Abstract
Purpose
To determine rate of upgrading to Gleason score (GS) ≥ 4+3, using targeted biopsy for diagnosis and monitoring, in men undergoing active surveillance (AS) of prostate cancer (CaP).
Materials and Methods
Subjects were all 259 men (196 with GS 3+3 and 63 with GS 3+4) who were diagnosed by MRI/US fusion-guided biopsy (2009–2015) and who underwent subsequent fusion biopsy for as long as 4 years of AS. Primary endpoint was discovery of GS ≥ 4+3 CaP. Follow-up biopsies included targeting of positive sites, which were tracked in an Artemis device. Kaplan-Meier curves were generated to determine rates of upgrading, stratified by initial GS and PSA density.
Results
Based on a Cox proportional hazard model, men with GS 3+4 were 4.65 more likely to have upgrading than men with initial GS 3+3 (3yr, p < 0.01). 63% of men with GS 3+4 had upgraded by the third surveillance year, compared with 18.0% of men starting with GS 3+3 (p < 0.01). 97% of all upgrades (32/33) occurred within an MRI-visible or a tracked site of tumor, rather than a previously-negative systematic site. Independent predictors of upgrading were GS 3+4, PSA density ≥ 0.15 ng/ml/cm3, and a grade 5 lesion on MRI. The incidence-rate ratio of upgrading (GS 3+4 vs. GS 3+3) was 4.25 per year of patient follow-up (p < 0.01).
Conclusions
During AS of CaP, targeting of tracked tumor foci by MRI/US fusion biopsy allows heightened detection of GS ≥ 4+3 cancers. Baseline variables directly related to important upgrading and warranting increased vigilance include GS 3+4, PSA density ≥ 0.15, and grade 5 lesions on MRI.
Keywords: prostate, prostate cancer, magnetic resonance imaging, ultrasonography, image-guided biopsy
Introduction
Biopsy criteria for active surveillance (AS) vary from one program to another1. In the original Epstein criteria, AS was offered only for men with small Gleason score (GS) 3+3 prostate cancers (CaP)2, but in various programs, the criteria now include men with more extensive GS 3+3 lesions and even some with GS 3+41,3,4. In the latter men (GS 3+4, intermediate risk), pathologic upgrading has been observed more often than in men with low risk lesions (GS 3+3)1. In virtually all AS programs, GS ≥ 4+3 would be considered exclusionary and would ordinarily, if detected during the surveillance period, trigger active intervention. Biopsy GS is the most important factor for both initial selection and continued eligibility of AS4.
However, in most AS programs, biopsy has been performed using conventional ultrasound (US) guidance. In 2009, an AS registry at UCLA was initiated using MRI-guided biopsy5,6 in all participants at baseline and during follow-up. MRI-guided biopsy more accurately characterizes whole-organ pathology than conventional US-guided biopsy7–9. In previous studies, we and others have found that MRI-guided biopsy including target and template samples10, helps to detect clinically significant disease (csCaP) in men being screened for AS11,12.
In the present study, we sought to determine continued AS eligibility for men entering the registry with low (GS 3+3) vs. intermediate risk (GS 3+4) lesions. MRI guidance was used for all biopsies and appearance of GS ≥ 4+3 was used as a clear point of AS termination. The main finding—that the subseqent detection of potentially aggressive disease is much more common in men starting AS with a Gleason 4 component than in men without it—may have important implications for men with such lesions considering AS.
Methods
STUDY COHORT
The study design is shown in Figure 1. Subjects were all men with clinical stage T1c and an initial diagnosis of GS 3+3 or 3+4 CaP—i.e. low or intermediate risk by D’Amico histological criteria13—made by fusion biopsy who entered AS at UCLA (2009–2015) and received one or more follow-up fusion biopsies within one to four years. Serum PSA level and digital rectal examination were obtained every six months; a fusion biopsy was performed within one year after the confirmatory biopsy; thereafter, fusion biopsy with repeat multiparametric MRI (mpMRI) was performed at 2-year intervals. All patients who had not been seen for biopsy in the previous 15 months (individuals possibly lost to follow-up) were contacted by phone to verify that definitive treatment had not occurred outside the study. Pathologic upgrading, the primary outcome, was defined as appearance of Gleason primary pattern ≥ 4 or secondary pattern 5 on any follow-up surveillance biopsy. All data collection was performed within a UCLA IRB-approved registry.
Figure 1.
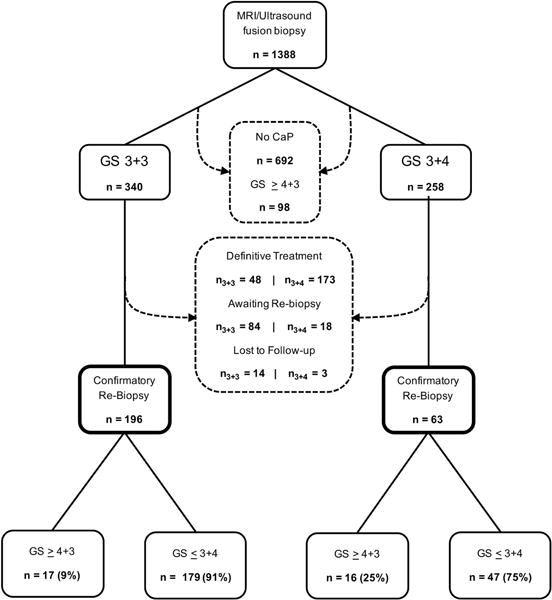
Study design and flow of patients. All patients undergoing MRI/ultrasound fusion biopsy from September 2009–November 2015 were screened for inclusion. Patients excluded from the study and the reason for their exclusion are identified by dashed arrows and text boxes. Upgrading to GS 3+5 was found in 5/17 men who started with GS 3+3 and in 3/16 men who started with GS 3+4. All other upgrades were to GS ≥ 4+3.
MRI/ULTRASOUND-GUIDED TARGETED BIOPSY
The fusion biopsy method, which has been previously described, was unchanged throughout the study period5,14. Briefly, within 2 months of biopsy, patients underwent a 3T mpMRI (body coil). MRI interpretation was conducted under the direction of a dedicated uro-radiologist (DJM), and regions of interest (ROI) were graded according to UCLA and Prostate Imaging-Reporting and Data System criteria5,15. MRI grading was based on the UCLA scoring system5, which pre-dates PI-RADS-1, and after PI-RADS-2 was established, by both systems using highest grade found. At biopsy, images were registered and fused with real-time transrectal US (Noblus, Hitachi Aloka, Wallingford, CT) to generate a 3-dimensional image of the prostate with delineated ROIs (targets).
Template and target samples were taken by a single urologist (LSM) at UCLA Clark Urology Center under local anesthesia using the Artemis device (Eigen; Grass Valley, CA)5,14. Intra-prostatic location of biopsy cores with CaP were tracked and specifically re-sampled on follow-up biopsies as described previously16. A dedicated uro-pathologist interpreted all biopsy cores (JH).
STATISTICAL ANALYSIS
Kaplan-Meier curves were generated to determine time to appearance of GS ≥ 4+3 in years from time zero, defined as the time of initial fusion biopsy showing cancer. Men who withdrew from AS, were lost to follow-up, or who died while on AS were censored at the date of the last surveillance visit. Follow-up is reported in years between time zero and the time to patient censoring or reclassification. Log-rank test was used to determine whether the probability of upgrading between GS 3+3 and 3+4 groups was statistically significant. Cox proportional hazard analysis was used for all other time-dependent co-variates to identify independent predictors of histological reclassification. Statistical significance was considered at p < 0.05 for all analyses. Statistical analyses were performed by a co-author (FJD) using Stata® software, version 13.1.
Results
Of 1388 men undergoing MRI/US fusion biopsy during the study period, 340 were diagnosed with GS 3+3 and 268 with GS 3+4 CaP. In the GS 3+3 arm, 196 (57.6%) entered AS, 48 (14.1%) were definitively treated, and 14 (4.1%) were lost to follow-up. In the GS 3+4 arm, 63 (23.5%) entered AS, 173 (67.1%) were treated, and 3 (1.2%) were lost to follow-up. For individual men, guideines for choosing active treatment or entering AS was not structured, except that Gleason primary pattern ≥ 4 or secondary pattern 5 was exclusionary for AS. Baseline characteristics for men who entered AS are shown in Table 1.
Table 1.
Patient characteristics at baseline.
| GS 3+3 (n = 196) |
GS 3+4 (n = 63) |
p | |
|---|---|---|---|
| Age at Diagnostic Biopsy | NS | ||
| Mean ± S.D. | 63.2 ± 7.6 | 64.5 ± 6.9 | |
| Ethnicity, n (%) | NS | ||
| Caucasian | 167 (85.2 %) | 47, (74.6 %) | |
| African American | 9 (4.6 %) | 3 (4.8 %) | |
| Latin American | 10 (5.1 %) | 7 (11.1 %) | |
| Asian American | 9 (4.6 %) | 5 (7.9 %) | |
| Other | 1 (0.5 %) | 1 (1.6 %) | |
| Total serum PSA (ng/mL) | < 0.01 | ||
| Median ± S.D. | 4.5 ± 3.0 | 6.0 ± 4.4 | |
| Range | 0.1–15 | 0.1–19.4 | |
| Percentage Free PSA | < 0.01 | ||
| Mean ± S.D. | 18.8 ± 8.1 | 15.0 ± 8.4 | |
| Median, Range | 18, 0.4–46 | 14, 3–43 | |
| PSA Density (ng/mL/cm3) | < 0.01 | ||
| Mean ± S.D. | 0.10 ± 0.07 | 0.16 ± 0.12 | |
| Range | 0.002–0.49 | 0.006–0.53 | |
| MRI Grade, n (%) | NS | ||
| 0–2 (negative) | 43 (22.0 %) | 12 (19.0 %) | |
| 3 (intermediate suspicion) | 96 (49.0 %) | 31 (49.2 %) | |
| 4 (high suspicion) | 50 (25.5 %) | 15 (23.8 %) | |
| 5 (very high suspicion) | 7 (3.5 %) | 5 (8.0 %) | |
| Maximum Cancer Core Length (mm) | < 0.01 | ||
| Mean ± S.D. | 2.1 ± 1.8 | 3.0 ± 1.9 | |
| Range | 0.1–8.0 | 0.2–9.0 | |
| Percent tumor involvement (%) | < 0.01 | ||
| Mean ± S.D. | 16.4 ± 18.7 | 25.1 ± 17.8 | |
| Range | 1–95 | 2–70 | |
| Number of positive cores | < 0.01 | ||
| Mean ± S.D. | 1.7 ± 1.2 | 2.2 ± 1.1 | |
| Range | 1–9 | 1–5 |
Overall, pathological upgrading to GS ≥ 4+3 was found in 17/196 men initially presenting with GS 3+3 (8.7%) and 16/63 initially presenting with GS 3+4 (25.4%) (p < 0.01). Annual reclassification rate was 5.5% (17 upgrades over 305.2 years of patient follow-up) for GS 3+3 and 23.4% (16 upgrades over 68.2 years of patient follow-up) for GS 3+4 (p < 0.01). The incidence-rate ratio of upgrading for GS 3+4 vs. GS 3+3 was 4.25 per year of patient follow-up (p < 0.01). Table 2 shows the distribution of tumor locations (within a ROI vs. template) at baseline and at time of upgrading. Overall, 94% of upgrades in GS 3+3 patients (n = 16/17) and all upgrades in GS 3+4 patients (n = 16) occurred within an MRI-defined ROI or a prior positive tracked site. Of tracked site upgrades, none were found within the same quadrant as the MRI-visible target. An example of MRI-guided biopsy and tracking of positive sites is shown in Figure 2.
Table 2.
Distribution of tumor locations at baseline and at time of histological progression.
| Baseline tumor (n = 259) | Upgraded tumor (n = 33) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| ROI | Template | Both | ROI | Tracked* | Template | |
|
| ||||||
| Gleason score 3+3 | 39 | 124 | 33 | 12 | 4 | 1 |
| Gleason score 3+4 | 15 | 29 | 19 | 9 | 7 | 0 |
Tracked sites refers to positive biopsy sites outside of an MRI-identified target. Of the 11 men who upgraded, MRI showed no target in 4, a Grade 3 lesion in 6, and a Grade 4 lesion in 1. In all 11, the tracked biopsy site was outside of an MRI visible target.
Figure 2.
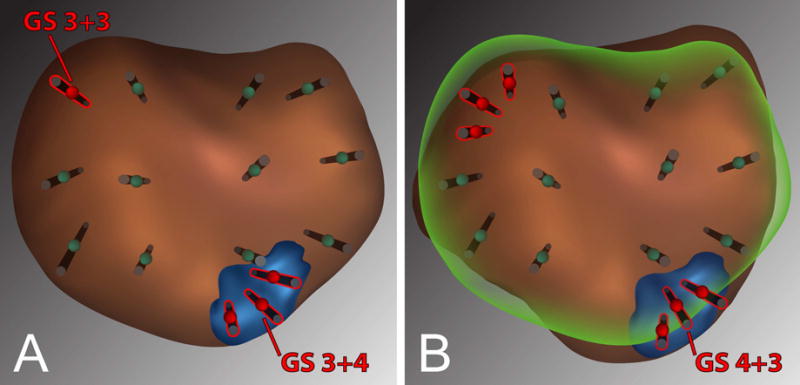
Example patient showing prostate images and 12-point template from Artemis device at time of initial MRI/ultrasound fusion biopsy (A) and at follow-up biopsy (B). Biopsy protocol followed throughout the study includes sampling of MRI-visible targets with cores obtained every 3 mm of the longest axis and sampling of tracked sites by triangulating with 3–5 cores10.
In the example (A) GS 3+4 tumor was found upon target biopsy of an MRI-identified ROI (blue), and GS 3+3 tumor was found in a systematic point from the template. At follow-up, (B) co-registration of prior imaging is performed (green outline), enabling tracking of prior positive sites. On follow-up, targeted biopsies showed GS 4+3 tumor in the ROI and no tumor in the prior GS 3+3 site.
For 32 of 33 men in this series, when upgrading was found it was in a previously positive site, either within a ROI or in a tracked site outside a ROI (Table 2).
A univariate analysis of baseline characteristics of the two groups is shown in Table 3, with upgrading as the primary outcome. Based on a Cox proportional hazards model, men with GS 3+4 were 4.65 times more likely to have upgrading than men with GS 3+3 (HR 2.4, p < 0.01). Other biopsy characteristics, such as maximum cancer core length ≥ 4 mm, maximum percentage of tumor involvement ≥ 60%, and number of positive biopsy cores > 3 were not significantly associated with an increased reclassification risk. Total serum PSA ≥ 10 ng/ml was associated with an approximately 2-fold increase in reclassification risk (p = NS). Men with percentage free PSA ≤ 10 and PSAD ≥ 0.15 were 3.75 (p < 0.01) and 3.43 (p < 0.01) times more likely to undergo pathological upgrading in AS compared to men with more favorable values.
Table 3.
Univariate analysis of baseline clinical and histopathologic variables.
| Rate of Upgrading per year of follow-up (95% CI) | HR (95% CI) | p | |
|---|---|---|---|
| Gleason score | |||
| 3+3 | 0.06 (0.03–0.09) | ||
| 3+4 | 0.23 (0.14–0.38) | 4.65 (2.32–9.34) | < 0.01 |
|
| |||
| PSA | |||
| <10 ng/mL | 0.08 (0.06–0.12) | ||
| ≥10 ng/mL | 0.15 (0.06–0.41) | 1.97 (0.69–5.64) | NS |
|
| |||
| PSA % free | |||
| >10 % | 0.06 (0.04–0.10) | ||
| ≤10% | 0.22 (0.13–0.39) | 3.75 (1.85–7.60) | < 0.01 |
|
| |||
| PSA density | |||
| < 0.15 ng/mL/cm3 | 0.06 (0.04–0.10) | ||
| ≥ 0.15 ng/mL/cm3 | 0.20 (0.12–0.35) | 3.43 (1.68–7.05) | < 0.01 |
|
| |||
| MRI grade | |||
| < 3 (referent) | 0.08 (0.03–0.17) | ||
| 3, 4, or 5 | 0.09 (0.06–0.13) | 1.23 (0.51–2.98) | NS |
| 5 | 0.35 (0.13–0.94) | 5.16 (1.79–14.87) | < 0.01 |
|
| |||
| Maximum cancer core length | |||
| <4 mm | 0.08 (0.05–0.12) | ||
| ≥4 mm | 0.12 (0.06–0.24) | 1.53 (0.71–3.30) | NS |
|
| |||
| Maximum % tumor involvement in any core | |||
| < 60% | 0.09 (0.06–0.13) | ||
| ≥60% | 0.05 (0.01–0.34) | 1.99 (0.27–14.59) | NS |
|
| |||
| Number of positive cores | |||
| ≤ 3 | 0.08 (0.06–0.11) | ||
| > 3 | 0.17 (0.06–0.44) | 2.26 (0.78–6.54) | NS |
Baseline mpMRI did not predict risk of reclassification, except in the 12 men with a grade 5 lesion, in whom risk of future reclassification was great (HR 5.16, p < 0.01). All men with grade 5 ROIs were reclassified within 2.5 years. Of GS 3+3 patients with a grade 5 ROI (n=7), only one remained stable in AS beyond two years. All five GS 3+4 patients with a baseline grade 5 ROI had shown upgrading by one year. Annual rate of upgrading for all other MRI grades combined (0–4) was approximately 8% without evidence of risk stratification by grade.
Kaplan-Meier estimates of the probability of upgrading over three years was determined (Figure 3). 63.0% of GS 3+4 patients were reclassified by the third surveillance year, compared to 18.0% of GS 3+3 patients (p < 0.01, log rank test) (Figure 3A). The annual hazard ratio was 5.23, 4.12, and 4.65 by the first, second, and third surveillance years (p < 0.01). The combination of GS and baseline PSAD was further analyzed (Figure 3B). Patients with the least favorable clinical-histological profile (GS 3+4 and PSAD ≥ 0.15) had the greatest incidence of upgrading; 33% had upgraded to GS ≥ 4+3 by 1.3 years. Among men with GS 3+4 CaP and PSAD < 0.15, 34% had upgraded by the third year. In the group with lowest risk, men with GS 3+3 and PSAD < 0.15, only 15% of had upgraded by the third surveillance year. Men in the two groups had similar numbers of total biopsy cores (mean 14.3 vs. 14.1, p = NS) and targeted cores (mean 4.3 vs. 4.4, p = NS) taken at each session. Survival functions and 95% confidence intervals (CI) are shown in Tables 4 and 5.
Figure 3.
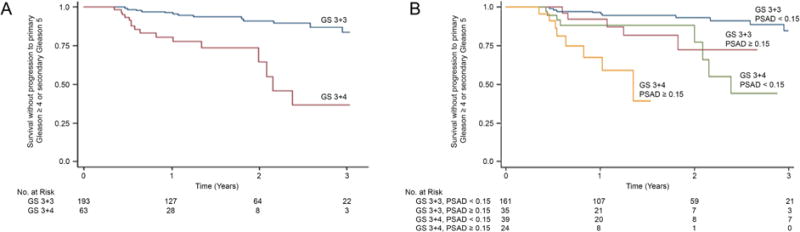
(A) Kaplan-Meier curves showing survival without histological upgrading in men starting AS with Gleason score 3+3 or 3+4. (B) Kaplan-Meier curves stratified by both initial GS and PSAD. Average number of follow-up biopsy sessions per patient was the same in both GS 3+3 and 3+4 groups (1.48 vs. 1.42); men entering AS with GS 3+4 were re-biopsied 3 months earlier than men entering with GS 3+3 (9 vs. 12 months).
Table 4.
Kaplan-Meier survivor functions by GS.
| Group | Time (months) | Number at Risk (n) | Survivor Function | 95% CI |
|---|---|---|---|---|
| GS 3+3 | 0 | 193 | 1.00 ± 0 | 0.00 |
| 12 | 127 | 0.96 ± 0.02 | 0.91–0.98 | |
| 24 | 64 | 0.90 ± 0.03 | 0.83–0.94 | |
| 36 | 22 | 0.82 ± 0.05 | 0.70–0.90 | |
|
| ||||
| GS 3+4 | 0 | 63 | 1.00 ± 0 | 0.00 |
| 12 | 28 | 0.78 ± 0.06 | 0.63–0.87 | |
| 24 | 8 | 0.64 ± 0.10 | 0.40–0.81 | |
| 36 | 3 | 0.37 ± 0.13 | 0.13–0.61 | |
Table 5.
Kaplan-Meier survivor functions by GS and PSAD (ng/ml/cm3).
| Group | Time (months) | Number at Risk (n) | Survivor Function | 95% CI |
|---|---|---|---|---|
| 0 | 161 | 1.00 ± 0 | 0.00 | |
| GS 3+3 | 12 | 107 | 0.97 ± 0.02 | 0.92–0.99 |
| PSAD < 0.15 | 24 | 59 | 0.93 ± 0.02 | 0.86–0.97 |
| 36 | 21 | 0.85 ± 0.05 | 0.71–0.93 | |
|
| ||||
| 0 | 35 | 1.00 ± 0 | 0.00 | |
| GS 3+3 | 12 | 21 | 0.92 ± 0.05 | 0.73–0.98 |
| PSAD ≥ 0.15 | 24 | 7 | 0.73 ± 0.11 | 0.43–0.89 |
| 36 | 3 | 0.73 ± 0.11 | 0.43–0.89 | |
|
| ||||
| 0 | 39 | 1.00 ± 0 | 0.00 | |
| GS 3+4 | 12 | 20 | 0.88 ± 0.06 | 0.72–0.95 |
| PSAD < 0.15 | 24 | 8 | 0.77 ± 0.11 | 0.45–0.92 |
| 36 | 7 | 0.66 ± 0.14 | 0.32–0.86 | |
|
| ||||
| 0 | 24 | 1.00 ± 0 | 0.00 | |
| GS 3+4 | 12 | 8 | 0.59 ± 0.13 | 0.31–0.79 |
| PSAD ≥ 0.15 | 24 | 1 | 0.39 ± 0.18 | 0.09–0.70 |
A stepwise multivariate analysis of the strongest predictors of reclassification (Table 6) confirmed that GS 3+4 on initial fusion biopsy, a grade 5 ROI on mpMRI, and PSAD ≥ 0.15 were significant independent predictors of future reclassification risk (p < 0.005). Total PSA level and percent free PSA did not have a statistically significant association with reclassification risk in the multivariate analysis.
Table 6.
Multivariable comparison of GS 3+4 vs. 3+3, adjusted for PSA, % free PSA, and MRI grade over the length of the study.
| HR | 95% CI | p | |
|---|---|---|---|
| Gleason score 3+4 | 4.58 | 2.14–9.80 | < 0.01 |
| Grade 5 on mpMRI | 5.06 | 1.65–15.52 | < 0.01 |
| PSA density ≥ 0.15 ng/mL/cm3 | 2.38 | 1.01–5.60 | < 0.05 |
| PSA % free ≤ 10 % | 2.15 | 0.92–5.01 | NS |
Discussion
The present study of AS differs from others in that all men were initially diagnosed by MRI/US fusion biopsy and all were followed with serial fusion biopsies. The central findings are (1) that men with GS 3+4 who enter AS had more than four times the likelihood of later showing potentially aggressive disease (GS ≥ 4+3) than men entering AS with GS3+3; and (2) that PSA density (PSAD) and a grade 5 ROI on mpMRI were important predictors of subsequent upgrading. Of 24 men entering AS with the combination of both GS3+4 and PSAD ≥ 0.15, 8 were found upon subsquent fusion biopsy to have upgraded within 1.3 years of follow-up. In addition, all 12 men with a grade 5 ROI on mpMRI were likewise upgraded within the study period, regardless of initial GS.
The discovery of primary GS ≥ 4 or secondary GS5 was used as the main outcome of interest, because such lesions carry substantial metastatic potential17–19. Crossing the threshold from primary pattern three, where immediate risk appears to be limited20, to primary four or secondary pattern five, where risk is appreciable, thus was used here as a discrete point for discontinuance of AS. The increased sensitivity of MRI-guided biopsy for detection of clinically significant prostate cancer (csCaP), widely reported for other situations7–9,21, also appears valuable when the new modality is employed for men in AS. 97% of upgrades (32/33) were detected only by targeted biopsy of an MRI-defined ROI (n = 21) or a tracked site (n = 11). Both targeted and template biopsies were obtained in all patients at baseline because of the possibility of csCaP outside of an MRI visible target10.
In large, well-established AS programs, the rate of patient discontinuance, which includes a variety of reasons in addition to upgrading, ranges from 24–40% over 5 years of follow-up22–24. In these studies most men entered with GS3+3. However, among men entering with elements of Gleason pattern 4 in programs at Sunnybrook and Royal Marsden and diagnosed with conventional biopsy, the risk of progressive disease was found to be increased23,25. In fact, a recent report documents the possibility of metastatic disease in men entering AS with secondary Gleason pattern 426. The present data confirm and quantify that GS-related risk using MRI-guided biopsies (Kaplan-Meier curves): upgrading to unfavorable pathology over 3 years was found in 63% of men entering with GS3+4 vs. 18% of men entering with GS3+3 (p<0.01). Risk of upgrading may be further stratified by factoring in PSA density (Figure 3B), an important variable first identified more than 20 years ago by Epstein et al.2.
According to the modified Epstein criteria, men with GS 3+3 lesions are acceptable for AS if no more than 3 positive cores are present and no core contains more than 60% tumor involvement27. However, in the present study many men whose pathology exceeded those strict criteria remained stable for up to four years of surveillance. Of the 11 men with initial GS 3+3 and greater than 60% core involvement, only 1 progressed to GS ≥ 4+3. Through increased detection and exclusion of high-grade cancer at baseline, the new biopsy method may help explain the relatively limited amount of ugrading from GS3+3 seen in this study during AS follow-up.
Limitations of the present study include the retrospective nature of data analysis, though data were collected prospectively in an IRB-approved registry. Not all eligible patients were followed for the entirety of the study period because of various interventions; few (n = 2) were lost to follow-up during the course of the study. These patients were censored at the time of their last surveillance biopsy. The length of GS4 components on biopsy cores (mm) was not recorded initially and could be a factor in outcomes. Follow-up was relatively short, but outcomes were clear by the end of the third year and longer follow-up would not have materially influenced near-term group differences. Numbers were relatively small, but the number of men entering AS with GS3+4 (N=63) compared favorably with other available reports. Cost-effectiveness has not yet been established. Biopsies and MRI interpretations were performed by individuals with considerable experience, perhaps limiting how much these data can be generalized to less experienced personnel. However, the difference in outcomes between the two matched groups is dramatic and unlikely to disappear in a study lacking the above limitations.
The data presented herein should not be used to establish criteria for entry into AS or to set rules of termination. However, the present findings do suggest (1) the importance of continued vigilance in follow-up, especially of GS3+4 lesions; (2) and the advantage of MRI-guided biopsy in detecting potentially aggressive disease. The AS protocol outlined here includes initial diagnostic biopsy, confirmatory biopsy within a year, a follow-up biopsy a year later, and then a biopsy every two years thereafter, all employing MRI guidance and biopsy-site tracking. With continued evolution of AS, such protocols will likely change in the future, but the utility of MRI-guided biopsy in any management strategy appears to offer substantial advantage over blind biopsy.
Conclusions
Men entering AS with GS 3+4 lesions have a much greater chance of subsequent upgrading to GS ≥ 4+3 than men entering with GS 3+3 (63% vs 18% at 3 years). Addition of PSA density helps to stratify chances of upgrading. One-third of all 24 patients with GS 3+4 and PSAD ≥ 0.15 were upgraded by 1.3 years of surveillance. Use of MRI-guided fusion biopsy, especially the tracking utility to re-sample prior positive sites, appears to provide added value in detection of potentially aggressive cancer in men undergoing AS.
Acknowledgments
Supported by Award R01CA158627 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Supported by UCLA Clinical and Translational Sciences Institute Grant No. UL1TR000124, the Beckman Coulter Foundation, the Jean Perkins Foundation, the Andre Agassi Foundation and the Steven C. Gordon Family Foundation. Study received UCLA institutional review board approval at the outset (IRB#11-001580-AM-00014).
Abbreviations and Acronyms
- AS
active surveillance
- CaP
prostate cancer
- CI
confidence interval
- GS
Gleason score
- mpMRI
multiparametric MRI
- PSA
prostate specific antigen
- PSAD
prostate specific antigen density (serum PSA level, ng/ml)/prostate volume, cc determined on mpMRI)
- ROI(s)
region(s) of interest defined by mpMRI (grade 3,4, or 5 by UCLA and PI-RADS criteria)
- US
ultrasound
- HR
hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tosoian JJ, Carter HB, Lepor A, et al. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol. 2016 doi: 10.1038/nrurol.2016.45. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26954332, accessed March 12, 2016. [DOI] [PMC free article] [PubMed]
- 2.Epstein JI. Pathologic and Clinical Findings to Predict Tumor Extent of Nonpalpable (Stage T1 c) Prostate Cancer. JAMA J Am Med Assoc. 1994;271:368. Available at: http://jama.jamanetwork.com/article.aspx?articleid=363820, accessed January 23, 2016. [PubMed] [Google Scholar]
- 3.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23541457, accessed March 15, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26733552, accessed April 6, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Natarajan S, Marks LS, Margolis DJA, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29:334–42. doi: 10.1016/j.urolonc.2011.02.014. Available at: http://www.sciencedirect.com/science/article/pii/S107814391100072X, accessed March 30, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonn GA, Natarajan S, Margolis DJA, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86–91. doi: 10.1016/j.juro.2012.08.095. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3561472&tool=pmcentrez&rendertype=abstract, accessed November 5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7. doi: 10.1001/jama.2014.17942. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4572575&tool=pmcentrez&rendertype=abstract, accessed September 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le JD, Stephenson S, Brugger M, et al. Magnetic resonance imaging-ultrasound fusion biopsy for prediction of final prostate pathology. J Urol. 2014;192:1367–73. doi: 10.1016/j.juro.2014.04.094. Available at: http://www.sciencedirect.com/science/article/pii/S0022534714035095, accessed January 25, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radtke JP, Schwab C, Wolf MB, et al. Multiparametric Magnetic Resonance Imaging (MRI) and MRI-Transrectal Ultrasound Fusion Biopsy for Index Tumor Detection: Correlation with Radical Prostatectomy Specimen. Eur Urol. 2016 doi: 10.1016/j.eururo.2015.12.052. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26810346, accessed February 4, 2016. [DOI] [PubMed]
- 10.Filson CP, Natarajan S, Margolis DJA, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016 doi: 10.1002/cncr.29874. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26749141, accessed January 12, 2016. [DOI] [PMC free article] [PubMed]
- 11.Hu JC, Chang E, Natarajan S, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol. 2014;192:385–90. doi: 10.1016/j.juro.2014.02.005. Available at: http://www.sciencedirect.com/science/article/pii/S002253471400250X, accessed January 23, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarpato KR, Barocas DA. Use of mpMRI in active surveillance for localized prostate cancer. Urol Oncol. 2016 doi: 10.1016/j.urolonc.2016.02.020. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27036218, accessed April 11, 2016. [DOI] [PubMed]
- 13.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9749478, accessed January 19, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–15. doi: 10.1016/j.eururo.2013.03.025. Available at: http://www.sciencedirect.com/science/article/pii/S0302283813002492, accessed October 17, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging -Reporting and Data System: 2015, Version 2. Eur Urol. 2015;69:16–40. doi: 10.1016/j.eururo.2015.08.052. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26427566, accessed October 20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonn GA, Filson CP, Chang E, et al. Initial experience with electronic tracking of specific tumor sites in men undergoing active surveillance of prostate cancer. Urol Oncol. 2014;32:952–7. doi: 10.1016/j.urolonc.2014.04.003. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4254112&tool=pmcentrez&rendertype=abstract, accessed April 6, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeal JE, Villers AA, Redwine EA, et al. Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer. 1990;66:1225–33. doi: 10.1002/1097-0142(19900915)66:6<1225::aid-cncr2820660624>3.0.co;2-x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2400973, accessed March 16, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Stamey TA. Biological Determinants of Cancer Progression in Men With Prostate Cancer. JAMA. 1999;281:1395. doi: 10.1001/jama.281.15.1395. Available at: http://jama.jamanetwork.com/article.aspx?articleid=189523, accessed April 6, 2016. [DOI] [PubMed] [Google Scholar]
- 19.Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score (GS) ≤6 have the potential to metastasize to lymph nodes? Am J Surg Pathol. 2012;36:1346–52. doi: 10.1097/PAS.0b013e3182556dcd. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3421030&tool=pmcentrez&rendertype=abstract, accessed April 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter HB, Partin AW, Walsh PC, et al. Gleason score 6 adenocarcinoma: should it be labeled as cancer? J Clin Oncol. 2012;30:4294–6. doi: 10.1200/JCO.2012.44.0586. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3515766&tool=pmcentrez&rendertype=abstract, accessed February 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornud F, Khoury G, Bouazza N, et al. Tumor target volume for focal therapy of prostate cancer-does multiparametric magnetic resonance imaging allow for a reliable estimation? J Urol. 2014;191:1272–9. doi: 10.1016/j.juro.2013.12.006. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24333516, accessed January 22, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol. 2015;33:3379–85. doi: 10.1200/JCO.2015.62.5764. Available at: http://jco.ascopubs.org/content/33/30/3379.long, accessed January 17, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7. doi: 10.1200/JCO.2014.55.1192. Available at: http://jco.ascopubs.org/content/33/3/272.long, accessed February 22, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Welty CJ, Cowan JE, Nguyen H, et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015;193:807–11. doi: 10.1016/j.juro.2014.09.094. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25261803, accessed March 30, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Selvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol. 2013;64:981–7. doi: 10.1016/j.eururo.2013.02.020. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23473579, accessed April 11, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, Musunuru B, Vesprini D, et al. Metastatic Prostate Cancer in Men Initially Treated with Active Surveillance. J Urol. 2015 doi: 10.1016/j.juro.2015.11.075. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26707510, accessed April 11, 2016. [DOI] [PubMed]
- 27.Reese AC, Landis P, Han M, et al. Expanded criteria to identify men eligible for active surveillance of low risk prostate cancer at Johns Hopkins: a preliminary analysis. J Urol. 2013;190:2033–8. doi: 10.1016/j.juro.2013.05.015. Available at: http://www.sciencedirect.com/science/article/pii/S0022534713043516, accessed January 23, 2016. [DOI] [PubMed] [Google Scholar]


