Abstract
Objective
To longitudinally evaluate the association of post-ICU muscle weakness and associated trajectories of weakness over time with 5-year survival.
Design
Longitudinal prospective cohort study over 5 years of follow-up
Patients
156 acute respiratory distress syndrome (ARDS) survivors
Setting
13 ICUs in 4 hospitals in Baltimore, MD
Interventions
None
Measurements and Main Results
Strength was evaluated with standardized manual muscle testing using the Medical Research Council sumscore (range: 0–60, higher is better), with post-ICU weakness defined as sumscore <48. Muscle strength was assessed at hospital discharge and at 3, 6, 12, 24, 36, and 48 months after ARDS. At discharge, 38% of patients had muscle weakness. Every 1 point increase in sumscore at discharge was associated with improved survival (hazard ratio (95% confidence interval) 0.96 (0.94–0.98)), with similar findings longitudinally (0.95, 0.93–0.98). Having weakness at discharge was associated with worse 5-year survival (1.75, 1.01–3.03); but the association was attenuated (1.54, 0.82–2.89) when evaluated longitudinally over follow-up. Occurring in 50% of patients during follow-up, persisting and resolving trajectories of muscle weakness were associated with worse survival (3.01, 1.12–8.04; and 3.14, 1.40–7.03, respectively) compared to a trajectory of maintaining no muscle weakness.
Conclusions
At hospital discharge, >1/3 of ARDS survivors had muscle weakness. Greater strength at discharge and throughout follow-up was associated with improved 5-year survival. In patients with post-ICU weakness, both persisting and resolving trajectories, were commonly experienced and associated with worse survival during follow-up.
Keywords: Muscle Weakness/mortality, Respiratory Distress Syndrome, Adult, Muscle Strength, Patient Outcome Assessment, Longitudinal Studies
INTRODUCTION
Despite improving in-patient mortality for patients with acute respiratory distress syndrome (ARDS) and other critical illnesses,(1–3) survivors face increased mortality after hospital discharge.(4–8) Survivors also face muscle weakness and related physical impairments that persist for months and years after ARDS,(9–11) with recent studies demonstrating that objective in-patient measures of muscle weakness were associated with hospital mortality(12;13) and worse survival up to 1-year later.(14;15)
These findings justify the need to better understand longer-term patient outcomes beyond the initial months of recovery, including patient survival and associations of muscle weakness with long-term survival. Our objective, therefore, was to longitudinally evaluate the association of post-ICU muscle weakness with long-term survival until 5-years after ARDS, specifically evaluating muscle strength measured prior to hospital discharge and longitudinally thereafter. Additionally, we evaluate the association of trajectories in patients’ recovery from muscle weakness in the years after ARDS and their subsequent survival.
METHODS
Study Design and Patients
In this cohort study, conducted in 13 medical, surgical and trauma ICUs at four teaching hospitals in Baltimore, MD, we prospectively screened and enrolled consecutive mechanically ventilated patients meeting the American-European Consensus Conference criteria for acute lung injury(16) that were in effect during the time of enrollment. Throughout this report, we use the term ARDS, rather than acute lung injury, to be consistent with the more recent Berlin definition.(17) Relevant exclusion criteria included: 1) high short-term mortality unrelated to ARDS (life expectancy <6 months due to pre-existing illness or a physician order limiting the use of life support therapies at onset of ARDS); 2) important barrier to exposure or outcome assessment (pre-existing cognitive impairment, communication/language barrier, or homelessness); and 3) substantial exposure to critical illness/critical care services prior to enrollment (mechanically ventilated for >5 days before ARDS onset or transfer from another hospital with pre-existing ARDS of >24 hours duration). Institutional review board approval was obtained from all participating sites and written informed consent was obtained from all participants.
Primary Outcome: 5-Year Survival
Consenting patients were prospectively evaluated at 3, 6, 12, 24, 36, 48, and 60 months after ARDS onset. Vital status along with date and cause of death were ascertained from family members, with death date verified via a commercial version of the Social Security Death Master File.(18) Patients for whom vital status could not be determined were censored at their last day known to be alive.
Primary Exposure: Muscle Strength
As commonly done in prior critical care research,(12;15;19–21) patients’ muscle strength was evaluated via standardized physical examination using manual muscle testing and the Medical Research Council (MRC) sumscore (range 0–60, higher score is better). All strength assessors underwent initial training and ongoing quality assurance review by a reference-rater (co-author NDC) with high inter-rater reliability.(22) Patients’ strength was evaluated prior to hospital discharge and at each follow-up assessment. Muscle strength was evaluated both as a continuous (MRC sumscore) and binary (MRC sumscore <48) exposure variable. The binary evaluation of muscle strength, referred to hereafter as post-ICU-weakness, was defined as per prior research.(10;13;15;20)
Potential Confounders
Based on a prior publication,(23) a subset of risk factors for mortality after ARDS was considered for inclusion in this analysis as potential confounders of the exposure-outcome relationship. These variables included patient factors: age, sex, body mass index, and baseline comorbidity (Charlson comorbidity index(24) >1), and the following ICU factors: Acute Physiology and Chronic Health Evaluation II (APACHE II)(25) score within the first 24 hours of ICU admission, ARDS risk factor (sepsis vs. non-sepsis), type of ICU (medical ICU vs. other), source of admission (e.g. emergency room), mean daily Sequential Organ Failure Assessment (SOFA)(26) score in the ICU, daily sedation (Richmond Assessment of Sedation Scale)(27) and delirium (Confusion Assessment Method for the ICU)(28) status, cumulative dose of systemic corticosteroids and neuromuscular blocking medications, and the number of days with these medications, cumulative fluid balance, mean daily positive end-expiratory pressure (PEEP), mean daily ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen (PaO2/FiO2), mean daily static lung compliance, mean tidal volume (per kilogram of predicted body weight),(29) mean daily respiratory rate (breaths per minute), total days of mechanical ventilation, and ICU length of stay. Missing data among potential confounders was rare (<1%) except for sedation and delirium. Multiple imputation was used for the missing sedation and delirium assessments as described elsewhere.(30)
Statistical Methods
Descriptive statistics included median and interquartile range (IQR) for continuous variables, and percentages for categorical variables, with comparisons conducted using Wilcoxon rank-sum and Fisher’s exact tests, respectively. Locally weighted regression (LOESS) was used to assess for linearity of association between continuous variable and survival, and if not linear, then they were categorized, as appropriate, based on the LOESS (e.g. Charlson Comorbidity Index, see Supplementary Digital Content). A Kaplan-Meier curve was used to describe the survival from hospital discharge until 5-years after ARDS onset, and a lasagna plot (31) was created to visualize muscle weakness trajectories over 5-year follow-up (Supplementary Digital Content Figure 1).
To evaluate the longitudinal association of muscle weakness with survival until 5-years post-ARDS, we used Cox proportional hazards regression models. To prevent potential over-fitting of the regression model, an initial multivariable model was created that included only potential confounders that had a significant (p<0.05) unadjusted association with 5-year survival in simple Cox regression analyses. From this initial multivariable model, variables with p<0.2 were retained to create the minimum set of potential confounders for adjustment in the subsequent models evaluating exposure-outcome association; these variables were age, Charlson comorbidity index >1, and mean daily SOFA score (Supplementary Digital Content Table 1). Post-hoc analyses included evaluation of baseline activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as potential confounders. Neither variable met inclusion into the minimum set of potential confounders (Supplementary Digital Content Table 1). Variance inflation factors were evaluated to confirm the absence of multicollinearity of these potential confounders. Both continuous and binary measures of muscle strength at hospital discharge were then evaluated in separate multivariable Cox regression models adjusting for these three potential confounders. Additionally, both measures of muscle strength were evaluated as time-varying exposures in separate multivariable Cox regression models adjusting for the same potential confounders. For each multivariable model, Martingale residual plots were assessed to confirm that there were no departures from linearity for continuous exposure and confounder variables. In addition, Schoenfeld residual plots were assessed to evaluate the proportional hazards assumption which demonstrated some evidence of non-proportional hazards over time for MRC sumscore and post-ICU weakness indicator; however, since the estimated hazard ratios within each year of follow-up were qualitatively similar, a single hazard ratio is reported for each exposure variable.
In addition to evaluating muscle strength at hospital discharge and each follow-up assessment, changes in post-ICU muscle weakness over time (i.e., trajectories of muscle weakness) were evaluated longitudinally, in a time-varying manner, using the same multivariable Cox regression model described above. For this analysis, patients needed to survive for at least 2 strength assessments to evaluate change over time (i.e. trajectory). This time-varying exposure of change in weakness from one time point to the next was categorized as “No weakness” (i.e., consecutive assessments of No Weakness and No Weakness), “Persisting weakness” (i.e., from Weakness to Weakness), “Resolving weakness” (i.e., from Weakness to No Weakness), and “New weakness” (i.e., from No weakness at or after hospital discharge to subsequent Weakness).
RESULTS
There were 156 consenting and eligible patients for evaluation in this analysis (Figure 1). Of these 156 patients surviving until hospital discharge, 53 (34%) died after hospital discharge during 5-year follow-up (Figure 2), with the following categories for causes of death: infection 16 (30%), cardiovascular 11 (21%), cancer 6 (11%), gastrointestinal 4 (8%), respiratory 4 (8%), renal 3 (6%), other causes 3 (6%), and unknown cause 6 (11%) (see Supplemental Digital Content Table 2 for trends over time for top 2 causes of death). Those who died were significantly older with greater comorbidity, organ failure and days of delirium in the ICU, and hospital length of stay (Table 1). At hospital discharge, 38% of patients had post-ICU muscle weakness, and the median (interquartile range) MRC sumscore was significantly lower for those who died during 5-year follow-up vs. survivors (44 (36–52) vs. 51 (46–58), p<0.001), with 55% versus 30% (p= 0.003) having post-ICU muscle weakness (Table 1).
Figure 1.
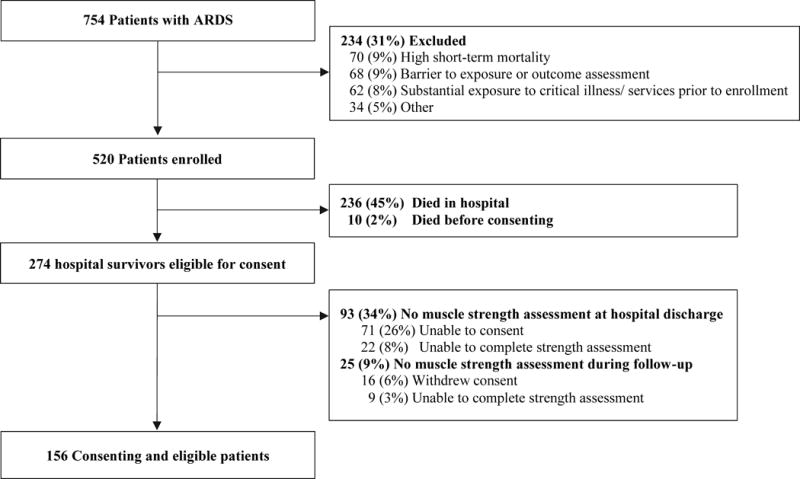
Patient Flow Chart
Figure 2.
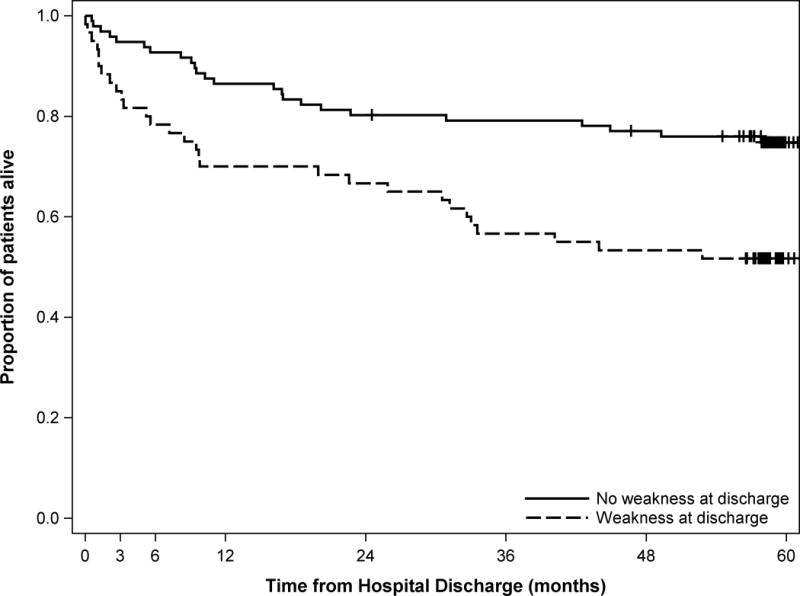
Kaplan-Meier curve of 5-year survival after hospital discharge for 156 ARDS patients comparing those with muscle weakness vs. those without muscle weakness at hospital discharge*
* Log-rank test p-value = 0.002
Table 1.
Characteristics of 156 ARDS survivors, by subsequent mortality status at 5 years after ARDS onset
| Characteristics | All patients (n=156) |
Alive (n=103) |
Dead (n=53) |
p-value* |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Male, No. (%) | 84 (54) | 57 (55) | 27 (51) | 0.615 |
| Age (years) | 47 (40–57) | 45 (38–51) | 56 (44–64) | <0.001 |
| Body Mass Index, No. (%) | 0.754 | |||
| Underweight (<18.5 kg/m2) | 5 (3) | 3 (3) | 2 (4) | |
| Normal weight (18.5 – 24.9 kg/m2) | 44 (28) | 28 (27) | 16 (30) | |
| Overweight (25–30 kg/m2) | 50 (32) | 36 (35) | 14 (26) | |
| Obese (>30 kg/m2) | 57 (37) | 36 (35) | 21 (40) | |
| Charlson comorbidity index | 1 (1–3) | 1 (0–2) | 3 (1–5) | <0.001 |
| Critical illness/ICU characteristics | ||||
| APACHE II score | 23 (17–29) | 23 (17–28) | 24 (17–30) | 0.310 |
| Non-pulmonary sepsis as ARDS risk factor | 32 (21) | 24 (23) | 8 (15) | 0.296 |
| Admission from emergency department | 74 (47) | 50 (49) | 24 (45) | 0.737 |
| Admission to medical ICU | 127 (81) | 80 (78) | 47 (89) | 0.128 |
| Mean daily SOFA score | 5 (4–7) | 5 (4–7) | 6 (5–8) | 0.006 |
| Days of delirium | 5 (3–9) | 5 (3–8) | 7 (4–10) | 0.009 |
| Days of deep sedation | 4 (2–8) | 2 (2–9) | 3 (0–8) | 0.203 |
| Corticosteroids use (ever), No. (%) | 87 (56) | 52 (50) | 35 (66) | 0.088 |
| Days of corticosteroid use, if any | 7 (4–13) | 7 (3–11) | 8 (5–15) | 0.097 |
| Cumulative prednisone dose, if any (mg)** | 366 (193–1079) | 403 (156–1169) | 363 (288–840) | 0.382 |
| Neuromuscular blocker use (ever), No. (%) | 32 (21) | 27 (26) | 5 (9) | 0.020 |
| Days of neuromuscular blocker use, if any | 1 (1–2) | 1 (1–2) | 1 (1–1) | 0.489 |
| Cumulative vecuronium dose, if any (mg) | 10 (10–37) | 16 (10–39) | 10 (8–10) | 0.235 |
| Cumulative fluid balance (liters) | 3 (−2 to 10) | 3 (−2 to 9) | 5 (−3 to 14) | 0.688 |
| Days of mechanical ventilation | 10 (6–17) | 9 (6–16) | 10 (7–21) | 0.141 |
| Length of stay in ICU (days) | 15 (10–23) | 14 (9–21) | 17 (11–30) | 0.068 |
| Length of stay in hospital (days) | 26 (17–36) | 23 (16–34) | 30 (21–45) | 0.014 |
| Hospital discharge muscle strength | ||||
| Muscle strength MRC sumscore (range: 0 – 60) | 50 (42–55) | 51 (46–58) | 44 (36–52) | <0.001 |
| Post-ICU weakness (MRC sumscore <48), No. (%) | 60 (38) | 31 (30) | 29 (55) | 0.003 |
Abbreviations: ARDS, acute respiratory distress syndrome; APACHE II=Acute Physiology And Chronic Health Evaluation II; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment; MRC, Medical Research Council
Data are presented as median (interquartile range), unless stated otherwise. Proportions may not add to 100% because of rounding.
Wilcoxon rank-sum and Fisher’s exact tests were used for continuous variables (presented as median [inter-quartile range]) and categorical variables (presented as numbers [percentage]), respectively
Corticosteroids doses converted to prednisone-equivalents using standard conversions(43)
For continuous measures, the mean of all available measures for each patient were averaged, and these averages were summarized as median (interquartile range).
After adjusting for age, Charlson comorbidity index, and mean daily SOFA score, increased muscle strength at hospital discharge was associated with long-term survival (Table 2), with every 1 point increase in MRC sumscore (range: 0 to 60) associated with improved survival (hazard ratio for death (HR) 0.96, 95% confidence interval (95% CI) 0.94 – 0.98; p<0.001). When analyzed longitudinally as a time-varying exposure over 5-year follow-up, each 1 point increase in muscle strength sumscore demonstrated a similar association with survival (0.95, 0.93 – 0.98; p<0.001). Having post-ICU muscle weakness (i.e., strength score <48) at hospital discharge was associated with worse survival (1.75, 1.01 – 3.03; p=0.004), with attenuation of this association, to become non-significant, when analyzed longitudinally as a time-varying exposure over the 5-year follow-up (1.54, 0.82 – 2.89; p=0.174) (Table 2).
Table 2.
Continuous and binary assessments of muscle strength and 5-year survival for 156 ARDS patients
| Predictor | 5-year Survival and Strength Assessment at Hospital Discharge* | 5-year Survival and Strength Assessment over Longitudinal Follow-Up** | ||
|---|---|---|---|---|
|
| ||||
| Adjusted hazard ratio (95% CI) | p-value* | Adjusted hazard ratio (95% CI) | p-value** | |
| Multivariable Regression Model 1 (continuous) | ||||
| Manual muscle strength sumscore (for 1 point increase) | 0.96 (0.94 – 0.98) | <0.001 | 0.95 (0.93 – 0.98) | <0.001 |
| Age at enrollment | 1.04 (1.02 – 1.06) | <0.001 | 1.04 (1.02 – 1.06) | <0.001 |
| Comorbidity prior to ARDS (Charlson index(24) >1) | 2.52 (1.35 – 4.73) | 0.004 | 2.97 (1.53 – 5.74) | 0.001 |
| Mean daily SOFA score in ICU | 1.08 (0.97 – 1.21) | 0.154 | 1.09 (0.98 – 1.21) | 0.125 |
| Multivariable Model 2 (binary) | ||||
| Post-ICU weakness (strength score <48) | 1.75 (1.01 – 3.03) | 0.044 | 1.54 (0.82 – 2.89) | 0.174 |
| Age at enrollment | 1.04 (1.02 – 1.07) | <0.001 | 1.04 (1.02 – 1.06) | <0.001 |
| Comorbidity prior to ARDS (Charlson index(24) >1) | 2.36 (1.25 – 4.46) | 0.008 | 2.88 (1.48 – 5.59) | 0.002 |
| Mean daily SOFA score in ICU | 1.10 (0.99 – 1.23) | 0.079 | 1.10 (0.99 – 1.22) | 0.086 |
Abbreviations: CI, confidence interval; SOFA, Sequential Organ Failure Assessment
Muscle strength was assessed prior to hospital and evaluated in a multivariable Cox regression model adjusting for age, comorbidity, and daily SOFA score
Muscle strength was assessed prior to hospital discharge and at 3, 6, 12, 24, 36, and 48-month follow-up points and evaluated as a time-varying exposure in a multivariable Cox regression model adjusting for age, comorbidity, and daily SOFA score
There were 588 consecutive assessments from 140 patients in this study, including, 38 (7%) assessments from 22 (16%) patients demonstrating persisting weakness, and 70 (12%) assessments from 63 (45%) patients demonstrating resolving weakness (Table 3, see Supplemental Digital Content Figure 1). Compared to a trajectory of maintaining no weakness, both persistent and resolving weakness trajectories were associated with significantly worse survival (3.01, 1.12 – 8.04; p=0.028, and 3.14, 1.40 – 7.03; p=0.005, respectively, Table 3), while a trajectory of “New weakness” (i.e. going from “No weakness” at or after hospital discharge to subsequent “Weakness”) was not associated with subsequent survival (0.99, 0.22 – 4.43; p=0.985).
Table 3.
Trajectory of muscle weakness and subsequent survival over 5-year follow-up*
| Trajectory pattern (weakness status at consecutive assessments) | No. (%) of patients** | No. (%) of consecutive assessments over 5-year follow-up | Adjusted Hazard ratio*** (95% CI) | p-value |
|---|---|---|---|---|
| No weakness (from No weakness to No weakness) | 121 (86%) | 443 (75%) | 1.00 | |
| Persisting weakness (from Weakness to Weakness) | 22 (16%) | 38 (7%) | 3.01 (1.12 – 8.04) | 0.028 |
| Resolving weakness (from Weakness to No weakness) | 63 (45%) | 70 (12%) | 3.14 (1.40 – 7.03) | 0.005 |
| New weakness (from No weakness to Weakness) | 35 (25%) | 37 (6%) | 0.99 (0.22 – 4.43) | 0.985 |
Abbreviations: ICU, intensive care unit; CI, confidence interval
Change in post-ICU weakness status (i.e., MRC sumscore <48) from one longitudinal follow-up assessment to the next available assessment and its association with survival time after the second assessment during this 5-year prospective follow-up study. Longitudinal follow-up assessments were performed prior to hospital discharge and at 3, 6, 12, 24, 36, and 48-month follow-up
Proportions will not add up to 100% since a patient can be in more than 1 category. For this analyses, patients must have at least 2 strength assessments for which 140 (90%) of 156 patients were eligible.
Each trajectory pattern was modeled as a time-varying exposure in a multivariable Cox model that adjusted for age at enrollment, Charlson comorbidity index, and mean daily Sequential Organ Failure Assessment score in the ICU
DISCUSSION
This 5-year, prospective multi-site longitudinal follow-up study of 156 ARDS survivors found that 38% had muscle weakness at hospital discharge, and that greater muscle strength at both hospital discharge and longitudinally during follow-up was independently associated with improved survival. Trajectories of persistent and resolving weakness were experienced by 50% of patients and both trajectories were associated with a >3 times greater hazard of subsequent death.
Given improving in-patient survival for patients with ARDS and other critical illnesses,(1–3) but ongoing mortality after hospital discharge,(4–8) understanding potential risk factors or markers for decreased long-term survival can help identify potential interventions to evaluate in future studies. For example, muscle weakness is known to be associated with short-term in-hospital mortality, as shown in a study of mechanically ventilated patients (odds ratio [95% confidence interval], 7.8 [2.4, 25.3]),(12) and a study of surgical ICU patients (odds ratio 0.95 [0.90, 0.99] for 1 point increase in muscle strength sumscore).(13) Another study of mechanically ventilated patients reported decreased 90-day survival in those with post-ICU weakness at ICU discharge,(14) and a study of mostly surgical patients with ICU stay >7 days reported that weakness at ICU discharge was associated with 1-year survival (HR [95% CI] of 2.1 [1.1, 3.9] for those with MRC sumscore of 36 – 47, and 4.3 [2.1, 8.8] for those with sumscore <36).(15) We build on these prior studies by evaluating the association of post-ICU weakness with long-term survival over 5-years; where roughly half of the deaths occurred during years 2 through 5. In our study, we found that at hospital discharge, increased muscle strength is associated with improved survival and having muscle weakness (i.e., a sumscore <48) is associated with worse survival over 5-year follow-up.
Additionally, our study is novel in that muscle strength was assessed not only in-hospital, but also longitudinally over 5-year follow-up. Post-discharge muscle weakness is an important aspect of survivorship since it is associated with impaired respiratory muscle strength, physical functioning, and quality of life.(10) In our longitudinal analyses, greater muscle strength was associated with improved survival; however, the association with binary modelling of muscle weakness (i.e., sumscore <48) was attenuated, becoming non-significant, perhaps due to decreased prevalence of muscle weakness and mortality in the cohort over the duration of follow-up. Lastly, longitudinal evaluation of muscle strength allowed for a novel exploration of the association of trajectories of muscle weakness over time with subsequent survival, with our study demonstrating that both persistent weakness and recovering from weakness, which occurred in 50% of patients during follow-up, were similarly associated with worse survival compared to trajectories of no weakness.
Across the multivariable regression analyses in our study, age and baseline comorbidity remained significantly associated with post-hospital survival, while acute severity of illness in the ICU (e.g., daily SOFA score) was not; a finding that is consistent with previous studies.(32;33) Even after adjusting for these variables, post-ICU muscle weakness was associated with worse 5-year survival. Future studies should aim to elucidate the mechanisms that link muscle weakness with mortality. For example, muscle weakness in survivors may be a marker for new-onset frailty or pre-existing frailty worsened by critical illness,(34;35) The pathogenesis of frailty is hypothesized to involve multi-system dysfunction, including the musculoskeletal system, and be related to chronic inflammation, rendering patients vulnerable to deleterious health outcomes and setting them to a path of progressive decline(36;37) and decreased survival.(38)
Our analyses of post-ICU trajectories of muscle weakness yielded clues that there are likely complex associations of weakness with mortality. In our study, the trajectory of new weakness was not associated with worse survival, signaling that it may represent a period similar to that of a “pre-frail” state, a state in which patients are still resistant to physiologic challenges.(37) However, once weak, the patient becomes vulnerable resulting in adverse outcomes, including death. This vulnerability may continue for some time in those with a trajectory of recovery. The risk of adverse outcomes may be higher in ICU survivors, even in the recovery process, since they often experience profound and persistent muscle wasting and weakness in the ICU, along other complications of bed rest.(10;39;40) These ideas may explain our finding that even a trajectory of resolving weakness was associated with worse survival.
Understanding the mechanism(s) linking muscle weakness and mortality will help refine potential interventions to improve long-term survival, but also clarify if a multi-faceted approach is necessary to mitigate the post-hospital mortality in ICU survivors. For now, with currently available evidence, prevention of muscle weakness via starting rehabilitation interventions shortly after start of mechanical ventilation may be an important consideration for improving long-term outcomes.(41–43) For example, one study demonstrated that early ICU rehabilitation was associated with decreased 1-year re-hospitalization and mortality.(44)
This study has several strengths, including being a multisite, prospective study with long-term, longitudinal follow-up until 5-years after ARDS. This study also has potential limitations. First, this study could not prospectively measure pre-ICU muscle strength as these ARDS patients were typically admitted emergently; hence, just as in routine clinical practice, we are unable to determine if the post-ICU weakness is incident or prevalent. Nonetheless, these findings have clinical value in indicating that post-ICU weakness, irrespective of baseline status, has prognostic significance for long-term survival, even after accounting for patient age, comorbidity and severity of organ failure in the ICU. Second, 1/3 of patients could not be assessed for muscle strength prior to discharge, excluding them from analyses. However, these excluded patients were generally similar to the cohort of patients retained in this study, except for older age and greater dependency in activities of daily living at hospital discharge (Digital Supplement Content Table 3). Hence, the observed associations with survival may be conservative. Furthermore, only 3% of patients were unable to complete strength assessment at least once during the course of follow-up, helping minimize bias in our longitudinal assessments. Third, this study could not adjust for other factors (e.g. pre-ICU frailty, chronic inflammation, any interventions such as physical rehabilitation)(45) and potential time-varying confounders (e.g. re-hospitalizations,) that may have affected the association of muscle weakness with survival. Future studies of long-term survival should account for these issues to potentially refine these associations. Fourth, as an observational study, causality cannot be proven; hence, more research is needed to understand if post-ICU weakness is directly associated with mortality or if post-ICU weakness is a marker for another mechanism more directly responsible for mortality. Lastly, the results of this study may not be generalizable to other ICU populations since only ARDS patients, enrolled from 4 hospitals in a single city, were studied.
In conclusion, this multi-site, prospective longitudinal study found that over 1/3 of ARDS survivors had muscle weakness at hospital discharge. Increased strength at hospital discharge and longitudinally over follow-up was consistently, independently, and significantly associated with improved 5-year survival. Once weak, both resolving and persistent trajectories of weakness were associated with worse subsequent survival, with most patients experiencing these two trajectories during follow-up. Given a growing number of survivors of critical illness, future research should include larger-sized, multi-center studies to confirm these findings. Moreover, given these findings and complementary results of prior studies evaluating shorter-term survival outcomes, future research should evaluate potential mechanisms underlying the association between muscle weakness and survival, and whether early interventions can reduce post-ICU weakness and improve both physical function and survival.
Supplementary Material
Acknowledgments
The authors thank all patients who participated in the study and the dedicated research staff who assisted with data collection, management and analysis for the study, including Dr. Nardos Belayneh, Ms. Kimberly Boucher, Dr. Abdulla Damluji, Mrs. Rachel Evans, Ms. Thelma Harrington, Dr. Praveen Kondreddi, Ms. Stacey Murray, Dr. Mohammed Nabeel, Ms. Arabela Sampaio, Ms. Kristin Sepulveda, Dr. Shabana Shahid, Dr. Faisal Siddiqi, Ms. Michelle MacDonna, Ms. Jennifer Titus, and Ms. Amy Wozniak.
Funding/Support: This research was supported by the National Institutes of Health (P050HL73994, R01HL088045, and K24HL088551), along with the Johns Hopkins Institute for Clinical and Translational Research (ICTR) (UL1 TR 000424-06).
Footnotes
Reprints: No reprint will be ordered.
Copyright Form Disclosure: Mr. Dinglas received support for article research from the National Institutes of Health (NIH). Dr. Friedman received support for article research from the NIH. Dr. Colantuoni received support for article research from the NIH. Her institution received funding from the NIH. Dr. Mendez-Tellez received support for article research from the NIH. Dr. Shanholtz received support for article research from the NIH. His institution received funding from the NIH. Dr. Ciesla received support for article research from the NIH. Dr. Pronovost received money from Johns Hopkins University (employee), Lehigh Speakers Bureau (speaker), and Penguin Publishin (book royalties). Dr. Needham received support for article research from the NIH. His institution received funding from AHRQ, the Gordon & Betty Moore Foundation, and NHMRC (Australia).
Author Contributions
DMN, LAF, and VDD had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the conception and/or design of this study. VDD, NDC, PAM-T, DMN contributed to the acquisition of data. All authors contributed to the analysis and interpretation of data. VDD drafted the manuscript, and all authors critically revised it for important intellectual content and approved the final version to be submitted.
Reference List
- 1.Lilly CM, Zuckerman IH, Badawi O, Riker RR. Benchmark data from more than 240,000 adults that reflect the current practice of critical care in the United States. Chest. 2011 Nov;140(5):1232–42. doi: 10.1378/chest.11-0718. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care. 2013;17(2):R81. doi: 10.1186/cc12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008 May;133(5):1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 4.Sigurdsson MI, Sigvaldason K, Gunnarsson TS, Moller A, Sigurdsson GH. Acute respiratory distress syndrome: nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol Scand. 2013 Jan;57(1):37–45. doi: 10.1111/aas.12001. [DOI] [PubMed] [Google Scholar]
- 5.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010 Mar 3;303(9):849–56. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 6.Brinkman S, Bakhshi-Raiez F, Abu-Hanna A, de JE, de Keizer NF. Determinants of mortality after hospital discharge in ICU patients: literature review and Dutch cohort study. Crit Care Med. 2013 May;41(5):1237–51. doi: 10.1097/CCM.0b013e31827ca4f9. [DOI] [PubMed] [Google Scholar]
- 7.Chen HC, Wu HD, Liu HW, et al. Acute Respiratory Distress Syndrome in Patients Discharged From a Tertiary Hospital in Taiwan: Long-Term Survival and Prognostic Factors. Nurs Res. 2015 Sep;64(5):402–8. doi: 10.1097/NNR.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 8.Lone NI, Gillies MA, Haddow C, et al. Five Year Mortality and Hospital Costs Associated With Surviving Intensive Care. Am J Respir Crit Care Med. 2016 Jan 27; doi: 10.1164/rccm.201511-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012 Mar 1;185(5):517–24. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014 Apr;42(4):849–59. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011 Apr 7;364(14):1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 12.Ali NA, O’Brien JM, Jr, Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008 Aug 1;178(3):261–8. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 13.Lee JJ, Waak K, Grosse-Sundrup M, et al. Global muscle strength but not grip strength predicts mortality and length of stay in a general population in a surgical intensive care unit. Phys Ther. 2012 Dec;92(12):1546–55. doi: 10.2522/ptj.20110403. [DOI] [PubMed] [Google Scholar]
- 14.Hodgson C, Bellomo R, Berney S, et al. Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-centre, prospective cohort study. Crit Care. 2015;19:81. doi: 10.1186/s13054-015-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermans G, Van MH, Clerckx B, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014 Aug 15;190(4):410–20. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 17.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012 Jun 20;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 18.Social security death index. http://ssdi.rootsweb.ancestry.com/cgi-bin/ssdi.cg. Last Access November 5. 2012.
- 19.Medical Research Council. Aids to the Investigation of the Peripheral Nervous System. London: Her Majesty’s Stationary Office; 1976. [Google Scholar]
- 20.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002 Dec 11;288(22):2859–67. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 21.Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009 Oct;37(10 Suppl):S299–S308. doi: 10.1097/CCM.0b013e3181b6ef67. [DOI] [PubMed] [Google Scholar]
- 22.Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med. 2010 Jun;36(6):1038–43. doi: 10.1007/s00134-010-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13(10):818–29. [PubMed] [Google Scholar]
- 26.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996 Jul;22(7):707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 27.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003 Jun 11;289(22):2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 28.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001 Dec 5;286(21):2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 29.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000 May 4;342(18):1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 30.Bienvenu OJ, Gellar J, Althouse BM, et al. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med. 2013 Feb;26:1–15. doi: 10.1017/S0033291713000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swihart BJ, Caffo B, James BD, Strand M, Schwartz BS, Punjabi NM. Lasagna plots: a saucy alternative to spaghetti plots. Epidemiology. 2010 Sep;21(5):621–5. doi: 10.1097/EDE.0b013e3181e5b06a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garland A, Olafson K, Ramsey CD, Yogendran M, Fransoo R. Distinct determinants of long-term and short-term survival in critical illness. Intensive Care Med. 2014 Aug;40(8):1097–105. doi: 10.1007/s00134-014-3348-y. [DOI] [PubMed] [Google Scholar]
- 33.Wang CY, Calfee CS, Paul DW, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med. 2014 Mar;40(3):388–96. doi: 10.1007/s00134-013-3186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hope AA, Gong MN, Guerra C, Wunsch H. Frailty Before Critical Illness and Mortality for Elderly Medicare Beneficiaries. J Am Geriatr Soc. 2015 Jun;63(6):1121–8. doi: 10.1111/jgs.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagshaw SM, McDermid RC. The role of frailty in outcomes from critical illness. Curr Opin Crit Care. 2013 Oct;19(5):496–503. doi: 10.1097/MCC.0b013e328364d570. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–41. doi: 10.2147/CIA.S45300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55(5):539–49. doi: 10.1159/000211949. [DOI] [PubMed] [Google Scholar]
- 38.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013 Mar;12(2):719–36. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013 Oct 16;310(15):1591–600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 40.Brower RG. Consequences of bed rest. Crit Care Med. 2009 Oct;37(10 Suppl):S422–S428. doi: 10.1097/CCM.0b013e3181b6e30a. [DOI] [PubMed] [Google Scholar]
- 41.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009 May 30;373(9678):1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010 Apr;91(4):536–42. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008 Oct 8;300(14):1685–90. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 44.Morris PE, Griffin L, Berry M, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011 May;341(5):373–7. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao PW, Shih CJ, Lee YJ, et al. Association of postdischarge rehabilitation with mortality in intensive care unit survivors of sepsis. Am J Respir Crit Care Med. 2014 Nov 1;190(9):1003–11. doi: 10.1164/rccm.201406-1170OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


