be that VQI participation can result in survival benefits by participating in a quality initiative collaborative, providing the framework for improving medications, technique, and follow-up protocols. Yet, due to the addition of patients without extended follow-up in our final analysis, it is possible that our data contain a type II error. As more follow-up for newer centers becomes available, we may be able to identify this difference and attempt to separate this from other site-specific variations in patient care.
Our study has several limitations. Medication adherence is recorded in VQI at the 1-year follow-up patient encounter to assess AP and statin use. Medication is not assessed after 1 year. However, 85% of those discharged on both medications remained on them at 1 year, so perhaps once these medications are prescribed, they are likely to be continued. In addition, the statin medication dose, lipid profiles, or other patient-level biomarkers, such as C-reactive protein, are not available to assess the effect of statin use. Thus, we cannot assess if low-density lipoprotein or C-reactive protein values are within goal limits. Yet, it is more likely that patients may be undertreated. If patients are not on the maximal dose of statins or within the targeted low-density lipoprotein range, we may have actually underestimated the mortality benefit of these medications. However, our data do not permit us to determine this definitively.
Our findings are limited by shorter long-term followup from newer VQI centers, and re-evaluation of these data after follow-up data from other centers accrues will provide generalizability of our findings across more centers and regions. Various socioeconomic or practice patterns may affect the probability of patients being on MM that we cannot account for, which could also influence survival. Yet, we have shown that despite these factors, participation in VQI has a positive effect that may overcome some of these prior perceived factors. Finally, due to the observational nature of the data, we can only demonstrate association, not causation, in our findings.
CONCLUSIONS
The present study demonstrates that participation in the VQI is associated with improved use of AP and statin medication for patients undergoing vascular procedures, and treatment with AP and statins is associated with a 14% survival advantage at 5 years. The process of collecting data within the VQI permits provider feedback with national and regional benchmarks and the ability to initiate quality projects. These factors are likely responsible for the improvements seen across the VQI to date. Future work will seek to increase AP and statin medication use and eliminate potential disparities in care. The VQI Arterial Quality Committee benchmark target that 90% of appropriate patients be discharged on AP and statin medications represents an important goal for optimizing survival after vascular surgical procedures, which may be comparable to the survival benefit from the procedure itself.
Footnotes
Author conflict of interest: none.
Presented at the 2014 Vascular Annual Meeting of the Society for Vascular Surgery, Boston, Mass; June 4–7, 2014.
AUTHOR CONTRIBUTIONS
Conception and design: RD, AB, JE, JC, PG
Analysis and interpretation: RD, AH, AB, JE, JH, JC, PG
Data collection: RD, AH, AB, JE, JH, GU, JC, PG
Writing the article: RD, AH, AB, JH, GU, JC, PG
Critical revision of the article: RD, AH, AB, JE, JH, GU, JC, PG
Final approval of the article: RD, AH, AB, JE, JH, GU, JC, PG
Statistical analysis: RD
Obtained funding: Not applicable
Overall responsibility: RD
REFERENCES
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 3.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. J Vasc Surg. 2011;54:e32–e58. doi: 10.1016/j.jvs.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 5.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/ SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Neuro-Interventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:489–532. doi: 10.1161/CIR.0b013e31820d8d78. [DOI] [PubMed] [Google Scholar]
- 6.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 7.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. doi: 10.1161/CIRCULATIONAHA.110.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Martino RR, Eldrup-Jorgensen J, Nolan BW, Stone DH, Adams J, Bertges DJ, et al. Perioperative management with antiplatelet and statin medication is associated with reduced mortality following vascular surgery. J Vasc Surg. 2014;59:1615–1621. e1. doi: 10.1016/j.jvs.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–1537. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–1101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101-2. [DOI] [PubMed] [Google Scholar]
- 11.Sobel M, Verhaeghe R. Antithrombotic therapy for peripheral artery occlusive disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133(6 Suppl):815S–843S. doi: 10.1378/chest.08-0686. [DOI] [PubMed] [Google Scholar]
- 12.De Martino RR, Nolan BW, Goodney PP, Chang CK, Schanzer A, Cambria R, et al. Outcomes of symptomatic abdominal aortic aneurysm repair. J Vasc Surg. 2010;52:5–12. e1. doi: 10.1016/j.jvs.2010.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bruin JL, Baas AF, Heymans MW, Buimer MG, Prinssen M, Grobbee DE, et al. Statin therapy is associated with improved survival after endovascular and open aneurysm repair. J Vasc Surg. 2014;59:39–44. e1. doi: 10.1016/j.jvs.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Wang TY, Ventura HO, Pina IL, Vijayaraghavan K, Ferdinand KC, et al. The coalition to reduce racial and ethnic disparities in cardiovascular disease outcomes (credo): why credo matters to cardiologists. J Am Coll Cardiol. 2011;57:245–252. doi: 10.1016/j.jacc.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MG, Fonarow GC, Peterson ED, Moscucci M, Dai D, Hernandez AF, et al. Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines-Coronary Artery Disease program. Circulation. 2010;121:2294–2301. doi: 10.1161/CIRCULATIONAHA.109.922286. [DOI] [PubMed] [Google Scholar]
- 16.Vascular Quality Initiative 2013 Annual Report. [Accessed July 13, 2014]; Available at: http://www.vascularqualityinitiative.org/wp-content/uploads/VQI_2013_Annual_Report.pdf. [Google Scholar]