A recent meta-analysis and meta-regression of 13 randomized clinical trials by Mocking et al.1 concluded that supplementation with omega-3 fatty acids, found naturally in fatty fish, has a beneficial effect in patients with major depressive disorder (MDD), especially for higher doses of the eicosapentaenoic acid (EPA) and in patients taking antidepressants. Novel treatments for MDD are certainly desired. However, in our view the evidence in this study does not solve the academic debate on the efficacy of omega-3 fatty acids for MDD. Some food for thought.
Meta-analysis: not more than the sum of its parts
On the basis of the widely accepted GRADE system, a recent Cochrane review evaluated the overall quality of the evidence of studies on omega-3 fatty acids and depressive symptomatology (n=26) as very low,2 and the body of evidence as composed of a limited number of predominantly small studies at high risk of selection, performance or attrition bias. Poor evidence quality downgrades the credibility of overall effect size estimates, particularly when the evidence for an effect appears to be driven by poorer quality studies.
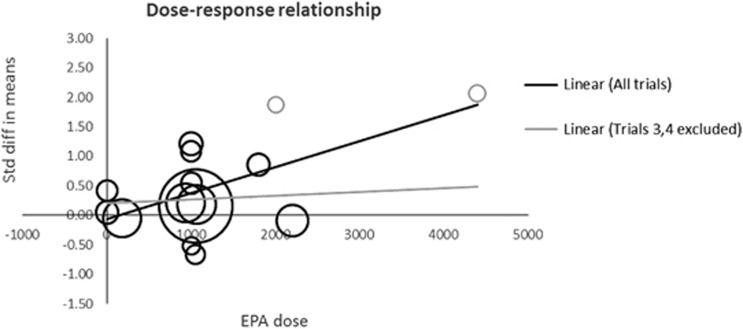
Study selection concerns aside, Mocking et al.1 found no association between study effect size and study quality as operationalized by the 5-point Jadad score in their subset of 13 studies. Jadad scores, however, simply indicate whether a study reports a double-blind randomized trial and reports drop-outs and withdrawals, resulting in a maximal score for 9 of the 13 reviewed studies. This minimal variation largely reduced the power to detect associations with study effect size. More importantly, the Jadad score ignores highly relevant aspects such as risk of bias and study precision (1/s.e.). Analyses conducted on studies with low risk of bias have consistently produced nonsignificant effect estimates.2 Moreover, based on the mean standardized differences and s.e.'s reported in their Figure 1, we found that the studies included in Mocking et al.1 show an inverse association between study effect size and study precision (r=−0.344): less precise trials produced larger effect sizes. To illustrate the impact that less precise studies can have on meta-analytic results, we repeated the meta-analysis (based on the data provided in Mocking et al.1) but without the least precise study3 (N=20), which reduced the overall effect size from a standardized mean difference (SMD) of 0.398 (95% confidence interval (CI): 0.114, 0.681, P=0.006) to 0.317 (95% CI: 0.051, 0.582, P=0.019). In addition, excluding the second-least precise study4 (N=22) further reduces the effect size to 0.227 (95% CIs: 0.001, 0.453, P=0.049). Thus, the observed effect of omega-3 fatty-acid supplementation on depression seems largely driven by the most imprecise studies.
Figure 1.
Dose–response relationship. Circles represent the effect size of the individual trials scaled by their sample size. The gray circles represent the studies by Nemets et al.3 and Su et al.4 (top right), which have the smallest sample sizes and the largest effect sizes. The dose–response relationship is depicted as a solid line for the linear trend based on all trials (r=0.6) and a gray line discarding Nemets et al.3 and Su et al.4 (r=0.1). EPA, eicosapentaenoic acid.
Meta-regression: the more trials the merrier
Based on nine univariate meta-regressions (one for each study characteristic) across 13 trials, Mocking et al.1 concluded that omega-3 fatty-acid supplementation in MDD patients is especially beneficial in patients using antidepressants and for higher doses of EPA. A low number of trials reduces the probability of a true-negative finding. Whereas the number of trials here may not be exceptionally low compared with other meta-regressions, detecting moderator effects requires more powerful analyses than are employed in most published studies.5 Especially when high heterogeneity is present across studies, as is the case in Mocking et al.1 (I2=73%, t2=0.171), power of 80% to detect even the largest of the modest moderator effects reported in Mocking et al.1 may not be achieved except with a much larger number of trials.5 Perhaps counterintuitively, low statistical power also decreases the probability that an observed effect that reaches nominal statistical significance actually reflects a true effect.6, 7 The risk of false-positive findings is further increased by the substantial number of statistical tests conducted in this study.7, 8 Indeed, neither of the results (antidepressants, P=0.044; EPA dose, P=0.009) survives correction for multiple comparisons (Bonferroni P-value=0.05/9 =0.006), and the EPA dose–response relationship is mainly attributable to the two least precise studies3, 4 (Figure 1).
Meta-regression: correlation is not causation
The conclusions on EPA dose and antidepressant use were not based on randomization of these characteristics. Meta-regression is observational and, therefore, susceptible to confounding; it does not allow causal inference.8 Hence, the associations found with EPA dose and antidepressant use could be due to other, known or unknown, trial characteristics. That findings from this meta-regression do not necessarily align with results from intervention studies is illustrated by the largest clinical trial available to date (N=432),9 which stratified randomization by antidepressant use and found evidence for neither an interaction between treatment group and antidepressant use, nor benefit from EPA supplementation among the subgroup of patients also taking antidepressants (n=174).
Meta-analyses are critical to evidence-based medicine, but may lead to biased conclusions if the quality of available evidence is not adequately considered. Findings from meta-regression should be interpreted with particular caution, especially when suggesting clinical implications. Even if unbiased, a statistically significant result is not necessarily clinically relevant, and one may wonder whether, for instance, a decrease of 0.04 on the 17-item Hamilton Depression Rating Scale with every 100 mg increase in EPA dose is meaningful. In our view, the current evidence supporting the use of omega-3 fatty-acid supplementation in depression remains weak and clinical implications should be tempered.
Footnotes
The authors declare no conflict of interest.
References
- Mocking RJT, Harmsen I, Assies J, Koeter MWJ, Ruhé HG, Schene AH. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry 2016; 6: e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton KM, Perry R, Sallis HM, Ness AR, Churchill R Omega-3 fatty acids for depression in adults. Cochrane Database Syst 2015; (11): CD004692. [DOI] [PMC free article] [PubMed]
- Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002; 159: 477–479. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2003; 13: 267–271. [DOI] [PubMed] [Google Scholar]
- Hempel S, Miles JN, Booth MJ, Wang Z, Morton SC, Shekelle PG. Risk of bias: a simulation study of power to detect study-level moderator effects in meta-analysis. Syst Rev 2013; 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14: 365–376. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA, Tarone R, McLaughlin JK. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology 2011; 22: 450–456. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Higgins J. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002; 21: 1559–1573. [DOI] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N, St-Andre E, Turecki G, Lesperance P, Wisniewski SR. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry 2011; 72: 1054–1062. [DOI] [PubMed] [Google Scholar]