Abstract
Migraine is one of the most common and most disabling disorders. Between attacks, migraine patients are otherwise normal but are sensitized to non-noxious events known as triggers. The purpose of these studies was to investigate whether a headache-like event causes sensitization, or priming, to subsequent subthreshold events. Interleukin-6 (IL-6) was applied to the rat cranial dura mater which produced cutaneous facial and hindpaw allodynia that lasted 24 hours. At 72-hours, IL-6 treated rats developed allodynia in response to dural stimulation with either a pH 6.8 or pH 7.0 solution and to a systemic nitric oxide (NO) donor, a well-known migraine trigger. Vehicle-treated rats did not respond to either pH stimulus nor to the NO donor, demonstrating that IL-6 exposure primes rats to subthreshold stimuli. Inhibitors of brain-derived neurotrophic factor (BDNF) signaling given either systemically or intracisternally 24-hours after IL-6 eliminated responses to dural pH stimulation at 72 hours. Additionally, intracisternal administration of BDNF without prior dural stimulation produced allodynia and once resolved, animals were primed to dural pH 6.8/pH 7.0 and a systemic NO donor. Finally, hindpaw IL-6 produced paw allodynia but not priming to paw injection of pH 7.0 at 72 hours demonstrating differences in priming depending on location. These data indicate that afferent input from the meninges produces BDNF-dependent priming of the dural nociceptive system. This primed state mimics the interictal period of migraine where attacks can be triggered by normally non-noxious events and suggests that BDNF-dependent plasticity may contribute to migraine.
Keywords: headache, migraine, BDNF, cutaneous allodynia, TrkB-fc, ANA-12
Introduction
Migraine is the 3rd most common and 8th most disabling disease worldwide [53] but the underlying pathophysiology is poorly understood. Biomarkers for migraine do not yet exist and few changes can be found in patients that potentially explain the disorder. Although structural changes in the brain have been identified [26], it is not clear whether these changes contribute to or whether they are a consequence of migraine. Migraine is often triggered by stimuli such as stress, skipping meals, foods/scents, excess/inadequate sleep, and changes in hormone levels [1; 38; 46]. However, these stimuli are innocuous in non-migraineurs, suggesting that changes in the nervous system of migraine patients enhance their susceptibility to triggers. The location and mechanism of these changes are not well understood.
The frequency of migraine attacks can increase over time. Episodic migraine (0–14 migraine days/month) can progress to chronic migraine (15 or more days/month) at a rate of 2.5% per year [9], although a transition from chronic to episodic migraine can also occur [31], and higher frequency is associated with higher risk of progression [29; 47]. Another risk factor for progression is adequacy of treatment of acute migraines. When maximum treatment efficacy of acute attacks was achieved, the progression to chronic migraine was 1.9% while progression increased to 2.7%, 4.4%, and 6.8% with moderate, poor, and very poor treatment efficacy, respectively [30]. These data indicate that migraine attacks and the severity of attacks can increase the likelihood of future migraines. Although mechanisms contributing to progression of migraine are not known, plasticity within the nervous system due to events occurring during attacks may play a role [7].
Several animal behavioral models have been developed that allow investigation of potential mechanisms contributing to headache disorders. Acute stimulation of the dura mater of rats produces behavioral responses consistent with headache, including generalized allodynia and decreased rearing [19; 22; 23; 55] . Repetitive dural stimulation promotes increased responses over time and animals eventually transition into chronic cutaneous allodynia [36] that is dependent on neuronal sensitization in the trigeminal nucleus caudalis (TNC) [13]. Repetitive administration of sumatriptan to rats [20] or nitric oxide donors to mice [41] also sensitizes these animals to migraine triggers, even well after drug administration has ceased. Although these behavioral models cause sensitization to migraine triggers and mimic migraine progression, the underlying mechanisms have not been fully uncovered.
Previously, we showed that interleukin-6 (IL-6) administration to the rat dura produced cutaneous allodynia [57]. Though IL-6 has been implicated in other pain conditions, elevated levels of IL-6 are present in migraine patients [45; 54] and a recent study found elevated IL-6 RNA in the cranial periosteum of chronic migraine patients [39]. IL-6 administration to other tissues produces a state of hyperalgesic priming where, after resolution of IL-6 allodynia, animals display enhanced responses to non-noxious or mildly noxious stimuli [32; 42]. The purpose of the current studies was to determine whether meningeal IL-6 administration primes the dural afferent system to later subthreshold stimuli and to investigate the potential mechanisms by which this state of sensitization is mediated.
Methods
Animals
Adult male and female Sprague-Dawley rats (250–300g, Harlan) were kept in a temperature-controlled room on 12-hr light/dark cycle with food and water ad libitum. All procedures were performed in accordance with the policies of the IASP and the NIH guidelines for use of laboratory animals. The Institutional Animal Care and Use Committee (IACUC) of both the University of Arizona and The University of Texas at Dallas approved all procedures.
Surgeries
As previously described [22; 56], dura cannulation surgeries were performed on rats (250–300) grams. Animals were anesthetized with a combination of ketamine and xylazine (80 mg/kg and 12 mg/kg) and an incision exposing the skull was made to the top of the skull. Once the skull was exposed, a 1-mm hole was made in the skull to expose the dura (1mm left of midline, 1mm anterior to lambda). A 1mm guide cannula (Plastics One) was then inserted into the hole and secured with Vetbond™ (3M™). Two screws (Small Parts) were placed rostral to the cannula biparietal to sagittal suture and posterior to bregma. Dental acrylic was used to secure the cannula to both the screws and the skull. A dummy cannula (Plastics One) was placed into the cannula to ensure patency. Animals received gentamicin (8 mg/kg) to minimize infection following surgery. Rats were housed separately and allowed 6–8 days for recovery prior to behavioral testing.
Behavioral Testing
The animals were habituated in the testing chambers for a minimum of one hour prior to baseline. Animal weights were recorded and dural injections were given post baseline. Testing of both facial and hind paw allodynia was conducted every hour for 5 hours using calibrated Von Frey filaments thresholds were determined by the “up-down” method6. The Von Frey filaments were applied to the peri-orbital region of the face or to the plantar surface of the hind paw perpendicularly until the entire force was applied, and held for approximately 5 seconds or until animals withdrew. Maximum filaments used were 8 g for the peri-orbital region and 15 g for the hindpaw. Upon completion of allodynia testing, all animals’ cannula patency was verified by ink injection into the cannula and dissection post mortem. The experimenter collecting measurements was blinded to the experimental conditions.
Intracisternal Administration
Curved 25 gauge needles 1.5 inches long (BD Needles) were bent at approximately 40° to no more than 45° at a distance 7 mm from the bevel of the tip. These needles were then used, 1 per animal, while attached to a 25 μL micro-syringe (Hamilton Syringes). Intracisternal injections were performed under light isoflurane anesthesia administered via nose cone from a vaporizer. Animals were anesthetized for <2 min. Animal heads were tilted forward to allow for exposure of the cisterna magna in order to perform injections. The curved needle was then positioned at the back of the neck just below the occiput and above the C1 vertebrae. The needle was inserted through the cisterna magna at the midline. Central placement of the needle was verified by drawing cerebrospinal fluid back into the needle prior to infusion of a given solution. Injections of 10 μL volume are made over a 10 sec period after which the needle is removed. Animals recover from anesthesia for 5 min before being returned to cages or testing chambers[18].
Rotarod
Animals were tested for motor impairments following intracisternal injection by their ability to balance and walk on a slowly rotating rod (Rotamex 4/8, 6.5 cm diameter for rats, respectively; rate of rotation, 10 revolutions per min, Columbus Instruments, Columbus, Ohio). Animals were trained for three consecutive days prior to challenge with a maximal cut-off time of 180 seconds 8. Training consisted of a habituation to the rod while stationary for 180 seconds, as well as while rotating for a maximum of 180 seconds. Animals unable to stay on the rod for the full training period were excluded. On the fourth day the length of time that an animals were able to stay on the rotating rod during a 10 minute period was recorded as a baseline. Compounds were then administered via intracisternal injections and rats were assessed for time spent on the rod at peak behavioral response times (for allodynia) for the compound (3 hours post injection) and no changes were observed (data not shown). Vehicle-treated rats were also tested for time spent on the rod.
Solution Preparation
Rat recombinant IL-6 (R&D Systems) stock solution (10 μg/ml) was prepared in sterile 0.1% bovine serum albumin (BSA) and diluted to final concentrations of 10 ng/ml in synthetic interstitial fluid (SIF) (pH 7.4, 310 Osmolality). Human recombinant BDNF and TrkB/Fc (R&D Systems) were made into stock solutions (50ng/ml & 25ug/ml respectively). BDNF stock solution was made in sterile phosphate buffered saline (PBS) containing 0.1% BSA and TrkB/Fc stock solution was made in sterile PBS. Both BDNF and TrkB/Fc were dissolved in artificial cerebrospinal fluid (aCSF) for intracisternal administration. Vehicle control was aCSF. ANA-12 (Maybridge) was diluted to final concentration of .5 mg/kg in 10% polyethyl glycol 300 (PEG 300) and administered via intraperitoneal injection. APETx2 (Alomone Labs) was diluted in SIF solution at pH 6.8 to a final concentration of 20 μM/mL.
Data analysis
All behavioral data are graphed as means ± SEM. Statistical evaluations of allodynia studies were conducted using GraphPad Prism Version 6.0 (GraphPad Software Inc., La Jolla, CA, USA). Data was analyzed among groups and across time by one- or two-way analysis of variance (ANOVA) for treatment and time followed by Bonferroni post-test where appropriate. Data were also converted to area over the time-effect curve to allow for analysis of multiple treatment groups and analyzed with a 1-factor ANOVA and Bonferroni post-test. In experiments where differences in allodynia at the 72-hour time point and 3 hours following this time point are being compared, t-tests were used. Significance was set at P < 0.05 for all data analysis.
Results
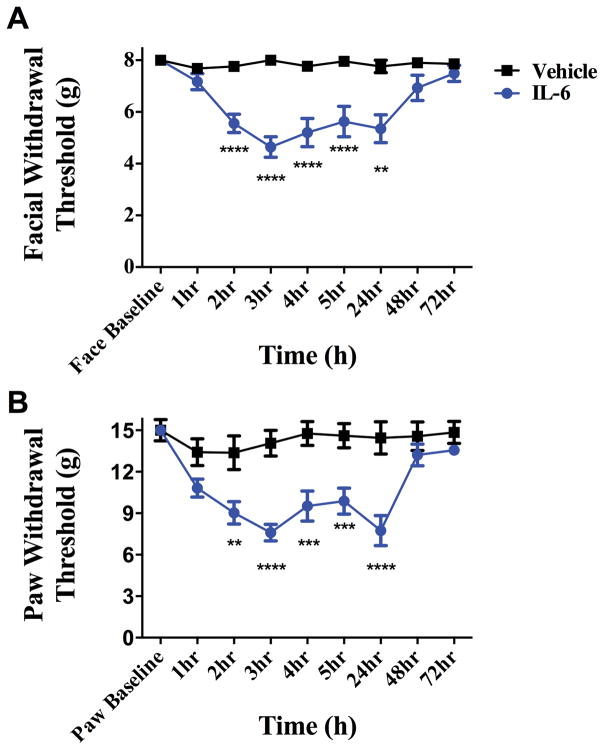
We have shown previously that application of IL-6 to the rat dura mater produced cutaneous facial and hindpaw allodynia that remained significantly different from baseline at 24 hours post injection [57]. IL-6 was applied in the current studies to determine the duration of the allodynic response. Dural application of IL-6 (0.1 ng) produced both facial and hindpaw allodynia that resolved by 48 hours post application (Figure 1) and remained at baseline at 72 hours. In order to ensure that the responses to dural IL-6 are due to initiation of afferent activity at the site of injection on the dura, the local anesthetic bupivacaine (0.5% w/v) was administered with IL-6 (0.1 ng) in the same injection. In the presence of dural bupivacaine, no allodynia of the facial or hindpaw region was observed at any timepoint following IL-6 injection (Supplementary Figure 1).
Figure 1.
Dural application of IL-6 produces headache-related behaviors. Withdrawal thresholds to tactile stimuli applied to the face (A) and hindpaws (B) were measured in animals prior to and after dural application of .1 ng IL-6 (n = 39) or Vehicle(pH 7.4) (n = 28). Administration of IL-6 produced significant allodynia that lasted 24 hours in both face and hindpaw. Two-factor analysis of variance (ANOVA) indicated a significant effect of both treatment and time of both the face and hindpaws. Significant differences among means for each group were determined by analysis of variance followed by Bonferroni post hoc test. (A) Facial: time F (8, 418) = 5.029, P < 0.0001, treatment F (1, 418) = 49.79,P < 0.0001; (B) Hindpaw: time F (8, 347) = 7.818, P < 0.0001, treatment F (1, 347) = 81.33, P < 0.0001.
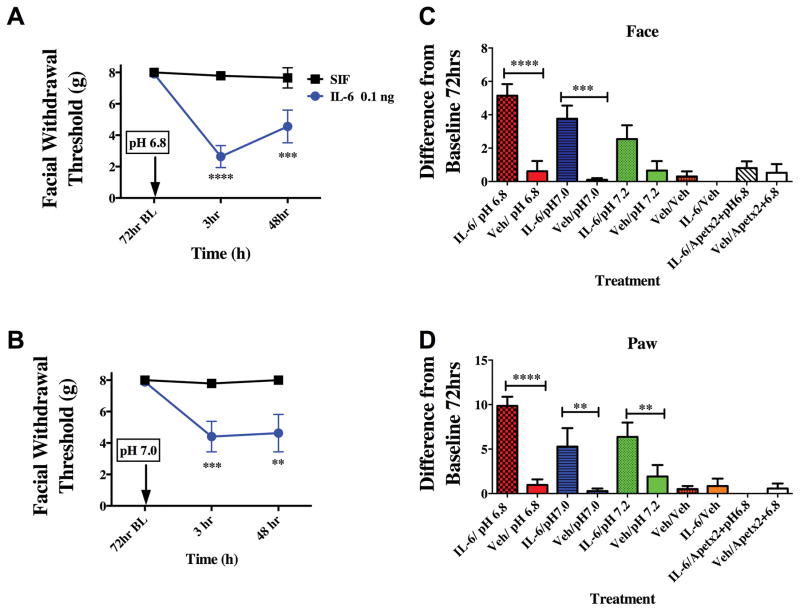
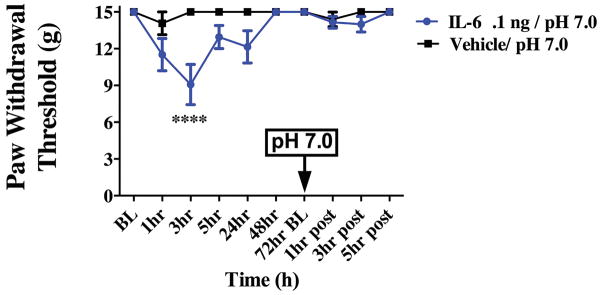
At the 72-hour time point, when animals had remained at baseline for 24 hours, they were tested for responses to pH 6.8 and pH 7.0 application to the dura mater. Previously, pH 6.8 was shown to not produce significant facial and hindpaw allodynia [58] indicating that this pH value is subthreshold in normal animals. At 72 hours following IL-6, stimulation of the dura with the normally subthreshold pH values of pH 6.8 or pH 7.0 produced robust facial and hindpaw allodynia at 3 hours post administration (Figure 2). Only animals that had previously received IL-6, but not vehicle-treated animals (vehicle is pH 7.4 SIF), responded to the subsequent low pH stimulus. Higher pH values (pH 7.2) did not produce significant facial allodynia in either IL-6 or vehicle-treated rats (Figure 2C). In order to determine whether acid-sensing ion channels (ASICs) contribute to the behavioral response to low pH after sensitization (as we have shown previously in the absence of sensitization [56; 58]), the ASIC3 blocker APETx2 was given with pH 6.8 at the 72-hour time point following IL-6. In the presence of APETx2, pH 6.8 did not produce significant facial allodynia indicating that ASICs still detect the decrease in pH even after sensitization with IL-6. Additionally, stimulation of the dura with pH 7.4 at the 72-hour time point following IL-6 did not produce facial allodynia, indicating that the animals are not simply responding to the pressure of the injection but that they are responding to decreased pH.
Figure 2.
Dural application of IL-6 primes animals to sub-threshold stimuli. Animals that received a prior application of IL-6 exhibit cutaneous allodynia when dural pH 6.8 (A, n=9 for all groups ) or pH 7.0 (B, n=10 for all groups) is applied after the resolution of allodynia at 72 hours. Withdrawal thresholds to tactile stimuli applied to the face (A & B) were measured in animals after application of dural pH 6.8 or pH 7.0 72 hours post IL-6. Significant differences among means for each group were determined by analysis of variance followed by Bonferroni post hoc test. Increasing the pH value to pH 7.2 did not produce significant facial allodynia in either IL-6 or vehicle-treated rats (C). ASIC3 blocker APETx2 was able to attenuate facial (C) and hindpaw (D) allodynia when administered with pH 6.8 at 72 hours post IL-6. Stimulation of the dura with Vehicle at 72 hours post IL-6 did not produce facial allodynia (C). Significant (**p < 0.01) differences among change of means at 72 hours for each group were determined using a t-test (C & D). (A) Facial: time F (2, 36) = 26.53, P <0.0001, treatment F (1, 87) = 0.0046,P =0.0001; (B) Facial: time F (2, 36) = 5.112, P =0.0111, treatment F (1, 36) = 19.70, P < 0.0001.
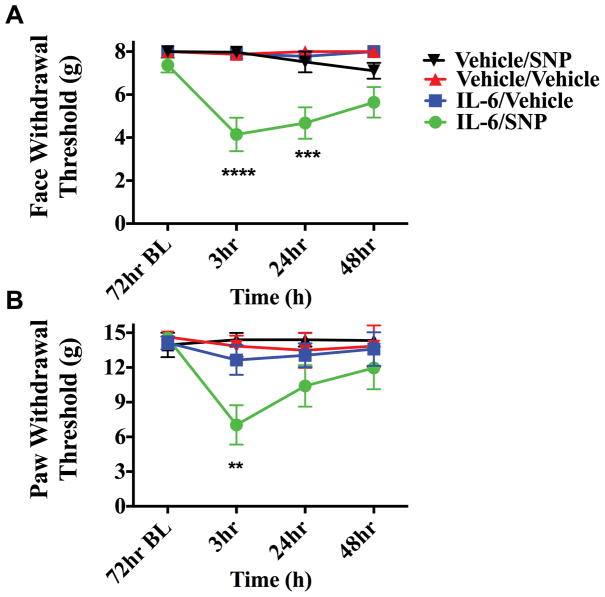
In order to determine whether IL-6 primes the dural afferent system only to subthreshold pH stimulation or whether they are sensitive to other stimuli, primed rats were given sodium nitroprusside (SNP, 3 mg/kg) at 72 hours following IL-6. NO donors are consistent migraine triggers in humans susceptible to migraine, but they do not trigger attacks in non-migraine patients [35], suggesting sensitivity to these stimuli contributes to the pathophysiology of the disorder. This dose of SNP was chosen as it has been shown to be below threshold in normal rats [20]. In rats treated with dural IL-6, systemic SNP produced allodynia when given 72-hours later while animals treated with dural vehicle did not respond to SNP (Figure 3). These data demonstrate that dural stimulation with IL-6 produces changes in the nervous system that prime animals to normally subthreshold stimuli including common triggers of migraine.
Figure 3.
Dural application of IL-6 primes animals to systemic NO donors. Animals treated with meningeal IL-6 (n=8 IL-6/SNP 3mg/kg , n=7 IL-6/Vehicle) respond to systemic SNP; producing both facial (A) and hindpaw (B) allodynia at 72 hours post IL-6 while animals treated with Vehicle (n=5 for all groups)did not respond to SNP. Withdrawal thresholds to tactile stimuli applied to the face (A) and the hindpaw (B) after systemic SNP given 72 hours post IL-6. Significant differences among means for each group were determined by analysis of variance followed by Bonferroni post hoc test. Facial: time F (3, 88) = 1.578, P =0.2004, treatment F (3, 88) = 9.588,P <0.0001; Hind paw: time F (3, 80) = 1.172, P =0.3256 treatment F (3, 80) = 20.31, P < 0.0001.
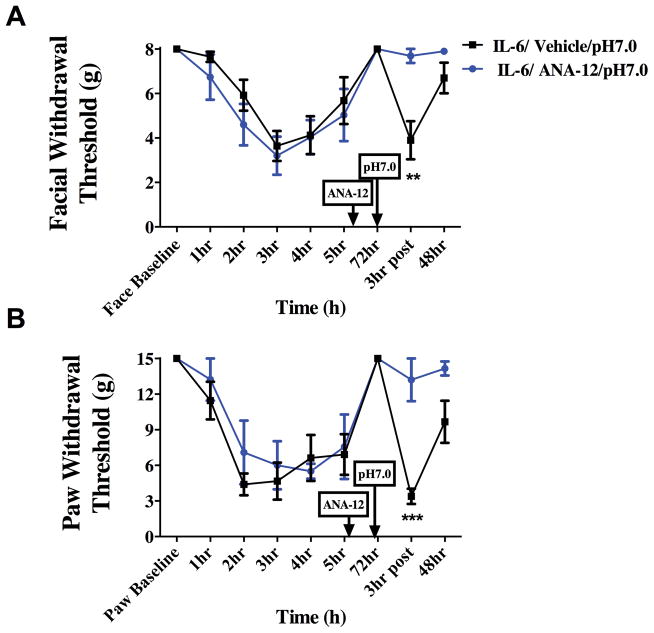
Prior studies have shown that IL-6 injection into the mouse hindpaw primes animals to a subsequent injection of subthreshold prostaglandin E2 (PGE2) and that the primed state is dependent on brain-derived neurotrophic factor (BDNF) in the spinal cord [32]. In order to test whether priming in the context of headache is dependent on BDNF, rats were treated systemically with ANA-12, previously characterized as a selective and CNS-penetrant antagonist of the BDNF receptor TrkB [17]. Rats were given i.p. injections of ANA-12 (0.5 mg/kg) 24 hours following IL-6 administration on the dura (Figure 4). Systemic administration of ANA-12 blocked both the facial and hindpaw response to dural pH 7.0 at the 72-hour time point. These data implicate BDNF in the development and/or maintenance of the primed state but do not provide information regarding the location of BDNF signaling necessary for priming.
Figure 4.
Priming following dural IL-6 stimulation is dependent on BDNF signaling. Systemic administration of ANA-12(0.5 mg/kg) 24 hours following dural IL-6 application attenuated allodynia in the face (A) and the hindpaw (B) at 72 hours Animals given ANA-12 did not show significant responses to subsequent application of dural pH 7.0 vehicle (n=11) at 72 hours post IL-6 when compared with animals given dural Vehicle (n=7)(A &B). Significant differences among means for each group were determined with a one-way ANOVA followed by Bonferroni post hoc test. Facial: time F (8, 72) = 10.88, P < 0.0001, treatment F (1, 72) = .2654,P =0.6080; Hind paw: time F (8, 72) = 13.51, P < 0.0001, treatment F (1, 72) = 9.33, P = 0.0032.
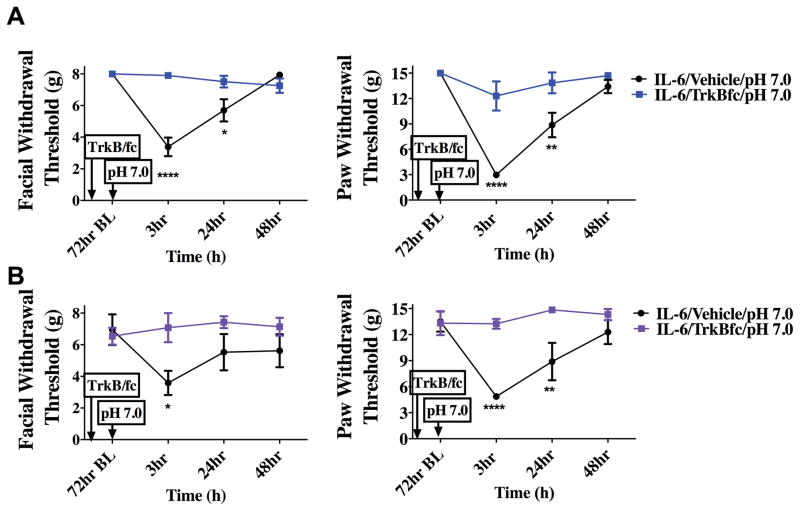
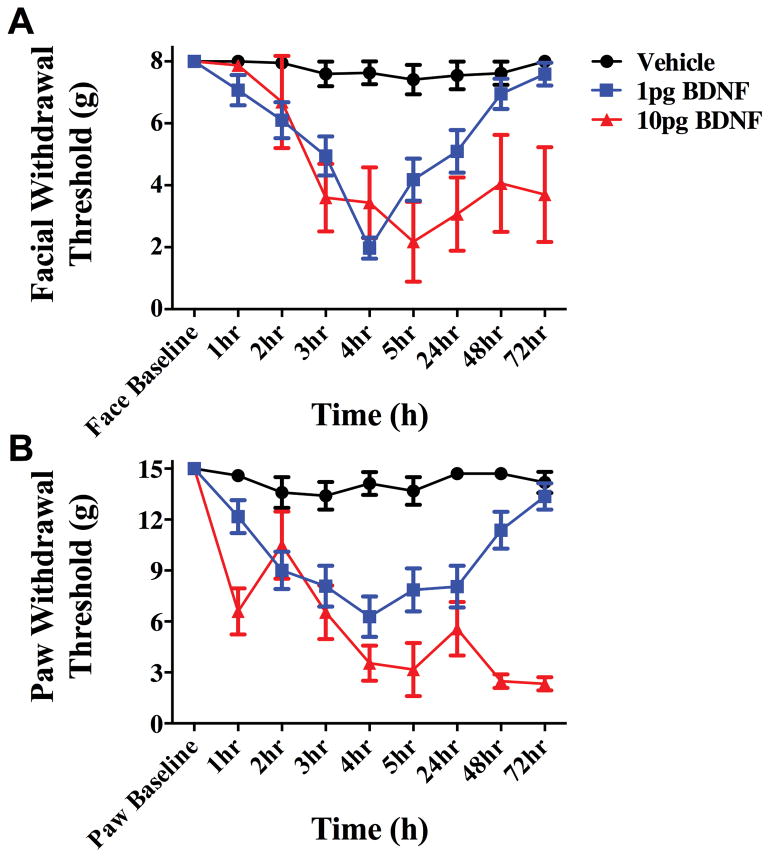
In order to test whether BDNF signaling at central terminals of dural afferents in the TNC contributes to priming in this headache behavioral model, the BDNF sequestering agent TrkB-Fc was given via intracisternal injection to decrease extracellular levels of BDNF in the TNC. When TrkB-Fc was given 24 hours following dural IL-6, rats did not respond to dural pH 7.0 at the 72-hour time point (Figure 5). Animals treated with i.c. vehicle after IL-6 displayed allodynia 3 hours after dural pH 7.0 administration, similar to data shown in Figure 2. This effect occurred regardless of the sex of the animal as both males and females were tested. These data demonstrate a role for central BNDF release in the maintenance of the primed state. If central BDNF is released following dural IL-6, and BDNF release contributes to the sensitivity to subsequent exposure to dural pH 7.0, then direct central administration of BDNF should recapitulate some or all of the features present in the primed state. As shown in Figure 6, i.c. administration of 1 or 10 picograms (pg) of BDNF produced cutaneous facial allodynia that in the case of 1 pg is resolved by 72 hours. Since 1 pg i.c. BDNF produced a time course of allodynia similar to dural IL-6, this dose was chosen for future studies.
Figure 5.
Priming following dural IL-6 stimulation is dependent on BDNF signaling in the brainstem. TrkB-Fc was given i.c. 24 hours after dural application of IL-6. Only animals given i.c. vehicle (males n=12 A & females n=7 B) respond to dural pH 7.0 at 72 hours post IL6. Males treated with IL-6/i.c vehicle/ dural pH 7.0 showed significant facial and hindpaw responses at 3 hours and 24 hours. However, females treated with IL-6/i.c vehicle/dural pH 7.0 had significant facial responses at 3 hours and hindpaw responses at 3 hours and 24 hours. Both male(n=8 A) and female (n=7 B) animals treated with i.c. TrkB-Fc after IL-6 failed to display allodynia 3 hours post administration of pH 7.0 on to the dura. Significant differences among means for each group were determined one-way ANOVA followed by Bonferroni post hoc test. Males facial: time F (3, 72) = 11.74, P <0.0001, treatment F (1,72) = 10.29, P < 0.0001; Hind paw: time F (3, 64) = 1.944, P =0.134, treatment F (3, 64) = 13.21, P < 0.0001. Females Facial: time F (3, 64) = 20.92, P <0.0001, treatment F (3, 72) = 1.109, P =0.0013; Hind paw: time F (1, 72) = 6.152, P =0.0012, treatment F (3, 64) = 24.51, P < 0.0001
Figure 6.
Intracisternal BDNF produces cutaneous allodynia. Administration of 1 or 10 picograms (pg) of BDNF i.c produces cutaneous facial (A) and hindpaw (B) allodynia. Allodynia induced by BDNF at 1 pg (n=20) resolved within 72 hours, whereas that induced by 10pg (n=6) of BDNF persisted beyond 72 hours. Vehicle (n=5) i.c. showed no significant results at any time point. Facial: time F (8, 252) = 5.85, P < 0.0001, treatment F (2,252) = 31.02, P < 0.0001; Hind paw: time F (3, 64) = 4.937 P < 0.0001, treatment F (3, 64) = 60.07, P < 0.0001
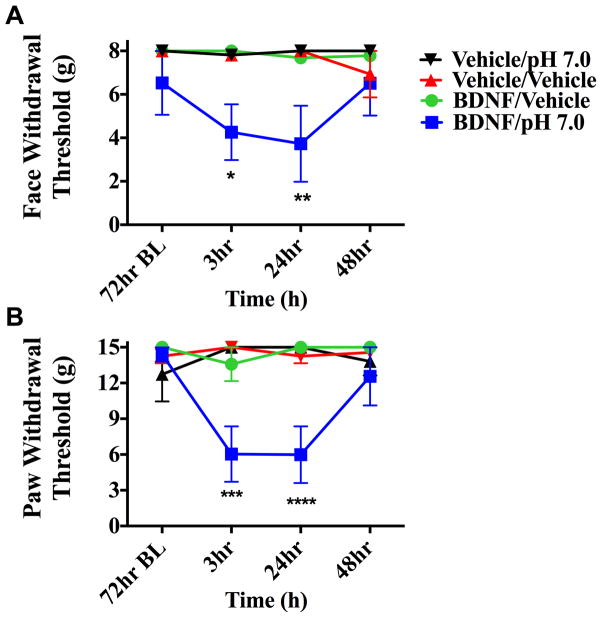
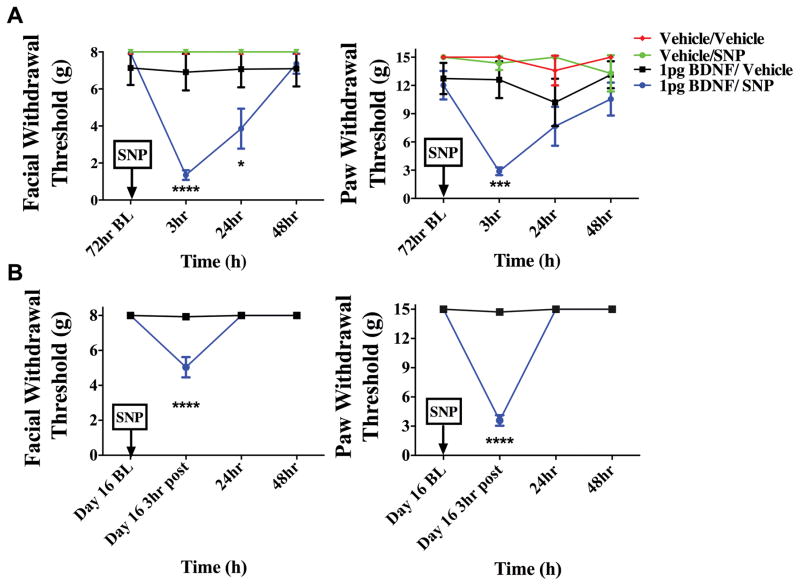
To determine whether i.c. administration of BDNF is capable of producing priming in the absence of dural stimulation, pH 7.0 was applied to the dura at the 72-hour time point after i.c. BDNF (1 pg). In animals treated with i.c. BDNF, pH 7.0 application to the dura 72 hours later produced facial allodynia while dural vehicle given to i.c. BDNF animals produced no response at this time point (Figure 7). Prior administration of i.c. vehicle did not prime rats as neither pH 7.0 nor vehicle on the dura produced allodynia at 72 hours. Additionally, i.c. BDNF-treated animals were tested for responses to systemic NO donor administration after resolution of allodynia due to BDNF (Figure 8). At the 72-hour time point after i.c. BDNF, only BDNF-treated rats developed facial allodynia when given i.p SNP. Transient allodynia was observed in i.c. BDNF animals given vehicle at 72 hours but i.c. vehicle–treated animals did not respond to later administration of SNP or vehicle. In order to examine whether BDNF priming lasts beyond the 72-hour time point, animals were given i.c. BDNF or vehicle followed by systemic (i.p.) SNP at day 16 following BDNF (Figure 8). BDNF-treated rats developed allodynia to SNP at this late time point while there was no response to vehicle in these rats, indicating that BDNF priming lasts for weeks. Together, these data show that similar to stimulation of the dura with IL-6, direct central administration of BDNF is capable of producing priming to subsequent subthreshold stimuli including common migraine triggers.
Figure 7.
Intracisternal BDNF primes animals to sub-threshold dural pH stimulation. Animals treated with i.c. BDNF followed by pH 7.0(n=5 for all groups) application to the dura 72 hours later displayed facial allodynia (A &B) while BDNF-treated animals given i.c dural vehicle showed no response at this time point. Significance was determined by one-way ANOVA analysis if the differences of the means for each group, (****p < 0.0001, *p < 0.01) . Facial: time F (3, 64) = 0.7616, P <=0.5198, treatment F (3, 64) = 10.29, P < 0.0001; Hind paw: time F (3, 64) = 1.944, P =0.134, treatment F (3, 64) = 13.21, P < 0.0001
Figure 8.
Priming following intracisternal BDNF lasts for at least several weeks. BDNF-treated animals developed facial allodynia when given i.p SNP at 72 hours (A) (BDNF/SNP n=10, BDNF/vehicle n=8, Vehicle/Vehicle n=5, Vehicle/SNP n=5) or day 16 (B) (n=8 for all groups) post BDNF treatment. A significant attenuation of withdrawal thresholds to tactile stimuli applied to the face were observed at both 3 hours and 24 hours, whereas hindpaw responses were only significant 3 hours after 72 hour SNP administration. However, on day 16 a significant attenuation of withdrawal thresholds was observed 3 hours after SNP administration for facial and hindpaw responses. Allodynia was not observed in i.c. BDNF-treated animals given vehicle at 72 hours or day 16(. Significant (****p < 0.0001) differences among means for each group were determined one-way ANOVA followed by Bonferroni post hoc test. (A) Facial: time F (3, 64) = 8.347, P =< 0.0001, treatment F (1, 64) = 12.30, P =0.0008; Hind paw: time F (3, 96) = 1.789, P =0.1544, treatment F (3, 96) = 11.55, P < 0.0001(B)Facial: time F (3, 56 = 26.59, P < 0.0001, treatment F (1,56) = 24.15, P < 0.0001; Hind paw: time F (8, 406) = 8.027, P < 0.0001, treatment F (1, 406) = 87.69, P < 0.0001
Finally, to investigate whether priming to pH 7.0 application following IL-6 is unique to the dura or whether this is a generalizable effect in any part of the body, we gave IL-6 subcutaneously into the rat hindpaw and tested for priming 72 hours later. Hindpaw injection of IL-6 caused significant paw allodynia at 3 hours post injection (Figure 9). However, at 72 hours following IL-6, animals were not sensitized to pH 7.0 administered into the hindpaw. These data demonstrate that although IL-6 is capable of priming other parts of the body to subthreshold stimulation, there may be unique mechanisms occurring within the dura and trigeminal afferent system that enable priming to specific stimuli.
Figure 9.
Hindpaw application of IL-6 fails to prime animals to subthreshold pH stimulation of the hindpaw. Animals received application of IL-6 in the hindpaw and show paw allodynia. Animals do not exhibit cutaneous allodynia when pH 7.0 is applied to the hindpaw (n=5 for all groups) after the resolution of IL-6 allodynia at 72 hours. Two-factor analysis of variance (ANOVA) indicated a significant effect of both treatment and time of the hindpaws. Significant differences among means for each group were determined by analysis of variance followed by Bonferroni post hoc test. Hind paw: time F (9, 90) = 4.11, P=0.0002, treatment F (1, 90) = 20.47, P < 0.0001.
Discussion
Migraine in most commonly an episodic disorder where, between attacks, patients are otherwise normal. However, during interictal periods, migraine patients likely carry underlying neuroplasticity that causes susceptibility to mild triggering events. The findings from these studies show that afferent input from the meninges, such as during the headache phase of migraine, can prime the nociceptive system to future subthreshold stimuli. Importantly, this primed state is present in rats that display no outward signs of hypersensitivity (e.g. no cutaneous allodynia). Despite an otherwise normal phenotype, exposure to stimuli such as moderate changes in dural pH or systemic delivery of subthreshold doses of NO donors can provoke headache-related behavior. These studies also show that this primed state is dependent on central BDNF within the brainstem. Although migraine is a complex neurological disorder, the findings presented here implicate BDNF-dependent mechanisms as a contributing factor to the sensitivity to events and stimuli that are subthreshold in non-migraineurs.
The presence of a primed state in otherwise normal rats where typically subthreshold events cause headache-related behaviors (cutaneous allodynia) mimics the phenotype of common episodic migraine. Given the presence of allodynia in other primary headache disorders, these data may also have implications for conditions such as cluster and tension-type headache [2; 8]. Our findings show that the primed state can develop following a single exposure of the dura to IL-6 or a single injection of i.c. BDNF. This suggests that a primed state in episodic migraine patients may be present after a minimal number of prior events. While previous studies have shown that animals transition into a state of persistent allodynia following repetitive exposure to dural inflammatory mediators [36], triptans [20], or NO donors [41], these models more closely mimic chronic migraine where headaches and allodynia are present most of the time [29]. Our study demonstrates that the neuroplasticity contributing to these chronic states may develop early and with repetitive exposure, may facilitate the transition from episodic to chronic migraine. Identification of plasticity mechanisms present during the episodic phase may allow these mechanisms to be targeted early, before the disorder progresses to a chronic state.
These studies demonstrate a role for central BDNF in priming of the dural afferent system, consistent with a pain-promoting role for this neuropeptide [49]. First, we show that the maintenance of priming requires BDNF signaling since priming was not present in IL-6 treated rats that were given the TrkB antagonist ANA-12 24 hours after IL-6. Second, the maintenance of priming requires central BDNF since sequestration of BDNF via i.c. injection of TrkB-Fc 24 hours following dural IL-6 can reverse the primed state. Third, we show that direct central administration of BDNF can both produce cutaneous allodynia and induce priming following resolution of allodynia. These actions of BDNF are consistent with a similar role for this neuropeptide in priming in the spinal system [32] and imply that BDNF may play a similar role in priming throughout the pain system. Our findings suggest that input from the meninges promotes the release of BDNF, contributing to neuroplasticity that underlies the primed state. Other studies have shown that dural stimulation leads to sensitization within the TNC (for example see [4; 16]), and our current data suggest that BDNF may play a role in these findings. Additionally, BDNF signaling may contribute to the sensitized state in models of chronic migraine (e.g. due to repeated dural inflammatory mediators or triptan/NO donor administration). Repetitive dural stimulation with inflammatory mediators was shown to both sensitize TNC neurons and to impair diffuse noxious inhibitory control (DNIC, a form of central descending modulation) in the TNC [13]. Chronic morphine administration also sensitized dural input into the TNC and impaired DNIC [34]. Although BDNF was not examined in these DNIC studies, chronic morphine exposure is known to increase spinal BDNF [24] providing a possible mechanistic link between BDNF and the loss of TNC DNIC. Our current work suggests that BDNF may be a unifying factor in the generation of plasticity in the TNC that contributes to migraine and other forms of primary headache.
An important observation from these studies is that central administration of BDNF, without any prior dural stimulation, promotes headache-like behavior following subsequent stimulation of the dura with pH 7.0. Dural stimulation with IL-6 produced similar priming to pH 7.0 and while we have shown previously that IL-6 can sensitize dural afferents [57], central BDNF administration is not likely to sensitize nerve endings in the dura mater. Thus, this central mechanism can exist independent of any hypersensitivity present in peripheral terminals before or during headache. Central sensitization in the TNC may enhance transmission of background levels of input from the meninges. Additionally, hypersensitivity in dural nerve endings would likely further enhance afferent trafficking. A variety of activating/sensitizing mechanisms within the dura have been proposed to contribute to migraine [7; 15] and these may occur in addition to central sensitization creating efficient afferent signaling in response to otherwise non-noxious events.
IL-6 injection into the hindpaw or gastrocnemius muscle causes priming to later injections of prostaglandin E2 (PGE2) that is also dependent on central BDNF [21; 32]. These studies suggest that BDNF-dependent IL-6 priming is not unique to migraine but may exist throughout the pain system. However, specific mechanistic differences may also exist depending on location, priming stimulus, and what stimuli animals become primed to. We show here that while dural IL-6 causes priming to later injection of a pH 7.0 solution, the same is not true in the hindpaw as rats did not respond to pH 7.0 after injection of the same dose of IL-6 into the paw. We have shown previously that dural afferents are sensitive to subtle changes in pH [56; 58] so this stimulus may be particularly important in the dura and not in the paw skin. Thus, while priming may be common in the pain system, nociceptive pathways may ultimately become more effectively primed to stimuli that are most important in the local environment.
BDNF can be released from both primary afferents [28; 33; 59] and microglia [5]. Calcitonin gene-related peptide (CGRP), which is closely linked to migraine [10; 44], promotes the release of BDNF from trigeminal neurons [14] and this may be one downstream mechanism by which administration of CGRP triggers migraine. Levels of serum BDNF are elevated in migraine patients during attacks compared to between attacks [25; 52] (although see [11]), further implicating BDNF in migraine in humans. However, recent work in several mouse pain models found that release of BDNF from spinal microglia does not contribute to pain in female mice [50]. Our data show that sequestration of central BDNF reverses priming in both males and females. This may indicate that the source of BDNF in this primed state is not microglia but that BDNF released from afferents contributes to priming. Future work is necessary to identify the source of BDNF for priming, potentially using tools such as mice where BDNF is deleted from primary afferents [59] or deleted from microglia [37].
Although our findings suggest that targeting BDNF/TrkB may have potential for the treatment of migraine, BDNF is thought to contribute to a variety of beneficial neurological processes and decreased BDNF may contribute to several disorders. Among these are learning/memory, major depressive disorder, anxiety, and addiction [3; 6; 27]. Thus, a more likely therapeutic strategy will be to identify downstream mechanisms by which BDNF and TrkB signaling contribute to priming of the dural nociceptive system. BDNF increases excitability and signaling of pain pathways via altered Cl− gradients, increased glutamate (AMPA/NMDA), acid-sensing ion channel (ASIC1a), and voltage-gated Na+ currents [12; 49] and can regulate the synthesis and activity of atypical protein kinase C (PKC) [32]. In the trigeminal system, BDNF application increased expression of the ATP-gated ion channel P2X3 [48] and decreased voltage-gated K+ currents in neurons projecting to the nucleus interpolaris/caudalis transition zone [51], a region known to contribute to the development of persistent orofacial pain [43]. Activation of PKCγ-expressing interneurons in the medullary dorsal horn also contributes to pain states in the trigeminal system [40], and this mechanism may be engaged downstream of BDNF/TrkB signaling. The above mechanisms may contribute to the primed state and it will be important to identify BDNF mechanisms that are unique to nociceptive priming that could be exploited for novel migraine therapeutics.
Together, these studies indicate that afferent input from the meninges, which occurs during headache, primes the dural nociceptive system using central release of BDNF. This suggests that headache events themselves contribute to future headaches. Our data demonstrate that a single round of either meningeal input or BDNF intracisternally can induce neuroplasticity leading to priming. Although other preclinical migraine models use repetitive stimulation to produce sensitization [20; 36; 41], BDNF in the TNC may provide a common link between these models as it is a critical mediator of plasticity related to pain [49]. Assessment of a role for BDNF in the variety of stimuli that contribute to migraine, and identification of downstream events following activation of TrkB, could lead to novel therapeutics targeting the neuroplasticity present in migraine patients.
Supplementary Material
Supplementary Figure 1- Dural IL-6 does not produce cutaneous allodynia in the facial or hindpaw regions when the dura is anesthetized. The dura was anesthetized with 0.5% w/v Bupivacaine (Hospira), given at the same time and in the same injection as 0.1 ng IL-6. Withdrawal thresholds to tactile stimuli applied to the face (A) and hindpaws (B) were measured in animals prior to and after dural application of 0.1 ng IL-6 (n = 5), Bupivacaine/IL-6 (n=5), and Bupivacaine/Vehicle (n = 3). Administration of IL-6 produced significant allodynia lasting 24 hours. However, animals given Bupivacaine/IL-6 or Bupivacaine/Vehicle show no significant response at any timepoint. Two-factor analysis of variance (ANOVA) indicated a significant effect of both treatment and time of both the face and hindpaws for IL-6 alone. Significant differences among means for each group were determined by analysis of variance followed by Bonferroni post hoc test. (A) Facial: time F (4, 50) = 4.190, P =0.0053, treatment F (2, 50) = 54.30, P < 0.0001; (B) Hindpaw: time F (4, 50) = 9.435, P < 0.0001, treatment F (2, 50) = 73.08, P < 0.0001. No impairment of motor function/coordination or any signs of neurological deficits (apart from the lack of allodynia) or distress were observed in the 24 hours following bupivacaine administration.
Acknowledgments
Funding from the NIH (NS072204 and NS065926) and the Migraine Research Foundation supported this work.
Footnotes
Potential Conflicts of Interest
The authors declare no conflicts of interest
References
- 1.Andress-Rothrock D, King W, Rothrock J. An analysis of migraine triggers in a clinic-based population. Headache. 2010;50(8):1366–1370. doi: 10.1111/j.1526-4610.2010.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A. Allodynia in cluster headache. Current pain and headache reports. 2010;14(2):140–144. doi: 10.1007/s11916-010-0097-7. [DOI] [PubMed] [Google Scholar]
- 3.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64(2):238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003;126(Pt 8):1801–1813. doi: 10.1093/brain/awg190. [DOI] [PubMed] [Google Scholar]
- 5.Beggs S, Salter MW. The known knowns of microglia-neuronal signalling in neuropathic pain. Neurosci Lett. 2013;557(Pt A):37–42. doi: 10.1016/j.neulet.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 2014;76(Pt C):677–683. doi: 10.1016/j.neuropharm.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol. 2012;8(2):89–99. doi: 10.3988/jcn.2012.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezov D, Ashina S, Jensen R, Bendtsen L. Pain perception studies in tension-type headache. Headache. 2011;51(2):262–271. doi: 10.1111/j.1526-4610.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- 9.Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157–1168. doi: 10.1111/j.1526-4610.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 10.Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache. 2013;53(8):1230–1244. doi: 10.1111/head.12179. [DOI] [PubMed] [Google Scholar]
- 11.Blandini F, Rinaldi L, Tassorelli C, Sances G, Motta M, Samuele A, Fancellu R, Nappi G, Leon A. Peripheral levels of BDNF and NGF in primary headaches. Cephalalgia. 2006;26(2):136–142. doi: 10.1111/j.1468-2982.2005.01006.x. [DOI] [PubMed] [Google Scholar]
- 12.Boyce VS, Mendell LM. Neurotrophins and spinal circuit function. Frontiers in neural circuits. 2014;8:59. doi: 10.3389/fncir.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer N, Dallel R, Artola A, Monconduit L. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain. 2014;155(7):1196–1205. doi: 10.1016/j.pain.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. Journal of neurochemistry. 2006;99(5):1338–1350. doi: 10.1111/j.1471-4159.2006.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgos-Vega C, Moy J, Dussor G. Meningeal afferent signaling and the pathophysiology of migraine. Progress in molecular biology and translational science. 2015;131:537–564. doi: 10.1016/bs.pmbts.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79(2):964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 17.Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121(5):1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Imai H, Ito A, Saito N. Novel modified method for injection into the cerebrospinal fluid via the cerebellomedullary cistern in mice. Acta Neurobiol Exp (Wars) 2013;73(2):304–311. doi: 10.55782/ane-2013-1938. [DOI] [PubMed] [Google Scholar]
- 19.De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Ann Neurol. 2013;74(2):257–265. doi: 10.1002/ana.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Felice M, Ossipov MH, Wang R, Lai J, Chichorro J, Meng I, Dodick DW, Vanderah TW, Dussor G, Porreca F. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann Neurol. 2010;67(3):325–337. doi: 10.1002/ana.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152(2):521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Activation of TRPA1 on dural afferents: A potential mechanism of headache pain. Pain. 2012;153(9):1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65(2):184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrini F, Trang T, Mattioli TA, Laffray S, Del’Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(−) homeostasis. Nat Neurosci. 2013;16(2):183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer M, Wille G, Klien S, Shanib H, Holle D, Gaul C, Broessner G. Brain-derived neurotrophic factor in primary headaches. The journal of headache and pain. 2012;13(6):469–475. doi: 10.1007/s10194-012-0454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hougaard A, Amin FM, Ashina M. Migraine and structural abnormalities in the brain. Curr Opin Neurol. 2014;27(3):309–314. doi: 10.1097/WCO.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 27.Jiang C, Salton SR. The Role of Neurotrophins in Major Depressive Disorder. Translational neuroscience. 2013;4(1):46–58. doi: 10.2478/s13380-013-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19(12):5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurology. 2009;72(5 Suppl):S3–7. doi: 10.1212/WNL.0b013e3181974b19. [DOI] [PubMed] [Google Scholar]
- 30.Lipton RB, Fanning KM, Serrano D, Reed ML, Cady R, Buse DC. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology. 2015;84(7):688–695. doi: 10.1212/WNL.0000000000001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manack A, Buse DC, Serrano D, Turkel CC, Lipton RB. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology. 2011;76(8):711–718. doi: 10.1212/WNL.0b013e31820d8af2. [DOI] [PubMed] [Google Scholar]
- 32.Melemedjian OK, Tillu DV, Asiedu MN, Mandell EK, Moy JK, Blute VM, Taylor CJ, Ghosh S, Price TJ. BDNF regulates atypical PKC at spinal synapses to initiate and maintain a centralized chronic pain state. Mol Pain. 2013;9:12. doi: 10.1186/1744-8069-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. The Journal of cell biology. 1992;119(1):45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada-Ogawa A, Porreca F, Meng ID. Sustained morphine-induced sensitization and loss of diffuse noxious inhibitory controls in dura-sensitive medullary dorsal horn neurons. J Neurosci. 2009;29(50):15828–15835. doi: 10.1523/JNEUROSCI.3623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther. 2008;120(2):157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47(7):1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peroutka SJ. What turns on a migraine? A systematic review of migraine precipitating factors. Curr Pain Headache Rep. 2014;18(10):454. doi: 10.1007/s11916-014-0454-z. [DOI] [PubMed] [Google Scholar]
- 39.Perry CJ, Blake P, Buettner C, Papavassiliou E, Schain AJ, Bhasin MK, Burstein R. Upregulation of inflammatory gene transcripts in periosteum of chronic migraineurs: Implications for extracranial origin of headache. Ann Neurol. 2016;79(6):1000–1013. doi: 10.1002/ana.24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pham Dang N, Descheemaeker A, Dallel R, Artola A. Activation of medullary dorsal horn PKCgamma Interneurons is essential to the development of both static and dynamic facial mechanical allodynia. Eur J Neurosci. 2016 doi: 10.1111/ejn.13165. [DOI] [PubMed] [Google Scholar]
- 41.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2013 doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price TJ, Inyang KE. Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog Mol Biol Transl Sci. 2015;131:409–434. doi: 10.1016/bs.pmbts.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren K, Dubner R. The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. International review of neurobiology. 2011;97:207–225. doi: 10.1016/B978-0-12-385198-7.00008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annual review of pharmacology and toxicology. 2015;55:533–552. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, Floridi A, Calabresi P. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46(2):200–207. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 46.Sauro KM, Becker WJ. The stress and migraine interaction. Headache. 2009;49(9):1378–1386. doi: 10.1111/j.1526-4610.2009.01486.x. [DOI] [PubMed] [Google Scholar]
- 47.Scher AI, Stewart WF, Ricci JA, Lipton RB. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain. 2003;106(1–2):81–89. doi: 10.1016/s0304-3959(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 48.Simonetti M, Giniatullin R, Fabbretti E. Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J Biol Chem. 2008;283(27):18743–18752. doi: 10.1074/jbc.M800296200. [DOI] [PubMed] [Google Scholar]
- 49.Smith PA. BDNF: no gain without pain? Neuroscience. 2014;283:107–123. doi: 10.1016/j.neuroscience.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 50.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature neuroscience. 2015;18(8):1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda M, Takahashi M, Matsumoto S. Inflammation enhanced brain-derived neurotrophic factor-induced suppression of the voltage-gated potassium currents in small-diameter trigeminal ganglion neurons projecting to the trigeminal nucleus interpolaris/caudalis transition zone. Neuroscience. 2014;261:223–231. doi: 10.1016/j.neuroscience.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 52.Tanure MT, Gomez RS, Hurtado RC, Teixeira AL, Domingues RB. Increased serum levels of brain-derived neurotropic factor during migraine attacks: a pilot study. The journal of headache and pain. 2010;11(5):427–430. doi: 10.1007/s10194-010-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005;64(10 Suppl 2):S9–15. doi: 10.1212/wnl.64.10_suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- 55.Wieseler J, Ellis A, Sprunger D, Brown K, McFadden A, Mahoney J, Rezvani N, Maier SF, Watkins LR. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. J Neurosci Methods. 2009 doi: 10.1016/j.jneumeth.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan J, Edelmayer RM, Wei X, De Felice M, Porreca F, Dussor G. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain. 2011;152(1):106–113. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6) Molecular pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan J, Wei X, Bischoff C, Edelmayer RM, Dussor G. pH-Evoked Dural Afferent Signaling Is Mediated by ASIC3 and Is Sensitized by Mast Cell Mediators. Headache. 2013 doi: 10.1111/head.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN, London Pain C. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci. 2006;31(3):539–548. doi: 10.1016/j.mcn.2005.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1- Dural IL-6 does not produce cutaneous allodynia in the facial or hindpaw regions when the dura is anesthetized. The dura was anesthetized with 0.5% w/v Bupivacaine (Hospira), given at the same time and in the same injection as 0.1 ng IL-6. Withdrawal thresholds to tactile stimuli applied to the face (A) and hindpaws (B) were measured in animals prior to and after dural application of 0.1 ng IL-6 (n = 5), Bupivacaine/IL-6 (n=5), and Bupivacaine/Vehicle (n = 3). Administration of IL-6 produced significant allodynia lasting 24 hours. However, animals given Bupivacaine/IL-6 or Bupivacaine/Vehicle show no significant response at any timepoint. Two-factor analysis of variance (ANOVA) indicated a significant effect of both treatment and time of both the face and hindpaws for IL-6 alone. Significant differences among means for each group were determined by analysis of variance followed by Bonferroni post hoc test. (A) Facial: time F (4, 50) = 4.190, P =0.0053, treatment F (2, 50) = 54.30, P < 0.0001; (B) Hindpaw: time F (4, 50) = 9.435, P < 0.0001, treatment F (2, 50) = 73.08, P < 0.0001. No impairment of motor function/coordination or any signs of neurological deficits (apart from the lack of allodynia) or distress were observed in the 24 hours following bupivacaine administration.