Abstract
Exposure to therapeutic doses of ionizing radiation is associated with damage to the heart and coronary arteries. However, only recently have studies with high-quality individual dosimetry data allowed this risk to be quantified while also adjusting for concomitant chemotherapy, and medical and lifestyle risk factors. At lower levels of exposure the evidence is less clear. In this article we review radiation-associated risks of circulatory disease in groups treated with radiotherapy for malignant and non-malignant disease, and in occupationally- or environmentally-exposed groups receiving rather lower levels of radiation dose, also for medical diagnostic purposes.
Results of a meta-analysis suggest that excess relative risks per unit dose for various types of heart disease do not differ significantly (p>0.2) between studies. In particular, there are no marked discrepancies between risks derived from the high-dose therapeutic and medical diagnostic studies and from the moderate/low dose occupational and environmental studies. However, risk for stroke and other types of circulatory disease are significantly more variable (p<0.0001), possibly resulting from confounding and effect-modification by well known (but unobserved) risk factors. Adjustment for any of mean dose, dose fractionation or age at exposure results in the residual heterogeneity for cerebrovascular disease becoming non-significant. The review provides strong evidence in support of a causal association between both low and high dose radiation exposure and most types of circulatory disease.
Keywords: circulatory disease, radiation, heart disease, stroke, review
1. INTRODUCTION
Circulatory disease, which is customarily defined as those causes of mortality and morbidity with International Classification of Diseases 10th revision (ICD10) codes I00-I99 (or equivalently the International Classification of Diseases 8th or 9th revision (ICD8, ICD9) codes 390-459), is the leading cause of death in the developed world [1,2] There are many types of circulatory disease [3]; the main types are listed in Table 1. Circulatory disease accounts for 30.8% of the 2.6 million deaths in the USA in 2014, of which the two leading components are ischemic heart disease (IHD), accounting for 23.4%, and stroke accounting for 5.1%, of all deaths [2]; worldwide IHD and stroke rank first and third in years of life lost [4]. Consistently identified independent risk factors include cigarette smoking, diabetes, high blood pressure, obesity, and increased total and low-density-lipoprotein cholesterol [5]. Of emerging importance are certain maternal reproductive factors [6,7]. Circulatory disease has also been shown to aggregate in families, so that children of parents with cardiovascular disease are more likely to develop it themselves. Relative risk (RR) for coronary heart disease in first-degree relatives has been reported to range from 2 to 12 times higher than that of the general population [8-11]. Advances in genetic epidemiology over the past few years have helped to identify several genetic polymorphisms that increase or decrease an individual’s chance of developing circulatory disease [12,13]. Such genetic polymorphisms have so far been associated with small effects on cardiovascular risk.
Table 1.
Major types of circulatory disease.
| Disease endpoint | International Classification of Diseases 10th revision (ICD10) coding | Description |
|---|---|---|
| Arteriosclerosis | I25.0, I25.1, I70 | Arteriosclerosis is characterized by a thickening, hardening and loss of elasticity of the walls of arteries. This process gradually restricts the blood flow to organs and tissues. and comprises three main types, (a) Monckeberg (medial calcific) sclerosis, (b) arteriolosclerosis, and (c) atherosclerosis. Monckeberg sclerosis is caused by calcium build-up in the arterial walls, and results in them becoming stiffer, and is often asymptomatic. Arteriolosclerosis is the process of artery thickening and hardening in the small arteries and arterioles. Hyaline arteriolosclerosis results from (a) lumenal protein leakage into and build-up in the arterial walls, resulting in thickening and stiffening of the arterial wall and reduced blood flow through the lumen or (b) diabetes, which causes high levels of blood sugar that directly damages the endothelial cell layer, likely via alterations in carbohydrate and fat metabolism, resulting in damage to the basement membrane of the blood vessels. Hyperplastic arteriolosclerosis results from extreme hypertension and compensatory thickening, via build up of smooth-muscle cells in the arterial wall. In contrast, atherosclerosis is caused by build-up of cholesterol-rich atheromatous plaques in the tunica intima (the part of the arterial wall immediately behind the endothelial cell layer) and is a disease of the large arteries (e.g., coronary, carotid). Plaque build-up and rupture, which results in clotting of the blood at the site of rupture, reduces blood-flow in the affected arteries. If blood flow to the kidneys is reduced for whatever reason (whether due to atherosclerosis or arteriolosclerosis), the kidney interprets this as low blood pressure and activates the renin-angiotensin-aldosterone system, raising blood volume and so blood pressure, causing hypertension (high blood pressure). When arteriolosclerosis leads to chronically reduced blood flow to the kidney arteriolonephrosclerosis is produced, which if untreated can lead to chronic renal failure. Atherosclerosis is also caused by hypertension, as well as by smoking, by elevated levels of low density lipoprotein (LDL) cholesterol, or by reduced levels of high density lipoprotein (HDL) cholesterol. The weakening of the arterial wall that results from atherosclerosis can lead to aneurysms in many parts of the body, in particular the intestine (e.g., abdominal aortic aneurysms). The term arteriosclerosis is sometimes (incorrectly) used interchangeably with the term atherosclerosis. Arteriosclerosis is mostly subsumed within IHD, but a substantial component (atherosclerosis, ICD10 I70) is independent of that. It is a relatively common type of cardiovascular disease, and the substantial part subsumed within IHD accounts for about a third of all IHD deaths, so about 4% of all deaths in the UK [104,105]. |
| Cardiac valve diseases | I05-I09, I34-I39 | This rubric includes a variety of abnormalities to one or more of the heart valves (tricuspid, pulmonary, mitral, and aortic valves). Problems in all four valves are typically of three types (a) regurgitation or backflow - when the valve doesn’t close properly (b) stenosis - when the valve flaps stiffen or fuse and (c) atresia - when a valve lacks an opening for blood to flow through. Cardiac valve disease can be congenital, but can also be acquired over the course of life. This is a less common type of circulatory disease mortality, and accounts for about 0.6% of all deaths in the UK [104,105]. |
| Cardiac arrythmias | I47-I49 | Cardiac arrhythmia, also known as cardiac dysrhythmia or irregular heartbeat, is a group of conditions in which the heartbeat is irregular, too fast, or too slow. This is a less common type of circulatory disease mortality, and accounts for about 0.6% of all deaths in the UK [104,105]. A heart rate that is too fast - above 100 beats per minute in adults - is called tachycardia and a heart rate that is too slow - below 60 beats per minute - is called bradycardia. Many types of arrhythmia have no symptoms. When symptoms are present these may include palpitations or feeling a pause between heartbeats. More seriously there may be lightheadedness, fainting, shortness of breath, or angina. While most types of arrhythmia are not serious, some predispose a person to complications such as stroke or heart failure. Others may result in cardiac arrest. There are four main types of arrhythmia: (a) extra beats, (b) supraventricular tachycardias, (c) ventricular arrhythmias, and (d) bradyarrhythmias. Extra beats include premature atrial contractions and premature ventricular contractions. Supraventricular tachycardias include atrial fibrillation, atrial flutter, and paroxysmal supraventricular tachycardia. Ventricular arrhythmias include ventricular fibrillation and ventricular tachycardia. Arrhythmias are due to problems with the electrical conduction system of the heart. Arrhythmias may occur in children; however, the normal range for the heart rate is different and depends on age. |
| Cardiomyopathy | I25.5, I42-I43 | Cardiomyopathy is characterized by the heart muscle becoming enlarged, thick, or rigid. In rare cases, the muscle tissue in the heart is replaced with scar tissue. This is a less common type of circulatory disease mortality, and accounts for about 0.3% of all deaths in the UK [104,105]. As cardiomyopathy worsens, the heart becomes weaker, and less able to pump blood through the body and maintain a normal electrical rhythm. This can lead to heart failure or irregular heartbeats called arrhythmias. In turn, heart failure can cause fluid to build up in the lungs, ankles, feet, legs, or abdomen. The weakening of the heart also can cause other complications, such as heart valve problems. The four main types of cardiomyopathy are (a) hypertrophic cardiomyopathy, (b) dilated cardiomyopathy, (c) restrictive cardiomyopathy, and (d) arrhythmogenic right ventricular dysplasia. Cardiomyopathy can be congenital or acquired over the course of life. |
| Cerebrovascular disease (CeVD) | I60-I69 | CeVD, commonly termed stroke, arises because of problems with the circulation of blood in the blood vessels of the brain. This is the second most common type of circulatory disease mortality, and accounts for about 7% of all deaths in the UK [104,105]. A blockage with effects lasting less than 24 hours is referred to as a transient ischemic attack (TIA). Loss of blood and oxygen to areas of the brain can lead to cell death and consequently permanent brain dysfunction. Two major forms of stroke are recognised (a) ischemic stroke, caused by narrowing of blood vessels, and (b) hemorrhagic stroke, cause by bursting of a blood vessel in the brain. Ischemic stroke is divided into those caused (a) by blockage due to blood clots forming locally (thrombotic stroke) or (b) fragments from distant clots lodging in the brain vasculature (embolic stroke). |
| Hypertensive disease | I10-I15 | Hypertension (high blood pressure) has a number of adverse effects on the circulatory system; in particular, as the heart pumps against this pressure, it must work harder, causing the heart muscle to thicken, and eventually heart failure may develop. This is a less common type of circulatory disease mortality, and accounts for about 0.7% of all deaths in the UK [104,105]. With increasing blood pressure risk of hemorrhagic stroke increases. The major types of hypertensive disease include (a) hypertensive heart disease, (b) hypertensive chronic kidney disease, and (c) hypertensive heart and chronic kidney disease. Hypertension also results in damage to and thickening of the arterial walls, resulting in arteriosclerosis (see above), also increased prevalence of atheromatous plaques (degenerative cholesterol deposits) in the large arterial walls, resulting in increased risk of myocardial infarction and stroke. Hypertensive heart disease is the leading cause of illness and death from hypertension. |
| Ischemic heart disease (IHD) | I20-I25 | IHD, also known as coronary artery disease (CAD), is the most common type of cardiovascular disease in most developed countries. This is the most common type of circulatory disease, and accounts for about 12% of all deaths in the UK [104,105]. It is characterized by problems with the arterial blood supply to the heart. A partial blockage of one or more of the coronary arteries (e.g. resulting from atheromatous plaque rupture and consequent blood clotting) can result in myocardial ischemia (oxygen starvation of myocardial (heart muscle) cells) thus causing symptoms such as angina (chest pain) and dyspnea (shortness of breath). A partial or complete blockage of an artery causes necrosis (damage to the myocardial cells) and if sufficiently severe a myocardial infarction (heart attack). The underlying mechanism involves atherosclerosis of the arteries of the heart (see above). Risk factors for IHD include: hypertension, smoking, diabetes, lack of exercise, obesity, high levels of LDL cholesterol, low levels of HDL cholesterol, poor diet, excessive alcohol consumption, and depression. This rubric includes a number of common types of heart disease, including arteriosclerosis (stiffening/thickening of arterial walls - see above), and angina (chest pain). |
| Pericarditis | I01.0, I09.2, I30-I32 | Inflammation of the pericardium, the membrane that surrounds the heart, is most frequently attributable to infectious agents but is also well established to be caused by high doses of ionizing radiation (> 35 Gy to heart) [14]. This is a very uncommon type of circulatory disease mortality, and accounts for about 0.04% of all deaths in the UK [104,105]. |
Environmental agents may also contribute to circulatory disease risk and it has long been recognized that human exposure to ionizing radiation during radiotherapy can damage the heart [14]. Radiotherapeutic (RT) doses to the heart and other organs/tissues of relevance to the circulatory system can be very high, as for example in the treatment of Hodgkin’s lymphoma (HL) where doses to some regions of the heart from mediastinal exposure can exceed 40 Gy 1 [15]; however, doses after treatment of some other cancers, for example breast cancer, are often lower than this [16]. Heart and coronary arterial doses associated with RT treatment tend to be lower among groups treated for non-malignant disease [17]. Many of the earlier studies lack individual radiation dosimetry (e.g., [18-22]). There is also generally little information on concomitant chemotherapy (CT), some types of which (e.g., vincristine, anthracyclines) are cardiotoxic, irrespective of the administration of concomitant RT [21]. Since concomitant CT is often correlated with RT dose there is potential for serious confounding of the dose response.
The Life Span Study (LSS) of the Japanese atomic-bomb survivors provides evidence of increased risk of myocardial infarction and stroke at rather lower levels of dose, under 5 Gy, and with mean doses of somewhat less than 0.5 Gy [23,24]. There is no appreciable nonlinearity in the radiation dose response for circulatory disease in the LSS data, although the form of the dose-response relationship, particularly at lower doses, is uncertain [24]. Therefore the magnitude of risk of circulatory disease in the low dose region where issues of radiation protection usually operate is not clear. There is emerging, and still controversial, evidence that exposure to much lower doses and dose rates of radiation, in particular associated with occupational and diagnostic exposure [25], may be associated with excess risk of circulatory disease. Claims have been made of no-effect thresholds for circulatory diseases in the LSS [26], although this has been disputed [27]. Epidemiological studies are likely to have difficulty in detecting increased risk at low dose levels as the main circulatory diseases of concern are very common in the population as a whole and, as above, there are multiple potentially confounding contributory risk factors. The International Commission on Radiological Protection (ICRP) has classified circulatory disease as a tissue reaction effect, with an approximate threshold dose of about 0.5 Gy [28]. The threshold was derived by fitting a linear model to epidemiologic data and selecting the dose below which there was less than a 1% chance of an effect. As such this does not represent a true no-effect dose threshold.
In the present review I shall consider in turn the risks of radiation-associated circulatory disease that have been observed in therapeutically- or diagnostically-exposed cohorts. Risks among groups exposed to generally lower levels of radiation dose will also be assessed, specifically in the LSS and in occupationally- and environmentally-exposed groups. Attention will generally be concentrated on studies with high quality individual organ dosimetry, based on those of previous systematic reviews [25,29], which have been updated for the present paper, based in part on updates also on previously reported (non-systematic) reviews of the moderate/low-dose literature [30,31]; unlike all these previous reviews the organ or tissue dose range that is to be considered is not constrained. As part of the review a meta-analysis of the eligible studies will be performed, similar to that conducted by Little et al [29]; meta-regression will be used to assess the effect of certain explanatory variables as a means of accounting for possible inter-study heterogeneity.
2. DATA SELECTION AND STATISTICAL METHODS FOR META-ANALYSIS
When both mortality and morbidity data are available for a particular cohort, preference will generally be given to use of the morbidity data in the meta-analysis, because of the generally greater diagnostic accuracy of the former, and to minimize the possibility of double-counting circulatory disease counts. However, in the LSS data both endpoints will be analyzed, since there is likely not much overlap in the endpoints being considered, and both are likely to be informative. For the Mayak worker cohort, as above, preference is given to use of the morbidity data in analyses of the two main endpoints, IHD [32] and cerebrovascular disease (CeVD) [33]; nevertheless, to assess differences made by this assumption, for certain subsidiary analyses (presented in Tables 6 and 7) analysis will be presented based on the mortality data.
Table 6. Results of meta-regression analyses adjusting for age at exposure, dose fractionation/dose rate, mean dose.
Random effects models are fitted via maximum likelihood. Optimal model (with lowest AIC) is given in boldface and underlined. For ischemic heart disease and cerebrovascular disease models are fitted using Mayak morbidity data (main analysis) or Mayak mortality data (subsidiary analysis)
| Ischemic heart disease (Mayak morbidity) | Ischemic heart disease (Mayak mortality) | Heart disease other than ischemic heart disease | Cerebrovascular disease (Mayak morbidity) | Cerebrovascular disease (Mayak mortality) | Other circulatory disease | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| AIC | p-valuea | I2 (%) | AIC | p-valuea | I2 (%) | AIC | p-valuea | I2 (%) | AIC | p-valuea | I2 (%) | AIC | p-valuea | I2 (%) | AIC | p-valuea | I2 (%) | |
| Unadjusted | -20.28 | 0.4430 | 3.17 | -24.09 | 0.7443 | 0.00 | -14.63 | 0.2041 | 7.52 | 18.69 | <0.0001 | 73.10 | 16.04 | 0.0226 | 57.00 | 4.75 | <0.0001 | 88.99 |
| Adjusted for age at exposure | -18.82 | 0.6654 | 0.00 | -17.29 | 0.5380 | 0.00 | -11.04 | 0.1305 | 8.76 | 11.09b | 0.3717 | 0.02 | 17.22 | 0.0184 | 39.77 | 6.75 | <0.0001 | 89.98 |
| Adjusted for radiation dose fractionation | -22.95c | 0.8161 | 0.00 | -20.67 | 0.6494 | 0.00 | -12.77 | 0.1834 | 9.81 | 15.44d | 0.1545 | 0.00 | 19.36 | 0.0111 | 42.24 | 8.41 | <0.0001 | 90.14 |
| Adjusted for mean dose | -20.63 | 0.5483 | 0.00 | -22.28 | 0.6918 | 0.00 | -13.70 | 0.1972 | 6.49 | 15.97e | 0.0858 | 24.83 | 17.82 | 0.0226 | 41.10 | 6.43 | <0.0001 | 88.98 |
| Adjusted for age at exposure, radiation dose fractionation | -18.11 | 0.8132 | 0.00 | -16.22 | 0.6384 | 0.00 | -11.43 | 0.1917 | 9.19 | 14.67f | 0.2372 | 0.00 | 20.75 | 0.0075 | 43.51 | 8.41 | <0.0001 | 90.14 |
| Adjusted for age at exposure, mean dose | -17.48 | 0.6416 | 0.00 | -15.38 | 0.4558 | 0.00 | -11.43 | 0.1919 | 9.19 | 11.07g | 0.4557 | 0.01 | 13.61h | 0.2517 | 0.00 | 8.41 | <0.0001 | 90.14 |
| Adjusted for radiation dose fractionation, mean dose | -21.65 | 0.8077 | 0.00 | -19.38 | 0.6318 | 0.00 | -13.26 | 0.2812 | 7.73 | 14.35i | 0.2592 | 0.01 | 16.94 | 0.1232 | 0.01 | 8.41 | <0.0001 | 90.14 |
| Adjusted for age at exposure, radiation dose fractionation, mean dose | -16.44 | 0.7668 | 0.00 | -16.47 | 0.7856 | 0.00 | -9.43 | 0.1071 | 11.99 | 14.77j | 0.2904 | 0.01 | 16.65 | 0.1672 | 0.01 | 0.41 | <0.0001 | 90.14 |
p-value of residual heterogeneity
significant improvement in fit over null model (p=0.0019);
significant improvement in fit over null model (p=0.0357);
significant improvement in fit over null model (p=0.0267);
significant improvement in fit over null model (p=0.0299);
significant improvement in fit over null model (p=0.0184);
significant improvement in fit over null model (p=0.0030);
significant improvement in fit over null model (p=0.0402);
significant improvement in fit over null model (p=0.0159);
significant improvement in fit over null model (p=0.0179).
Table 7. Results of meta-regression analyses for ischemic heart disease and cerebrovascular disease.
Random effects models are fitted via restricted maximum likelihood (REML). Analysis uses either Mayak morbidity data (main analysis) or Mayak mortality data (subsidiary analysis)
| Main analysis: using data | Mayak morbidity | Subsidiary analysis: using data | Mayak mortality | |
|---|---|---|---|---|
| Subset | ERR / Gy (+95% CI) | p-value | ERR / Gy (+95% CI) | p-value |
|
| ||||
| Ischemic heart disease (IHD) | ||||
|
| ||||
| Analysis adjusted for mean dose | ||||
| Adjusted to 2.4 Gya | 0.092 (0.063, 0.121) | <0.0001 | 0.073 (0.045, 0.101) | <0.0001 |
|
| ||||
| Analysis by exposure dose-rate group | ||||
| Acute high dose-rate exposure | 0.038 (-0.043, 0.118) | 0.1797 | 0.038 (-0.043, 0.118) | 0.1797 |
| Acute moderate/high dose-rate fractionated exposure | 0.069 (0.050, 0.088) | <0.0001 | 0.069 (0.050, 0.088) | <0.0001 |
| Low dose-rate exposure | 0.147 (0.087, 0.207) | <0.0001 | 0.114 (-0.003, 0.232) | 0.0278 |
|
| ||||
| Analysis by age at exposure group | ||||
| Childhood and younger adult exposure | 0.064 (0.043, 0.084) | <0.0001 | 0.064 (0.043, 0.084) | <0.0001 |
| Adult and older adult exposure | 0.111 (0.075, 0.148) | <0.0001 | 0.085 (0.047, 0.122) | <0.0001 |
| All ages at exposure | 0.055 (-0.023, 0.132) | 0.0837 | 0.055 (-0.023, 0.132) | 0.0837 |
|
| ||||
| Cerebrovascular disease (CeVD) | ||||
|
| ||||
| Analysis adjusted for mean dose | ||||
| Adjusted to 0.2 Gyb | 0.238 (0.105, 0.371) | 0.0002 | 0.154 (0.000, 0.307) | 0.0247 |
|
| ||||
| Analysis by exposure dose-rate group | ||||
| Acute moderate/high dose-rate exposure | 0.112 (0.048, 0.176) | 0.0003 | 0.112 (0.048, 0.176) | 0.0003 |
| Low dose-rate exposure | 0.308 (0.075, 0.542) | 0.0048 | 0.175 (-0.058, 0.408) | 0.0700 |
|
| ||||
| Analysis by age at exposure group | ||||
| Adult exposure | 0.111 (0.047, 0.175) | 0.0003 | 0.111 (0.047, 0.175) | 0.0003 |
| All ages at exposure | 0.382 (0.188, 0.576) | <0.0001 | 0.205 (-0.047, 0.457) | 0.0553 |
mean dose over all studies of IHD;
mean dose over all studies of CeVD.
The basis of all estimations of radiation risk is the value of the excess relative risk (ERR) per unit (Sv / Gy) of radiation exposure (ERR per Sv / ERR per Gy). [Most publications employ unweighted radiation dose (Gy), but some (e.g., LSS) use weighted (equivalent) dose (Sv).] Wherever possible the ERR was taken directly from the relevant publication, which are reproduced in Tables 2-4. For the studies of Cutter et al [34] and Mulrooney et al [35] subsidiary analysis was performed to derive useful risk estimates, described in Appendix A.
Table 2.
Estimated excess relative risk of circulatory disease in various therapeutically treated groups, exposed at high doses, with mean dose generally > 0.5 Gy. All data are in relation to underlying cause of death, unless otherwise indicated.
| Study | Reference | Average Dose to Heart (Gy) (mean, range) | Persons (person years of follow-up) | Endpoint (mortality unless otherwise indicated) | Excess relative risk Sv-1 (95% CI) |
|---|---|---|---|---|---|
| US Childhood Cancer Survivor Study | Mulrooney et al [35] | NA (<5 – >35) | 14,358 (NA) | Congestive heart failure incidence | 0.084 (0.062, 0.106)a |
| Myocardial infarction incidence | 0.062 (0.040, 0.084)a | ||||
| Pericardial disease incidence | 0.091 (0.068, 0.115)a | ||||
| Valvular heart disease incidence | 0.109 (0.091, 0.127)a | ||||
|
| |||||
| French–UK childhood cancer study | Tukenova et al [43] | 11.1b (<1 – >15) | 4122 (NA) | All cardiac disease | 0.6 (0.2, 2.5) |
|
| |||||
| Peptic ulcer study | Little et al [17] | 1.01 (0.0 – 6.20) | 3600 (76,571.7) | Ischemic heart disease ICD9 410-414 using heart dose | 0.102 (0.039, 0.174) |
| Ischemic heart disease ICD9 410-414 using thyroid dose | 1.696 (0.651, 2.907) | ||||
| Ischemic heart disease ICD9 410-414 using kidney dose | 0.033 (0.012, 0.056) | ||||
| Ischemic heart disease ICD9 410-414 using pancreas dose | 0.020 (0.008, 0.035) | ||||
| Cerebrovascular disease ICD9 430-438 using heart dose | 0.028 (-0.085, 0.186) | ||||
| Cerebrovascular disease ICD9 430-438 using thyroid dose | 0.422 (-1.455, 3.039) | ||||
| Cerebrovascular disease ICD9 430- 438 using brain dose | 2.649 (-8.912, 18.740) | ||||
| All other circulatory disease ICD9 390-409, 415-429, 439-459 using heart dose | 0.050 (-0.053, 0.194) | ||||
| All circulatory disease ICD9 390-459 using heart dose | 0.082 (0.031, 0.140) | ||||
|
| |||||
| Nordic breast cancer case–control study | Darby et al [46] | 4.9 (0.03 – 27.72)c | 963 cases, 1205 controls | schemic heart disease incidence ICD10 I20-I25 | 0.074 (0.029, 0.145)c |
| 3.9 (0.1 – 30.4)d | 0.084 (0.036, 0.159)d | ||||
|
| |||||
| Netherlands Hodgkin lymphoma valvular disease case-control study | Cutter et al [34] | 30.7e (0 - >40) | 89 cases, 200 controls | Valvular heart disease incidence | 0.141 (0.024, 1.013)d, f |
|
| |||||
| Netherlands Hodgkin lymphoma heart disease case-control study | van Nimwegen et al [47] | 20.4g (0 - >35) | 325 cases, 1204 controls | Coronary heart disease incidence | 0.074 (0.033, 0.148)d |
estimate derived by fitting a linear model by weighted least squares, applied to the aggregate data provided in Table 5 of Mulrooney et al. [35]. Average cardiac doses of 0, 2.5, 10, 25, and 40 Gy were assumed for the respective groups with the following specified ranges of cardiac doses: 0, 0-4, 5-14, 15-34 Gy, ≥35 Gy.
mean dose to heart in 21 persons who died of cardiovascular disease.
cumulative mean dose to heart.
equivalent dose to heart in 2 Gy fractions (EQD2).
mean EQD2 dose to heart valves in controls.
estimate derived by fitting a linear binomial odds model to aggregate numbers of cases and controls, and employing the median EQD2 heart-valve doses by dose group given in Table 4 of Cutter et al [34].
mean EQD2 dose to heart in controls.
Table 4.
Estimated excess relative risks of circulatory disease in the Japanese atomic bomb survivors and in other groups with moderate-or low-dose radiation exposure, with mean dose generally < 0.5 Gy. (Adapted from Little and Lipshultz [31]). All data are in relation to underlying cause of death, unless otherwise indicated.
| Cohort/Study | Reference | Mean (range) heart/brain dose, Sv | Persons (person years of follow-up) | Endpoint (mortality unless otherwise indicated) | Excess relative risk Sv-1 (95% CI) |
|---|---|---|---|---|---|
| Japanese atomic bomb survivors | |||||
|
| |||||
| Japanese atomic bomb survivors | Shimizu et al. [24] | 0.1 (0 - 4)a | 86,611 (n.a.) | Ischemic heart disease (ICD9 410-414) | 0.02 (-0.10, 0.15) |
| Myocardial infarction (ICD9 410) | 0.00 (-0.15, 0.18) | ||||
| Hypertensive heart disease (ICD9 402, 404) | 0.37 (0.08, 0.72) | ||||
| Rheumatic heart disease (ICD9 393-398) | 0.86 (0.25, 1.72) | ||||
| Heart failure (ICD9 428) | 0.22 (0.07, 0.39) | ||||
| Other heart disease (ICD9 390-392, 415-427, 429) | -0.01 (-0.21, 0.24) | ||||
| Hypertensive disease without heart disease (ICD9 401, 403, 405) | 0.07 (-0.22, 0.55) | ||||
| Heart disease total (ICD9 393-429 excluding 401, 403, 405) | 0.18 (0.11, 0.25)b | ||||
| Cerebral infarction (ICD9 433,434) | 0.04 (-0.10, 0.20) | ||||
| Cerebral hemorrhage (ICD9 431) | 0.05 (-0.06, 0.17) | ||||
| Subarachnoid hemorrhage (ICD9 430) | 0.30 (-0.04, 0.76) | ||||
| Other or unspecified cerebrovascular disease | 0.16 (0.01, 0.34) | ||||
| Cerebrovascular disease total (ICD9 430-438) | 0.12 (0.05, 0.19)b | ||||
| Circulatory disease apart from heart disease and stroke (ICD9 390-392, 401, 403, 405, 439-459) | 0.58 (0.45, 0.72)b | ||||
| Other circulatory disease (ICD9 399-400, 406-409, 439-459) | -0.01 (<-0.01, 0.34) | ||||
| All circulatory disease (ICD9 390-459) | 0.15 (0.10, 0.20)b | ||||
|
| |||||
| Japanese atomic bomb survivors | Yamada et al. [23] | 0.1 (0 - 4)b | 10,339 (n.a.) | Hypertension incidence, 1958-1998 (ICD9 401) | 0.05 (-0.01, 0.10)c |
| Hypertensive heart disease incidence, 1958-1998 (ICD9 402, 404) | -0.01 (-0.09, 0.09)c | ||||
| Ischemic heart disease incidence, 1958-1998 (ICD9 410-414) | 0.05 (-0.05, 0.16)c | ||||
| Myocardial infarction incidence, 1964-1998 (ICD9 410) | 0.12 (-0.16, 0.60)c | ||||
| Occlusion incidence, 1958-1998 (ICD9 433, 434) | 0.06 (-0.11, 0.30)c | ||||
| Aortic aneurysm incidence, 1958-1998 (ICD9 441, 442) | 0.02 (-0.22, 0.41)c | ||||
| Stroke incidence, 1958-1998 (ICD9 430, 431, 433, 434, 436) | 0.07 (-0.08, 0.24)c | ||||
|
| |||||
| Japanese atomic bomb survivors | Tatsukawa et al. [54] | 0.001 (0-1.79) | 506 (9,265) | Morbidity in utero: hypertension | 0.20 (-0.39, 1.38) |
| Morbidity in utero: nonfatal stroke or myocardial infarction | -0.91 (-1.00, 79.3) | ||||
|
| |||||
| Japanese atomic bomb survivors | Tatsukawa et al. [54] | 0.13 (0-3.53) | 1,053 (20,216) | Morbidity: hypertension | 0.15 (-0.01, 0.34) |
| Morbidity: stroke or myocardial infarction | 0.72 (0.24, 1.40) | ||||
|
| |||||
| Occupational studies | |||||
|
| |||||
| Mayak workers | Moseeva et al. [33] | 0.62 ± 0.80 (males)d | 22,377 (447,281) | Ischemic heart disease morbidity (ICD9 410-414) | 0.14 (0.08, 0.21)e |
| Azizova et al. [32] | 0.51 ± 0.68 (females)d | Ischemic heart disease morbidity (ICD9 410-414) | 0.14 (0.08, 0.21)f | ||
| Ischemic heart disease morbidity (ICD9 410-414) | 0.16 (0.10, 0.24)g | ||||
| 22,377 (836,048) | Ischemic heart disease mortality (ICD9 410-414) | 0.05 (-0.01, 0.13)e | |||
| Ischemic heart disease mortality (ICD9 410-414) | 0.05 (-0.01, 0.13)f | ||||
| Ischemic heart disease mortality (ICD9 410-414) | 0.05 (-0.01, 0.13)g | ||||
| 18,856 (341,663) | Cerebrovascular disease morbidity (ICD9 430-438) | 0.497 (0.393, 0.601) e | |||
| Cerebrovascular disease morbidity (ICD9 430-438) | 0.529 (0.415, 0.642) f | ||||
| Cerebrovascular disease morbidity (ICD9 430-438) | 0.572 (0.450, 0.695) g | ||||
|
| |||||
| 18,856 (272,525) | Cerebrovascular disease mortality (ICD9 430-438) | 0.057 (-0.046, 0.161) e | |||
|
| |||||
| Cerebrovascular disease mortality (ICD9 430-438) | 0.064 (-0.042, 0.170) f | ||||
|
| |||||
| Cerebrovascular disease mortality (ICD9 430-438) | 0.076 (-0.033, 0.186) g | ||||
|
| |||||
| Chernobyl emergency workers | Ivanov et al. [60] | 0.109 (0 - >0.5) | 61,017 (n.a.) | Hypertension (ICD10 I10-I15) morbidity | 0.26 (-0.04, 0.56) |
| Essential hypertension (ICD10 I10) morbidity | 0.36 (0.005, 0.71) | ||||
| Hypertensive heart disease (ICD10 I11) morbidity | 0.04 (-0.36, 0.44) | ||||
| Ischemic heart disease (ICD10 I20-I25) morbidity | 0.41 (0.05, 0.78) | ||||
| Acute myocardial infarction (ICD10 I21) morbidity | 0.19 (-0.99, 1.37) | ||||
| Other acute ischemic heart disease (ICD10 I24) morbidity | 0.82 (-0.62, 2.26) | ||||
| Angina pectoris (ICD10 I20) morbidity | 0.26 (-0.19, 0.71) | ||||
| Chronic ischemic heart disease (ICD10 I25) morbidity | 0.20 (-0.23, 0.63) | ||||
| Other heart disease (ICD10 I30-I52) morbidity | -0.26 (-0.81, 0.28) | ||||
| Cerebrovascular disease (ICD10 I60-I69) morbidity | 0.45 (0.11, 0.80) | ||||
| Morbidity from diseases of arteries, arterioles and capillaries (ICD10 I70-I79) | 0.47 (-0.15, 1.09) | ||||
| Morbidity from diseases of veins, lymphatic vessels and lymph nodes (ICD10 I80-I89) | -0.26 (-0.70, 0.18) | ||||
| All circulatory disease (ICD10 I00-I99) morbidity | 0.18 (-0.03, 0.39) | ||||
|
| |||||
| Chernobyl emergency workers | Kaschcheev et al. [61] | 0.161 (0.0001 - 1.24) | 53,772 (958,540.5) | Cerebrovascular disease (ICD10 I60-I69) morbidity after no diabetes | 0.35 (0.18, 0.53) |
| Cerebrovascular disease (ICD10 I60-I69) morbidity after diabetes | 1.29 (0.63, 1.94) | ||||
| Cerebrovascular disease (ICD10 I60-I69) morbidity after no atherosclerosis | 0.43 (0.25, 0.62) | ||||
| Cerebrovascular disease (ICD10 I60-I69) morbidity after atherosclerosis | 0.50 (0.09, 0.90) | ||||
| Cerebrovascular disease (ICD10 I60-I69) morbidity after no hypertensive disease | 0.38 (0.08, 0.68) | ||||
| Cerebrovascular disease (ICD10 I60-I69) morbidity after hypertensive disease | 0.48 (0.27, 0.68) | ||||
| Cerebrovascular disease (ICD10 I60-I69) morbidity after no IHD | 0.41 (0.14, 0.68) | ||||
| Cerebrovascular disease (ICD10 I60-I69) morbidity after IHD | 0.47 (0.25, 0.69) | ||||
| Cerebrovascular disease (ICD10 I60-I69) morbidity after no concomitant disease | 0.19 (-0.99, 1.37) | ||||
| Cerebrovascular disease (ICD10 I60-I69) morbidity | 0.45 (0.28, 0.62) | ||||
|
| |||||
| German uranium miner study | Kreuzer et al. [75] | 0.047 (0.0002 – 0.909) | 58,982 (2,180,639) | All circulatory disease (ICD10 I00-I99) | -0.13 (-0.38, 0.12)f |
| Ischemic heart disease (ICD10 I20-I25) | -0.03 (-0.38, 0.32)f | ||||
| Cerebrovascular disease (ICD10 I60-I69) | 0.44 (-0.16, 1.04)f | ||||
|
| |||||
| Électricité de France workers | Laurent et al. [77] | 0.0215 (0 – 0.6) | 22,393 (440,984) | Ischemic heart disease | 4.1 (-2.9, 13.7)h |
| Cerebrovascular disease | 17.4 (0.2, 43.9)h | ||||
| All circulatory disease | 2.7 (-2.3, 9.1)h | ||||
|
| |||||
| Eldorado uranium miners and processing (male) workers | Lane et al. [76] | 0.0522 (<0.0234 – >0.1215) | 16,236 (508,673) | Ischemic heart disease | 0.15 (-0.14, 0.58) |
| Stroke | -0.29 (<-0.29, 0.27) | ||||
| All other circulatory disease | 0.07 (<-0.33, 0.77) | ||||
|
| |||||
| British Nuclear Fuels plc. workers | McGeoghegan et al. [78] | 0.0569 (0 – >0.729) | 38,779 (1,081,570) | Ischemic heart disease (ICD9 410-414) | 0.70 (0.37, 1.07)b, g, h |
| Cerebrovascular disease (ICD9 430-438) | 0.66 (0.17, 1.27)b, g, h | ||||
| Other circulatory diseases (ICD9 390-398, 415-429, 440-459) | 0.83 (-0.10, 1.12)g, h | ||||
| Circulatory diseases apart from cerebrovascular (ICD9 390-429, 439-459) | 0.72 (0.39, 1.10)g, h | ||||
| All circulatory disease (ICD9 390-459) | 0.54 (0.30, 0.82)b, g, h | ||||
|
| |||||
| 3rd Analysis of UK National Registry for Radiation Workers | Muirhead et al. [73] | 0.0249 (<0.01 - >0.4) | 174,541 (3.9 × 106) | All circulatory disease (ICD9 390-459) | 0.251 (-0.01, 0.54)f |
| Circulatory disease not strongly related to smoking (ICD9 390-409, 415-440, 442-459) | 0.280 (-0.19, 0.85)f | ||||
| Aortic aneurysm (ICD9 441) | -0.132 (-1.29, 1.92)f | ||||
| Ischemic heart disease (ICD9 410-414) | 0.259 (-0.05, 0.61)f | ||||
| Cerebrovascular disease (ICD9 430-438) | 0.161 (-0.42, 0.91)f | ||||
|
| |||||
| US Oak Ridge workers | Richardson and Wing [106] | NA (0 – >0.1) | 14,095 (425,486) | Ischemic heart disease (ICD8 410-414) | -2.39 (-5.94, 1.16)e |
| Ischemic heart disease (ICD8 410-414) | -2.86 (-6.90, 1.18)f | ||||
| Ischemic heart disease (ICD8 410-414) | -0.16 (-6.02, 5.70) i | ||||
|
| |||||
| International Agency for Research on Cancer15-country nuclear worker study | Vrijheid et al. [59] | 0.0207 (0.0 - >0.5) | 275,312 (4,067,861) | Circulatory disease (ICD10 I00-I99, J60-J69, O88.2, R00-R02, R57) | 0.09 (-0.43, 0.70)f |
| Ischemic heart disease (ICD10 I20-I25) | -0.01 (-0.59, 0.69)f | ||||
| Heart failure (ICD10 I50) | -0.03 (<0, 4.91)f | ||||
| Deep vein thrombosis and pulmonary embolism (ICD10 I26, I80, I82, O88.2) | -0.95 (-1.00, 9.09)f, j | ||||
| Cerebrovascular disease (ICD10 I60-I69) | 0.88 (-0.67, 3.16)f | ||||
| All other circulatory disease (ICD10 R00-R02, R57, I00-I99 excluding I20-26, I50, I60-69, I80, I82) | 0.29 (<0, 2.40)f | ||||
|
| |||||
| Environmental studies | |||||
|
| |||||
| Techa River study | Krestinina et al. [79] | 0.035 (0-0.51)k | 29,735 (901,563) | All circulatory disease mortality (ICD9 390-459) | 0.18 (-0.13, 0.52)k, e |
| All circulatory disease mortality (ICD9 390-459) | 0.24 (-0.08, 0.59)k, f | ||||
| All circulatory disease mortality (ICD9 390-459) | 0.36 (0.02, 0.75)k, g | ||||
| Ischemic heart disease mortality (ICD9 410-414) | 0.26 (-0.22, 0.81)k, e | ||||
| Ischemic heart disease mortality (ICD9 410-414) | 0.40 (-0.11, 0.99)k, f | ||||
| Ischemic heart disease mortality (ICD9 410-414) | 0.56 (0.01, 1.19) k, g | ||||
|
| |||||
| Semipalatinsk nuclear test study | Grosche et al. [80] | 0.09 (0-0.63) | 19,545 (582,656) | Heart disease (ICD9 410-429): all settlements | 3.22 (2.33, 4.10)f |
| Heart disease (ICD9 410-429): exposed settlements | 0.06 (-0.39, 0.52)f | ||||
| Stroke (ICD9 430-438): all settlements | 2.96 (1.77, 4.14)f | ||||
| Stroke (ICD9 430-438): exposed settlements | -0.06 (-0.65, 0.54)f | ||||
| Cardiovascular disease (ICD9 390-459): all settlements | 3.15 (2.48, 3.81)f | ||||
| Cardiovascular disease (ICD9 390-459): exposed | 0.02 (-0.32, 0.37)f | ||||
CI, Confidence Interval; ICD, International Classification of Diseases
Analysis based on colon dose.
Analysis using underlying or contributing cause of death.
Analysis based on stomach dose, derived from Table 3 of Yamada et al. [23] with smoking and drinking in the stratification.
Risk estimates in relation to cumulative whole body external gamma dose; doses given here are from Moseeva et al. [33].
Assuming a lag period of 5 years.
Assuming a lag period of 10 years.
Assuming a lag period of 15 years.
90% CI
Assuming a lag period of 20 years.
Estimate derived from log-linear model, evaluated at 1 Sv.
Analysis based on dose to muscle.
An aggregate estimate of ERR per Gy is computed across subsets of these studies using random effects models, using standard statistical methods. Random effects models are fitted by restricted maximum likelihood (REML) because of the theoretically superior performance, in particular the absence of bias in the estimates of variance [36]. However, for certain analyses (Table 6) maximum-likelihood and the one-step variance estimate of DerSimonian and Laird [37] are also used to estimate residual heterogeneity; the fixed-effect parameter estimates, and parameter estimates of these alternative model fits were generally within 5% of those obtained via REML. Maximum likelihood methods had to be used to assess comparative goodness of fit of models with various sets of fixed-effect variables, reported in Table 6. Residual heterogeneity was assessed using Cochran’s Q-statistic:
| (1) |
the significance of which was assessed by comparing it against centiles of the χ2 distribution with the relevant number of degrees of freedom (= N − 1). Random effects models are fitted to subsets of the studies in Tables 2-4 selected so as to be more or less disjoint, as previously discussed [25]. The 1-sided p-values in Tables 5 and 7 are calculated in the standard way from the mean, μ, and standard deviation, σ, derived from the meta-analysis for each circulatory disease endpoint, as P [N (0 , 1) < − μ / σ]. [I give 1-sided rather than 2-sided p-values since I judge that the hypothesis being tested is of detrimental effects.] Statistical significance was defined by p<0.05. In order to assess the contribution of the heterogeneity to the aggregate data the I2 statistic of Higgins and Thompson [38] is computed. This is expressed as a percentage, so that a value near 0% implies little estimated inter-study heterogeneity relative to the intra-study variance, and values near 100% that the inter-study heterogeneity dominates the intra study variance [38]. Values of ERR per Sv derived from the meta-analysis are given in Table 5 for four major subtypes of circulatory disease determined a priori, and as used in a previous meta-analysis [25], namely: (a) IHD (ICD10 I20-I25); (b) heart disease apart from IHD (ICD10 I26-I52); (c) CeVD (ICD10 I60-I69); and (d) all other circulatory diseases (ICD10 I00-I19, I53-59, I70-I99). All statistical models were fitted using the metafor package [39] in R [40]. Forest plots were prepared using the forestplot package [41] in R [40]. Results of the meta-analysis are generally based on the data given in Appendix B Table B1.
Table 5. Excess relative risk coefficients for circulatory diseases as a result of radiation exposure, by disease endpoint.
Values for the analysis are from Tables 1-3, using 5-year lag whenever possible, and restricting to <0.5 Gy for the TB fluoroscopy cohorts, whenever possible. Thyroid dose (a surrogate for dose to the carotid artery) is used for cerebrovascular disease, whenever possible. Random effects models are fitted via restricted maximum likelihood (REML).
| Disease (ICD Code) | Studies/detailed endpoints | Random-effect estimate ERR Sv-1 (95% CI) | 1-sided significance, p-value | Heterogeneity p-value |
|---|---|---|---|---|
| Ischemic heart disease (ICD10 I20-I25) | Yamada et al [23], Ivanov et al [60], Vrijheid et al [59], Muirhead et al [73], Mulrooney et al [35] [myocardial infarction], Lane et al [76], Laurent et al [77], Shimizu et al [24] [underlying cause], Little et al[17], Darby et al [46] [EQD2 heart dose], Krestinina et al [79] [5 year lag], Kreuzer et al [75], Zablotska et al [50] [<0.5 Gy], Azizova et al [32] [5 year lag, morbidity data], Little et al [51][< 0.5 Gy], van Nimwegen et al [47] | 0.082 (0.057, 0.106) | <0.0001 | 0.4430 |
|
| ||||
| Non-ischemic heart disease (ICD10 I26-I52) | Ivanov et al [60], Vrijheid et al [59] [heart failure], Mulrooney et al [35] [congestive heart failure, pericardial disease, valvular disease], Shimizu et al [24] [heart failure (ICD9 428)(underlying cause), other heart disease (ICD9 390-392, 415-427, 429)(underlying cause)], Cutter et al [34][valvular heart disease], Little et al [51] | 0.094 (0.078, 0.111) | <0.0001 | 0.2041 |
|
| ||||
| Cerebrovascular disease (ICD10 I60-I69) | Yamada et al [23], Vrijheid et al [59], Muirhead et al [73], Lane et al [76], Laurent et al [77], Shimizu et al [24] [underlying or contributing cause], Grosche et al [80][exposed settlements only, 10 year lag], Little et al [17] [thyroid dose], Kreuzer et al [75], Moseeva et al [33][5 year lag, morbidity data], Kashcheev et al [61], Little et al [51] [thyroid dose, <0.5 Gy] | 0.236 (0.062, 0.410) | 0.0040 | <0.0001 |
|
| ||||
| Circulatory disease apart from heart disease and stroke (ICD10 I00-I19, I53-I59, I70-I99) | Yamada et al [23] [hypertension (linear model), hypertensive heart disease, aortic aneurysm], Ivanov et al [60] [diseases of arteries, arterioles and capillaries (ICD10 I70-I79), hypertension (ICD10 I10-I15), disease of veins, lymphatic vessels and lymph nodes (ICD10 I80-I89)], Shimizu et al [24] [underlying or contributing cause], Little et al [51] | 0.137 (-0.049, 0.322) | 0.0745 | <0.0001 |
|
| ||||
| All circulatory disease (ICD10 I00-I99) | Yamada et al [23] [hypertension (linear model), hypertensive heart disease, IHD, CeVD, aortic aneurysm], Ivanov et al [60] [hypertension (ICD10 I10-I15), IHD, other heart disease (ICD10 I30-I52), diseases of arteries, arterioles and capillaries (ICD10 I70-I79), disease of veins, lymphatic vessels and lymph nodes (ICD10 I80-I89)], Vrijheid et al [59], Muirhead et al [73], Mulrooney et al [35] [congestive heart failure, pericardial disease, valvular disease, myocardial infarction], Lane et al [76] [IHD, CeVD, other circulatory disease], Laurent et al [77], Shimizu et al [24] [underlying or contributing cause], Tukenova et al (2010)[cardiac disease], Grosche et al [80] [exposed settlements only, 10 year lag], Little et al [17], Darby et al [46] [EQD2 heart dose], Krestinina et al [79] [5 year lag], Kreuzer et al [75], Moseeva et al [33] [5 year lag, morbidity data], Zablotska et al [50] [5 year lag], Azizova et al [32] [5 year lag, morbidity data], Cutter et al [34], Kashcheev et al [61] [CeVD], Little et al [51] [< 0.5 Gy], van Nimwegen et al [47] | 0.115 (0.064, 0.167) | <0.0001 | <0.0001 |
3. RESULTS
3.1 Therapeutically exposed groups
The study of Mulrooney et al [35], a largely US-based cohort of persons treated for cancer in childhood, documented significant excess risk for heart doses above 15 Gy for each of the four main endpoints studied (congestive heart failure, myocardial infarction, pericardial disease, valvular disease); there are also significant increasing trends in risk with dose (Table 2). The heart dosimetry in the study, which relied on measurements in physical phantoms, was not fully individualized, in that treatment blocking data was not taken into account [42]. It was also reliant on self-reported information on circulatory disease outcomes. However, treatment information, in particular relating to the RT and concomitant CT is derived from medical records. Oddly, risk of myocardial infarction was not modified by anthracycline dose, although there was significant modification of risk of pericardial disease [35]. The French-UK study of Tukenova et al [43], of mortality in childhood cancer survivors, does not have the weaknesses of the study of Mulrooney et al [35], in that diagnostic information is obtained via national mortality registers (in France and UK). The RT organ dosimetry is also of somewhat higher quality, in that it is fully individualized, based on Monte Carlo reconstructions derived from individual treatment records [44,45]. There was a strong and highly significant increasing trend of cardiac risk with dose to the heart, 0.6 Gy-1 (95% confidence intervals (CI) 0.2, 2.5) (Table 2); there was also significant risk associated with anthracyclines or vinca alkaloids, but there was no significant statistical interaction of radiation dose with anthracycline score, nor with any other type of concomitant CT. The US study adjusted for tobacco use [35], but otherwise neither study corrected for standard risk factors for circulatory disease. A significant weakness of the study of Mulrooney et al is that for an appreciable fraction the “cardiac event was reported but the participant did not report the age at which the event occurred. Age at first cardiac condition was imputed for 9% and 14% of survivors and siblings, respectively, who reported a specific condition” [35].
The US study of patients treated for peptic ulcer, who were given mostly a single treatment course of X-rays to the stomach, of Little et al [17] documented significant excess mortality risks for all circulatory disease, with an ERR Gy-1 of 0.082 (95% CI 0.031, 0.140), and IHD, with an ERR Gy-1 of 0.102 (95% CI 0.039, 0.174) (both p<0.01), and indications of excess risk for stroke. There were no statistically significant (p>0.2) differences between risks by endpoint, and few indications of curvature in the dose response, or of confounding effects of smoking or alcohol consumption [17]. There were significant decreasing trends of ERR with increasing time since exposure for all circulatory disease, IHD and CeVD (p<0.01), the magnitude of which does not vary between endpoints (p>0.2). Risk modifications were similar if analysis was restricted to those receiving radiation, although ERRs are slightly larger and the risk of stroke failed to be significant. Doses to a number of different target tissues, specifically heart, thyroid, kidney, pancreas, and brain, were used to assess radiation effects. Using thyroid dose (a surrogate for dose to the carotid artery) for CeVD and heart dose for other circulatory disease endpoints resulted in significant heterogeneity of risk (p=0.011) between endpoints, which was not the case when heart dose was used throughout (p=0.283) [17]. Using brain or thyroid (a surrogate for the carotid artery) dose resulted in somewhat higher risks for CeVD, the risk being particularly high for brain dose. As noted by Little et al “one limitation of the study is that the radiation dosimetry, although of high quality in many respects, fails to account for variability in patient anatomy, e.g., the heart size/shape/position and its relation to the diaphragm and stomach.” [17]
The Nordic case-control study of Darby et al [46] assessed IHD incidence in a group of women treated for breast cancer. Doses to the heart and left anterior descending artery were assessed. A major strength of the study is that national morbidity registers in Sweden and Denmark were used to assess incidence of IHD. Dosimetry reconstruction was also based on individual RT charts; both cumulative dose and equivalent dose in 2-Gy fractions (EQD2) was calculated. Another strength of the study is the rich covariate lifestyle and medical information, in particular the standard risk factors for circulatory disease such as diabetes, obesity and smoking status, that is available and used for the analysis. Adjustment for these variables did not modify the (significant) trend of IHD with heart dose, nor was there any significant modification by age at treatment.
The two Netherlands case-control studies, of Cutter et al [34] and van Nimwegen et al [47], assessed morbidity from valvular disease and IHD, respectively, in a group of survivors of HL. EQD2 doses to the affected heart valve and to the whole heart were estimated using patient treatment records. Morbidity was assessed in both studies via a postal questionnaire completed by the patients’ general practitioner (GP) and/or cardiologist. As such there may be variation in ascertainment over time, also by whether a cardiologist or GP responded to the questionnaire; as case-control matching was by year of HL diagnosis, at least the variation in ascertainment over time should not affect the derived risks. Oddly, there was no significant modification of circulatory disease risk by concomitant CT (vincristine, procarbazine, anthracyclines) in either study once the effects of RT were accounted for. Other lifestyle risk factors (smoking, obesity, diabetes mellitus, hypertension, hypercholesterolemia) did not appreciably modify the radiation risk in either study. There was borderline significant (p=0.03) upward curvature in the dose-response for valvular disease [34] but no significant curvature for IHD (p=0.356) [47].
The risks suggested by these six studies are generally consistent with each other, and with those in the diagnostically and other, lower-dose, studies; a possible exception is the French-UK study [43], where risk is much higher than for many of the other studies considered (Table 2). The discrepancy with some other studies (e.g., of adult exposure) may reflect the younger exposure age, also the younger age at follow-up in this group, although this would not explain the discrepancy with risks in the US childhood-cancer survivor study [35]. As discussed below, ERR of circulatory disease in the Japanese atomic bomb survivors Life Span Study (LSS) cohort are significantly modified by attained age [24,25]. The fact that the ERR in relation to cumulative heart dose, 0.074 Gy-1 (95% CI 0.029, 0.145), or in relation to EQD2, 0.084 Gy-1 (95% CI 0.036, 0.159), in the Nordic study [46] agrees well with those in many other radiation-exposed groups (Tables 2-4), suggests that either of these measures (cumulative heart dose, EQD2 heart dose) may be relevant for this endpoint (IHD) [48]. The fact that risks evaluated using brain dose for CeVD in the US peptic ulcer study yielded much higher risks than those observed using heart or thyroid dose, or in the LSS [24,25] suggests that this organ may not be the most relevant one for this endpoint.
Although not otherwise reported here, because only a mean heart and brain dose for this cohort have been reported, there is no radiation-associated excess mortality from circulatory disease in a study of UK ankylosing spondylitis patients [49].
3.2 Diagnostically exposed groups
The two major studies of circulatory disease mortality in relation to medical diagnostic exposure are both of groups that received repeated fluoroscopic doses as part of the lung collapse treatment for tuberculosis (TB), in Canada [50] and in Massachusetts [51]. In both groups the lung dose was used as a surrogate for heart dose. In the Massachusetts cohort there were additional analyses employing thyroid dose (a surrogate for dose to the carotid artery) and red bone marrow dose. As discussed by Little et al “one would expect carotid artery dose to be higher than thyroid dose, but that lung dose is probably lower than heart dose; estimates of both the heart and carotid dose may be wrong by a factor of 2” [51]. A novel finding in the Canadian data was a significant inverse dose rate effect for IHD, after adjustment for which the IHD dose-response was significant [50]. However, this was only the case when a 10-year lag was used; when 5- or 15-year lags were employed the effect ceased to be significant. There are no indications of such effects in the Massachusetts data, in which a 5-year lag was the default [51]. Although there is no dose-response overall in the Massachusetts data, if analysis is restricted to persons with < 0.5 Gy the dose response trends for all circulatory disease and IHD become much steeper, and borderline significant (p=0.0743, p=0.0682, respectively) (Table 3). Interestingly, there is also evidence of a steeper dose-response slope under 0.5 Gy for IHD in the Canadian data [50] (Table 3). In both cohorts there is limited medical and lifestyle information. This is more extensive in the Massachusetts data, and includes smoking and alcohol consumption, thoracoplasty, and pneumolobectomy; some of these variables were included in baseline models for certain disease endpoints [51].
Table 3.
Estimated excess relative risk of circulatory disease in various diagnostically treated groups, exposed at moderate to high doses, with mean dose generally > 0.5 Gy. All data are in relation to underlying cause of death, unless otherwise indicated.
| Study | Reference | Average Dose to Heart (Gy) (mean, range) | Persons (person years of follow-up) | Endpoint (mortality unless otherwise indicated) | Excess relative risk Sv-1 (95% CI) |
|---|---|---|---|---|---|
| Canadian TB fluoroscopy cohort | Zablotska et al [50] | 0.79 (0 – 11.60)a | 63,707 (1,902,252) | Ischemic heart disease ICD9 410-414, 429.2 | 0.011 (-0.040, 0.075)a |
| Ischemic heart disease ICD9 410-414, 429.2 | 0.007 (-0.044, 0.072)b | ||||
| Ischemic heart disease ICD9 410-414, 429.2: < 0.5 Gy | 0.149 (-0.284, 0.670)b | ||||
| Ischemic heart disease ICD9 410-414, 429.2 | 0.003 (-0.047, 0.067)c | ||||
| Hypertensive and other non-stroke circulatory disease ICD9 390-409, 415-429.1, 429.3-429.9, 439-448 | 0.034 (-0.059, 0.179)a | ||||
| Hypertensive and other non-stroke circulatory disease ICD9 390-409, 415-429.1, 429.3-429.9, 439-448 | 0.027 (-0.064, 0.167)b | ||||
| Hypertensive and other non-stroke circulatory disease ICD9 390-409, 415-429.1, 429.3-429.9, 439-448 | 0.056 (-0.049, 0.219)c | ||||
| All circulatory disease ICD9 390-448 | 0.024 (-0.020, 0.079)a | ||||
| All circulatory disease ICD9 390-448 | 0.020 (-0.025, 0.074)b | ||||
| All circulatory disease ICD9 390-448 | 0.023 (-0.023, 0.079)c | ||||
|
| |||||
| Massachusetts TB fluoroscopy cohort | Little et al [51] | 0.36 (0.00 – 8.56)a 0.20 (0.00 – 4.61)d | 13,568 (345,948) | All circulatory disease ICD9 390-459 | -0.020 (-0.067, 0.028)a |
| All circulatory disease ICD9 390-459: < 0.5 Gy | 0.345 (-0.032, 0.764)a | ||||
| Cerebrovascular disease ICD9 430-438 | 0.075 (-0.050, 0.237)a | ||||
| Cerebrovascular disease ICD9 430-438 | 0.132 (-0.088, 0.415)d | ||||
| Cerebrovascular disease ICD9 430-438: < 0.5 Gy | 0.343 (-0.536, 1.473)d | ||||
| All heart disease ICD9 390-429 | -0.042 (-0.088, -0.013)a | ||||
| Ischemic heart disease ICD9 410-414 | -0.077 (-0.130, -0.012)a | ||||
| Ischemic heart disease ICD9 410-414: < 0.5 Gy | 0.465 (-0.032, 1.034)a | ||||
| Heart disease excluding IHD ICD9 390-409, 415-429 | 0.013 (-0.072, 0.127)a | ||||
| Hypertensive heart disease ICD9 401-405 | 0.357 (-0.043, 1.030)a | ||||
| Hypertensive heart disease ICD9 401-405: < 0.5 Gy | 0.801 (-1.226, 4.638)a | ||||
| All circulatory disease apart from heart and cerebrovascular ICD9 439-459 | 0.015 (-0.183, 0.243)a | ||||
based on 5-year lagged lung dose.
based on 10-year lagged lung dose.
based on 15-year lagged lung dose.
based on 5-year lagged thyroid dose.
Although not reported in Table 3, there have been a number of groups exposed to internally deposited radionuclides, in particular α-particles from the diagnostic contrast medium Thorotrast. Among the largest of these is a cohort of US, Danish and Swedish patients [52] which reported marginally significant elevations in risk from cardiac disease [for males RR = 1.0 (95% CI 0.8, 1.2), for females RR =1.2 (95% CI 1.0, 1.6), total RR = 1.1 (95% CI 0.9, 1.3)) although for CeVD there was more substantial (and statistically significant) elevations (for males RR = 1.4 (95% CI 1.0, 2.0), for females RR =1.8 (95% CI 1.3, 2.5), total RR = 1.6 (95% CI 1.2, 2.0)]. In a somewhat smaller Portuguese series risks of circulatory disease were not significantly elevated (for males RR = 1.11 (95% CI 0.76, 1.62), for females RR =0.97 (95% CI 0.53, 7.70), total RR = 1.08 (95% CI 0.79, 1.46)) [53]. The findings in relation to CeVD in the international series should be treated with caution, since a frequent reason for use of Thorotrast was investigation of cerebral vascular anomalies, as pointed out by Travis et al. [52]. Thorotrast deposits α-particle dose primarily to the liver. Unfortunately, to the best of my knowledge, evaluation of these health endpoints in relation to liver dosimetry has not been performed.
3.3 Moderate/low-dose exposed groups
3.3.1 Japanese Atomic Bomb Survivors
Excess radiation-associated mortality from heart disease and stroke has been observed in the LSS cohort (Table 4) [24]. In the latest follow-up of the Adult Health Study (AHS), a subset of the LSS cohort subject to biennial clinical examinations, Yamada et al. [23] observed generally non-statistically significant, radiation-associated excess risks of hypertension and myocardial infarction morbidity (Table 4). Analysis within the AHS of those exposed in early childhood showed a significantly increased incidence of non-fatal stroke or myocardial infarction, although there was no excess risk among those exposed in utero for whom the average exposures were much lower [54] (Table 4).
Some aspects of the Japanese atomic bomb survivor data imply that risks may not necessarily apply to other exposed populations. Survivors suffered from burns, epilation, and other acute injuries caused by the radiation, heat, and blast of the bombs, respectively, and these injuries, in addition to radiation, may have contributed to the development of non-cancer diseases in later life. In addition to the direct effect of the injuries, these and other trauma might introduce selection bias. Evidence of such bias has been presented by Stewart and Kneale [55], who documented the heterogeneity of risk for various endpoints, in particular cardiovascular disease mortality, among the various acute-injury groups. However, Stewart and Kneale [55] did not consider the effects of dose error. Analysis considering this error provided much reduced and generally not statistically significant evidence for a differential effect among those survivors, especially for cardiovascular disease [56]. Although selection bias cannot be entirely discounted, the general consistency of risks in the Japanese and other groups suggests that it does not have a major impact (Tables 2-4). (For a more formal analysis see reference [25].) Perhaps more than in most other radiation-exposed groups there have been substantial changes in circulatory disease morbidity and mortality in the underlying cohort in the period since the two atomic bombings, a consequence of the partial westernization of the Japanese diet, also substantial increases in prevalence of cigarette smoking [57]. However, the major risk factor for circulatory disease in the Japanese population, and in the Japanese atomic bomb survivors, remains as it has been, hypertension [57]; hypercholesterolemia, which is a risk factor of some significance in western populations (see Table 1), is relatively unimportant in the older Japanese population [58]. There have been other changes in disease coding in Japan, consequent on introduction of the 10th revision to the International Classification of Diseases (ICD-10), so that after 1995 heart failure became much less commonly diagnosed [57].
3.3.2 Occupationally Exposed Groups
The International Agency for Research on Cancer 15-country study of radiation workers found increasing dose-related trends for mortality from all circulatory disease, CeVD, and other circulatory diseases and decreasing trends for IHD, heart failure, deep vein thrombosis, and pulmonary embolism [59] (Table 4), although none of these trends was statistically significant (1-sided p≥0.20).
Radiation-associated excess IHD and CeVD morbidity were observed in Chernobyl recovery workers, although morbidity from hypertensive heart disease and other heart disease was not increased [60,61] (Table 4). There has been analysis of circulatory disease mortality in this cohort, but based only on comparison with external circulatory disease rates, via use of standardized mortality ratios [62]. As such this analysis almost certainly yields biased estimates of risk, as the general Russian population is very likely not representative of the Chernobyl recovery workers, because of generally observed healthy-worker selection effects [63,64]. A remarkable feature of this cohort is the relatively high rates of circulatory disease, including for example 23,264 cases of CeVD in a cohort of 53,772 people [61], reflecting the substantially elevated circulatory disease mortality and morbidity rates in the Russian population relative to those in other developed countries [1].
A highly statistically significant trend with dose was seen for IHD and CeVD morbidity in the Mayak workers, although the trend of IHD and CeVD mortality is much lower, and generally not statistically significant (Table 4). There have been a number of analyses of the Mayak worker cohort in the last few years [32,33,65-68], based on a similar underlying dataset characterized by: (a) cohort (18,797 - 22,377 workers first employed by the Mayak Production Association (PA) 1948-1972 or 1948-1982); (b) disease endpoints (all circulatory disease, IHD, CeVD morbidity/mortality); (c) years of follow-up (to end 2005 or end 2008); and (d) dosimetry system (all MWDS 2008), which yield slightly different risk estimates, because of variations in these (and possibly other) criteria. Risk estimates are also of course somewhat discrepant in other analysis of this cohort which differ more significantly with respect to criteria (a)-(d) [69-71]. Here the most recent studies of IHD and CeVD are used, in particular the studies of Azizova et al [32] and Moseeva et al [33], which are cited in Table 4 and used as the basis of the meta-analysis. The study is unusual in that doses to certain internal organs, especially the lung and liver, were dominated by doses from internally deposited radionuclides; in particular, the α-particle-emitting radioisotopes of plutonium. Doses in this study are among the highest among the occupationally-exposed groups considered in this section, and arguably more comparable with at least the medical-diagnostic or even the RT-exposed groups considered above: average whole body doses for external γ rays were 0.5 to 0.6 Gy (Table 4). However, unlike the partial-body doses received from RT (Table 2), or even those in the TB fluoroscopy cohorts (Table 3), the external whole-body doses received by the Mayak workers generally accumulated over a long time, and average <5 mGy/hour, so must be considered a low dose-rate exposure [72].
Nonetheless, interpreting the results of the Mayak cohort is complicated by the large and highly heterogeneous internal α-particle dose from plutonium. The dose response was significant, both in relation to the external γ dose and the internal (α-particle) dose to the liver [33,68]. Apart from these workers, few cohorts with α-particle liver dose have individual organ dose estimates, or are large enough to merit analysis of this endpoint.
In the latest analysis of the United Kingdom National Registry for Radiation Workers [73], circulatory disease mortality had a borderline significant trend with dose, with an ERR of 0.25 Sv-1 (95% CI, -0.01, 0.54) (Table 4). In most other workforces [74-77], there were generally no statistically significant trends of circulatory disease with dose (Table 4). Some of these studies overlap and, in particular, substantial portions of the study populations of Muirhead et al. [73] are included in the International Agency for Research on Cancer study [59]. The highly significant excess risks of circulatory disease in a study of British Nuclear Fuels plc workers should also be noted [78] (Table 4); however, this study is largely subsumed within the study by Muirhead et al. [73] (Table 4) and has only 4 more years of follow-up (to December 31, 2005 versus December 31, 2001 for Muirhead et al. [73]).
3.3.3 Environmentally Exposed Groups
A study of a cohort of environmentally exposed individuals in the Southern Ural Mountains reported a statistically significant, or borderline significant, increase (depending on the latent period used) of both all circulatory disease mortality, with an ERR of 0.24 Gy-1 (95% CI, -0.08, 0.59), and IHD mortality, with an ERR of 0.40 Gy-1 (95% CI, -0.11, 0.99) with a 10-year lag [79] (Table 4). The trends were statistically significant (p≤0.05) with lags of 15 to 20 years, but not significant (p>0.1) with lags of 0 to 10 years [79].
Grosche et al. [80] studied circulatory disease mortality in a Kazakhstan group exposed to fallout from nuclear weapons tests at the Semipalatinsk site (Table 4). No excess circulatory disease risk was reported in the group of exposed settlements, with an ERR of 0.02 Gy-1 (95% CI, -0.32, 0.37) for cardiovascular disease, an ERR of 0.06 Gy-1 (95% CI, -0.39, 0.52) for heart disease, and an ERR of -0.06 Gy-1 (95% CI, -0.65, 0.54) for stroke. On the other hand, if exposed and unexposed settlements were analyzed together, the excess risks were highly statistically significant and implausibly large, an ERR of 3.15 Gy-1 (95% CI, 2.48, 3.81) for circulatory disease, an ERR of 3.22 Gy-1 (95% CI, 2.33, 4.10) for heart disease, and an ERR of 2.96 Gy-1 (95% CI, 1.77, 4.14) for stroke. The dosimetry in this cohort is problematic because it is based on assessments of residence, estimates of time spent outdoors, and diet, all of which were collected by interviews more than 30 years after the bomb tests. As such, the results of this study may be less informative than others considered here.
3.4 Risk modifying factors
In the LSS radiation-associated ERR for circulatory disease decreases with increasing age at exposure [25] and there are borderline significant decreasing trends with attained age [24, 25]; however, risk does not substantially vary by sex, or time since exposure [24]. Increasing time trends have been observed in other groups [59], but decreasing trends in others [17].
3.5 Results of meta-analysis
Tables 5-7 and Fig. 1, also Appendix B Figs. B1-B2 report the results of the meta-analysis. This is largely based on the summary table given in Appendix B Table B1. The funnel plots given in Appendix B Fig. B2 do not suggest any material selection or publication bias. The meta-analysis demonstrates that there is a statistically significant ERR per Sv (one-sided p<0.001) for all circulatory disease endpoints considered except all circulatory disease apart from heart disease and CeVD (p=0.0745; Table 5). The heterogeneity in ERR between the various studies and endpoints for IHD and non-ischemic heart disease is not statistically significant (p>0.2), although it is significant for CeVD or all circulatory disease excluding heart and CeVD (p<0.001; Table 5). At least for CeVD, adjustment for any of mean dose, age at exposure, or radiation results in the residual heterogeneity becoming non-significant (p>0.2), but for the remainder endpoint (all circulatory disease excluding heart and CeVD) the heterogeneity remains highly statistically significant (p<0.0001) irrespective of the adjustments made (Table 6). Despite the presence of heterogeneity for certain endpoints, only for the group of all circulatory disease excluding heart and CeVD is the heterogeneity substantial, with values of I2 generally in excess of 85% (Table 6). For most other endpoints the I2 is near 0 (Table 6). Fig. 1 illustrates the variation in risk of CeVD with mean dose, and the lack of such variation for other endpoints. Adjustment for each of exposure age, dose fractionation and mean dose improve the fit of the model for ERR in relation to CeVD over the null model (p=0.0019, p=0.0267, p=0.0299, respectively) (Table 6), and there is a significant improvement in fit for this endpoint (p=0.0086) if adjustment is made for exposure age while allowing for dose, also borderline significant improvements in fit resulting from adjusting for dose fractionation while allowing for the effects of dose, and vice versa (p=0.0600, p=0.0785, respectively) (results not shown). Other than that the only significant effect is in relation to dose fraction for IHD, adjustment for which results in significant (p=0.0357) improvement in fit (Table 6); other tests generally do not even approach borderline levels of significance (p>0.1). Use of the Mayak mortality rather than morbidity data generally somewhat weakens evidence for such modifying effects, although there is a borderline significant joint effect (p=0.0402) of age at exposure and dose for CeVD (Table 6). Both for CeVD and IHD the ERR coefficients are largest for groups exposed at lower dose rates, and among persons exposed at older ages (Table 7). Use of Mayak mortality rather than morbidity data in this analysis generally reduces the magnitude of these differences (Table 7).
Fig. 1.
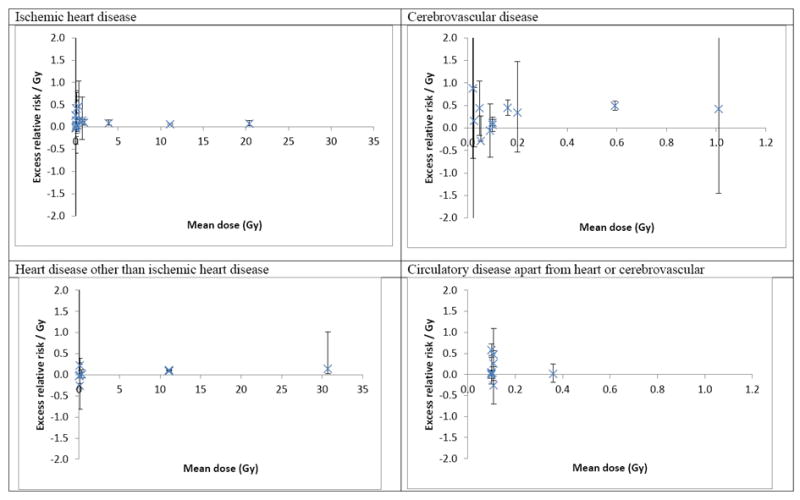
Excess relative risk / Gy (+95% CI) in relation to mean dose by circulatory disease endpoints.
Within each exposure age and dose fractionation group risks for CeVD generally exceed those for IHD by a factor of two or more (Table 7). For IHD ERR in the various subgroups range from 0.038 to 0.147 per Gy; however, the ERR coefficient for CeVD is somewhat higher, ranging from 0.112 to 0.382 per Gy (Table 7). The ERR associated with low-dose-rate radiation exposure is highly significant both for IHD (ERR = 0.147 Gy-1, 95% CI 0.087, 0.207, p<0.0001) and CeVD (ERR = 0.308 Gy-1, 95% CI 0.075, 0.542, p=0.0048) (Table 7).
4. DISCUSSION
Compelling increases in circulatory disease risk are observed after RT, and there are strong indications of increased risk among groups receiving fluoroscopic doses, also among occupationally- or environmentally-exposed groups. The cohorts treated with RT generally received substantial doses, with mean organ doses generally exceeding 1 Gy (Table 2). An intermediate category are the two TB fluoroscopy cohorts, with mean doses between 0.2 and 1.0 Gy (Table 3). Most of the other studies considered here involved low-to-moderate mean cumulative radiation doses (0.2 Gy or less), with participants in the occupational studies exposed at near-background dose rates (Table 4). Nevertheless, the small numbers of participants exposed at high cumulative doses (0.5 Gy or above) drive the observed trends in most cohorts with these higher dose groups (Tables 2-4).
The findings in the meta-analysis (Table 7 and Fig. 1) that increasing dose fractionation or reducing mean cumulative dose increases ERR is consistent with findings elsewhere. In particular, analysis of the Canadian TB fluoroscopy cohort suggested that risk per unit dose of IHD increased with increasing fractionation of dose [50]. Both in the Canadian [50] and in the Massachusetts [51] TB fluoroscopy cohorts there are indications that for IHD and other circulatory disease endpoints risk at doses below 0.5 Gy is elevated compared with risk over the full range of exposure, consistent with the pattern observed in our meta-analysis (Fig. 1). However, there is evidence of ERR reducing with increasing age at exposure in the LSS [25], in the opposite direction to the trend suggested by our meta-analysis (Table 7).
The ERRs that are derived (Table 5, 7) are generally consistent with those of a previous systematic review and meta-analysis of moderate/low dose studies [25]. In particular the risks of IHD, non-ischemic heart disease, CeVD, and all other circulatory disease estimated from the present analysis, namely 0.082 Gy-1 (95% CI 0.057, 0.106) [or for low dose-rate exposure 0.147 Gy-1 (95% CI 0.087, 0.207)], 0.094 Gy-1 (95% CI 0.078, 0.111), 0.236 Gy-1 (95% CI 0.062, 0.410) [or for low dose-rate exposure 0.308 Gy-1 (95% CI 0.075, 0.542)], and 0.137 Gy-1 (95% CI -0.049, 0.322), respectively, can be compared with the previously derived risks for the same endpoints of 0.10 Gy-1 (95% CI 0.04, 0.15), 0.08 Gy-1 (95% CI -0.12, 0.28), 0.21 Gy-1 (95% CI 0.02, 0.39), and 0.19 Gy-1 (95% CI -0.00, 0.38) [25], respectively. Given the overlap in the moderate/low dose studies considered here and previously this is perhaps unsurprising, but the analysis nevertheless confirms that there are no marked discrepancies between risks derived from the high-dose therapeutic and medical diagnostic studies (Tables 2, 3) and from the moderate/low dose occupational and environmental studies (Table 4). However, as suggested by the results of the meta-regression analysis (Tables 6, 7), even if the differences between risks at low and moderate/high dose rate are not substantial, they are nevertheless statistically significant.
Many of the studies of RT or of medical diagnostic exposure that are considered here (Tables 2, 3) have a substantial amount of information on the standard lifestyle and medical risk factors for circulatory disease. This is in contrast to many of the lower dose occupational/environmental studies that are considered (Table 4), in which such information is more limited. Of the lower dose studies considered only those of the Japanese atomic bomb survivors [24] and Mayak workers [33,68] had information on lifestyle factors, in particular cigarette smoking, alcohol consumption, obesity and (in the LSS) a few other variables associated with circulatory disease (diabetes mellitus, education, household occupation). The substantial heterogeneity that was observed for CeVD and circulatory disease apart from heart disease and CeVD in the previous meta-analysis [25] and also here (Table 5) may not be surprising given the variation in the distributions of different risk factors across populations, but it limits interpretation of the observed associations for these endpoints. Heterogeneity of all circulatory disease radiation risk by industrial grouping has also been observed within the Sellafield workers [78]. However, in most radiation-exposed groups there is little or no evidence that these lifestyle risk factors, when available, interact with radiation-associated circulatory disease risk [17,23,24,33,34,46,47,50,51,68].
Although preference is given to use of morbidity rather than mortality data, because of the likely greater diagnostic accuracy of the former, a case could be made for preferring mortality data, particularly because of the possibility that disease ascertainment might vary with dose within a cohort, as for example might be the case if the investigating medical professional was aware of the radiation history of the subject. This may be an issue with the Mayak worker data [32,33] and Chernobyl recovery workers [60,61] analyzed here. On the other hand, Russian national mortality data is likely to be particularly unreliable, with major variations in disease coding practices across the country [81,82], and should therefore probably not be used for epidemiologic analysis, in particular for the Russian worker studies considered here [32,33,60,61].
There have been a number of recent reviews of candidate biological mechanisms [3,29,83]. After high (5–15 Gy) or very high (>15 Gy) doses a variety of so-called tissue reaction (deterministic) effects are observed. There are plausible, if not completely understood, inflammatory mechanisms by which high doses of radiation affect the blood circulatory system [83]. Among such effects are direct damage to the structures of the heart – including marked diffuse fibrotic damage, especially of the pericardium and myocardium, pericardial adhesions, microvascular damage and stenosis of the valves – and to the coronary arteries; these sorts of damage occur both in patients receiving RT and in experimental animals [14,84]. With the exception of pericarditis, which occurs on timescales of months, most of these endpoints occur 10 or more years after irradiation [14].
At lower doses (0.5–5 Gy), in humans and in experimental studies, many inflammatory markers are up-regulated long after exposure to radiation. However, for exposures less than about 0.5 Gy, the balance shifts toward anti-inflammatory effects [29,85]. Interestingly, there is evidence of a steeper dose-response slope for various types of circulatory disease under 0.5 Gy in the two groups given highly fractionated fluoroscopic X-ray exposures [50,51]. This may reflect some particular medical issues associated with the group given high doses, who were also treated for longer, and were likely to have a more serious underlying TB.
As discussed above, there is evidence from the RT cohorts that heart dose, whether cumulative or EQD2, may be the most relevant for IHD [46]. Heart dose and thyroid dose (a surrogate for dose to the carotid artery) may also be relevant for CeVD; however, brain dose is unlikely to be so associated [17]. The generally uniform whole-body, low linear energy transfer radiation in the lower-dose cohorts that is assessed here is uninformative as to specific target tissues. In many occupational studies effective dose is used (e.g., Hp(10)), in which absorbed dose to each organ is weighted by appropriate tissue-weighting factors; this contrasts with the absorbed organ dose that is used elsewhere. However, these different dose metrics would not be expected to be markedly different for the penetrating ionizing radiations considered here, so would not substantially contribute to heterogeneity in radiation risk. At least for heart disease and CeVD, the consistency of risks, across a wide range of doses (Tables 2-4, 5, 7) suggests that target tissues and associated mechanisms may be the same for all levels of dose; however, this may not be the case for circulatory disease other than heart and CeVD.
Dose-related variations in T-cell and B-cell populations in Japanese atomic bomb survivors suggest that the immune system may be adversely affected [86]. There is at best conflicting evidence for involvement of the immune system in cardiovascular disease [87-91]; to the extent that it might be, whole-body or bone-marrow dose could be the most relevant to radiation effects. Monocyte cell killing in the arterial intima has been proposed as a mechanism, based on predictions of a bio-mathematical model [92]; however, this mechanism remains speculative. There is nevertheless suggestive evidence for radiation-induced endothelial cell senescence and associated monocyte adhesion [93,94]. Endothelial cells, because of their strategic anatomic position between the circulating blood and the vessel wall, regulate vascular function and structure; dysfunctions in endothelial cells are thought to be a critical initiating stage in many types of circulatory disease [95]. The critical role of vascular endothelial cells in circulatory disease suggests that the large arteries (e.g., aorta, carotid), may also be an etiologically relevant target.
There are indications in the LSS that the kidney may be a target tissue for hypertension [96], and there is some support for this from experimental animal data [97]. The consistency of IHD risk in the peptic ulcer cohort in relation to kidney dose, 0.033 Gy-1 (95% CI 0.012, 0.056) [17] (Table 2), with that in the LSS, 0.02 Sv-1 (95% CI -0.10, 0.15) [24] (Table 4) also suggests that this may be a target tissue.
Diabetes and obesity are major risk factors for circulatory disease [5], the former suggesting that the pancreas may be an etiologically relevant target tissue. Many of the metabolic derangements known to occur in diabetes, including hyperglycemia, excess free fatty acid liberation, and insulin resistance, mediate abnormalities in endothelial cell function [95]. There is other evidence suggesting a role for RT, and specifically dose to the pancreas, in causing diabetes, both in the peptic ulcer cohort [98], in the French-UK childhood cancer cohort [99] and in the Netherlands HL cohort [100]; however, the role of ionizing radiation in inducing diabetes at the lower doses remains uncertain, since, based on an early report, no increase has been observed in the AHS [101]. Parathyroid hormone increases with dose in the Japanese atomic bomb survivors, suggesting that there may be radiation-associated hyperparathyroidism [102]. Parathyroid hormone (PTH) has a central role in well-regulated calcium homeostasis and its release is triggered by a decrease in serum calcium levels. Primary hyperparathyroidism results in overproduction of PTH, mobilizing excess calcium to the bloodstream [103]. This elevation results in hypertension (via disturbances in the renin-angiotensin-aldosterone system), cardiac hypertrophy, and myocardial dysfunction [103]. PTH receptors are present in the myocardium and exert hypertrophic effects on cardiomyocytes [103]. These associations suggest plausible mechanisms whereby the elevated PTH concentrations that result from hyperparathyroidism may be involved in various pathological processes that lead to circulatory disease. However, the relatively low level prevalence of hyperparathyroidism (7/1459) in the AHS [102] suggests that even if this were a mechanism, it cannot account for more than a small fraction of the LSS circulatory disease cases.
4.1 CONCLUSIONS
The review provides strong evidence in support of a causal association between acute high dose and chronic low dose radiation exposure and most types of circulatory disease, in particular for the two main types of circulatory disease, namely IHD and CeVD. These findings confirm the results of a previous systematic review and meta-analysis of moderate and low dose groups [25]. The lack of heterogeneity for three out of the four endpoints considered, namely IHD, non-ischemic heart disease, and CeVD (after adjustment for any of dose, dose fractionation or age at exposure), strengthens the case for the associations to be considered causal. The association is less certain for circulatory diseases other than heart disease and CeVD given the only marginal level of statistical significance (p=0.0745) and the highly significant (p<0.0001) inter-study heterogeneity of risks in studies of this endpoint. The previous meta-analysis suggested that if the association between low-level exposure to radiation and the risk of circulatory disease reflects an underlying causal relationship, linear in dose, then the overall excess risk of mortality after exposure to low doses or low dose-rates of radiation may therefore be about twice that currently assumed [25]. Since the risks that are derived here using a somewhat larger body of data, that includes exposures at all levels of dose, are consistent with those, the implications for low dose radiation risk are unaltered. Nevertheless, the possible mechanisms for risk at low doses and low dose rates are, in contrast to the situation at higher doses and dose rates, relatively little understood. There is an urgent need for further research in this area [4].
Supplementary Material
Acknowledgments
The Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics supported this work. The author is grateful for the detailed and helpful comments of the two referees.
Abbreviations
- AHS
Adult Health Study
- CAD
coronary artery disease
- CeVD
cerebrovascular disease
- CT
chemotherapeutic/chemotherapy
- CI
confidence intervals
- EQD2
equivalent dose in 2 Gy fractions
- ERR
excess relative risk
- GP
general practitioner
- Gy
gray
- HDL
high density lipoprotein
- HL
Hodgkin’s lymphoma
- ICD10
International Classification of Diseases 10th revision
- ICD8
International Classification of Diseases 8th revision
- ICD9
International Classification of Diseases 9th revision
- ICRP
International Commission on Radiological Protection
- IHD
ischemic heart disease
- LDL
low density lipoprotein
- OR
odds ratio
- PA
Production Association
- REML
restricted maximum likelihood
- RR
relative risk
- RT
radiotherapeutic/radiotherapy
- Sv
sievert
- TB
tuberculosis
Appendix A
Details of preliminary analyses performed to derive risk estimates in the Netherlands valvular disease study of Cutter et al [34] and in the Childhood Cancer Survivor Study of Mulrooney et al [35]
In the Netherlands valvular disease case-control study [34] ERR was estimated from tabulations of numbers of cases and controls in the associated paper. To make such estimations a simple linear odds ratio (OR) model was fitted, in which the OR in dose group i with average organ dose Di, relative to group 0, with organ dose D0 = 0 , is assumed to be given by:
| (A1) |
where α is the excess OR per Gy. +Assuming binomially-distributed numbers of n1,i cases and n0,i controls in each dose group i for i = 0 ,1, …, N , the prospective likelihood (known to be equivalent to the retrospective likelihood [107]) is given by:
| (A2) |
where the parameter λ0 is the baseline odds. Fitting of this model is performed by maximum likelihood [108] using Epicure [109]. Central (maximum likelihood) estimates and 95% profile likelihood confidence intervals (CI) [108] are given in Table 2. As is well known, when disease rates are low the OR is approximately equal to the RR [110], so that the parameter α that we estimate in this way is approximately equal to the ERR per Gy.
For the study of Mulrooney et al [35] the most useful information given are estimates of the (adjusted) relative risk, RRi (and associated 95% CI (CIli , CIui)) in each dose group i ; estimates of α and associated CI are obtained by weighted least squares, i.e., by minimizing the inverse-variance-weighted sum of squares:
| (A3) |
where wi is the inverse-variance weight attached to dose group i , which is approximately given by:
| (A5) |
[N0.975 ≈ 1.96 is the 97.5% percentile point of the standard normal distribution: 0.975 = P[N(0,1) < N0.975].] The regression was performed using R[40].
Footnotes
For radiation protection purposes, the evaluation of risk for adverse effects typically considers the radiation energy deposited per unit mass of tissue, with units of gray (Gy) = 1 J kg-1. Stochastic effects such as cancer and hereditary effects are known to depend on the radiation energy, and so in estimation of radiation effects for a given organ/tissue the physical radiation dose (in Gy) is multiplied by a tissue weighting factor wR to yield the equivalent dose in sievert (Sv).
Authors’ contributions MPL wrote this review.
CONFLICT OF INTEREST STATEMENT.
The author (MPL) has no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization (WHO) World Health Organization Statistical Information System (WHOSIS) 2015 (updated 17 November 2015)( http://www.who.int/gho/en/)
- 2.Centers for Disease Control and Prevention (CDC) Underlying Cause of Death 1999-2014 on CDC WONDER Online Database. CDC WONDER; 2016. [Google Scholar]
- 3.McMillan TJ, Bennett MR, Bridges BA, Darby SC, Hendry J, Jones B, Kanthou C, Little MP, Taylor A, Tzoulaki I, Bouffler SD, Zhang W, Walker H, Keep P. Report of the independent Advisory Group on Ionising Radiation. Health Protection Agency; Holborn Gate, 330 High Holborn, London: 2010. Circulatory disease risk; pp. 1–116. [Google Scholar]
- 4.Kreuzer M, Auvinen A, Cardis E, Hall J, Jourdain JR, Laurier D, Little MP, Peters A, Raj K, Russell NS, Tapio S, Zhang W, Gomolka M. Low-dose ionising radiation and cardiovascular diseases--Strategies for molecular epidemiological studies in Europe. Mutat Res Rev Mutat Res. 2015;764:90–100. doi: 10.1016/j.mrrev.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 6.Clifton VL, Stark MJ, Osei-Kumah A, Hodyl NA. Review: The feto-placental unit, pregnancy pathology and impact on long term maternal health. Placenta. 2012;33(Suppl):S37–41. doi: 10.1016/j.placenta.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Dior UP, Hochner H, Friedlander Y, Calderon-Margalit R, Jaffe D, Burger A, Avgil M, Manor O, Elchalal U. Association between number of children and mortality of mothers: results of a 37-year follow-up study. Ann Epidemiol. 2013;23:13–18. doi: 10.1016/j.annepidem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slack J. Risks of ischaemic heart-disease in familial hyperlipoproteinaemic states. Lancet. 1969;2:1380–1382. doi: 10.1016/s0140-6736(69)90930-1. [DOI] [PubMed] [Google Scholar]
- 9.Rissanen AM, Nikkila EA. Aggregation of coronary risk factors in families of men with fatal and non-fatal coronary heart disease. Br Heart J. 1979;42:373–380. doi: 10.1136/hrt.42.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rissanen AM. Familial aggregation of coronary heart disease in a high incidence area (North Karelia, Finland) Br Heart J. 1979;42:294–303. doi: 10.1136/hrt.42.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rissanen AM. Familial occurrence of coronary heart disease: effect of age at diagnosis. The American journal of cardiology. 1979;44:60–66. doi: 10.1016/0002-9149(79)90251-0. [DOI] [PubMed] [Google Scholar]
- 12.Ioannidis JP. Prediction of cardiovascular disease outcomes and established cardiovascular risk factors by genome-wide association markers. Circ Cardiovasc Genet. 2009;2:7–15. doi: 10.1161/CIRCGENETICS.108.833392. [DOI] [PubMed] [Google Scholar]
- 13.Cambien F, Tiret L. Genetics of cardiovascular diseases: from single mutations to the whole genome. Circulation. 2007;116:1714–1724. doi: 10.1161/CIRCULATIONAHA.106.661751. [DOI] [PubMed] [Google Scholar]
- 14.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 15.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 16.Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiat Oncol Biol Phys. 2007;69:1484–1495. doi: 10.1016/j.ijrobp.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Little MP, Kleinerman RA, Stovall M, Smith SA, Mabuchi K. Analysis of dose response for circulatory disease after radiotherapy for benign disease. Int J Radiat Oncol Biol Phys. 2012;84:1101–1109. doi: 10.1016/j.ijrobp.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darby S, McGale P, Peto R, Granath F, Hall P, Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ. 2003;326:256–257. doi: 10.1136/bmj.326.7383.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 20.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y G. Early Breast Cancer Trialists’ Collaborative. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 21.Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, Hoskin PJ, Lister A, Radford JA, Rohatiner AZ, Linch DC. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 22.Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol. 1993;11:1208–1215. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- 23.Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958-1998. Radiat Res. 2004;161:622–632. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, Grant EJ, Sugiyama H, Sakata R, Moriwaki H, Hayashi M, Konda M, Shore RE. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F, Hall P, Harrison JD, Hildebrandt G, Ivanov V, Kashcheev VV, Klymenko SV, Kreuzer M, Laurent O, Ozasa K, Schneider T, Tapio S, Taylor AM, Tzoulaki I, Vandoolaeghe WL, Wakeford R, Zablotska LB, Zhang W, Lipshultz SE. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012;120:1503–1511. doi: 10.1289/ehp.1204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schöllnberger H, Kaiser JC, Jacob P, Walsh L. Dose-responses from multi-model inference for the non-cancer disease mortality of atomic bomb survivors. Radiat Environ Biophys. 2012;51:165–178. doi: 10.1007/s00411-012-0410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F, Hall P, Harrison JD, Hildebrandt G, Ivanov V, Kashcheev VV, Klymenko SV, Laurent O, Ozasa K, Tapio S, Taylor AM, Tzoulaki I, Vandoolaeghe WL, Wakeford R, Zablotska L, Zhang W, Lipshultz SE. Comment on “Dose-responses from multi-model inference for the non-cancer disease mortality of atomic bomb survivors” (Radiat. Environ. Biophys (2012) 51:165-178) by Schöllnberger et al. Radiat Environ Biophys. 2013;52:157–159. doi: 10.1007/s00411-012-0453-6. [DOI] [PubMed] [Google Scholar]
- 28.International Commission on Radiological Protection. ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs - threshold doses for tissue reactions in a radiation protection context. ICRP publication 118. Ann ICRP. 2012;41:1–322. doi: 10.1016/j.icrp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F, Tapio S, Elliott P. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res. 2008;169:99–109. doi: 10.1667/RR1070.1. [DOI] [PubMed] [Google Scholar]
- 30.Little MP. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat Environ Biophys. 2013;52:435–449. doi: 10.1007/s00411-013-0484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little MP, Lipshultz SE. Low dose radiation and circulatory diseases: a brief narrative review. Cardio-Oncology. 2015;1:1–10. doi: 10.1186/s40959-015-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azizova TV, Grigoryeva ES, Haylock RG, Pikulina MV, Moseeva MB. Ischaemic heart disease incidence and mortality in an extended cohort of Mayak workers first employed in 1948-1982. Br J Radiol. 2015;88:20150169. doi: 10.1259/bjr.20150169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moseeva MB, Azizova TV, Grigoryeva ES, Haylock R. Risks of circulatory diseases among Mayak PA workers with radiation doses estimated using the improved Mayak Worker Dosimetry System 2008. Radiat Environ Biophys. 2014;53:469–477. doi: 10.1007/s00411-014-0517-x. [DOI] [PubMed] [Google Scholar]
- 34.Cutter DJ, Schaapveld M, Darby SC, Hauptmann M, van Nimwegen FA, Krol AD, Janus CP, van Leeuwen FE, Aleman BM. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, Leisenring WM. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behavioral Stat. 2005;30:261–293. [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 39.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Statist Software. 2010;36:1–48. [Google Scholar]
- 40.R Project version 3.2.2. R version 3.2.2. Comprehensive R Archive Network (CRAN); 2015. http://www.r-project.org/ [Google Scholar]
- 41.Lumley T. Package rmeta version 2.16. Comprehensive R Archive Network (CRAN); Seattle, Washington, USA: 2015. [Google Scholar]
- 42.Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, Kleinerman R. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiation Research. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 43.Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, Guérin S, Pacquement H, Aouba A, Hawkins M, Winter D, Bourhis J, Lefkopoulos D, Diallo I, de Vathaire F. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 44.Shamsaldin A, Grimaud E, Hardiman C, Diallo I, de Vathaire F, Chavaudra J. Dose distribution throughout the body from radiotherapy for Hodgkin’s disease in childhood. Radiother Oncol. 1998;49:85–90. doi: 10.1016/s0167-8140(98)00110-8. [DOI] [PubMed] [Google Scholar]
- 45.Diallo I, Lamon A, Shamsaldin A, Grimaud E, de VF, Chavaudra J. Estimation of the radiation dose delivered to any point outside the target volume per patient treated with external beam radiotherapy. Radiother Oncol. 1996;38:269–271. doi: 10.1016/0167-8140(96)01713-6. [DOI] [PubMed] [Google Scholar]
- 46.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 47.van Nimwegen FA, Schaapveld M, Cutter DJ, Janus CP, Krol AD, Hauptmann M, Kooijman K, Roesink J, van der Maazen R, Darby SC, Aleman BM, van Leeuwen FE. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 48.Little MP, Zablotska LB, Lipshultz SE. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med. 2013;368:2523–2524. doi: 10.1056/NEJMc1304601. [DOI] [PubMed] [Google Scholar]
- 49.Darby SC, Doll R, Gill SK, Smith PG. Long term mortality after a single treatment course with X-rays in patients treated for ankylosing spondylitis. Br J Cancer. 1987;55:179–190. doi: 10.1038/bjc.1987.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zablotska LB, Little MP, Cornett RJ. Potential increased risk of ischemic heart disease mortality with significant dose fractionation in the Canadian fluoroscopy cohort study. Am J Epidemiol. 2014:179. 120–131. doi: 10.1093/aje/kwt244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Little MP, Zablotska LB, Brenner AV, Lipshultz SE. Circulatory disease mortality in the Massachusetts tuberculosis fluoroscopy cohort study. Eur J Epidemiol. 2016;31:287–309. doi: 10.1007/s10654-015-0075-9. [DOI] [PubMed] [Google Scholar]
- 52.Travis LB, Land CE, Andersson M, Nyberg U, Goldman MB, Knudson GL, Berger E, Storm HH, Hall P, Auvinen A, Janower ML, Holm LE, Monson RR, Schottenfeld D, Boice JD., Jr Mortality after cerebral angiography with or without radioactive Thorotrast: an international cohort of 3,143 two-year survivors. Radiat Res. 2001;156:136–150. doi: 10.1667/0033-7587(2001)156[0136:macawo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 53.dos Santos Silva I, Malveiro F, Jones ME, Swerdlow AJ. Mortality after radiological investigation with radioactive Thorotrast: a follow-up study of up to fifty years in Portugal. Radiat Res. 2003;159:521–534. doi: 10.1667/0033-7587(2003)159[0521:mariwr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Tatsukawa Y, Nakashima E, Yamada M, Funamoto S, Hida A, Akahoshi M, Sakata R, Ross NP, Kasagi F, Fujiwara S, Shore RE. Cardiovascular disease risk among atomic bomb survivors exposed in utero, 1978-2003. Radiat Res. 2008;170:269–274. doi: 10.1667/RR1434.1. [DOI] [PubMed] [Google Scholar]
- 55.Stewart AM, Kneale GW. A-bomb survivors: factors that may lead to a re-assessment of the radiation hazard. Int J Epidemiol. 2000;29:708–714. doi: 10.1093/ije/29.4.708. [DOI] [PubMed] [Google Scholar]
- 56.Little MP. Absence of evidence for differences in the dose-response for cancer and non-cancer endpoints by acute injury status in the Japanese atomic-bomb survivors. Int J Radiat Biol. 2002;78:1001–1010. doi: 10.1080/0955300021000013803. [DOI] [PubMed] [Google Scholar]
- 57.Ozasa K, Takahashi I, Grant EJ. Radiation-related risks of non-cancer outcomes in the atomic bomb survivors. Ann ICRP. 2016;45:253–261. doi: 10.1177/0146645316629318. [DOI] [PubMed] [Google Scholar]
- 58.Ueshima H. Explanation for the Japanese paradox: prevention of increase in coronary heart disease and reduction in stroke. J Atheroscler Thromb. 2007;14:278–286. doi: 10.5551/jat.e529. [DOI] [PubMed] [Google Scholar]
- 59.Vrijheid M, Cardis E, Ashmore P, Auvinen A, Bae JM, Engels H, Gilbert E, Gulis G, Habib R, Howe G, Kurtinaitis J, Malker H, Muirhead C, Richardson D, Rodriguez-Artalejo F, Rogel A, Schubauer-Berigan M, Tardy H, Telle-Lamberton M, Usel M, Veress K. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol. 2007;36:1126–1135. doi: 10.1093/ije/dym138. [DOI] [PubMed] [Google Scholar]
- 60.Ivanov VK, Maksioutov MA, Chekin SY, Petrov AV, Biryukov AP, Kruglova ZG, Matyash VA, Tsyb AF, Manton KG, Kravchenko JS. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- 61.Kashcheev VV, Chekin SY, Maksioutov MA, Tumanov KA, Menyaylo AN, Kochergina EV, Kashcheeva PV, Gorsky AI, Shchukina NV, Karpenko SV, Ivanov VK. Radiation-epidemiological Study of Cerebrovascular Diseases in the Cohort of Russian Recovery Operation Workers of the Chernobyl Accident. Health Phys. 2016;111:192–197. doi: 10.1097/HP.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 62.Ivanov VK, Gorski AI, Maksioutov MA, Tsyb AF, Souchkevitch GN. Mortality among the Chernobyl emergency workers: estimation of radiation risks (preliminary analysis) Health Phys. 2001;81:514–521. doi: 10.1097/00004032-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Doll R, Fisher RE, Gammon EJ, Gunn W, Hughes GO, Tyrer FH, Wilson W. Mortality of Gasworkers with Special Reference to Cancers of the Lung and Bladder, Chronic Bronchitis, and Pneumoconiosis. Br J Ind Med. 1965;22:1–12. doi: 10.1136/oem.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bell CM, Coleman DA. Models of the healthy worker effect in industrial cohorts. Stat Med. 1987;6:901–909. doi: 10.1002/sim.4780060805. [DOI] [PubMed] [Google Scholar]
- 65.Simonetto C, Azizova TV, Grigoryeva ES, Kaiser JC, Schollnberger H, Eidemuller M. Ischemic heart disease in workers at Mayak PA: latency of incidence risk after radiation exposure. PloS one. 2014;9:e96309. doi: 10.1371/journal.pone.0096309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simonetto C, Schollnberger H, Azizova TV, Grigoryeva ES, Pikulina MV, Eidemuller M. Cerebrovascular Diseases in Workers at Mayak PA: The Difference in Radiation Risk between Incidence and Mortality. PloS one. 2015;10:e0125904. doi: 10.1371/journal.pone.0125904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azizova TV, Grigorieva ES, Hunter N, Pikulina MV, Moseeva MB. Risk of mortality from circulatory diseases in Mayak workers cohort following occupational radiation exposure. Journal of radiological protection : official journal of the Society for Radiological Protection. 2015;35:517–538. doi: 10.1088/0952-4746/35/3/517. [DOI] [PubMed] [Google Scholar]
- 68.Azizova TV, Haylock RGE, Moseeva MB, Bannikova MV, Grigoryeva ES. Cerebrovascular diseases incidence and mortality in an extended Mayak Worker Cohort 1948-1982. Radiat Res. 2014;182:529–544. doi: 10.1667/RR13680.1. [DOI] [PubMed] [Google Scholar]
- 69.Azizova TV, Bannikova MV, Grigorieva ES, Bagaeva YP, Azizova EV. Risk of lower extremity arterial disease in a cohort of workers occupationally exposed to ionizing radiation over a prolonged period. Radiat Environ Biophys. 2016;55:147–159. doi: 10.1007/s00411-016-0645-6. [DOI] [PubMed] [Google Scholar]
- 70.Azizova TV, Muirhead CR, Druzhinina MB, Grigoryeva ES, Vlasenko EV, Sumina MV, O’Hagan JA, Zhang W, Haylock RGE, Hunter N. Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948-1958. Radiat Res. 2010;174:155–168. doi: 10.1667/RR1789.1. [DOI] [PubMed] [Google Scholar]
- 71.Azizova TV, Muirhead CR, Druzhinina MB, Grigoryeva ES, Vlasenko EV, Sumina MV, O’Hagan JA, Zhang W, Haylock RGE, Hunter N. Cerebrovascular Diseases in the Cohort of Workers First Employed at Mayak PA in 1948-1958. Radiat Res. 2010;174:851–864. doi: 10.1667/RR1928.1. [DOI] [PubMed] [Google Scholar]
- 72.Wakeford R, Tawn EJ. The meaning of low dose and low dose-rate. J Radiol Prot. 2010;30:1–3. doi: 10.1088/0952-4746/30/1/E02. [DOI] [PubMed] [Google Scholar]
- 73.Muirhead CR, O’Hagan JA, Haylock RGE, Phillipson MA, Willcock T, Berridge GLC, Zhang W. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009;100:206–212. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kreuzer M, Kreisheimer M, Kandel M, Schnelzer M, Tschense A, Grosche B. Mortality from cardiovascular diseases in the German uranium miners cohort study, 1946-1998. Radiat Environ Biophys. 2006;45:159–166. doi: 10.1007/s00411-006-0056-1. [DOI] [PubMed] [Google Scholar]
- 75.Kreuzer M, Dufey F, Sogl M, Schnelzer M, Walsh L. External gamma radiation and mortality from cardiovascular diseases in the German WISMUT uranium miners cohort study, 1946-2008. Radiat Environ Biophys. 2013;52:37–46. doi: 10.1007/s00411-012-0446-5. [DOI] [PubMed] [Google Scholar]
- 76.Lane RSD, Frost SE, Howe GR, Zablotska LB. Mortality (1950-1999) and cancer incidence (1969-1999) in the cohort of Eldorado uranium workers. Radiat Res. 2010;174:773–785. doi: 10.1667/RR2237.1. [DOI] [PubMed] [Google Scholar]
- 77.Laurent O, Metz-Flamant C, Rogel A, Hubert D, Riedel A, Garcier Y, Laurier D. Relationship between occupational exposure to ionizing radiation and mortality at the French electricity company, period 1961-2003. Int Arch Occup Environ Health. 2010;83:935–944. doi: 10.1007/s00420-010-0509-3. [DOI] [PubMed] [Google Scholar]
- 78.McGeoghegan D, Binks K, Gillies M, Jones S, Whaley S. The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946-2005. Int J Epidemiol. 2008;37:506–518. doi: 10.1093/ije/dyn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krestinina LY, Epifanova S, Silkin S, Mikryukova L, Degteva M, Shagina N, Akleyev A. Chronic low-dose exposure in the Techa River Cohort: risk of mortality from circulatory diseases. Radiat Environ Biophys. 2013;52:47–57. doi: 10.1007/s00411-012-0438-5. [DOI] [PubMed] [Google Scholar]
- 80.Grosche B, Lackland DT, Land CE, Simon SL, Apsalikov KN, Pivina LM, Bauer S, Gusev BI. Mortality from cardiovascular diseases in the Semipalatinsk historical cohort, 1960-1999, and its relationship to radiation exposure. Radiat Res. 2011;176:660–669. doi: 10.1667/rr2211.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Danilova I, Shkolnikov VM, Jdanov DA, Mesle F, Vallin J. Identifying potential differences in cause-of-death coding practices across Russian regions. Popul Health Metr. 2016;14:8. doi: 10.1186/s12963-016-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wasserman D, Varnik A. Reliability of statistics on violent death and suicide in the former USSR, 1970-1990. Acta Psychiatr Scand Suppl. 1998;394:34–41. doi: 10.1111/j.1600-0447.1998.tb10763.x. [DOI] [PubMed] [Google Scholar]
- 83.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 84.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) UNSCEAR 2006 Report. Annex B. Epidemiological Evaluation of Cardiovascular Disease and other Non-Cancer Diseases following Radiation Exposure. United Nations; New York: 2008. pp. 325–383. [Google Scholar]
- 85.Mitchel REJ, Hasu M, Bugden M, Wyatt H, Little MP, Gola A, Hildebrandt G, Priest ND, Whitman SC. Low-dose radiation exposure and atherosclerosis in ApoE-/- mice. Radiat Res. 2011;175:665–676. doi: 10.1667/RR2176.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kusunoki Y, Kyoizumi S, Hirai Y, Suzuki T, Nakashima E, Kodama K, Seyama T. Flow cytometry measurements of subsets of T, B and NK cells in peripheral blood lymphocytes of atomic bomb survivors. Radiat Res. 1998;150:227–236. [PubMed] [Google Scholar]
- 87.Danesh J, Whincup P, Lewington S, Walker M, Lennon L, Thomson A, Wong YK, Zhou X, Ward M. Chlamydia pneumoniae IgA titres and coronary heart disease. Prospective study and meta-analysis. European Heart Journal. 2002;23:371–375. doi: 10.1053/euhj.2001.2801. [DOI] [PubMed] [Google Scholar]
- 88.Ridker PM. Inflammation, infection, and cardiovascular risk: how good is the clinical evidence? Circulation. 1998;97:1671–1674. doi: 10.1161/01.cir.97.17.1671. [DOI] [PubMed] [Google Scholar]
- 89.Whincup P, Danesh J, Walker M, Lennon L, Thomson A, Appleby P, Hawkey C, Atherton J. Prospective study of potentially virulent strains of Helicobacter pylori and coronary heart disease in middle-aged men. Circulation. 2000;101:1647–1652. doi: 10.1161/01.cir.101.14.1647. [DOI] [PubMed] [Google Scholar]
- 90.Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, Cairns R, Skene AM E. Pravastatin or Atorvastatin, I. Infection Therapy-Thrombolysis in Myocardial Infarction. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352:1646–1654. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- 91.Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, Rogers WJ, Crouse JR, Borrowdale SL, Schron E, Knirsch C A. Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–1645. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- 92.Little MP, Gola A, Tzoulaki I. A model of cardiovascular disease giving a plausible mechanism for the effect of fractionated low-dose ionizing radiation exposure. PLoS Comput Biol. 2009;5:e1000539. doi: 10.1371/journal.pcbi.1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lowe D, Raj K. Premature aging induced by radiation exhibits pro-atherosclerotic effects mediated by epigenetic activation of CD44 expression. Aging Cell. 2014;13:900–910. doi: 10.1111/acel.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rombouts C, Aerts A, Quintens R, Baselet B, El-Saghire H, Harms-Ringdahl M, Haghdoost S, Janssen A, Michaux A, Yentrapalli R, Benotmane MA, Van Oostveldt P, Baatout S. Transcriptomic profiling suggests a role for IGFBP5 in premature senescence of endothelial cells after chronic low dose rate irradiation. International Journal of Radiation Biology. 2014;90:560–574. doi: 10.3109/09553002.2014.905724. [DOI] [PubMed] [Google Scholar]
- 95.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 96.Adams MJ, Grant EJ, Kodama K, Shimizu Y, Kasagi F, Suyama A, Sakata R, Akahoshi M. Radiation dose associated with renal failure mortality: a potential pathway to partially explain increased cardiovascular disease mortality observed after whole-body irradiation. Radiat Res. 2012;177:220–228. doi: 10.1667/rr2746.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lenarczyk M, Lam V, Jensen E, Fish BL, Su J, Koprowski S, Komorowski RA, Harmann L, Migrino RQ, Li XA, Hopewell JW, Moulder JE, Baker JE. Cardiac injury after 10 Gy total body irradiation: indirect role of effects on abdominal organs. Radiat Res. 2013;180:247–258. doi: 10.1667/RR3292.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kleinerman RA, Weinstock RM, Mabuchi K. High-dose abdominal radiotherapy and risk of diabetes mellitus. Arch Intern Med. 2010;170:1506–1507. doi: 10.1001/archinternmed.2010.285. [DOI] [PubMed] [Google Scholar]
- 99.de Vathaire F, El-Fayech C, Ben Ayed FF, Haddy N, Guibout C, Winter D, Thomas-Teinturier C, Veres C, Jackson A, Pacquement H, Schlumberger M, Hawkins M, Diallo I, Oberlin O. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. The Lancet Oncology. 2012;13:1002–1010. doi: 10.1016/S1470-2045(12)70323-6. [DOI] [PubMed] [Google Scholar]
- 100.van Nimwegen FA, Schaapveld M, Janus CPM, Krol ADG, Raemaekers JMM, Kremer LCM, Stovall M, Aleman BMP, van Leeuwen FE. Risk of diabetes mellitus in long-term survivors of Hodgkin lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:3257–3263. doi: 10.1200/JCO.2013.54.4379. [DOI] [PubMed] [Google Scholar]
- 101.Belsky JL, Tachikawa K, Jablon S. The health of atomic bomb survivors: a decade of examinations in a fixed population. Yale J Biol Med. 1973;46:284–296. [PMC free article] [PubMed] [Google Scholar]
- 102.Fujiwara S, Sposto R, Shiraki M, Yokoyama N, Sasaki H, Kodama K, Shimaoka K. Levels of parathyroid hormone and calcitonin in serum among atomic bomb survivors. Radiat Res. 1994;137:96–103. [PubMed] [Google Scholar]
- 103.Andersson P, Rydberg E, Willenheimer R. Primary hyperparathyroidism and heart disease--a review. Eur Heart J. 2004;25:1776–1787. doi: 10.1016/j.ehj.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 104.Townsend N, Bhatnagar P, Wilkins E, Wickramasinghe K, Rayner M. Cardiovascular disease statistics 2015. British Heart Foundation; London, UK: 2015. [Google Scholar]
- 105.Office for National Statistics (ONS) Series DH2 no 30. Her Majesty’s Stationery Office; 2004. Mortality statistics cause. Review of the Registrar General on deaths by cause, sex and age, in England and Wales, 2003; pp. 1–291. [Google Scholar]
- 106.Richardson DB, Wing S. Radiation and mortality of workers at Oak Ridge National Laboratory: positive associations for doses received at older ages. Environ Health Perspect. 1999;107:649–656. doi: 10.1289/ehp.99107649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prentice RL, Pyke R. Logistic disease incidence models and case-control studies. Biometrika. 1979;66:403–411. [Google Scholar]
- 108.McCullagh P, Nelder JA. Monographs on statistics and applied probability. 2. Vol. 37. Chapman and Hall/CRC; Boca Raton, FL: 1989. Generalized linear models; pp. 1–526. [Google Scholar]
- 109.Risk Sciences International. Epicure version 2.0.1.0. Risk Sciences International; 55 Metcalfe, K1P 6L5, Canada: 2015. [Google Scholar]
- 110.Breslow NE, Day NE. Statistical methods in cancer research. The analysis of case-control studies. IARC Sci Publ. 1980;I:5–338. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


