Abstract
Background
Inflammatory bowel diseases lead to progressive bowel damage and need for surgery. While the increase in prevalence of other immune-mediated diseases in IBD is well recognized, the impact of this on the natural history of IBD is unknown.
Aim
To determine the impact of concomitant immune-mediate disease on phenotypes and outcomes in IBD
Methods
Patients with IBD enrolled in a prospective registry were queried about the presence of other immune-mediated diseases, defined as those where immune dysregulation plays a role in pathogenesis. Demographics and disease-related information were obtained. Subjects also completed measures of quality of life. Multivariable regression models compared disease phenotype and outcomes of IBD patients with and without other immune-mediated diseases.
Results
The cohort included 2,145 IBD patients among whom 458 (21%) had another immune-mediated disease. There was no difference in CD phenotype between the two groups. UC patients were more likely to have pancolitis in the presence of another immune-mediated disease (62%) compared to those without (52%, p=0.02). IBD patients with another immune-mediated disease had higher rates of needing anti-TNF biologics (Odds ratio (OR) 1.31, 95% CI 1.05–1.63) and surgery (OR 1.26, 95% CI 0.99–1.61). The presence of another immune-mediated disease was also associated with lower disease-specific and general physical quality of life.
Conclusions
The presence of another immune-mediated disease in IBD patients was associated with higher likelihood of pancolonic involvement in UC and a modest increase in need for IBD-related surgery and anti-TNF biologic therapy. Such patients also experienced worse quality of life.
Keywords: quality of life, immune mediated disease, pancolitis, biologics, surgery, inflammatory bowel disease
INTRODUCTION
Immune-mediated diseases affect up to 10% of the Western population1–3. Among these are inflammatory bowel diseases (IBD) comprising of Crohn’s disease (CD) and ulcerative colitis (UC) which are complex chronic immunologically mediated diseases affecting the gastrointestinal tract4–6. Characterized by a protracted course frequently leading to hospitalizations and surgery, they are a source of significant morbidity and impairment of health-related quality of life for the affected individuals4–8. Through advances in sequencing technology, over 200 distinct single nucleotide polymorphisms (SNP) contributing to an elevated or reduced risk of IBD have been identified with many shared between CD and UC9. Emerging literature suggests that a greater overall burden of these genetic risk alleles may favor earlier onset of disease in CD and predict disease sub-phenotypes, particularly ileal involvement10, 11. As well, several environmental factors have been identified that influence both risk of developing disease as well as subsequent course12. Despite this, prediction of natural history of disease and occurrence of complications remains challenging.
It is well recognized that there is an increased risk for individuals with one immune-mediated disease to develop other immune-mediated diseases3, 13, 14. For example, in a prospective study 17% of patients with IBD compared to 10% of patients without IBD had at least one other immune-mediated disease14. Such overlap may be due to the known clustering of susceptibility loci between the different immune mediated diseases and shared pathogenic pathways (such as the IL-12/23 pathway in both CD and psoriasis)9, 15, 16. The overlap may also be due to common environmental risk factors such as smoking that is known to modulate risk for several immune-mediated diseases including CD, rheumatoid arthritis, and psoriasis. While many studies have investigated the prevalence of concomitant immune-mediated diseases in IBD, few have looked at the impact this has on disease course and it is not known whether affected individuals who have multiple immune-mediated diseases have a more aggressive course of these diseases by virtue of a greater genetic burden13, 14, 17–20. Most of the data investigating this topic has been on the co-existence of primary sclerosing cholangitis and IBD21–24 with rare scattered reports on celiac disease19 and thyroid disorders20. However, investigating this correlation could allow prognostication and selection of therapy for the individual patient by predicting more complicated disease course, and shed light on the phenotypic implications of the shared genetic loci.
Consequently, we performed this study with the following aims: (1) to describe the prevalence of other immune-mediated diseases in a large, well-phenotyped IBD cohort; and (2) to analyze the effect of co-occurring immune mediated diseases on the phenotype and outcomes of patients with CD and UC.
METHODS
Study Population
Subjects enrolled in the Prospective Registry for IBD Study at Massachusetts General Hospital (PRISM) comprised the study population. Details of this cohort have been previously published11, 19. The subjects for this study include all patients with an established diagnosis of CD, UC, or IBD-unspecified (IBDU) receiving care at the Massachusetts General Hospital Crohn’s and Colitis center. Upon provision of informed consent, patients completed a detailed questionnaire with a trained research coordinator that included ascertainment of their IBD including age at diagnosis, presence of complications, existence of concomitant diseases, and current and past treatments. All information was confirmed by review of the medical records where available.
Variables
At the time of enrollment, all patients reported other physician-diagnosed immune-mediated diseases which were confirmed via electronic medical record review. Immune-mediated diseases were considered to be one of several organ-specific or systemic diseases where a dysregulated immune response or immune dysfunction is believed to play a role in its pathogenesis. However, we specifically excluded those that are known extra-intestinal manifestations of IBD (such as IBD-related arthritis) and immune-mediated adverse effects of therapy (such as anti-TNF associated psoriasis or lupus).This formed our main covariate of interest and comprised immune-mediated diseases that are not considered extra-intestinal manifestations of IBD (Figure 1). Subjects provided information on demographics, age at diagnosis, and smoking history. Disease phenotype was classified based on age at diagnosis, location of involvement, and behavior in patients according to the Montreal classification in CD, and disease extent in UC25. This information was either at enrollment (in patients who did not have surgery) or prior to the first resection. Patients also reported past and current medications including aminosalicylates, immunomodulators (thiopurines or methotrexate), anti-TNF biologics (infliximab, adalimumab, certolizumab, golimumab), and anti-integrin (natalizumab, vedolizumab) therapy. Disease activity according the Harvey-Bradshaw Index (HBI) for CD26 and the Simple Clinical Colitis Activity Index (SCCAI) for UC27 were calculated by the treating physician. Disease activity was further evaluated objectively through measurements of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) within 2 weeks of enrollment where available. To determine health-related quality of life (HRQoL), patients completed the short-form 12 (SF-12) Health Survey28, 29 and the Short Inflammatory Bowel Disease Questionnaire (SIBDQ), a validated measure for assessment of HRQoL in patients with IBD30. The primary outcomes for this study included need for anti-TNF biologic therapy and for IBD-related surgery.
Figure 1.
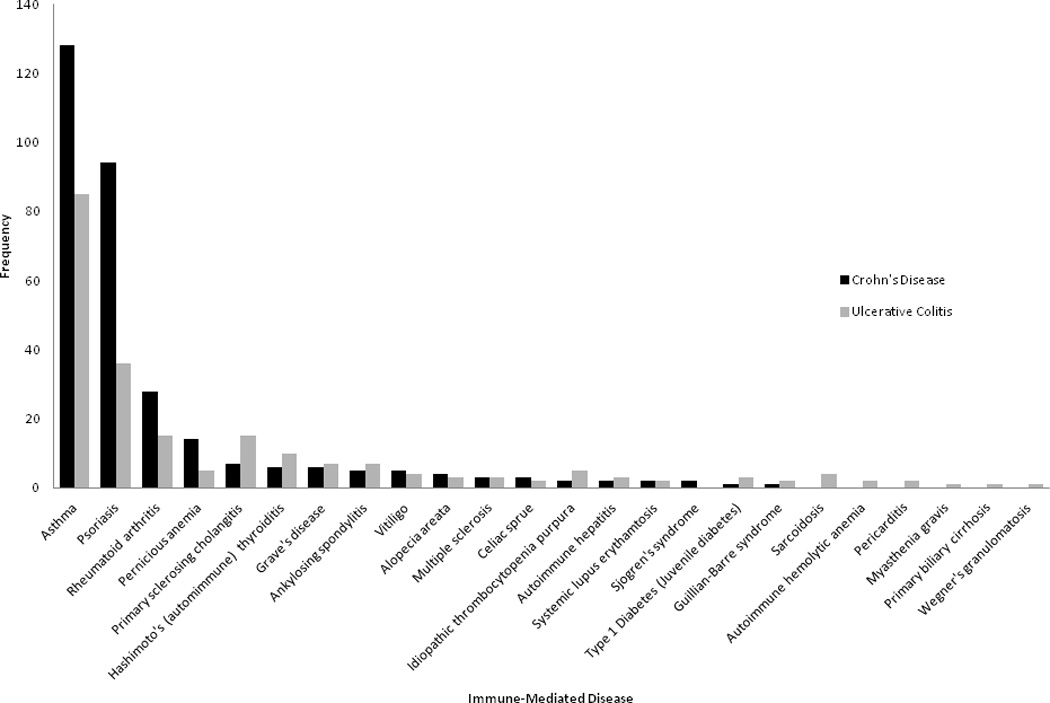
Prevalence of various immune-mediated diseases in patients with IBD, stratified by type of IBD
Statistical Analysis
Data was analyzed using Stata 13.1. Continuous variables were reported with means and standard deviations and compared using a two sample t-test. Categorical values were expressed as proportions and compared using the chi-square test. Variables found to be significant in the initial univariate analysis at p < 0.05 were analyzed using multivariate models. A multivariable logistic regression model was used to calculate the odds ratios (OR) and 95% confidence intervals (CI), investigating the independent association of co-existing immune-mediated diseases with need for biologic therapies and surgery, adjusting for potential confounders. For patients where interval between disease diagnosis and time of first resection was available, we used a Cox proportional hazards regression model to estimate hazard ratio (HR) and 95% CI for risk o surgery, stratifying by type of IBD. Survival curves were generated and compared using the log-rank test. A multivariable linear regression model was used to evaluate the regression coefficient and 95% confidence interval for the continuous outcome of quality of life (SF-12 scores and SIBDQ). A two-sided p-value < 0.05 in the multivariable model indicated independent statistical significance. The Institutional Review Board of Partners Healthcare approved this study.
RESULTS
Study Cohort
Of the 2,145 patients reviewed, 458 patients (21.4%) had one or more immune mediated diseases and the remaining 1,687 patients (78.6%) had only IBD. The immune-mediated diseases with the highest prevalence were asthma (9.9 %), psoriasis (6.1%), and rheumatoid arthritis (2.0%). Disease-specific analysis revealed there was a significant higher prevalence of psoriasis among those with CD compared to UC (8% vs. 4%, p < 0.0001) while the prevalence of PSC (2% vs. 0.5%, p=0.019) and sarcoidosis (0.4% vs. 0%, p=0.036) were higher in those with UC compared to CD (Figure 1). There was no significant difference in gender distribution, age at diagnosis, disease duration, or type of IBD between the two groups (Table 1). Differences in type of immune-mediated disease between men and women revealed no differences except for a higher prevalence of Hashimoto’s thyroiditis in women compared to men (1.1% vs. 0.3%, p=0.022) (Supplemental Figure 1). Specifically among those older than age 50, no differences were noted in the prevalence of any immune-mediated disease between men and women. There was no difference in family history of IBD in people with (33%) or without (32%) another immune-mediated disease (p=0.66).
Table 1.
Characteristics of the study population
| Characteristic | IBD with another immune-mediated disease (n=458) |
IBD only (n=1687) |
p-value |
|---|---|---|---|
|
Mean Age at Enrollment (in years) (Mean (SD)) |
40.9(15.7) | 40.3(14.9) | 0.83 |
| Gender % | 0.121 | ||
| Male | 44.8 | 48.8 | |
| Female | 55.2 | 51.2 | |
| Type of IBD % | 0.316 | ||
| CD | 58.5 | 55.9 | |
| UC and IC | 41.5 | 44.1 | |
|
Mean Age at Diagnosis (in years) (mean (SD)) |
29.2(14.4) | 29.1(13.9) | 0.835 |
|
Mean Disease Duration (in years) (mean (SD)) |
11.6(11.2) | 10.9(10.6) | 0.265 |
| Ever smoker % | 37.1 | 31.4 | 0.020 |
| CD location % | 0.466 | ||
| Terminal ileum | 27.5 | 23.7 | |
| Colon | 24.2 | 25.6 | |
| Ileocolonic | 48.3 | 50.3 | |
| Upper GI | 0 | 0.5 | |
| CD Behavior % | 0.945 | ||
| Inflammatory | 49.6 | 49.3 | |
| Stricturing | 21.5 | 20.8 | |
| Penetrating | 28.9 | 29.9 | |
| Perianal Disease % | 0.490 | ||
| Yes | 24.8 | 27.0 | |
| No | 75.2 | 73.0 | |
| UC Extent % | 0.061 | ||
| Procto/sigmoiditis | 13.8 | 13.5 | |
| Left-sided | 24.5 | 34.1 | |
| Pancolitis | 61.6 | 52.4 |
In patients with CD, disease extent and behavior were similar between the group with and without another immune-mediated disease. Specifically, there was no difference in prevalence between isolated colonic CD (22%) compared to those with small bowel disease (23%, p=0.66). However, in patients with UC, there was a trend towards a larger proportion of those with pancolonic inflammation in those with concomitant immune-mediated diseases (62%) compared to those without (52%, p=0.02). A history of smoking was also more common in patients with concomitant immune-mediated disease (37%) compared to those without (31%, p=0.02).
Disease outcome and activity associated with concomitant immune-mediated diseases
Table 2 presents univariate comparisons of IBD outcomes in patients with concomitant immune-mediated diseases compared to those without. Patients with at least one other immune-mediated disease were significantly more likely to undergo IBD-related surgery (37%) compared to those without (30%, p=0.04). They were also more likely to have needed anti-TNF biologic therapy (53% vs. 45%, p= 0.005) but no different in need for immunomodulators (64% vs. 60%, p=0.21). Very few patients were on anti-integrin therapy (17 patients (1%) with IBD alone and 3 (0.7%) with an immune-mediated disease, p=0.49). Cross-sectionally, patients with concomitant immune-mediated diseases were more likely to have elevated HBI (8.1 vs. 3.6, p=0.04) (but not SCCAI) and higher C-reactive protein (11.2 vs. 8.4, p=0.02) and ESR (21.0 vs. 17.5, p=0.01) levels than those without.
Table 2.
Comparison of disease outcome in patients with IBD and other immune-mediated diseases
| Outcome | IBD with another immune-mediated disease (n=458) |
IBD only (n=1687) |
p-value |
|---|---|---|---|
| Surgery (%) | 37.1 | 30.0 | 0.004 |
| Steroid use | 0.80 | ||
| Never | 18.0 | 19.3 | |
| Past | 69.3 | 67.8 | |
| Current | 12.7 | 12.9 | |
| ASA | 0.43 | ||
| Never | 14.8 | 12.8 | |
| Past/Current | 85.3 | 87.3 | |
| Immunomodulators | 0.21 | ||
| Never | 36.3 | 39.6 | |
| Past/Current | 63.7 | 60.4 | |
| Anti-TNF | 0.01 | ||
| Never | 47.4 | 54.7 | |
| Past/Current | 52.6 | 45.3 | |
|
Harvey Bradshaw index [Mean(SD)] |
8.1 (63.5) | 3.6 (4.1) | 0.04 |
|
Simple Clinical Colitis Activity index [Mean(SD)] |
2.5 (2.6) | 2.6 (2.9) | 0.59 |
|
C-reactive protein (mg/dL) [Mean(SD)] |
11.2 (18.4) | 8.4 (16.0) | 0.02 |
|
Erythrocyte sedimentation rate (mm/hr) [Mean(SD)] |
21.0 (20.5) | 17.5 (18.6) | 0.01 |
|
Hemoglobin (g/dL) [Mean(SD)] |
13.4 (2.4) | 13.6 (2.6) | 0.38 |
Multivariable regression analysis confirmed the increase in use of anti-TNF biologics in patients with another immune-mediated disease (OR 1.31, 95% CI 1.05 – 1.63) and a modest increase in need for intestinal surgery (OR 1.26, 95% CI 0.99 – 1.61) without a difference in the need for conventional immunomodulators (OR 1.11, 95% CI 0.89 –1.38) (Table 3). Subgroup associations by type of IBD (and hence small numbers of patients) demonstrated that the trend towards increased use of biologics remained for both CD and UC but the differences in need for surgery were no longer statistically significant. There were too few patients with each specific immune-mediated disease to permit robust comparisons. The presence of concomitant PSC was not associated with an increased risk of surgery (OR 0.88, 95% CI 0.29 – 2.65), immunomodulator (OR 1.31, 95% CI 0.51 – 3.32) or anti-TNF use (OR 1.15, 95% CI 0.47 – 2.82).
Table 3.
Multivariable analysis of the effect of concomitant immune mediated diseases on patients with inflammatory bowel diseases
| Adjusted Odds Ratio (95% CI) |
p-value | |
|---|---|---|
| All IBD, n=2,041† | ||
| Surgery | 1.26 (0.99–1.61) | 0.064 |
| Immunomodulator use | 1.11 (0.89–1.38) | 0.373 |
| Anti-TNF use | 1.31 (1.05–1.63) | 0.016 |
| Crohn’s disease, n=1,020‖ | ||
| Surgery | 1.22 (0.86–1.71) | 0.262 |
| Immunomodulator use | 0.97 (0.70–1.34) | 0.844 |
| Anti-TNF use | 1.30 (0.96–1.78) | 0.092 |
| Ulcerative Colitis, n=759¶ | ||
| Surgery | 1.36 (0.81–2.27) | 0.245 |
| Immunomodulator use | 1.22 (0.85–1.76) | 0.288 |
| Anti-TNF use | 1.43 (0.97–2.10) | 0.072 |
- Adjusted for disease duration, age at diagnosis, gender, smoking status and type of IBD
- Adjusted for age at diagnosis, disease duration, disease location, phenotype, gender, and smoking status
- Adjusted for age at diagnosis, disease duration, gender, extent of disease, and smoking status
Time from disease diagnosis to surgery was available for 526 out of 676 patients (78%). We performed a Cox hazards regression on risk of surgery in the presence of concomitant immune mediated disease, stratifying by type of IBD. While there was no increase in risk of surgery among patients with Crohn’s disease (HR 1.06, 95% CI 0.86 – 1.31), there was a significant increased risk for surgery in patients with ulcerative colitis in the presence of concomitant immune mediated diseases (HR 1.56, 95% CI 1.04 – 2.35) (Supplemental Figure 2).
We repeated our analysis in broad groupings of type of immune-mediated disease by organ system affected, recognizing that there are many ways to classify immune mediated diseases. For anti-TNF use, the strongest association with increased frequency of use was observed with concurrent musculoskeletal (rheumatoid arthritis, ankylosing spondylitis) (62% vs. 47%, p=0.025) and cutaneous immune-mediated diseases (psoriasis, vitiligo, alopecia areata) (58% vs. 46%, p=0.006) with a trend towards increased use with respiratory immune mediated diseases (asthma, Wegener’s granulomatosis) (52% vs. 46%, p=0.10). For the outcome of ever needing bowel resection surgery, a significant increase in risk was noted with cutaneous immune-mediated diseases (44% vs. 31%, p=0.001) with a trend towards increased risk with respiratory (p=0.10) and musculoskeletal (p=0.11) immune-mediated diseases.
Quality of life associations between patients with immune mediated diseases and IBD
A total of 1,828 patients completed the surveys related to quality of life, 417 patients with autoimmune disease and IBD and 1,411 patients with IBD alone. Figure 2 compares the HRQoL between those with and without another immune-mediated disease. Patients with other immune-mediated diseases had significantly lower SIBDQ (51.8 vs. 53.7), physical (46.8 vs. 48.8) and mental subscales of SF-12 (47.5 vs. 48.6) (p < 0.05 for all) when compared to those with IBD alone. Multivariable linear regression analysis confirmed the association between a lower SIBDQ score (regress coefficient (β) −1.04, 95%CI −2.14 to −0.06) in patients with another immune-mediated disease and IBD as well as a lower SF-12 physical health score (β −1.25, 95% CI (−2.29 to −0.22). There was no association with SF-12 mental health score when confounding variables were accounted for.
Figure 2.
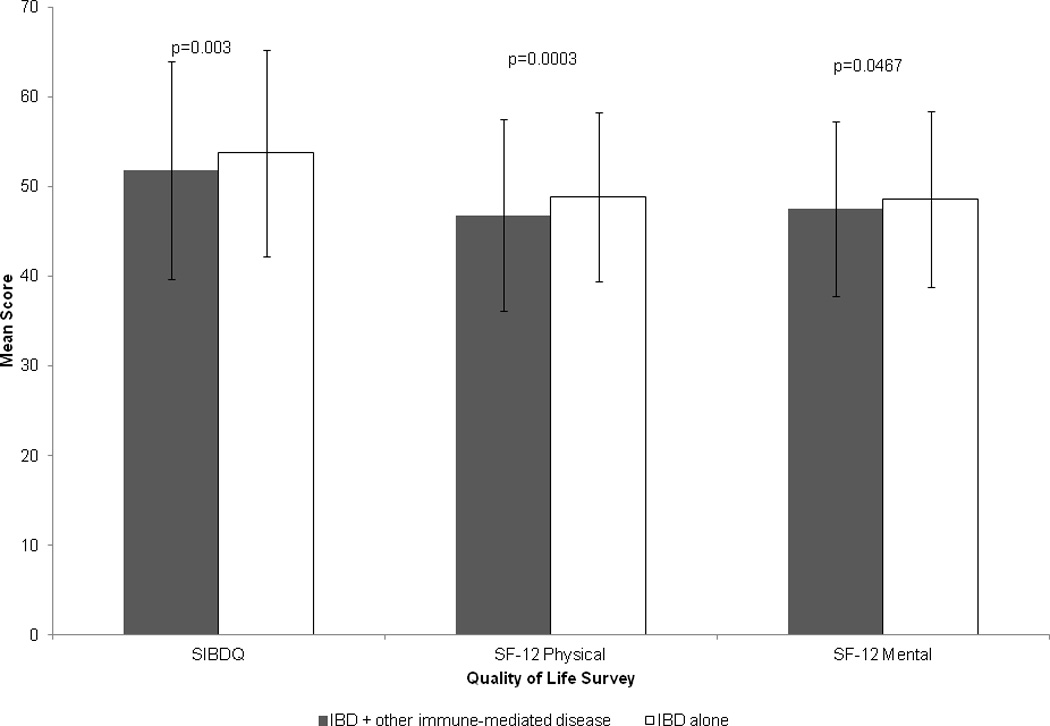
Health-related quality of life in patients with inflammatory bowel diseases, based on presence of other immune mediated diseases
DISCUSSION
Few studies have investigated disease outcomes in patients with IBD and other immunologically mediated diseases. While prior studies have looked to determine the prevalence of immune-mediated diseases in IBD, there is a lack of literature on whether the shared genetic burden or environmental factors such as smoking predispose to a more aggressive course of IBD when it occurs in conjunction with other immune mediated diseases. Using a large, well characterized IBD cohort, we demonstrate a modest increase in disease activity assessed cross-section as well as the cumulative need for anti-TNF biologic therapy or surgery in patients with concomitant immune mediated diseases. Importantly, even while adjusting for these factors, those with another immune-mediated disease demonstrated significantly lower disease-specific and general physical quality of life.
The prevalence of another immune-mediated disease in our cohort was 21%. This is comparable to the prevalence identified in other cohorts and supports an increase in the frequency of these diseases in those with IBD as has been demonstrated by several previous studies13–15, 17–21, 31. The most common immune-mediated diseases in our cohort were asthma and psoriasis, both of which have been commonly reported in other cohorts as well and to occur at an increased frequency in those with IBD. A population-based study from the University of Manitoba in Canada demonstrated an increased risk of several immune-mediated diseases in patients with IBD14. Among them, asthma was the most common comorbidity affecting 21% of UC patients and 20% of those with CD compared to 15% of controls. As well 9% of IBD patients had psoriasis, the second most common immune mediated disease, compared to 6% of controls. These estimates from cross-sectional studies have been confirmed in prospective cohorts. A large study from Taiwan comprising 5,260 patients with IBD demonstrated 1.5 fold increase in risk of asthma32. An analysis of the Nurses Health study showed a four-fold increase in risk of CD (but not UC) in those with psoriasis, suggesting bi-directional association33. However, despite shared genetics, not all immune-mediated diseases are more common in those with IBD. For example, prevalence of celiac disease in IBD is similar to the general population34, 35.
Few of these large studies examined if co-occurrence of immune-mediated diseases predict a more severe course of IBD. Our findings suggest more frequent pancolitis phenotype of UC in those with another immune-mediated disease, greater disease activity and an increase in need for anti-TNF and intestinal resection surgery. While the use of biologics may represent a shared therapeutic mechanism for both IBD and the immune-mediated disease, this is unlikely to be the main determinant as it would not explain the need for surgery in this population. Few studies have previously examined the impact of co-occurring immune mediated diseases on the natural history and phenotype of IBD. Most of the literature has focused on the impact of primary sclerosing cholangitis (PSC) on IBD21–24, 36, 37. IBD that occurs in the setting of PSC favors ulcerative pancolitis and infrequently demonstrates isolated ileal involvement in CD37, 38. In contrast with our findings of no effect of PSC on natural history of IBD, other studies have suggested that the behavior of underlying IBD tends to be milder, particularly in patients with severe PSC. In a cross-sectional study, the severity of IBD was greater when PSC occurred in conjunction with UC than CD, with a higher risk for colectomy and colon neoplasia in this population39. The need for colectomy is frequently lower in patients who required liver transplantation for their PSC22–24.
Less data exists examining the impact of other immune-mediated diseases on IBD. A single case report described a severe course of ulcerative colitis in a patient with rheumatoid arthritis40. In a study of 51 IBD patients with co-existing celiac disease, a trend towards more frequent pancolonic involvement and requirement for immunomodulators was noted in those with UC with no impact on those with CD19. A small case series of fewer than 10 patients each with celiac disease and IBD demonstrated high rates of surgical intervention but was limited by being referral center in origin and lacking a control group41. A small study of 67 children with UC did not demonstrate a difference in clinical course between those with and without concomitant autoimmune diseases though the small sample size limited statistical power and a majority of the patients had PSC as the underlying autoimmune disease18.
Importantly, our findings suggested that patient with concomitant immune mediated diseases experience significantly greater impairment in disease-specific and general physical quality of life in comparison to those without such diseases. Several factors are known to influence HRQoL in patients with IBD, importantly female gender, disease activity, and comorbidity42–44. While the impairment of quality of life in individuals with chronic immune-mediated diseases is well recognized few studies have examined whether co-occurrence further impairs HRQoL45–50. A single center study of patients with PSC and IBD did not find any impairment of HRQoL36. However general quality of life scores were not ascertained and PSC-IBD is usually milder. Our findings are similar to that Pizzi et al. who demonstrated that chronic co-morbidities including other autoimmune diseases exerted a significant negative impact on both physical and mental HRQoL in patients with IBD though a stronger effect was seen for comorbid depression and anxiety48.
This study examining the impact of the co-occurrence of IBD and immune-mediated diseases has a few implications. There are three potential mechanisms how co-occurrence of other immune-mediated diseases may influence phenotype and natural history of IBD. First, a wealth of literature highlights that some genes and genetic pathways are shared between different immune-mediated diseases. For example, the interleukin-12/23 pathway is pertinent to the development of both CD and psoriasis supported by the shared efficacy of monoclonal antibody targeting this pathway (ustekinumab) in both diseases. Thus, specific phenotypes that are enriched in this population with co-occurrence may be mediated by such shared genetic pathways. Second, environmental factors that are common to both diseases may contribute to the more severe natural history. For example, smoking is a known risk factor for asthma and IBD, and in turn, may lead to more severe disease course in patients who have both IBD and asthma. Some environmental factors such as non-steroidal anti-inflammatory drugs (NSAIDs) may be as a consequence of one immune-mediated disease (such as rheumatoid arthritis) and trigger relapses of IBD. Finally, studies have also suggested that many immune mediated diseases may share common gut microbial triggers2. Animal models in various immune mediated diseases including IBD, rheumatoid arthritis, lupus, multiple sclerosis, and ankylosing spondylitis all demonstrate a role for the microbiome in disease pathogenesis. While each disease appears to have distinct microbial signatures, there are some changes that may be common across diseases, for example reduction in faecalibacterium and increase in Escherichia in patients with Crohn’s disease and multiple sclerosis2. While the impact of gut microbial composition on disease phenotype is not well defined, such sharing of microbial triggers may also contribute to more severe disease in those with co-occurrence IBD and other immune-mediate diseases. Additionally, our findings highlight the deleterious impact of concomitant comorbid conditions on quality of life and the need to ascertain factors that impact such QoL and may be modified to improve patient outcomes.
There are several limitations to this study. First, since the study was conducted at a tertiary referral center, the disease severity in our study may be greater than in population-based cohorts. Assessment of disease activity was cross-sectional at a single time point. Though the size of our cohort was larger than many previous publications, the number of patients with each immune-mediated disease was small precluding an examination of the effect of each individual disease. Finally, certain immune mediated diseases may have treatment options that overlap with IBD, for example psoriasis and rheumatoid arthritis. Thus, it may not always be possible to discern which the primary determinant of escalating immunosuppression is. However, we did not exclude such patients from our primary analysis to avoid compromising the generalizability of the cohort. A trend towards increase rates of anti-TNF use and surgery remained on exclusion of such patients. Furthermore, recent analysis of Medicare data revealed that fewer than 5% each of patients with psoriasis were on infliximab or adalimumab therapy suggesting that in the majority of patients, psoriasis is unlikely to be the main factor determining need for anti-TNF use51. Disease activity indices and inflammatory markers were available at a single time point; cumulative burden of such measures over time may provide additional data regarding the longitudinal impact of concomitant immune-mediated diseases on natural history of IBD. We also did not have information on the sequence of diagnosis of IBD and other immune-mediated diseases and whether onset before or after the diagnosis of IBD differentially impacted natural history.
In conclusion, co-occurrence of another immune mediated disease in patients with IBD was associated with greater disease activity and a modest increase in need for anti-TNF medications and surgery, suggesting a potential impact on disease severity. The mechanism and impact of the clustering of immune-mediated diseases merits greater study to better understand the etiology of these diseases and develop therapeutic algorithms to improve patient outcomes.
Supplementary Material
(a) Crohn’s disease
(b) Ulcerative colitis
Acknowledgments
Conflicts of Interest: Ananthakrishnan has served on scientific advisory boards for Abbvie, Takeda, and Merck.
Source of funding: This work is supported by the National Institutes of Health (NIH) (P30 DK043351) to the Center for Study of Inflammatory Bowel Diseases. Ananthakrishnan is supported in part by a grant from the National Institutes of Health (K23 DK097142).
Footnotes
Authorship statement:
Conway: study design, data collection, analysis and interpretation of data, drafting of the manuscript
Velonias, Andrews, Garber, Yajnik: study design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content
Ananthakrishnan: study design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision
REFERENCES
- 1.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 2.Forbes JD, Van Domselaar G, Bernstein CN. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, Chen H, Xu H. The genomic landscape of human immune-mediated diseases. J Hum Genet. 2015;60:675–681. doi: 10.1038/jhg.2015.99. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein CN, Loftus EV, Jr, Ng SC, et al. Hospitalisations and surgery in Crohn's disease. Gut. 2012;61:622–629. doi: 10.1136/gutjnl-2011-301397. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein CN, Ng SC, Lakatos PL, et al. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19:2001–2010. doi: 10.1097/MIB.0b013e318281f3bb. [DOI] [PubMed] [Google Scholar]
- 6.Loftus EV, Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 7.Cohen RD. The quality of life in patients with Crohn's disease. Aliment Pharmacol Ther. 2002;16:1603–1609. doi: 10.1046/j.1365-2036.2002.01323.x. [DOI] [PubMed] [Google Scholar]
- 8.Floyd DN, Langham S, Severac HC, et al. The economic and quality-of-life burden of Crohn's disease in Europe and the United States, 2000 to 2013: a systematic review. Dig Dis Sci. 2015;60:299–312. doi: 10.1007/s10620-014-3368-z. [DOI] [PubMed] [Google Scholar]
- 9.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–986. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387:156–167. doi: 10.1016/S0140-6736(15)00465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananthakrishnan AN, Huang H, Nguyen DD, et al. Differential effect of genetic burden on disease phenotypes in Crohn's disease and ulcerative colitis: analysis of a North American cohort. Am J Gastroenterol. 2014;109:395–400. doi: 10.1038/ajg.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ananthakrishnan AN. Environmental risk factors for inflammatory bowel diseases: a review. Dig Dis Sci. 2015;60:290–298. doi: 10.1007/s10620-014-3350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng X, Liu L, Barcellos LF, et al. Clustering of inflammatory bowel disease with immune mediated diseases among members of a northern california-managed care organization. Am J Gastroenterol. 2007;102:1429–1435. doi: 10.1111/j.1572-0241.2007.01215.x. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet. 2009;10:43–55. doi: 10.1038/nrg2489. [DOI] [PubMed] [Google Scholar]
- 16.Li YR, Li J, Zhao SD, et al. Meta-analysis of shared genetic architecture across ten pediatric autoimmune diseases. Nat Med. 2015;21:1018–1027. doi: 10.1038/nm.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen R, Robinson D, Jr, Paramore C, et al. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001–2002. Inflamm Bowel Dis. 2008;14:738–743. doi: 10.1002/ibd.20406. [DOI] [PubMed] [Google Scholar]
- 18.Guinet-Charpentier C, Champigneulle J, Williet N, et al. The association of autoimmune diseases with pediatric ulcerative colitis does not influence its disease course. Scand J Gastroenterol. 2016;51:33–40. doi: 10.3109/00365521.2015.1058415. [DOI] [PubMed] [Google Scholar]
- 19.Oxford EC, Nguyen DD, Sauk J, et al. Impact of coexistent celiac disease on phenotype and natural history of inflammatory bowel diseases. Am J Gastroenterol. 2013;108:1123–1129. doi: 10.1038/ajg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shizuma T. Concomitant Thyroid Disorders and Inflammatory Bowel Disease: A Literature Review. Biomed Res Int. 2016;2016:5187061. doi: 10.1155/2016/5187061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jegadeesan R, Navaneethan U, Bharadwaj S, et al. Impact of Concurrent Non-IBD Immunological Diseases on the Outcome of Primary Sclerosing Cholangitis. Inflamm Bowel Dis. 2016;22:948–954. doi: 10.1097/MIB.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 22.Lundqvist K, Broome U. Differences in colonic disease activity in patients with ulcerative colitis with and without primary sclerosing cholangitis: a case control study. Dis Colon Rectum. 1997;40:451–456. doi: 10.1007/BF02258391. [DOI] [PubMed] [Google Scholar]
- 23.Marelli L, Xirouchakis E, Kalambokis G, et al. Does the severity of primary sclerosing cholangitis influence the clinical course of associated ulcerative colitis? Gut. 2011;60:1224–1228. doi: 10.1136/gut.2010.235408. [DOI] [PubMed] [Google Scholar]
- 24.Navaneethan U, Venkatesh PG, Mukewar S, et al. Progressive primary sclerosing cholangitis requiring liver transplantation is associated with reduced need for colectomy in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:540–546. doi: 10.1016/j.cgh.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 26.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 27.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assessment OoPH. Health Status in Utah: The Medical Outcomes Study SF-12 (2001 Utah Health Status Survey Report) Salt Lake City, UT: Utah Department of Health; 2004. [Google Scholar]
- 29.Ganz ML, Sugarman R, Wang R, et al. The Economic and Health-related Impact of Crohn's Disease in the United States: Evidence from a Nationally Representative Survey. Inflamm Bowel Dis. 2016;22:1032–1041. doi: 10.1097/MIB.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 30.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn's Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–1578. [PubMed] [Google Scholar]
- 31.Singh S, Kumar N, Loftus EV, Jr, et al. Neurologic complications in patients with inflammatory bowel disease: increasing relevance in the era of biologics. Inflamm Bowel Dis. 2013;19:864–872. doi: 10.1002/ibd.23011. [DOI] [PubMed] [Google Scholar]
- 32.Peng YH, Liao WC, Su CH, et al. Association of inflammatory bowel disease with asthma risk: A nationwide cohort study. Allergy Asthma Proc. 2015;36:e92–e98. doi: 10.2500/aap.2015.36.3869. [DOI] [PubMed] [Google Scholar]
- 33.Li WQ, Han JL, Chan AT, et al. Psoriasis, psoriatic arthritis and increased risk of incident Crohn's disease in US women. Ann Rheum Dis. 2013;72:1200–1205. doi: 10.1136/annrheumdis-2012-202143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leeds JS, Horoldt BS, Sidhu R, et al. Is there an association between coeliac disease and inflammatory bowel diseases? A study of relative prevalence in comparison with population controls. Scand J Gastroenterol. 2007;42:1214–1220. doi: 10.1080/00365520701365112. [DOI] [PubMed] [Google Scholar]
- 35.Casella G, D'Inca R, Oliva L, et al. Prevalence of celiac disease in inflammatory bowel diseases: An IG-IBD multicentre study. Dig Liver Dis. 2010;42:175–178. doi: 10.1016/j.dld.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Ananthakrishnan AN, Beaulieu DB, Ulitsky A, et al. Does primary sclerosing cholangitis impact quality of life in patients with inflammatory bowel disease? Inflamm Bowel Dis. 2010;16:494–500. doi: 10.1002/ibd.21051. [DOI] [PubMed] [Google Scholar]
- 37.Loftus EV, Jr, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinakos E, Samuel S, Enders F, et al. Inflammatory bowel disease in primary sclerosing cholangitis: a robust yet changing relationship. Inflamm Bowel Dis. 2013;19:1004–1009. doi: 10.1097/MIB.0b013e3182802893. [DOI] [PubMed] [Google Scholar]
- 39.Navaneethan U, Venkatesh PG, Jegadeesan R, et al. Comparison of outcomes for patients with primary sclerosing cholangitis associated with ulcerative colitis and Crohn's disease. Gastroenterol Rep (Oxf) 2016;4:43–49. doi: 10.1093/gastro/gou074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyer F, Fontanges E, Miossec P. Rheumatoid arthritis associated with ulcerative colitis: a case with severe flare of both diseases after delivery. Ann Rheum Dis. 2001;60:901. [PMC free article] [PubMed] [Google Scholar]
- 41.Yang A, Chen Y, Scherl E, et al. Inflammatory bowel disease in patients with celiac disease. Inflamm Bowel Dis. 2005;11:528–532. doi: 10.1097/01.mib.0000161308.65951.db. [DOI] [PubMed] [Google Scholar]
- 42.Casellas F, Lopez-Vivancos J, Vergara M, et al. Impact of inflammatory bowel disease on health-related quality of life. Dig Dis. 1999;17:208–218. doi: 10.1159/000016938. [DOI] [PubMed] [Google Scholar]
- 43.Hjortswang H, Strom M, Almer S. Health-related quality of life in Swedish patients with ulcerative colitis. Am J Gastroenterol. 1998;93:2203–2211. doi: 10.1111/j.1572-0241.1998.00537.x. [DOI] [PubMed] [Google Scholar]
- 44.Roman AL, Munoz F. Comorbidity in inflammatory bowel disease. World J Gastroenterol. 2011;17:2723–2733. doi: 10.3748/wjg.v17.i22.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1:10–20. doi: 10.1016/j.crohns.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18–ii23. doi: 10.1136/ard.2004.033217. discussion ii24-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nortvedt MW, Riise T, Myhr KM, et al. Performance of the SF-36, SF-12, and RAND-36 summary scales in a multiple sclerosis population. Med Care. 2000;38:1022–1028. doi: 10.1097/00005650-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Pizzi LT, Weston CM, Goldfarb NI, et al. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:47–52. doi: 10.1097/01.mib.0000191670.04605.e7. [DOI] [PubMed] [Google Scholar]
- 49.Arpinelli F, Carone M, Riccardo G, et al. Health-related quality of life measurement in asthma and chronic obstructive pulmonary disease: review of the 2009–2014 literature. Multidiscip Respir Med. 2015;11:5. doi: 10.1186/s40248-016-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor PC, Moore A, Vasilescu R, et al. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. 2016;36:685–695. doi: 10.1007/s00296-015-3415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare Population: Prevalence, Treatment, and Factors Associated with Biologic Use. J Invest Dermatol. 2015;135:2955–2963. doi: 10.1038/jid.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Crohn’s disease
(b) Ulcerative colitis


