Abstract
Background
Fat embolism (FE) and the consequent fat embolism syndrome (FES) occurring after trauma or surgery can lead to serious pulmonary injury, including ARDS and death. Current treatment of FES is limited to supportive therapy. We have shown in a rat model that the renin angiotensin system (RAS) plays a significant role in the pathophysiology of FE since drugs interfering with the RAS, captopril and losartan, reduce the histopathologic pulmonary damage. The purpose of the current study was to determine if inhibition of renin by aliskiren, an FDA-approved drug for treating hypertension, would produce effective protection in the same model.
Methods
The FE model used intravenous injection of the neutral fat triolein in unanesthetized rats. Intraperitoneal injections of saline or aliskiren at either 50 mg/kg or 100 mg/kg were performed one hour after FE induction via triolein. Rats were euthanized at 48 hours and various histological stains were used to examine the lungs.
Results
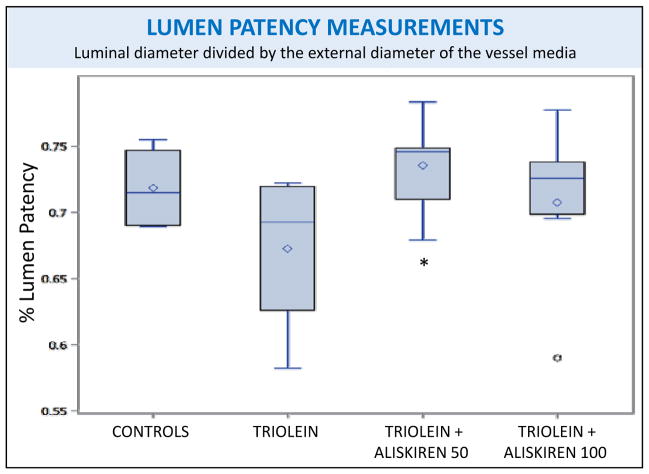
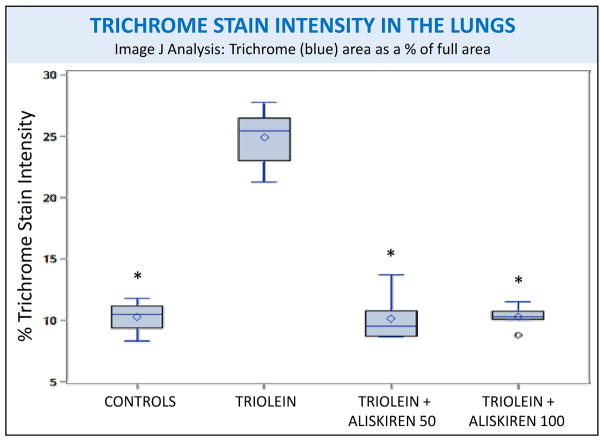
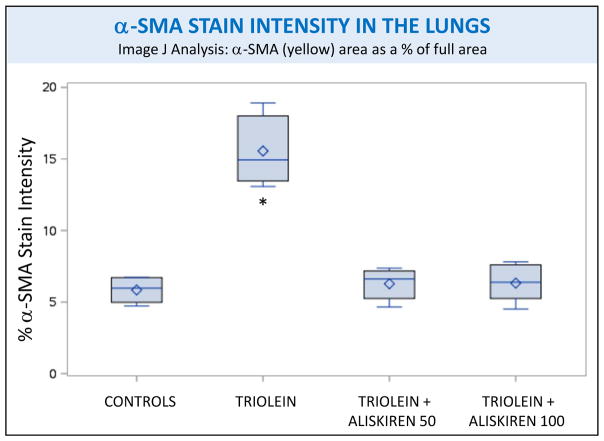
(1) Fibrosis: Rats treated with triolein showed significant fibrotic changes with increased collagen and myofibroblast activation (p < 0.0001 for both trichrome and α-SMA staining). Aliskiren blocked this inflammatory and profibrotic process to a level indistinguishable from the controls (p < 0.0001 for both trichrome and α-SMA staining). (2) Fat: Rats treated with triolein showed a statistically significant increase in fat (p= 0.0006). Subsequent aliskiren administration at both doses reduced the size, distribution, and amount of fat droplets (low dose p= 0.0095, high dose p= 0.0028). (3) Vessel Patency: The low dose of aliskiren blocked the reduction of lumen patency observed after triolein administration (p=0.0058).
Conclusions
Aliskiren protected the lungs of rats from gross and histopathologic FE-induced pulmonary damage at 48 hours. Clinical implications include the use of aliskiren both prophylactically (before certain orthopedic procedures) and therapeutically (after severe trauma) to prevent the consequent severe pulmonary pathological sequelae.
Level of Evidence
Therapeutic study, level V.
Keywords: Fat embolism, renin inhibitor, lung, histopathology, fibrosis
INTRODUCTION
Fat embolism (FE) and the consequent fat embolism syndrome (FES) occurring after trauma or surgery can lead to serious pulmonary injury, including ARDS and death.(1) FE has been estimated to occur in up to 90%(1, 2) of patients with traumatic injury and FES up to 35%(3) of patients with traumatic injury in prospective studies. While the incidence of clinical FES is lower than its subclinical precursor FE, both are thought to go undetected in cases of major trauma due to overshadowing by other illnesses or injuries. Additional risk factors for FE include burns, liposuction, pancreatitis, and bone marrow harvest.(4) Current treatment of FES is limited to supportive therapy.(5) We have shown in a rat model of FE that the renin angiotensin system (RAS) plays a significant role in the pathophysiology since drugs interfering with the RAS, captopril, an angiotensin I converting enzyme inhibitor, and losartan, an angiotensin II type I receptor antagonist, reduce the histopathologic pulmonary damage.(6) The lungs in this model demonstrate very similar pathological changes to those found in a recently described fatal case of fat embolism after caesarean section.(7) The purpose of the current study was to determine if inhibition of renin, the rate-limiting biochemical step in the RAS proximal to the sites of action of captopril and losartan, would produce effective protection in the same model. To examine this possibility, we utilized aliskiren (Tekturna), an FDA-approved renin inhibitor used for treating hypertension.
MATERIALS AND METHODS
Responsible Conduct of Research
This study was conducted under the approval of the University of Missouri at Kansas City Institutional Animal Care and Use Committee (IACUC), Protocol # 1507. Animal care and procedures were in accordance with institutional guidelines.
Overview
The model of FE utilizes intravenous injection of the neutral fat triolein in unanesthetized rats.(6, 8) Intraperitoneal injections of aliskiren at either 50 mg/kg or 100 mg/kg were performed one hour after FE induction via triolein. Rats were euthanized at 48 hours, and various pathology stains and methods were used to study and compare the lungs of these animals.
Experimental Design and Animals
22 male Sprague-Dawley rats (280–300 g) were obtained from Harlan Laboratories (Indianapolis, IN) and divided into four groups. Pure triolein (glyceryl trioleate, Sigma grade; Sigma Corp., St. Louis, MO) was used to simulate a FE. The control group (n=4) received 0.2 mL intravenous (i.v.) normal saline (NS) at 0 hours and 0.2 mL intraperitoneal (i.p.) NS at hour 1. The triolein-only group (n=6) received triolein 0.2 mL i.v. at 0 hours and NS 0.2 mL i.p at hour 1. The low dose aliskiren group (n=6) received the same dose of triolein at 0 hours and aliskiren 50 mg/kg at hour 1. The high dose aliskiren group (n=6) received the same dose of triolein at 0 hours and aliskiren 100 mg/kg at hour 1. These aliskiren doses have been employed in published experiments.(9, 10) All rats were given ad libitum access to food and water, and observed at various time points after the injections. No animals died before euthanasia at 48 hours using inhalation isoflurane (Sigma Corp., St. Louis, MO). The experiment was carried out to 48 hours post-triolein injection based on our previous studies demonstrating peak histopathological effects at this time point (8) and for comparison to our captopril and losartan results(6) also carried out to 48 hours.
Pathology Procedures
After euthanasia, rats were necropsied and the viscera of the thoracic and abdominal cavity removed and inspected for gross anatomic pathologic changes. The lower lobe of the right lung was collected and placed in 10% buffered formalin solution for fixation. After 10 days of fixation, the specimens were paraffin embedded, cut in sagittal sections (4 μm), and stained with hematoxylin and eosin (H&E) for histological evaluation and vessel measurements. Slides were also stained with Masson’s trichrome (trichrome) for collagen identification. Immunolabeling of lung alpha-smooth muscle actin (α-SMA) as a marker for myofibroblasts activation and differentiation was performed using previously described protocols.(11) The lower lobe of the left lung was frozen at −20°C, sectioned, and stained for fat using Oil Red O.
Histologic evaluation was performed by two researchers who were unaware of the slide identification. Histopathologic studies were done on slides stained for H&E, trichrome, α-SMA, and Oil Red O. Ten photographs of each H&E slide were taken at 400x magnification. From these photographs, the lumen patency and media to adventitia ratio of at least five small caliber pulmonary arteries and arterioles were measured per slide. The lumen patency of these vessels was evaluated by measuring the luminal diameter divided by the external diameter of the vessel media, and the media-to-adventitia ratios obtained by measuring the external diameter of the media divided by the external diameter of the adventitia. Eight photographs of each Oil Red O stained slide were taken at 100x magnification, and ten photographs of each trichrome and α-SMA stained slide were taken at 400x magnification. The slides stained with Oil Red O, trichrome, and α-SMA were then digitally analyzed using the Image J Software (NIH) to quantify the amount of fat, collagen, and myofibroblasts present in each slide.
Statistical Analysis
A power analysis using the G*power computer software (Faul & Erdfelder, 2009) indicated that a total sample size of 20 rats would be needed to detect large effects (f=0.9) with 85% power using a fixed effects one-way ANOVA with alpha set at 0.05. A sample size of 22 was chosen to account for 10% loss of animal subjects.
The assumption of normality was confirmed for each of our four primary outcome measures using the Shapiro-Wilk test and Q-Q plots. Heteroscedasticity was identified using Levene’s test for equality of variances for two outcome measures, Oil Red O and lumen patency. The data were then statistically analyzed using Fisher’s classic one-way ANOVA for trichrome and α-SMA and Welch’s ANOVA, appropriate when the assumption of homogeneity of variances has been violated, for Oil Red O and lumen patency. Where appropriate, comparisons were made using Fisher’s least significant differences. The level of statistical significance for comparison was set at p=0.01. The inter-rater reliability was calculated using intra-class correlation, appropriate for continuous data. The intra-class correlation coefficient was calculated under the assumption that all subjects were rated by the same raters who are assumed to be a random subset of all possible raters. The data analysis for this study was generated using SAS software, Version 9.2 (SAS Institute).
RESULTS
Behavior
All rats survived the injections. The rats injected with triolein became hypoactive immediately following the injections, but regained activity within a couple of hours and were indistinguishable from the controls as seen in our past studies.(6, 8, 12, 13) On days 1 and 2, the rats continued to show no apparent signs of distress.
Gross Organ Appearance at Necropsy
The surface of the lungs of the controls did not show any pathology. Triolein caused severe congestion with diffuse pleural hemorrhages and “cobblestone” appearance of the pleural surface. Aliskiren administration at both doses resulted in less organ congestion, few smaller pleural hemorrhages, and a smooth pleural surface.
Inflammation and Vascular Changes (H&E Staining)
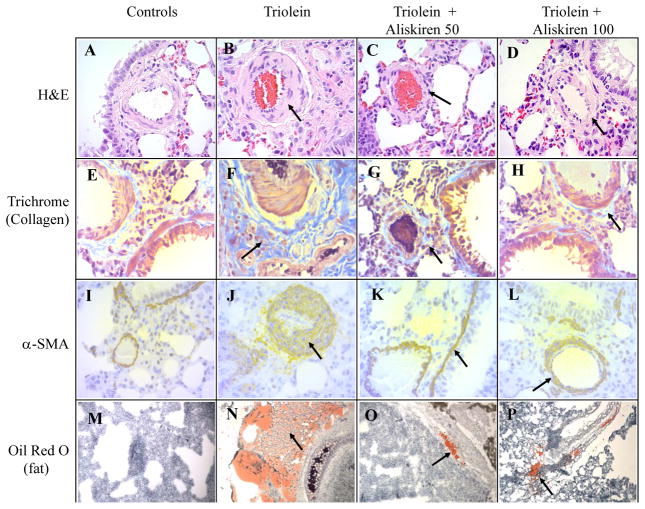
Representative H&E stained slides from the four different treatment groups are presented in figure 1. Rats of the control group demonstrated patent small caliber arteries and arterioles with few inflammatory cells throughout pulmonary parenchyma (Fig 1A). Histologic findings in the triolein-only treated animals mirrored those of past studies.(6, 8) These rats’ lungs presented with diffuse hemorrhages in the thickened septa, congestion, and diffuse inflammation with neutrophils, lymphocytes, macrophages, and erythrocytes (Fig 2A). Scattered areas of atelectasis and emphysema were also seen. Bronchial changes included thickening of the bronchial musculature, epithelial hypertrophy and inflammation, variable areas of disepithelialization, and loss of cilia. The lumina of large bronchi were also filled with edematous fluid, erythrocytes, and inflammatory cells.
Figure 1.
Representative slides of the lungs from the four treatment groups using four different staining methods. H&E (A–D): Hematoxylin and eosin stain, 400x. The triolein only group demonstrated marked thickening of the arterial walls with severe septal inflammation. The histopathology improved with increased lumen patency of the arteries after aliskiren administration. Trichrome (E–H): Stains collagen blue, 400x. α-SMA (I–L): Immunolabeling for alpha-smooth muscle actin which is a yellow-brown staining marker of myofibroblast activation and differentiation, 400x. Collagen and a-SMA staining demonstrate the severe inflammatory and fibrotic response with triolein treatment which was blocked in the aliskiren-treated animals. Oil Red O (M–P): Stains fat red, 100x. The triolein only group demonstrated large fat globules diffuse through the pulmonary parenchyma. Aliskiren treatment resulted in comparatively smaller sized droplets with limited distribution confined to the perivascular areas.
Figure 2.
Arterial lumen patency measurements of rat lungs subjected to different treatments. The only treatment groups that were significantly different from one another were the triolein and triolein + aliskiren 50 mg/kg groups, p=0.0058. However, no groups were significantly different from the control group. Boxplot labels-full box: interquartile range, upper box: upper quartile (75th percentile), lower box: lower quartile (25th percentile), diamond: mean, horizontal line within box: median, upper whisker: extends to maximum observation (excluding outliers), lower whisker: extends to minimum observation (excluding outliers), circle: outlier.
In the triolein-treated animals (Fig 1C and 1D), the small arteries and arterioles showed mild vasculitis with thickened media and reduced luminal patency. An improvement of the lumen patency was observed using both aliskiren doses with only the 50 mg/kg dose being statistically significant (p=0.0058) compared to the triolein-only treated rats. Periadventitial edema was also identified in these animals by qualitative assessment of the media-to-adventitia ratios, but the difference was not statistically significant (p= 0.4956).
The inter-rater reliability was very good with an intra-class correlation coefficient of 0.92 (95% confidence interval 0.87–0.95), indicating the blinded reviewers had a high degree of agreement and outcomes were measured similarly across the reviewers. The high intra-class correlation suggests that a minimal amount of measurement error was introduced by the independent reviewers, and therefore statistical power for subsequent analyses is not substantially reduced.
Markers of Collagen and myofibroblast activation (Trichrome and α-SMA Staining)
Representative slides stained with trichrome, a marker for collagen, and α-SMA, a marker for myofibroblast activation, from the four different groups are also shown in figure 1. A marked presence of trichrome is evident in the lungs of the rats injected with triolein in the septa, media and adventitia of small caliber arteries and arterioles, the peribronchial musculature, and in portions of the lungs close to the pleura (Fig 1F). α-SMA stain was more intense in the intraseptal and perivascular sections (Fig 1J). In the lungs of rats receiving both doses of aliskiren, the expression of trichrome (Fig 1G, 1H) and α-SMA (Fig 1K, 1L) was essentially the same as the controls. Quantification of both stains by Image J confirmed that aliskiren at both doses caused a statistically significant decrease in the fibrotic changes, to a level indistinguishable from the controls, when compared to the lungs of the triolein-only rats (p<0.0001 for both trichrome and α-SMA) as shown in figures 3 and 4.
Figure 3.
Image J analysis of the intensity of trichrome staining of rat lungs subjected to different treatments. Asterisks indicate significant difference from the control group, p<0.01.
Figure 4.
Image J analysis of the intensity of α-smooth muscle actin (α-SMA) staining of rat lungs subjected to different treatments. Asterisks indicate significant difference from the control group, p<0.01.
Fat Distribution in Lungs (Oil Red O Staining)
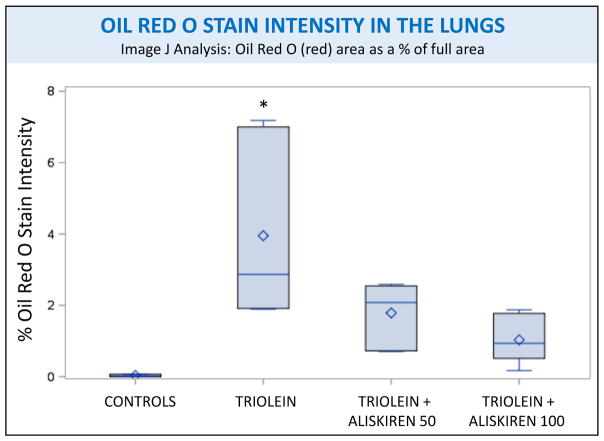
Representative Oil Red O stained slides from the four treatment groups are also shown in figure 1. Controls had a negligible amount of fat (Fig 1M) while the lungs of the triolein treated rats had a large amount of fat present as small droplets diffuse throughout the pulmonary interstitial space or as large droplets around the peribronchial veins (Fig 1N), confirming the findings of our previous studies conducted at the same time interval.(6, 8) Fat droplets present in the rats treated with aliskiren were comparatively small in size and mostly located around peribronchial veins, traversing the vascular musculature (Fig 1O, 1P). Few droplets were present throughout the alveolar septa. Rats treated with triolein showed a statistically significant increase in fat compared to the controls (p =0.0006) when evaluated by Image J as seen in figure 5. Subsequent aliskiren administration at both doses reduced the presence of fat (low dose p= 0.0095, high dose p= 0.0028) to a level indistinguishable from the controls as seen in figure 5
Figure 5.
Image J analysis of the intensity of Oil Red O staining of rat lungs subjected to different treatments. Asterisks indicate significant difference from the control group, p<0.01.
DISCUSSION
The current study extends the evidence for the central involvement of the RAS in the pathology of FE by showing that a direct renin inhibitor, aliskiren, reduced or prevented many of the histopathologic changes found in the lungs 48 hours post triolein administration. Some of these changes included: (1) reduction in inflammation and fibrosis as evidenced by the decrease in inflammatory cells and in fibrotic marker expression (collagen and α-SMA) in the aliskiren-treated animals compared to the triolein only group and (2) decrease in and limited distribution of fat compared to triolein only.
These results parallel those from our earlier experiments using agents acting at different sites in the RAS. Both captopril, a converting enzyme inhibitor (ACE1) and losartan, an inhibitor of the angiotensin II type 1 receptor (AT1), greatly reduced the effects of FE found at 48 hrs.(6) The previous results with losartan indicate that activation of AT1 receptors is critical for the histopathological effects but does not indicate what biochemical pathway or location the angiotensin II originated. It also argues against a role of renin or prorenin acting on the prorenin receptor which has been reported to cause angiotensin-independent profibrotic effects.(14, 15) It does not rule out the catalytic effects of activated prorenin or renin bound to the prorenin receptor.(16)
Since the effects have also been shown to be ameliorated by captopril(6), the results indicate that ACE1 activity is important for the generation of angiotensin II. This seems to rule out or diminish any important role for chymase or other enzymes capable of forming angiotensin II from angiotensin I. However, the activity of chymase in rat lung is very low.(17)
The enzymatic step responsible for angiotensin I generation is presumably renin. Since aliskiren is a specific renin inhibitor, the present results help delineate an earlier biochemical step in the histopathological changes found after FE. The source of this renin could be one or more of several cells that have been found to produce renin in the lung and other extrarenal tissues. These include mast cells in the lung that have been associated with pathological changes,(18) myofibroblasts,(19) or macrophages.(20)
Based on these reports and our studies, a suggested sequence of events after fat embolization would be: fat entrapment in pulmonary capillaries, passage into the lungs, followed by phagocytosis by macrophages. Activated macrophages have been shown to release a mast cell stimulating factor that can act on mast cells outside the lung(21) and stimulated mast cells can also release renin locally(18). The renin generates angiotensin I that ultimately leads to angiotensin II after local ACE1 activity. As mentioned above, activated macrophages(20) and myofibolasts(19) are other sources of local angiotensin generation in pathological conditions.
Angiotensin II can then initiate its well-known inflammatory and profibrotic actions which produce pathological changes, resulting in minimal symptoms or in some cases ARDS. Resolution of these changes by interference in the RAS by aliskiren (and the other drugs that we have studied) is mediated by blockade of production or actions of angiotensin II. Furthermore, we have reported that there is an increase in angiotensin peptides found in the lungs of animals weeks after triolein administration. (12)
The question arises why there was a decreased amount of fat found at 48 hrs in rats treated with aliskiren. One possibility is an increase in lipolysis. Endothelial lipase (EL) has been shown to be important in the breakdown of triolein by the rat lung.(22) Angiotensin has been shown to upregulate EL expression in several tissues in rat, including the lungs.(23) Thus, it is possible that some of the disappearance and redistribution of fat in the lungs could have been due to increased lipolysis during the 48 hours after injection.
Another possibility is increased clearance of fat by opening of constricted blood vessels by aliskiren. The protective effect of aliskiren on renal artery vasoconstriction has been shown in a model of ischemia reperfusion injury.(24) However, there is evidence that the RAS is not directly involved in pulmonary vasoconstriction after embolization with latex microspheres.(25) This does not rule out the possibility that aliskiren may mitigate the vasoconstrictive effects of FE on pulmonary vessels; biochemical pathways may be altered by FE compared to physical emboli such as the release of the pulmonary toxicant oleic acid.(26)
Studies by others in different species and different models of pulmonary injury have also implicated the RAS,(27–29) and thus it appears that activation of this system is a common early feature before the later stages where cytokines and other mediators can exert deleterious effects on cell structure and metabolism.(11)
Since current treatment is quite limited to supportive measures and mortality figures for FES ranges up to 20%,(3) there is a need for more specific therapy. The clinical implications of our studies are three-fold: (1) aliskiren or other drugs acting on the RAS could be useful for acute treatment of FES after trauma, (2) these agents could also be useful as prophylactic treatment before surgical procedures that are known to predispose to FES, (3) agents that stabilize mast cells, such as cromolyn, could also be of use to interfere with the sequence of events that follow the escape of fat into the circulation. To investigate these possibilities, future studies will be directed at examining the potential prior steps in activation of the RAS by testing the protective effects of stabilizing mast cells with cromolyn, depleting mast cells with compound 48/80, or depleting macrophages with established models.(30) We will also examine the expression of pulmonary renin after acute exposure to triolein just as we have done 6 weeks after triolein.(31)
Aliskiren administered one hour after triolein produced essentially complete protection against several histopathological markers, strengthening the suggestion that this drug, already approved by the FDA for treating hypertension, could be a good candidate for a clinical trial. In conclusion, since it has been suggested that FE without FES may have long term consequences possibly leading to progression to pulmonary fibrosis or COPD,(12, 13) the use of aliskiren and related agents could protect against the development of other pulmonary pathology at a time remote from the initial injury.
Acknowledgments
This work was supported by the Mary Katherine Geldmacher Research Foundation, St. Louis, MO. It was also supported by a CTSA grant from NCRR and NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # TL1TR000120. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, NCRR, or NCATS.
Footnotes
Conflicts of interest: No conflicts of interest declared.
Presentations: This study was presented at Experimental Biology 2016, April 2–5, 2016, in San Diego, California; the 2016 annual meeting of the Association of Clinical and Translational Science, April 13–15, 2016, in Washington, DC; and the International Colloquium of Lung and Airway Fibrosis, September 24–28, 2016, in Dublin, Ireland.
Author Contribution:
Amanda Fletcher: literature search, study design, animal care, pathology methods, data collection, data analysis, data interpretation, writing, critical revision
Agostino Molteni: literature search, study design, animal care, pathology methods, data collection, data analysis, data interpretation, writing, critical revision
Rakesh Ponnapureddy: animal care, pathology methods, data collection, data analysis
Chirag Patel: animal care, pathology methods, data collection
Mark Pluym: data collection
Alan Poisner: literature search, study design, data collection, data analysis, data interpretation, writing, critical revision
Contributor Information
Amanda N. Fletcher, University of Kansas Medical Center; University of Missouri Kansas City, Department of Pathology
Agostino Molteni, University of Missouri Kansas City, Department of Pathology
Rakesh Ponnapureddy, University of Missouri Kansas City, Department of Internal Medicine
Chirag Patel, University of Missouri Kansas City, Department of Pathology
Mark Pluym, University of Missouri Kansas City, Department of Internal Medicine
Alan M. Poisner, University of Kansas Medical Center, Department of Pharmacology and Toxicology
References
- 1.Levy D. The fat embolism syndrome. A review. Clinical orthopaedics and related research. 1990;(261):281–6. [PubMed] [Google Scholar]
- 2.Eriksson EA, Pellegrini DC, Vanderkolk WE, Minshall CT, Fakhry SM, Cohle SD. Incidence of pulmonary fat embolism at autopsy: an undiagnosed epidemic. J Trauma. 2011;71(2):312–5. doi: 10.1097/TA.0b013e3182208280. [DOI] [PubMed] [Google Scholar]
- 3.Gupta B, D’Souza N, Sawhney C, Farooque K, Kumar A, Agrawal P, Misra MC. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–41. doi: 10.4103/0974-2700.83859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145–54. doi: 10.1046/j.1365-2044.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41–8. doi: 10.3928/0147-7447-19960101-09. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 6.McIff TE, Poisner AM, Herndon B, Lankachandra K, Molteni A, Adler F. Mitigating effects of captopril and losartan on lung histopathology in a rat model of fat embolism. J Trauma. 2011;70(5):1186–91. doi: 10.1097/TA.0b013e3181e50df6. [DOI] [PubMed] [Google Scholar]
- 7.Ajemba O, Zia H, Lankachandra K, Singh G, Poisner A, Herndon B, Molteni A. Fat embolism syndrome following caesarean section in an obese patient and its histopathological similarity to an animal model of FE: a case report. Case Report in Clinical Pathology. 2015;2(3):30–35. [Google Scholar]
- 8.McIff TE, Poisner AM, Herndon B, Lankachandra K, Schutt S, Haileselassie B, Patel S, Quinn T, Adler F, Molteni A. Fat embolism: evolution of histopathological changes in the rat lung. J Orthop Res. 2010;28(2):191–7. doi: 10.1002/jor.20963. [DOI] [PubMed] [Google Scholar]
- 9.Marchionne EM, Diamond-Stanic MK, Prasonnarong M, Henriksen EJ. Chronic renin inhibition with aliskiren improves glucose tolerance, insulin sensitivity, and skeletal muscle glucose transport activity in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R137–42. doi: 10.1152/ajpregu.00448.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel RB, Pawar VD, Prajapati KD, Sonara BM, Deshpande SS, Shah GB, Jain MR. Anti-nociceptive and anti-allodynic activity of aliskiren in various pain models. Eur J Pharmacol. 2013;708(1–3):80–7. doi: 10.1016/j.ejphar.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Molteni A, Wolfe LF, Ward WF, Ts’ao CH, Molteni LB, Veno P, Fish BL, Taylor JM, Quintanilla N, Herndon B, et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13(13):1307–16. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 12.Poisner AM, Adler F, Uhal B, McIff TE, Schroeppel JP, Mehrer A, Herndon BL, Lankachandra KM, Molteni A. Persistent and progressive pulmonary fibrotic changes in a model of fat embolism. J Trauma Acute Care Surg. 2012;72(4):992–8. doi: 10.1097/TA.0b013e31823c96b0. [DOI] [PubMed] [Google Scholar]
- 13.Poisner A, Herndon B, Lankachandra K, Likhitsup A, Al Hariri A, Kesh S, Molteni A. Fat embolism sensitizes rats to a “second hit” with lipopolysaccharide: An animal model of pulmonary fibrosis. J Trauma Acute Care Surg. 2015;78(3):552–7. doi: 10.1097/TA.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 14.He M, Zhang L, Shao Y, Wang X, Huang Y, Yao T, Lu L. Inhibition of renin/prorenin receptor attenuated mesangial cell proliferation and reduced associated fibrotic factor release. Eur J Pharmacol. 2009;606(1–3):155–61. doi: 10.1016/j.ejphar.2008.12.050. [DOI] [PubMed] [Google Scholar]
- 15.Melnyk RA, Tam J, Boie Y, Kennedy BP, Percival MD. Renin and prorenin activate pathways implicated in organ damage in human mesangial cells independent of angiotensin II production. Am J Nephrol. 2009;30(3):232–43. doi: 10.1159/000220260. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen G, Danser AH. The (pro)renin receptor: therapeutic consequences. Expert Opin Investig Drugs. 2006;15(10):1131–5. doi: 10.1517/13543784.15.10.1131. [DOI] [PubMed] [Google Scholar]
- 17.Akasu M, Urata H, Kinoshita A, Sasaguri M, Ideishi M, Arakawa K. Differences in tissue angiotensin II-forming pathways by species and organs in vitro. Hypertension. 1998;32(3):514–20. doi: 10.1161/01.hyp.32.3.514. [DOI] [PubMed] [Google Scholar]
- 18.Veerappan A, Reid AC, Estephan R, O’Connor N, Thadani-Mulero M, Salazar-Rodriguez M, Levi R, Silver RB. Mast cell renin and a local renin-angiotensin system in the airway: role in bronchoconstriction. Proc Natl Acad Sci U S A. 2008;105(4):1315–20. doi: 10.1073/pnas.0709739105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber KT, Sun Y, Katwa LC. Myofibroblasts and local angiotensin II in rat cardiac tissue repair. The international journal of biochemistry & cell biology. 1997;29(1):31–42. doi: 10.1016/s1357-2725(96)00116-1. [DOI] [PubMed] [Google Scholar]
- 20.Dezso B, Nielsen AH, Poulsen K. Identification of renin in resident alveolar macrophages and monocytes: HPLC and immunohistochemical study. J Cell Sci. 1988;91(Pt 1):155–9. doi: 10.1242/jcs.91.1.155. [DOI] [PubMed] [Google Scholar]
- 21.Chao J, Blanco G, Wood JG, Gonzalez NC. Renin released from mast cells activated by circulating MCP-1 initiates the microvascular phase of the systemic inflammation of alveolar hypoxia. Am J Physiol Heart Circ Physiol. 2011;301(6):H2264–70. doi: 10.1152/ajpheart.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gal S, Bassett DJ, Hamosh M, Hamosh P. Triacylglycerol hyrolysis in the isolated, perfused rat lung. Biochim Biophys Acta. 1982;713(2):222–9. doi: 10.1016/0005-2760(82)90239-9. [DOI] [PubMed] [Google Scholar]
- 23.Shimokawa Y, Hirata K, Ishida T, Kojima Y, Inoue N, Quertermous T, Yokoyama M. Increased expression of endothelial lipase in rat models of hypertension. Cardiovascular research. 2005;66(3):594–600. doi: 10.1016/j.cardiores.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Hammad FT, Al-Salam S, Lubbad L. Does aliskiren protect the kidney following ischemia reperfusion injury? Physiol Res. 2013;62(6):681–90. doi: 10.33549/physiolres.932485. [DOI] [PubMed] [Google Scholar]
- 25.Riegger GA, Hoferer P. A new experimental model for measurement of pulmonary arterial haemodynamic variables in conscious rats before and after pulmonary embolism and during general anaesthesia. Cardiovasc Res. 1990;24(4):340–4. doi: 10.1093/cvr/24.4.340. [DOI] [PubMed] [Google Scholar]
- 26.Nakata Y, Tanaka H, Kuwagata Y, Yoshioka T, Sugimoto H. Triolein-induced pulmonary embolization and increased microvascular permeability in isolated perfused rat lungs. J Trauma. 1999;47(1):111–9. doi: 10.1097/00005373-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Zakheim RM, Mattioli L, Molteni A, Mullis KB, Bartley J. Prevention of pulmonary vascular changes of chronic alveolar hypoxia by inhibition of angiotensin I-converting enzyme in the rat. Lab Invest. 1975;33(1):57–61. [PubMed] [Google Scholar]
- 28.Molteni A, Ward WF, Ts’ao CH, Solliday NH, Dunne M. Monocrotaline-induced pulmonary fibrosis in rats: amelioration by captopril and penicillamine. Proc Soc Exp Biol Med. 1985;180(1):112–20. doi: 10.3181/00379727-180-42151. [DOI] [PubMed] [Google Scholar]
- 29.Ward WF, Molteni A, Ts’ao CH. Radiation-induced endothelial dysfunction and fibrosis in rat lung: modification by the angiotensin converting enzyme inhibitor CL242817. Radiation Res. 1989;117(2):342–50. [PubMed] [Google Scholar]
- 30.Berg JT, White JE, Tsan MF. Response of alveolar macrophage-depleted rats to hyperoxia. Exp Lung Res. 1995;21(1):175–85. doi: 10.3109/01902149509031752. [DOI] [PubMed] [Google Scholar]
- 31.Poisner A, Herndon B, Al Hariri A, Qin C, Quinn T, Molteni A. Renin as a mediator of pulmonary damage caused by fat embolism and LPS. FASEB J. 2013;27(1_MeetingAbstracts):lb444. [Google Scholar]