Abstract
Purpose
Previous work has shown nitric oxide (NO) contributes to ~15% of the hyperemic response to dynamic exercise in healthy humans. This NO-mediated vasodilation occurs, in part, via increases in intracellular cyclic guanosine monophosphate (cGMP), which is catabolized by phosphodiesterase. We sought to examine the effect of phosphodiesterase-5 (PDE-5) inhibition on forearm blood flow (FBF responses to dynamic handgrip exercise in healthy humans and the role of NO. We hypothesized exercise hyperemia would be augmented by sildenafil citrate (SDF, PDE-5 inhibitor). We further hypothesized any effect of SDF on exercise hyperemia would be abolished with intra-arterial infusion of the NO synthase (NOS) inhibitor L-NG-monomethyl arginine (L-NMMA).
Methods
FBF (Doppler ultrasound) was assessed at rest and during 5 minutes of dynamic forearm handgrip exercise at 15% of maximal voluntary contraction under control (saline) conditions and during 3 experimental protocols: 1) oral SDF (n=10), 2) intra-arterial L-NMMA (n=20), 3) SDF and L-NMMA (n=10). FBF responses to intra-arterial sodium nitroprusside (NTP, NO donor) were also assessed.
Results
FBF increased with exercise (p<0.01). Intra-arterial infusion of L-NMMA resulted in a reduction in exercise hyperemia (17±1 to 15±1 mL/dL/min, p<0.01). Although the hyperemic response to NTP was augmented by SDF (Area under the curve: 41±7 vs 61±11 AU, p<0.01), there was no effect of SDF on exercise hyperemia (p=0.33).
Conclusions
Despite improving NTP-mediated vasodilation, oral SDF failed to augment exercise hyperemia in young, healthy adults. These observations reflect a minor contribution of NO and the cGMP pathway during exercise hyperemia in healthy young humans.
Keywords: blood flow, regional, sildenafil, exercise, nitric oxide, cyclic GMP
INTRODUCTION
Blood flow to exercising skeletal muscle increases proportionally to match oxygen delivery with metabolic demand (Shepherd 1983). Extensive work from our group and others has shown that nitric oxide (NO), a potent vasodilator, contributes to ~15% of the hyperemic response to dynamic exercise in healthy young humans [Reviewed in (Joyner and Casey 2015)]. The majority of our mechanistic understanding has come from the use of NO synthase (NOS) inhibitors; however, the effects of these drugs have been shown to be dependent on the type of exercise (Green et al. 2005), the interpretation of the altered baseline flow (Bradley et al. 1999; Duffy et al. 1999; Dyke et al. 1995; Katz et al. 1996; Maiorana et al. 2003; Radegran and Saltin 1999), and the timing of measurements [e.g. steady-state exercise versus recovery from exercise (Dyke et al. 1995; Saltin et al. 1998; Shoemaker et al. 1997)]. Consistent with this, NOS has been shown to play a greater (Duffy et al. 1999; Dyke et al. 1995; Gilligan et al. 1994; Maxwell et al. 1998; Schrage et al. 2004) or lessor (Endo et al. 1994; Frandsenn et al. 2001; Heinonen et al. 2011; Martin et al. 2006; Radegran and Saltin 1999; Wilson and Kapoor 1993) role in the blood flow response to dynamic exercise.
NO activates the intracellular enzyme soluble guanylate cyclase in vascular smooth muscle, thereby increasing the formation of cyclic guanosine monophosphate (cGMP), leading to smooth muscle relaxation and vasodilation. The effect of NO is terminated by phosphodiesterase enzymes, including the cGMP-selective phosphodiesterase-5 (PDE-5). PDE-5 is the isozyme responsible for the majority of cGMP degradation in smooth muscle cells and PDE-5 inhibition has been shown previously to effectively increase resting blood flow to select vascular beds [e.g. corpus cavernosa, lungs (Ghofrani et al. 2006; Moreland et al. 1998)], including the skeletal muscle vasculature (Attina et al. 2008). PDE-5 inhibition with the pharmacological agent sildenafil citrate has also been shown to improve exercise tolerance in persons with pulmonary hypertension (Singh et al. 2006) and in healthy adults during hypoxia (Hsu et al. 2006). Furthermore, data from Attina and colleagues suggest that sildenafil citrate can improve post-exercise blood flow in hypertensive patients (Attina et al. 2008); however, the effect of PDE-5 inhibition on skeletal muscle blood flow responses during dynamic exercise has not been examined directly. With this, it is possible NO plays only a minor role (~15%) in exercise hyperemia because PDE-5 limits complete activation of the NO-cGMP pathway and there is evidence to suggest that the NO pathway can be potentiated in young healthy individuals (Ferguson et al. 2013; Lee et al. 2015). Understanding the role of PDE-5 in regulating peak NO-mediated vasodilation during exercise will provide important information that could be translated clinically to improve exercise tolerance in conditions with limited NO bioavailability. There are also intriguing new data that suggest blood flow during exercise may be uncoupled from metabolic demand (Shepherd et al. 2016); thus, studying the effect of PDE-5 inhibition on exercise hyperemia may also provide further insight into changes in blood flow in response to increased oxygen consumption.
Taken together, we proposed that inhibition of PDE-5 would increase skeletal muscle blood flow responses to dynamic exercise via potentiation of the NO-cGMP pathway. We examined the effect of the PDE-5 inhibitor sildenafil citrate on forearm blood flow responses to dynamic handgrip exercise in healthy humans. We hypothesized forearm blood flow responses to exercise would be increased with administration of sildenafil citrate. We further hypothesized any increase in forearm blood flow responses with sildenafil citrate would be absent with intra-arterial infusion of the NO synthase inhibitor L-NG-monomethyl arginine (L-NMMA) – demonstrating the specificity of sildenafil citrate for the NO pathway.
METHODS
Subjects
This study was approved by the Institutional Review Boards at the Mayo Clinic and University of Wisconsin – Madison and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Thirty subjects (24 females/16 males, age = 25±1 yrs, BMI = 22.4±0.3 kg/m2) gave informed, written consent prior to participation. Subjects were non-smokers, were free from pulmonary, cardiovascular, endocrine, or neurological diseases, and were not taking any medications with known cardiovascular effects. Subjects refrained from exercise, alcohol, and caffeine for 24 hours prior to the study.
Instrumentation
Subjects were positioned supine and heart rate was recorded continuously from a 3-lead electrocardiogram. A 20-gauge, 5-cm catheter was placed in the brachial artery of the non-dominant arm under aseptic conditions after local anesthesia. A three-port connector was placed in series with a pressure transducer to allow simultaneous administration of study drugs and continuous arterial blood pressure monitoring. Brachial artery blood velocity was measured using Doppler ultrasound (Vivid 7 Ultrasound, GE Healthcare, Chalfont St. Giles, UK) (Joyner and Dietz 1997). In Protocols 1 and 3, a commercial interface unit (Multigon Industries, Yonkers, NY) processed the angle-corrected, intensity-weighted Doppler audio information into a flow velocity signal that was sampled in real time. In Protocol 2, the Doppler audio information was converted into a real-time digital flow velocity signal using Fast Fourier Transform (Herr et al. 2010). This method of signal processing has been validated by both in vitro and in vivo methods (Herr et al. 2010). All data were recorded continuously on a computer and analyzed offline (PowerLab Chart5, ADInstruments, Colorado Springs, CO). Brachial artery diameter was measured digitally off-line from two-dimensional, longitudinal images. Forearm blood flow (hyperemia) was calculated as the product of blood velocity and vessel cross sectional area and is expressed relative to forearm size (mL·dL−1·min−1). To account for changes in blood pressure and to assess vasodilation, forearm vascular conductance (FVC) was calculated (mL·dL−1·min−1·100 mmHg−1).
For each protocol, measurements were made at baseline (rest), during forearm exercise, and during incremental infusion of sodium nitroprusside (NTP, Protocols 1 and 3). A minimum of 10 minutes was allowed between exercise and NTP trials to ensure blood flow returned to baseline levels. Subjects were enrolled in 3 separate protocols which included a control condition, followed by an experimental condition with addition of: 1) oral sildenafil citrate (n=10), 2) intra-arterial L-NMMA (n=20), 3) oral sildenafil citrate and intra-arterial L-NMMA (n=10). Our rationale for including multiple research cohorts was based on the half-life of sildenafil and L-NMMA. Both sildenafil and L-NMMA have half-lives >1 hour (Mayer et al. 1999; Nichols et al. 2002); which prohibit our ability to adequately study each independent condition in the same individuals on a single study day. Data from subjects in Protocol 2 were published previously (Harrell et al. 2015; Kellawan et al. 2015).
Forearm exercise
Forearm exercise consisted of a quiet resting period, followed by 5 minutes of submaximal, dynamic handgrip exercise as previously described (Tschakovsky et al. 2002). Briefly, subjects rhythmically squeezed a handgrip ergometer (20 contractions/min) using a load that was 15% of their maximal voluntary contraction (MVC). Blood velocity data were averaged across 30 seconds at the end of rest and steady-state (5-min) exercise. Two-dimensional images of the brachial artery were obtained at baseline and after 5 minutes of exercise and were saved for off-line measurements of brachial artery diameter and calculation of forearm blood flow.
Sodium nitroprusside (NTP)
Sodium nitroprusside is an exogenous, endothelial-independent NO donor which was administered to the forearm via the brachial artery catheter to activate the NO-cGMP pathway independent of endothelial NOS. Four incremental doses (0.25, 0.5, 1, and 2 µg·dL forearm vol−1·min−1) were infused at rates between 2–3 mL·min−1 for a minimum of 2-min to allow blood flow to reach steady state. The last 30-sec of blood velocity data for each infusion of sodium nitroprusside was averaged to obtain the velocity for that dose. Brachial artery diameter was measured immediately after the blood velocity measurements were completed.
Sildenafil citrate (SDF; Protocols 1 and 3)
After completion of control measurements, sildenafil citrate (100 mg) was administered orally with a sip of water. This dose is the maximum recommended oral dose for the treatment of erectile dysfunction and was similar to other studies examining the effect of sildenafil citrate on hemodynamics (Dishy et al. 2001; Kimura et al. 2003). One hour was allowed for sildenafil citrate to reach peak concentrations and have maximal effects on forearm blood flow (Schalcher et al. 2002).
L-NG-monomethyl arginine (L-NMMA; Protocols 2 and 3)
A loading dose (50 mg) of L-NMMA was administered intra-arterially in the instrumented forearm over 5–10 minutes to locally inhibit NO synthase. In Protocol 2, the loading dose began immediately after 5 minutes of handgrip exercise. In Protocol 3, the loading dose was administered one hour after sildenafil citrate administration. In both protocols, after the completion of the loading dose, a maintenance infusion of L-NMMA began (1 mg·min−1) and continued for the remainder of the study (Dinenno and Joyner 2003, 2004).
Statistical analysis
Subject demographics were compared between protocols using a one-way analysis of variance (ANOVA). Hemodynamic data were compared using two-way repeated measures ANOVA to examine the main effect of condition (SDF, L-NMMA, SDF + L-NMMA) and time (exercise) or dose (NTP), as well as the interaction between condition and time/dose. To account for potential changes in baseline hemodynamics, the hyperemic response to exercise was reported as a change from baseline (Δ, FBFCondition – FBFBaseline). Forearm blood flow responses to sodium nitroprusside infusions were also assessed using an area under the curve (AUC) analysis. Data are presented as mean ± standard error of the mean. P<0.05 was considered significant.
Sample size was determined by power test equations with α=0.05 and power=0.80 using differences reported from previously published data, which suggested that 6 subjects per group would be necessary to detect a 2.6±1.7 mL·dL−1·min−1 change in forearm blood flow with sildenafil citrate (Attina et al. 2008). Because little is known about the effect of sildenafil citrate on exercise hyperemia, we exceeded n=6 in each cohort.
RESULTS
Subject demographics did not differ significantly between protocols (See Table 1).
Table 1.
Subject demographics
| Protocol 1 (Sildenafil) |
Protocol 2 (L-NMMA) |
Protocol 3 (Sildenafil+L-NMMA) |
|
|---|---|---|---|
| Sex (M/F) | 4/6 | 11/9 | 1/9 |
| Age (years) | 26 ± 1 | 26 ± 1 | 24 ± 2 |
| Height (cm) | 169 ± 3 | 172 ± 2 | 171 ± 3 |
| Weight (kg) | 67 ± 3 | 68 ± 2 | 62 ± 4 |
| Body mass index (kg/m2) | 23 ± 1 | 23 ± 1 | 20 ± 1 |
| Forearm volume (mL) | 900 ± 75 | 895 ± 47 | 787 ± 71 |
| Maximal voluntary contraction (kg) | 35 ± 4 | 36 ± 2 | 33 ± 3 |
Data are from: Protocol 1 (n=10), Protocol 2 (n= 20), Protocol 3 (n=10), unless otherwise noted. Maximal Voluntary Contraction (Protocol 3, n=8). No significant differences between protocols (p>0.05 for all).
Exercise
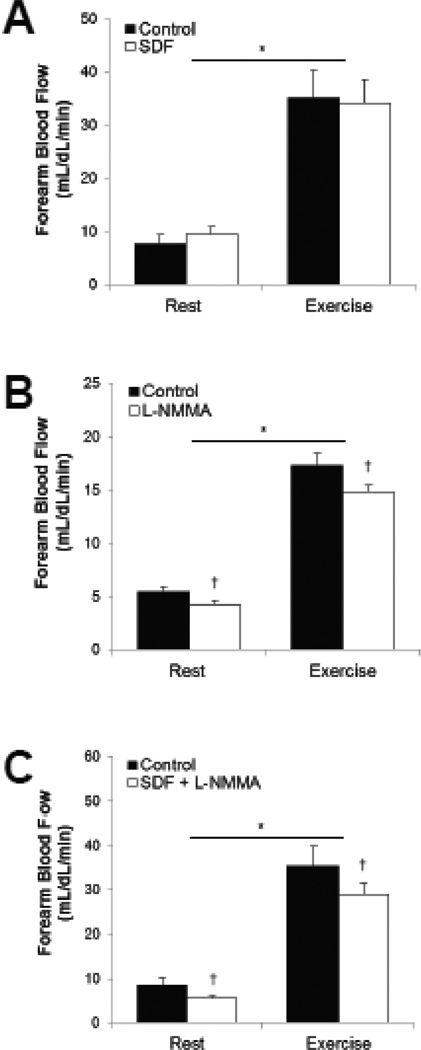
See Table 2 and Figure 1. Dynamic handgrip exercise resulted in an increase in heart rate (Main effect of exercise, p<0.05) and brachial artery diameter (Main effect of exercise, p≤0.05). No exercise-mediated changes in blood pressure were observed in Protocols 1 and 3 (Main effect of exercise, Protocol 1, p=0.15; Protocol 3, p=0.49), but blood pressure increased in Protocol 2 (Main effect of exercise, p<0.01). Heart rate was significantly increased following oral SDF when compared to control conditions (Main effect of SDF, p<0.01), but no changes in heart rate were observed when SDF was combined with L-NMMA (Main effect of SDF, p=0.18). There was no effect of SDF on blood pressure (Main effect of SDF, p=0.76; Main effect of SDF+L-NMMA, p=0.54), or brachial artery diameter (Main effect of SDF, p=0.83; Main effect of SDF+L-NMMA, p=0.66). There was no effect of L-NMMA on brachial artery diameter (Main effect of L-NMMA, p=0.06) or heart rate (Main effect of L-NMMA, p=0.44), although an increase in blood pressure was observed (Main effect of L-NMMA, p<0.01).
Table 2.
Hemodynamic responses to dynamic forearm exercise.
| Protocol 1 | Protocol 2 | Protocol 3 | ||||
|---|---|---|---|---|---|---|
| Control | SDF | Control | L-NMMA | Control | SDF+L-NMMA | |
| Heart Rate (beat/min) | ||||||
| Baseline | 60 ± 4 | 65 ± 4† | 61 ± 2 | 61 ± 2 | 62 ± 2 | 58 ± 3 |
| 5 minutes | 64 ± 3* | 70 ± 5*† | 64 ± 2* | 63 ± 2* | 65 ± 2* | 63 ± 3* |
| Δ | 4 ± 1 | 5 ± 1 | 3 ± 2 | 2 ± 1 | 3 ± 1 | 4 ± 1 |
| Mean Arterial Blood Pressure (mmHg) | ||||||
| Baseline | 91 ± 3 | 91 ± 3 | 88 ± 2 | 90 ± 1† | 92 ± 3 | 91 ± 3 |
| 5 minutes | 94 ± 2 | 93 ± 3 | 90 ± 2* | 92 ± 2*† | 91 ± 3 | 94 ± 4 |
| Δ | 3 ± 2 | 2 ± 1 | 2 ± 1 | 2 ± 1 | −1 ± 1 | 3 ± 1 |
| Brachial Artery Diameter (cm) | ||||||
| Baseline | 0.36 ± 0.02 | 0.36 ± 0.02 | 0.38 ± 0.01 | 0.38 ± 0.01 | 0.32 ± 0.01 | 0.32 ± 0.02 |
| 5 minutes | 0.38 ± 0.02* | 0.38 ± 0.02* | 0.40 ± 0.01* | 0.40 ± 0.01* | 0.34 ± 0.01* | 0.33 ± 0.02* |
| Δ | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.01 |
| Forearm Blood Flow (mL/dL forearm volume/min) | ||||||
| Baseline | 8±2 | 10±1 | 5±1 | 4±0† | 9±1 | 6±1† |
| 5 minutes | 35±5* | 34±4* | 17±1* | 15±1*† | 35±5* | 29±2*† |
| Δ | 27±4 | 25±4 | 12±1 | 11±1 | 27±3 | 23±2 |
| Forearm Vascular Conductance (mL/dL forearm volume/min/100 mmHg) | ||||||
| Baseline | 8±2 | 11±1 | 6±1 | 5±0† | 9±2 | 6±1† |
| 5 minutes | 37±5* | 37±5* | 19±1* | 16±1*† | 38±5* | 30±2*† |
| Δ | 29±4 | 26±4 | 13±1 | 12±1† | 29±4 | 24±2 |
p<0.05 vs Baseline;
p<0.05 vs Control
Fig. 1. Hyperemic response to dynamic handgrip exercise and role of the NO-cGMP pathway.
Forearm blood flow increased with exercise (Main effect of exercise, p<0.01). Blood flow was not altered by sildenafil citrate (Main effect of SDF, p=0.81). L-NMMA resulted in a reduction in blood flow alone (Main effect of L-NMMA, p<0.01) and when combined with sildenafil citrate (Main effect of SDF+L-NMMA, p=0.04). *p<0.05 vs Rest, †p<0.05 vs Control.
Forearm blood flow and forearm vascular conductance increased with exercise (Main effect of exercise, FBF: p<0.01; FVC: p<0.01). Blood flow and vascular conductance were not altered by sildenafil citrate (Main effect of SDF, FBF: p=0.81; FVC: p=0.59). L-NMMA resulted in a reduction in blood flow and vascular conductance alone (Main effect of L-NMMA, FBF: p<0.01; FVC: p=0.02) and when combined with sildenafil citrate (Main effect of SDF+L-NMMA, FBF: p=0.04; FVC: p=0.03). When responses were assessed as a change from baseline [Absolute (Δ)], any differences between forearm blood flow during control and experimental conditions (SDF, L-NMMA, SDF+L-NMMA) were no longer observed (Δ FBF p-value range 0.07–0.16). In regards to forearm vascular conductance, although there was no effect of SDF (p=0.33) or SDF+L-NMMA (p=0.053), there was a significant effect of L-NMMA (p=0.04) on the change in forearm vascular conductance (Δ FVC).
Sodium nitroprusside
Reductions in blood pressure (Main effect of NTP, p<0.01) and increases in brachial artery diameter (Main effect of NTP, p<0.01) and heart rate (Main effect of NTP, p<0.01) were observed with intra-arterial infusion of sodium nitroprusside. Blood pressure and brachial artery diameter were not altered by oral SDF (Main effect of SDF: Blood pressure, p=0.98; Diameter, p=0.48; Main effect of SDF+L-NMMA: Blood pressure, p=0.53; Diameter, p=0.87). Although heart rate increased following oral SDF when compared to control conditions (Main effect of SDF, p=0.02), there was no change in heart rate following SDF plus L-NMMA (Main effect of SDF+L-NMMA, p=0.65).
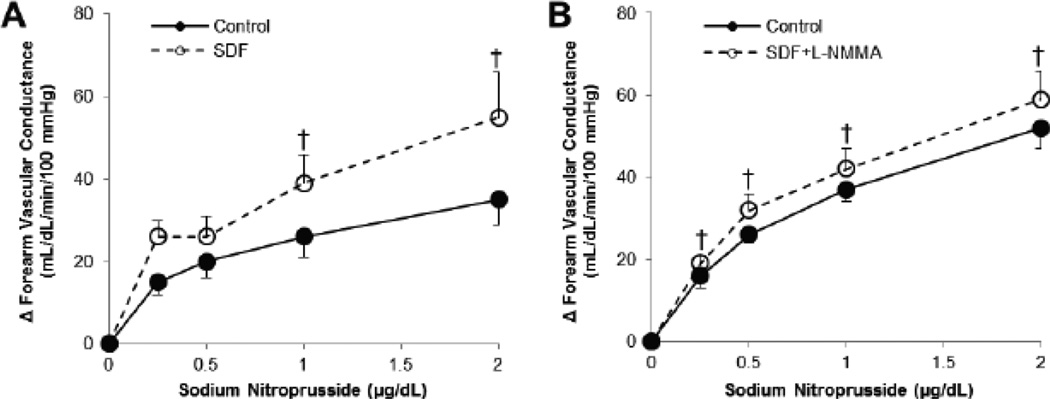
Forearm blood flow and vascular conductance increased with infusion of sodium nitroprusside (Main effect of NTP, FBF: p<0.01; FVC: p<0.01; Table 3). The increases in blood flow and conductance with sodium nitroprusside infusion were greater following oral sildenafil citrate when compared to control (Main effect of SDF, ΔFBF: p<0.01; ΔFVC: p<0.01; Figure 2). Blood flow and vascular conductance with infusion of sodium nitroprusside did not appear to be altered following sildenafil citrate plus L-NMMA (Main effect of SDF+L-NMMA, FBF: p=0.51; FVC: p=0.48; Table 3). However, when responses were assessed as a change from baseline (Δ), differences between control and sildenafil citrate plus L-NMMA were observed (Main effect of SDF+L-NMMA, ΔFBF: p=0.04; ΔFVC: p=0.049; Figure 2); thus L-NMMA did not alter the vasodilatory effect of sildenafil citrate on sodium nitroprusside-mediated increases in blood flow and vascular conductance.
Table 3.
Hemodynamic responses to intra-arterial infusion of sodium nitroprusside.
| Protocol 1 | Protocol 3 | |||
|---|---|---|---|---|
| Control | SDF | Control | SDF+LNMMA | |
| Heart Rate (beat/min) | ||||
| Baseline | 61 ± 3 | 63 ± 4 | 59 ± 2 | 59 ± 3 |
| 0.25 µg/dL/min | 61 ± 4 | 63 ± 5 | 59 ± 2 | 56 ± 2 |
| 0.5 µg/dL/min | 59 ± 4 | 62 ± 5 | 60 ± 2 | 56 ± 2 |
| 1.0 µg/dL/min | 61 ± 4 | 64 ± 5 | 60 ± 3 | 60 ± 3 |
| 2.0 µg/dL/min | 63 ± 5abcd | 67 ± 5abcd | 62 ± 3abc | 62 ± 2abc |
| Mean Arterial Blood Pressure (mmHg) | ||||
| Baseline | 90 ± 3 | 88 ± 2 | 89 ± 3 | 89 ± 3 |
| 0.25 µg/dL/min | 89 ± 2 | 86 ± 2 | 86 ± 3a | 87 ± 3a |
| 0.5 µg/dL/min | 87 ± 3a | 86 ± 2a | 84 ± 3ab | 85 ± 3ab |
| 1.0 µg/dL/min | 86 ± 2a | 84 ± 2a | 83 ± 3ab | 83 ± 3ab |
| 2.0 µg/dL/min | 85 ± 3abc | 84 ± 2abc | 80 ± 3abcd | 81 ± 3abcd |
| Brachial Artery Diameter (cm) | ||||
| Baseline | 0.36 ± 0.02 | 0.36 ± 0.02 | 0.32 ± 0.02 | 0.32 ± 0.02 |
| 0.25 /dL/min µg | 0.37 ± 0.02 | 0.37 ± 0.02 | 0.33 ± 0.01a | 0.33 ± 0.02a |
| 0.5 µg/dL/min | 0.37 ± 0.02a | 0.37 ± 0.02a | 0.34 ± 0.02ab | 0.33 ± 0.02ab |
| 1.0 µg/dL/min | 0.37 ± 0.02ab | 0.39 ± 0.02ab | 0.35 ± 0.02abc | 0.35 ± 0.02abc |
| 2.0 µg/dL/min | 0.38 ± 0.02abc | 0.39 ± 0.02abc | 0.35 ± 0.02abcd | 0.36 ± 0.02abcd |
| Forearm Blood Flow (mL/dL forearm volume/min) | ||||
| Baseline | 5±1 | 9±2 | 10±3 | 5±1 |
| 0.25 µg/dL/min | 19±3a | 31±4a† | 24±3a | 20±2a |
| 0.5 µg/dL/min | 22±4a | 31±6a | 31±4ab | 31±4ab |
| 1.0 µg/dL/min | 28±5a | 41±7a† | 39±5abc | 39±4abc |
| 2.0 µg/dL/min | 35±6abc | 54±10abcd† | 49±6abcd | 52±6abcd |
| AUC | 41±7 | 61±11† | 53±5 | 62±7† |
| Forearm Vascular Conductance (mL/dL forearm volume/min/100 mmHg) | ||||
| Baseline | 6±1 | 10±2 | 12±3 | 6±1 |
| 0.25 µg/dL/min | 21±4a | 36±5a† | 28±4a | 24±2a |
| 0.5 /dL/min µg | 26±5a | 36±7a | 38±4ab | 38±4ab |
| 1.0 µg/dL/min | 32±6a | 49±9a† | 49±5abc | 48±5abc |
| 2.0 µg/dL/min | 41±6abc | 65±13abcd† | 64±6abcd | 65±7abcd |
| AUC | 48±8 | 73±13† | 67±6 | 78±8† |
p<0.05 vs Baseline,
p<0.05 vs 0.25 µg/dL/min,
p<0.05 vs 0.5 µg/dL/min,
p<0.05 vs 1.0 µg/dL/min;
p<0.05 vs Control.
AUC = Area Under the Curve (AU).
Fig. 2. Vasodilatory responses to intra-arterial infusion of sodium nitroprusside.
The rise in forearm vascular conductance in response to intra-arterial sodium nitroprusside infusion was calculated (FVCinfusion – FVCbaseline). Sodium nitroprusside-mediated vasodilation was increased in oral SDF (A: Main effect of drug versus control, p<0.01) and remained high when sildenafil citrate was combined with L-NMMA (B: Main effect of drug versus control, p=0.049). †p<0.05 vs Control.
DISCUSSION
Results from the present study show that sildenafil citrate has no effect on the steady-state blood flow response to dynamic exercise in healthy humans. We also found that the NOS inhibitor, L-NMMA, had only a minor effect on exercise hyperemia. The lack of an effect of sildenafil citrate and a limited effect of L-NMMA on exercise hyperemia together support the idea of physiological redundancy such that activation of the NO-cGMP pathway in healthy humans plays a relatively minor role in normal exercise vasodilation (Endo et al. 1994; Gordon et al. 2002; Martin et al. 2006; Shoemaker et al. 1997). Our results further suggest that the role for NO in exercise hyperemia in healthy adults is not limited by PDE-5 activity. Consistent with this, other vasodilator pathways besides the NO-cGMP pathway have been shown contribute to exercise hyperemia, including: 1) prostacyclin, 2) adenosine triphosphate (ATP), and 3) K+ channels [Reviewed in (Joyner and Casey 2015)]. It is also likely that in healthy humans the NO-cGMP pathway is maximally activated in a way that further augmentation of its effects does not alter exercise responses (i.e. “ceiling effect”). Consistent with this, we did observe a trend for an increase in resting blood flow with sildenafil citrate in the healthy adults studied. These data suggest PDE-5 may be capable of keeping up with cGMP metabolism at rest; however, if the NO-cGMP pathway was maximally activated during exercise, further increases in cGMP levels might not appreciably affect forearm blood flow.
Despite no observable effect of sildenafil citrate on exercise hyperemia in healthy adults (Table 2, Figure 1), its administration may be effective in improving exercise hyperemia in older adults and/or clinical conditions. Consistent with this, other approaches to potentiate the NO pathway (e.g. dietary nitrate) have been effective at improving exercise hyperemia in such populations (Casey et al. 2015; Ferguson et al. 2013). In addition, sildenafil citrate has been shown previously to increase resting forearm blood flow in conditions with impaired endothelial function such as smoking (Kimura et al. 2003; Vlachopoulos et al. 2004) and cardiovascular disease (Halcox et al. 2002; Hryniewicz et al. 2005; Schofield et al. 2003). Additionally, Attina and colleagues have shown sildenafil citrate to improve post-exercise blood flow in hypertensive patients, despite little-to-no effect in normotensive controls (Attina et al. 2008). Thus, in the presence of impaired endothelial NOS (e.g. conditions exhibiting endothelial dysfunction) and/or upregulated PDE-5, sildenafil citrate may be a viable therapeutic option to restore blood flow via increases in cGMP (Dishy et al. 2004; Kimura et al. 2003; Robinson et al. 2006). Future work in this area is necessary.
As proof-of-principle, we also infused sodium nitroprusside to examine the effect of sildenafil citrate on exogenous NO-cGMP activation. Our results show that sildenafil citrate increases the hemodynamic response to sodium nitroprusside infusion approximately two-fold [Table 3; (Blaise et al. 2010; Dishy et al. 2001)]. After taking into consideration a baseline effect, the increase in sodium nitroprusside-mediated vasodilation with sildenafil citrate was not altered with co-infusion of L-NMMA (Figure 2). The lack of an effect of L-NMMA is to be expected given L-NMMA inhibits the activity of endothelial NOS and sodium nitroprusside-mediated vasodilation is achieved independent of the endothelium. These data thus highlight the ability of sildenafil citrate to increase forearm blood flow independent of endothelial NOS (eNOS), and also confirm effective dosing. With this, it is important to note that although we hypothesized any effect of sildenafil citrate on exercise hyperemia would be specific to the NO-pathway (with a primary focus on eNOS), there are other NOS isoforms, as well as non-NO sources of cGMP (e.g. natriuretic peptides), that should be considered in future work.
Experimental Considerations
First, sildenafil citrate was administered orally, resulting in systemic distribution of the drug. While sildenafil citrate is not associated with clinically significant changes in systemic hemodynamic parameters (Jackson et al. 1999), it may cause increases in muscle sympathetic nerve activity (MSNA) and plasma norepinephrine (Dopp et al. 2013; Phillips et al. 2000). Enhanced vasoconstriction due to an increase in MSNA could partially mask any increase in exercise hyperemia as a result of sildenafil citrate. However, an increase in sodium nitroprusside-mediated vasodilation with combined sildenafil citrate suggests this is unlikely to have a major effect on present findings. Second, our results are specific to the low (15% MVC) exercise workload studied. The contribution of NO-mediated vasodilation has been shown to be workload-specific and may be altered when combined with environmental stressors [e.g. hypoxia (Casey et al. 2011; Wilkins et al. 2008)]. Therefore we are unable to comment on whether conclusions would be altered with the use of sildenafil citrate under more physiological stressful conditions. Third, data were collected across three separate research cohorts in both men and women, thus limiting our ability to control for inter-subject variability. However, we have shown previously that the role of NO in exercise hyperemia is unlikely to be sex-dependent (Kellawan et al. 2015). In addition, the method of signal processing differed between protocols – resulting in lower absolute blood flow values in Protocol 2. Importantly, both methods of signal processing are commonly used, validated, and are consistent with flow velocity signals produced by the Doppler signal converter (Herr et al. 2010). Thus, differences in methodological approaches between protocols are unlikely to alter main conclusions.
CONCLUSIONS
Despite improvements in sodium nitroprusside-mediated vasodilation, oral sildenafil citrate failed to augment exercise hyperemia in young, healthy subjects. These observations most likely reflect a minor contribution of NO and the cGMP pathway during exercise hyperemia in healthy young humans. With this, we propose the importance of the NO-cGMP pathway may be greater in persons with underlying endothelial dysfunction where even modest increases in cGMP through sildenafil citrate may result in significant improvements in vasodilator responses and highlight this as an important area for follow-up work.
Acknowledgments
Many thanks to our research participants. Technical and other support was provided by Madhuri Somaraju, Christopher Johnson, Karen Krucker, Brandon Madery, Shelly Roberts, Branton Walker, and Brian Welch (Mayo Clinic), and Meghan Crain, Josh Sebranek, Marlowe Eldridge, Brad Walker, John Harrell, Rebecca Johansson, and Garrett Peltonen (University of Wisconsin).
FUNDING
Financial support was provided by the National Institutes of Health (NIH) HL46493 (MJJ), HL078019 (MJJ), HL105820 (WGS), RR17520 (TBC), UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), as well as the Mayo Clinic Department of Anesthesiology.
ABBREVIATIONS
- ACH
Acetylcholine
- ANOVA
Analysis of variance
- ATP
Adenosine triphosphate
- AU
Arbitrary units
- AUC
Area under the curve
- BMI
Body mass index
- cGMP
Cyclic guanosine monophosphate
- eNOS
Endothelial nitric oxide synthase
- FBF
Forearm blood flow
- FVC
Forearm vascular conductance
- L-NMMA
L-NG-monomethyl arginine
- MSNA
Muscle sympathetic nerve activity
- MVC
Maximal voluntary contraction
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- NTP
Sodium nitroprusside
- PDE-5
Phosphodiesterase-5
- SDF
Sildenafil citrate.
Footnotes
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
CONFLICT OF INTEREST
The authors declare no relevant conflicts of interest.
REFERENCES
- Attina TM, Malatino LS, Maxwell SR, Padfield PL, Webb DJ. Phosphodiesterase type 5 inhibition reverses impaired forearm exercise-induced vasodilatation in hypertensive patients. J Hypertens. 2008;26:501–507. doi: 10.1097/HJH.0b013e3282f382ff. [DOI] [PubMed] [Google Scholar]
- Blaise S, Hellmann M, Roustit M, Isnard S, Cracowski JL. Oral sildenafil increases skin hyperaemia induced by iontophoresis of sodium nitroprusside in healthy volunteers. Br J Pharmacol. 2010;160:1128–1134. doi: 10.1111/j.1476-5381.2010.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SJ, Kingwell BA, McConell GK. Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes. 1999;48:1815–1821. doi: 10.2337/diabetes.48.9.1815. [DOI] [PubMed] [Google Scholar]
- Casey DP, Curry TB, Wilkins BW, Joyner MJ. Nitric oxide-mediated vasodilation becomes independent of beta-adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol. 2011;110:687–694. doi: 10.1152/japplphysiol.00787.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Treichler DP, Ganger CTt, Schneider AC, Ueda K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J Appl Physiol. 2015;118(1985):178–186. doi: 10.1152/japplphysiol.00662.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:22. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Dishy V, Harris PA, Pierce R, Prasad HC, Sofowora G, Bonar HL, Wood AJ, Stein CM. Sildenafil does not improve nitric oxide-mediated endothelium-dependent vascular responses in smokers. Br J Clin Pharmacol. 2004;57:209–212. doi: 10.1046/j.1365-2125.2003.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishy V, Sofowora G, Harris PA, Kandcer M, Zhan F, Wood AJ, Stein CM. The effect of sildenafil on nitric oxide-mediated vasodilation in healthy men. Clin Pharmacol Ther. 2001;70:270–279. doi: 10.1067/mcp.2001.117995. [DOI] [PubMed] [Google Scholar]
- Dopp JM, Agapitov AV, Sinkey CA, Haynes WG, Phillips BG. Sildenafil increases sympathetically mediated vascular tone in humans. Am J Hypertens. 2013;26:762–769. doi: 10.1093/ajh/hpt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SJ, New G, Tran BT, Harper RW, Meredith IT. Relative contribution of vasodilator prostanoids and NO to metabolic vasodilation in the human forearm. Am J Physiol. 1999;276:H663–H670. doi: 10.1152/ajpheart.1999.276.2.H663. [DOI] [PubMed] [Google Scholar]
- Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol. 1995;488:259–265. doi: 10.1113/jphysiol.1995.sp020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation. 1994;90:2886–2890. doi: 10.1161/01.cir.90.6.2886. [DOI] [PubMed] [Google Scholar]
- Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. The Journal of physiology. 2013;591:547–557. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsenn U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with N(G)-nitro-L-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5:689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation. 1994;90:2853–2858. doi: 10.1161/01.cir.90.6.2853. [DOI] [PubMed] [Google Scholar]
- Gordon MB, Jain R, Beckman JA, Creager MA. The contribution of nitric oxide to exercise hyperemia in the human forearm. Vasc Med. 2002;7:163–168. doi: 10.1191/1358863x02vm439oa. [DOI] [PubMed] [Google Scholar]
- Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol. 2005;562:617–628. doi: 10.1113/jphysiol.2004.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcox JP, Nour KR, Zalos G, Mincemoyer RA, Waclawiw M, Rivera CE, Willie G, Ellahham S, Quyyumi AA. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol. 2002;40:1232–1240. doi: 10.1016/s0735-1097(02)02139-3. [DOI] [PubMed] [Google Scholar]
- Harrell JW, Johansson RE, Evans TD, Sebranek JJ, Walker BJ, Eldridge MW, Serlin RC, Schrage WG. Preserved Microvascular Endothelial Function in Young, Obese Adults with Functional Loss of Nitric Oxide Signaling. Front Physiol. 2015;6 doi: 10.3389/fphys.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen I, Saltin B, Kemppainen J, Sipila HT, Oikonen V, Nuutila P, Knuuti J, Kalliokoski K, Hellsten Y. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. Am J Physiol Heart Circ Physiol. 2011;300:21. doi: 10.1152/ajpheart.00996.2010. [DOI] [PubMed] [Google Scholar]
- Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol. 2010;298:19. doi: 10.1152/ajpheart.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniewicz K, Dimayuga C, Hudaihed A, Androne AS, Zheng H, Jankowski K, Katz SD. Inhibition of angiotensin-converting enzyme and phosphodiesterase type 5 improves endothelial function in heart failure. Clin Sci. 2005;108:331–338. doi: 10.1042/CS20040266. [DOI] [PubMed] [Google Scholar]
- Hsu AR, Barnholt KE, Grundmann NK, Lin JH, McCallum SW, Friedlander AL. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J Appl Physiol. 2006;100:2031–2040. doi: 10.1152/japplphysiol.00806.2005. [DOI] [PubMed] [Google Scholar]
- Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999;83:13C–20C. doi: 10.1016/s0002-9149(99)00043-0. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev. 2015;95:549–601. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol. 1997;83:1785–1796. doi: 10.1152/jappl.1997.83.6.1785. [DOI] [PubMed] [Google Scholar]
- Katz SD, Krum H, Khan T, Knecht M. Exercise-induced vasodilation in forearm circulation of normal subjects and patients with congestive heart failure: role of endothelium-derived nitric oxide. J Am Coll Cardiol. 1996;28:585–590. doi: 10.1016/0735-1097(96)00204-5. [DOI] [PubMed] [Google Scholar]
- Kellawan JM, Johansson RE, Harrell JW, Sebranek JJ, Walker BJ, Eldridge MW, Schrage WG. Exercise vasodilation is greater in women: contributions of nitric oxide synthase and cyclooxygenase. Eur J Appl Physiol. 2015;115:1735–1746. doi: 10.1007/s00421-015-3160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Higashi Y, Hara K, Noma K, Sasaki S, Nakagawa K, Goto C, Oshima T, Yoshizumi M, Chayama K. PDE5 inhibitor sildenafil citrate augments endothelium-dependent vasodilation in smokers. Hypertension. 2003;41:1106–1110. doi: 10.1161/01.HYP.0000068202.42431.CC. [DOI] [PubMed] [Google Scholar]
- Lee JS, Stebbins CL, Jung E, Nho H, Kim JK, Chang MJ, Choi HM. Effects of chronic dietary nitrate supplementation on the hemodynamic response to dynamic exercise. Am J Physiol Regul Integr Comp Physiol. 2015;309:R459–R466. doi: 10.1152/ajpregu.00099.2015. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports Med. 2003;33:1013–1035. doi: 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Bimodal distribution of vasodilator responsiveness to adenosine due to difference in nitric oxide contribution: implications for exercise hyperemia. J Appl Physiol. 2006;101:492–499. doi: 10.1152/japplphysiol.00684.2005. [DOI] [PubMed] [Google Scholar]
- Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–374. doi: 10.1161/01.cir.98.4.369. [DOI] [PubMed] [Google Scholar]
- Mayer BX, Mensik C, Krishnaswami S, Derendorf H, Eichler HG, Schmetterer L, Wolzt M. Pharmacokinetic-pharmacodynamic profile of systemic nitric oxide-synthase inhibition with L-NMMA in humans. British journal of clinical pharmacology. 1999;47:539–544. doi: 10.1046/j.1365-2125.1999.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland RB, Goldstein I, Traish A. Sildenafil, a novel inhibitor of phosphodiesterase type 5 in human corpus cavernosum smooth muscle cells. Life Sci. 1998;62:309–318. doi: 10.1016/s0024-3205(98)00158-1. [DOI] [PubMed] [Google Scholar]
- Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. British journal of clinical pharmacology. 2002;53(Suppl 1):5S–12S. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BG, Kato M, Pesek CA, Winnicki M, Narkiewicz K, Davison D, Somers VK. Sympathetic activation by sildenafil. Circulation. 2000;102:3068–3073. doi: 10.1161/01.cir.102.25.3068. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Ludlam CA, Boon NA, Newby DE. Phosphodiesterase type 5 inhibition does not reverse endothelial dysfunction in patients with coronary heart disease. Heart. 2006;92:170–176. doi: 10.1136/hrt.2004.059683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Schalcher C, Schad K, Brunner-La Rocca HP, Schindler R, Oechslin E, Scharf C, Suetsch G, Bertel O, Kiowski W. Interaction of sildenafil with cAMP-mediated vasodilation in vivo. Hypertension. 2002;40:763–767. doi: 10.1161/01.hyp.0000036027.71527.3e. [DOI] [PubMed] [Google Scholar]
- Schofield RS, Edwards DG, Schuler BT, Estrada J, Aranda JM, Pauly DF, Hill JA, Aggarwal R, Nichols WW. Vascular effects of sildenafil in hypertensive cardiac transplant recipients. Am J Hypertens. 2003;16:874–877. doi: 10.1016/s0895-7061(03)01006-9. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. Circulation to skeletal muscle. In: Abboud JSaF., editor. Handbook of Physiology The Cardiovascular System Peripheral Circulation and Blood Flow. Bethesda, MD: American Physiological Society; 1983. pp. 319–370. [Google Scholar]
- Shepherd JR, Joyner MJ, Dinenno FA, Curry TB, Ranadive SM. Prolonged adenosine triphosphate infusion and exercise hyperemia in humans. J Appl Physiol. 2016;121(1985):629–635. doi: 10.1152/japplphysiol.01034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol. 1997;273:H2388–H2395. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- Singh TP, Rohit M, Grover A, Malhotra S, Vijayvergiya R. A randomized, placebo-controlled, double-blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. Am Heart J. 2006;151:e1–e5. doi: 10.1016/j.ahj.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachopoulos C, Tsekoura D, Alexopoulos N, Panagiotakos D, Aznaouridis K, Stefanadis C. Type 5 phosphodiesterase inhibition by sildenafil abrogates acute smoking-induced endothelial dysfunction. Am J Hypertens. 2004;17:1040–1044. doi: 10.1016/j.amjhyper.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol. 2008;586:1195–1205. doi: 10.1113/jphysiol.2007.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Kapoor S. Contribution of endothelium-derived relaxing factor to exercise-induced vasodilation in humans. J Appl Physiol. 1993;75:2740–2744. doi: 10.1152/jappl.1993.75.6.2740. [DOI] [PubMed] [Google Scholar]