Abstract
Multiple sclerosis (MS) is a prototype autoimmune disease of the central nervous system (CNS). Currently, there is no drug that provides a cure for MS. To date, all immunotherapeutic drugs target relapsing remitting MS (RRMS); it remains a daunting medical challenge in MS to develop therapy for secondary progressive MS (SP-MS). Since the approval of the non-selective sphingosine-1-phosphate (S1P) receptor modulator FTY720 (fingolimod [Gilenya®]) for RR-MS in 2010, there have been many emerging studies with various selective S1P receptor modulators in other autoimmune conditions. In this article, we will review how S1P receptor may be a promising therapeutic target for SP-MS and other autoimmune diseases such as psoriasis, polymyositis and lupus.
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory disorder of the central nervous system (CNS) that results in damage to the myelin sheath (demyelination) and axons (axonopathy). MS pathogenesis not only involves autoreactive myelin-specific T cells, resulting in nerve axon demyelination. B-cells also play significant roles via cytokine secretion, autoantibody production and antigen presentation to T-cells [1,2]. MS most commonly presents with a relapsing remitting (RR) course, in which discrete episodes of neurologic dysfunction (referred to as relapses) are separated by clinically quiescent periods (referred to as remissions). However, most patients ultimately enter a progressive stage (secondary progressive MS, or SPMS) characterized by the inexorable accumulation of disability despite a reduction in the rate of relapses. Great strides have been made in the development of drugs that are therapeutically beneficial in RRMS, resulting in the introduction of 13 disease modifying agents approved by the Food and Drug Administration (FDA) for clinical use. In contrast, the treatment of SPMS remains suboptimal. The only FDA sanctioned drug for the management of progressive MS is mitoxantrone, a chemotherapeutic agent that is modestly effective and carries significant toxicities, including cardiomyopathy and leukemia. There is a dire need for novel agents that stabilize the disease course in patients with SPMS.
Although focal blood-brain barrier breakdown and perivascular infiltrates are relatively more common in RRMS, a substantial body of data indicates that autoimmune inflammation is still relevant, and contributes to CNS tissue damage, during the SP stage. However, there is evidence that the nature of the aberrant immune response evolves over time, such that the efficacy of individual disease modifying agents might differ between the RR and SP phases [1,2,3]. Furthermore, it has been postulated that, in parallel to the neuroinflammatory attack, a neurodegenerative process contributes to CNS tissue damage and clinical disability in progressive MS [4,5]. Hence, the ideal disease modifying agent for SPMS would combine novel immunomodulatory and neuroprotective (and even neuroregenerative) properties.
The sphingosine-1-phosphate receptor (S1PR) has emerged as a therapeutic target in MS. Fingolimod (FTY720; trade name Gilenya), a small molecule S1P modulator, is the first oral agent approved by the FDA for the reduction of clinical relapses and delay of clinical disability in relapsing forms of MS. Its putative mechanism of action is to block the egress of effector T cells from peripheral lymphoid tissues to the circulation and, ultimately, to the CNS. S1P modulators could, theoretically, also act to limit demyelination and axonopathy within the target organ. Preclinical studies suggest that antagonism of S1P on neurons and glial cells increases their resistance to toxic factors [6,7].
The goal of this reviewis to assess our understanding of the S1P systemin immune regulation and to review evidence on how targeting this system might be beneficial for treatment of MS and other autoimmune diseases.
2. Sphingosine-1-phosphate (S1P) receptor (S1PR) modulators for treatment of multiple sclerosis
2.1. Clinical features of multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory disorder of the central nervous system (CNS) that results in damage to the myelin sheath leading to paralytic disease. MS is the most common cause of non-traumatic neurological disability among young adults in the Western hemisphere. It affects over 400,000 individuals in the United States alone, approximately 2.5 million people worldwide, and its incidence is on the rise [8].
The symptoms of MS are protean, reflecting the multifocal nature of the disease. They include visual loss (due to involvement of the optic nerves), spastic paraparesis, numbness and bladder incontinence (due to spinal cord lesions), gait imbalance, double vision, and tremor (due to brainstem or cerebellar lesions) and dementia (due to cerebral involvement).
2.2. Multiple sclerosis disease modifying therapies
Interferon-beta, glatiramer acetate, natalizumab, daclizumab, alemtuzumab, and several oral agents are approved for use in relapsing remitting forms of MS. The effect of drug in such patients is presumed to be an effect on relapses, not disability progression, as efficacy on disability progression has not been confirmed in progressive patients. Mitoxantrone is the only therapy approved for the treatment of SPMS in the USA irrespective of relapses. However, there are no convincing data demonstrating the efficacy of mitoxantrone in SPMS patients without superimposed relapses, and the risks associated with mitoxantrone (heart failure, leukemia) limit the use of the product [9]. Of the SPMS trials with the three interferon-beta products, one Phase III study of subcutaneous IFNβ-1b in a European population of SPMS patients showed a significant reduction in disability as measured by Expanded Disability Status Scale (EDSS) [10]. However, a companion study of the same product in North American SPMS patients did not show a reduction in disability as measured by EDSS [11]. In the European Union (EU), IFNβ-1b carries an indication for SPMS with active disease, evidenced by relapses. Mitoxantrone is approved for use in only a few countries in Europe. Thus, there remains a considerable medical need to find therapies that will be effective in delaying disability progression in patients with SPMS.
2.3. Sphingosine 1-phosphate (S1P) receptor (S1PR) modulators
Fingolimod (FTY720; Gilenya™) (Novartis Pharma AG) is the first member of the new class of therapeutic compounds—the S1P receptor (S1PR) modulators. It was approved by the FDA in 2010 for treatment of relapsing MS based on its clinical and MRI efficacy [12,13,14]. Sphingosine is an essential structural component of the lipid bilayer of the plasma membrane and plays an important role in cell signaling [15]. Fingolimod is a structural analog of sphingosine; both sphingosine and fingolimod are phosphorylated by sphingosine kinase-1 (SphK1) or SphK2 into their active forms, which bind to specific S1P receptors [16,17]. There are five S1P receptors, named S1P1–5, in humans. They are differentially expressed by immune and central nervous system (CNS) cell types [18]. T and B cells mainly express S1P1; Natural Killer (NK) cells express S1P5; dendritic cells (DCs), mast cells and macrophages express S1P1 and S1P2 [19,20]. CNS glia and neurons express S1P1–3 and S1P5 [21].
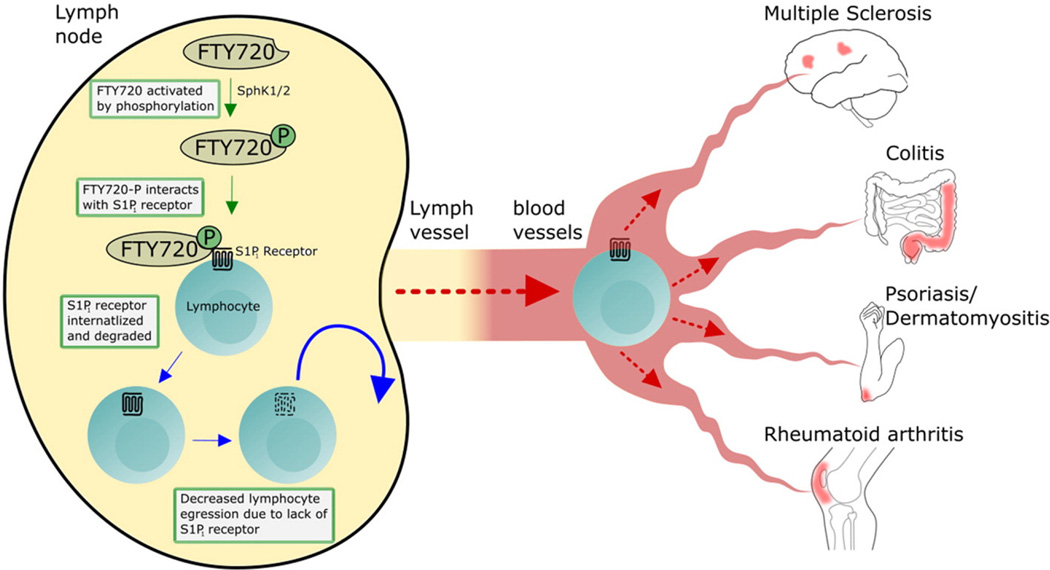
S1P is metabolized intra-cellularly and then excreted to exert autocrine or paracrine function. Under physiologic conditions, lymphocytes egress from the lymph node (LN) through migration along an S1P gradient [22,23]. When fingolimod is phosphorylated, it binds to all S1PRs, except S1P2, with an affinity comparable to the affinity of S1P [16,17]. S1P1 binding on lymphocytes results in internalization and ubiquitin-mediated degradation of the receptor (functional antagonism), as well as reduction in S1P1 mRNA levels, therefore blocking lymphocyte S1P1-mediated LN egress [23,24,25]. This includes potentially encephalitogenic T cells and their naïve progenitors that are thought to be primed in the LN in MS (Fig. 1).
Fig. 1.
S1P1 receptor modulator as therapeutic target for autoimmune diseases. The prototype S1P1 receptor modulator FTY720 was used to demonstrate this class of therapies. FTY720 is phosphorylated by sphingosine kinase (SphK) 1/2 to be activated. The phosphorylated FTY720 binds to S1P1 receptor which causes internalization of S1P1 on lymphocytes, thereby inhibiting the egress of lymphocytes from lymph nodes. The subsequent outcome is the reduction of recirculation of the auto-reactive lymphocytes to target organ in cases of multiple sclerosis, colitis, psoriasis, dermatomyositis and rheumatoid arthritis. Copyright Ali Mirza and Yang Mao-Draayer, reprint with permission.
Other S1PR modulators, including ozanimod and amiselimod, are also being investigated for the treatment of relapsing MS. Ozanimod is a S1P1 and S1P5 modulator and, unlike fingolimod, does not require phosphorylation for activation. It also has a significantly shorter half-life of 19 h. Phase 2 studies have shown reduction in MRI lesion activity where the number of gadolinium-enhancing MRI lesions after 12 weeks and 24 weeks was the primary endpoint. It is currently in an open label Phase 3 trial to further elucidate the safety profile, seen to be favorable in the Phase 2 trials [26]. Amiselimod has also shown efficacy with a favorable safety profile for relapsing MS patients. This compound is an S1P1 modulator with a half-life between 380 and 420 h. A Phase 2 trial with a primary endpoint of assessing the number of gadolinium enhanced T1-weighted lesions on monthly MRI revealed positive outcomes with significant improvement seen on imaging of those who received drug compared to placebo [27].
2.4. Siponimod (BAF312) as a potential therapy for SP-MS
Siponimod (BAF312) is a novel S1P receptor modulator that leads to the reduction of peripheral lymphocyte counts in blood [28]. The mechanism of action is similar to that of the S1P receptor modulator fingolimod, but siponimod belongs to a different chemical class. Whereas fingolimod acts as an agonist on four out of five S1P receptors (namely S1P1, S1P3, S1P4, and S1P5); siponimod is an S1P1/S1P5-selective agonist [29,30]. In contrast to fingolimod, siponimod does not require a phosphorylation step in vivo. The half-life of siponimod compared to fingolimod is shorter (approximately 30 h versus 200 h), therefore drug effects cease more rapidly after discontinuation. The siponimod receptor selectivity and shorter half-life has the potential to enable full efficacy in MS without targeting other S1P receptors (S1P3 and/or S1P4). These properties, together with the more rapid washout, may improve the safety profile. In SPMS, siponimod is CNS penetrant and therefore has the potential for synergistic anti-inflammatory effects in the periphery and neuroprotective effects in the target organ, making it an excellent candidate for a disease modifying therapy in SPMS [28].
In addition to the mode of action of siponimod through control of migratory and proinflammatory properties of T and B lymphocytes, siponimod may have direct beneficial effects in the CNS mediated by S1P1 and/or S1P5. Evidence from preclinical models suggests that siponimod may target S1P1 on astrocytes and/or S1P5 on oligodendrocytes [31]. Oligodendrocytes are responsible for myelinating axons, and failures in this system contribute to disease progression in MS patients. Preferential expression of S1P5 has been noted on mature oligodendrocytes, which may also express S1P1, S1P2 and S1P3 at lower levels [32,33,34]. In contrast, oligodendrocyte precursor cells (OPCs) show higher levels of S1P1 gene expression and lower levels of S1P5 and S1P3 expression [35,36]. The S1P receptors S1P1, S1P2, S1P3, and S1P5 are expressed by neural progenitor cells (NPCs) while neurons predominantly express S1P3 and S1P1 [37,38,39]. These receptors play an important role in neurogenesis, based on studies of mice with genetic deletions of S1P1 or SphK1/SphK2 double knockouts [40]. Sphingosine-1-phosphate is as effective as lysophosphatidic acid at enhancing survival and inducing proliferation and morphologic changes in primary cultures of NPCs [38,41,42]. S1P also triggers secretion of glutamate by primary hippocampal neurons as well as sensitizes the neuron cells to the depolarizing effects of glutamate secretion [39]. In cultured cortical neurons, fingolimod and S1P have been shown to protect against excitotoxic death [43]. S1P modulated neurite extension in cultured PC12 cells and dorsal root ganglion neurons, and enhanced nerve growth factor-induced excitability of adult sensory neurons [42,44].
2.5. A phase III multicenter randomized controlled trial of BAF312 in SP-MS
The EXPAND (EXploring the efficacy and safety of siponimod in PAtients with secoNDary progressive multiple sclerosis) trial is the largest double blind, placebo controlled Phase III study in SPMS. It is sponsored by Novartis to test the efficacy and safety of siponimod (BAF312), a second generation S1P1 modulator, in SP-MS. Currently it has enrolled over 1651 patients worldwide. The study population consists of ambulatory patients age 18 to 60 years with a diagnosis of MS defined by the 2010 Revised McDonald criteria and with a secondary progressive disease course. Progression denotes the continuous worsening of neurological impairment over at least 6 months not explained by incomplete recovery from relapses.
The primary objective is to demonstrate the efficacy of siponimod relative to placebo in delaying the time to 3-month confirmed disability progression as measured by EDSS in patients with SPMS. The first key secondary objective is to demonstrate the efficacy of siponimod relative to placebo in delaying in the time to six-month confirmed disability progression versus placebo as well as delaying the time to 3-month confirmed worsening of at least 20% from baseline in the timed 25-foot walk test (T25W). The second key secondary objective is to demonstrate the efficacy of siponimod relative to placebo in reducing the increase in T2 lesion volume on MRI from baseline to the end of the study. Recent results from the EXPAND trial demonstrate that the primary endpoint was met as siponimod reduced 3-month confirmed disability progression by 21% compared to placebo; in addition, its key secondary objective was met with a 26% reduction 6-month confirmed disability progression as well as significant reduction in the annualized relapse rate and MRI activities [45]. The positive EXPAND data are encouraging for a disease with such a high unmet need, as no other drug trial in SPMS has shown positive results.
In order to understand how siponimod works in SP-MS, the National Institute of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) sponsored the Autoimmunity Center of Excellence AMS04 study for University of Michigan in collaboration with 15 other US academic centers (Clinical Trials Number: NCT02330965). It is the first in-depth mechanistic study of its kind for SP-MS and involves a unique collaboration among academic centers, industry, and NIH. The ultimate goal is to understand how siponimod exerts its synergistic anti-inflammatory effects in the periphery and neuroprotective effects in the CNS, making it an effective treatment in SPMS. The ultimate goal is to develop future therapeutics and biomarkers to advance treatment of SP-MS.
3. S1P as a possible therapeutic target for other autoimmune conditions
While S1PR modulators (fingolimod and siponimod) have been demonstrated to have promising effects in MS, they also have the potential to be useful in inflammatory rheumatologic autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and idiopathic inflammatory myopathies such as polymyositis (PM) and dermatomyositis (DM). Fingolimod has been demonstrated to be effective in animal models of RA, while the use of siponimod is currently being explored in clinical trials for treatment of PM and DM [46,47]. Other clinical trials are ongoing for use of such compounds in SLE (Table 1).
Table 1.
Ongoing clinical trials with compounds targeting S1P receptors.
| Drug | S1P receptor activity | Target disease | Clinical trial phase | NCT number | Sponsor |
|---|---|---|---|---|---|
| Siponimod (BAF312) | S1P1,5 | SPMS | III-Active | 01665144 | Novartis Pharmaceuticals |
| RRMS | II-Active | 01185821 | |||
| Polymyositis | II-Recruiting | 01801917 | |||
| Dermatomyositis | II-Completed | 02029274 | |||
| SPMS | III-Active | 02330965 | NIH-NIAID | ||
| Fingolimod (FTY720) | S1P1,3,4,5 | RRMS | Approved | Novartis Pharmaceuticals | |
| Primary progressive MS | III-Completed | 00731692 | |||
| Stroke | II-Recruiting | 02002390 | Tianjin Medical University | ||
| Ponesimod (ACT128800) | S1P1,3,5 | RRMS | II-Active | 01093326 | Actelion |
| Chronic graft vs. host disease | II-Not yet recruiting | 02461134 | |||
| Plaque psoriasis | II-Completed | 01208090 | |||
| Relapsing MS | III-Recruiting | 02425644 | |||
| Ozanimod (RPC1063) | S1P1,5 | Relapsing MS | III-Active | 02294058 | Celgene |
| Ulcerative colitis | III-Recruiting | 02435992 | |||
| Crohn's disease | III-Recruiting | 02531113 | |||
| Ceralifimod (ONO-4641) | S1P1,5 | RRMS | II Extension-terminated | 01226745 | EMD Serono |
| Cenerimod (ACT-334441) | S1P1 | SLE | II-Recruiting | 02472795 | Actelion |
| Amiselimod (MT-1303) | S1P1 | RRMS | II-Completed | 01890655 | Mitsubishi Tanabe Pharma Corporation |
| Plaque psoriasis | II-Completed | 01987843 | |||
| SLE | I-Recruiting | 02307643 | |||
| Inflammatory bowel disease | I-Completed | 01666327 | |||
| Crohn's disease | II-Recruiting | 02378688 | |||
| LX3305 (LX2931) | S1P lyase inhibitor | RA | II-Completed | 00903383 | Lexicon Pharmaceuticals |
| AKP11 | S1P1 | Psoriasis | II-Recruiting | Unavailable | Akaal Pharma |
| KRP-203 | S1P1 | Subacute cutaneous lupus erythematous | II-Completed | 01294774 | Novartis Pharmaceuticals |
| Ulcerative colitis | II-Completed | 01375179 | |||
| ADP334 | S1P1 | Ulcerative colitis | II-Recruiting | Unavailable | Arena Pharmaceuticals |
| GSK2018682 | S1P1 | RRMS | I-Completed | 01466322 | GlaxoSmithKline |
3.1. S1P potential in autoimmunity: rheumatoid arthritis
While the etiology of RA is still unknown, it is a chronic autoimmune disease characterized by synovial hyperplasia and increased secretion of cytokines and chemokines from inflammatory cells into the synovium [48]. This leads to synovial inflammation and infiltration of inflammatory cells into the joint tissue, which ultimately leads to joint tissue destruction and systemic inflammatory burden [48]. S1P has been demonstrated to have a role in this disease process by causing the increased production of pro-inflammatory chemokines and cytokines, particularly Tumor Necrosis factor-alpha (TNF-α), a critical cytokine responsible for RA activity [49]. This in turn causes upregulation of S1PRs and may engender an exacerbation cycle that contributes to RA progression [49]. This upregulation of S1PR expression may also lead to increased inflammatory infiltration by lymphocytes and other inflammatory cells that further promote disease activity [49]. Fingolimod has been demonstrated to reduce the expression of interleukin 6 (IL-6) and TNF-α in the synovium as well as cause increased degradation of SP receptors in animal models, suggesting that S1Pmodulatorsmay have a place in treating this disease [46]. Degradation of S1P by spingosine-1-phosphate lyase (S1PL) can also alter inflammatory cell responses [50]. A previous double-blind, placebo-controlled, multi- dose study with LX3305, a S1P lyase (S1PL) developed by Lexicon Pharmaceuticals for patients who inadequately responded to methotrexate therapy alone was completed in 2011 (Clinical Trials Number: NCT00903383). The primary endpoint, which was the American College of Rheumatology 20% response criteria after 12 weeks, was achieved in 60% of patients, indicating that there is potential for benefit for patients [51,52]. Further investigations with these compounds may be forthcoming.
3.2. S1P potential in autoimmunity: systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is another chronic autoimmune disease with the potential to respond to S1PR modulators. It is characterized by the infiltration of inflammatory cells into target organs, increased production of cytokines, namely IL-6 and Interleukin-1 (IL-1), as well as the production of autoantibodies to multiple cytoplasmic and nuclear antigens in multiple organ systems [53,54,55]. The pathophysiology of this disease involves loss of tolerance to self-antigens, leading to aberrant activation of both B-cells and T-cells, with subsequent production of autoantibodies and direct tissue damage [55,56]. Preventing egress of these cells from the lymphatic tissue with S1PR modulators could therefore provide a new treatment modality. Currently, a double-blind, placebo controlled, dose-response study is underway with cenerimod, a S1PR modulator developed by Actelion Pharmaceuticals, Ltd., to assess biological activity, safety, and tolerability in subjects with SLE (Clinical Trials Number: NCT02472795).
3.3. S1P potential in autoimmunity: idiopathic inflammatory myopathies
Polymyositis (PM) is characterized by the infiltration of lymphocytes into the skeletal muscle, causing clinical symptoms such as symmetric proximal muscle weakness and elevated muscle enzymes due to damage to the endomysial tissues [57]. Specifically, this pathophysiology is related to infiltration by CD8+ T-cells into the endomysium [58,59]. Dermatomyositis (DM) has similar myopathic clinical findings as well as cutaneous manifestations, most commonly a heliotrope rash and Gottron's papules. Histopathologically, DM is characterized by both perifascicular atrophy and perivascular inflammation [58]. The pathophysiology of this disease is due to infiltration by CD4+ T-cells and B-cells, though it has been suggested that the CD4+ cells may actually represent plasmacytoid dendritic cells [58]. In both diseases, siponimod could inhibit the recirculation of these lymphocytes from secondary lymphoid organs into the tissues, providing a possible intervention point to reduce the inflammatory response within the tissues.
3.4. Potential clinical application of siponimod for polymyositis and dermatomyositis
Recently a randomized, double-blind, placebo-controlled, partial cross over Phase IIa, proof of concept study of siponimod (BAF312) in patients with PM and DM has demonstrated therapeutic potential [47]. All patients were given 10 mg of siponimod, and the outcome measures included improvement in manual muscle testing (MMT8) by 1–15% and improvement in International Myositis Assessment Study (IMACS) core set measures greater than 30% or an improvement in MMT8 by greater than 15% and in myositis disease global activity by greater than 10% [47].
Though no definitive conclusions can be drawn as only 18 patients were enrolled, a Bayesian analysis of the IMACS responder status at 12 weeks yielded a probability of 0.96 that siponimod was superior to placebo [47]. Ongoing efforts to develop this drug in DM and PM are being undertaken, and multi-dose Phase IIb trials are being conducted to assess the dose-responses in these populations (PM Clinical Trials Number: NCT01801917, DM Clinical Trials Number: NCT02029274).
4. Other potential applications
In addition to the potential uses of the S1PR modulators already described, further uses are currently being explored in diseases such as psoriasis, ulcerative colitis and uveitis [53], and rheumatoid arthritis [55]. There have even been investigations into their potential uses for cancer, due to the interference with angiogenesis and autoimmune diabetes, suggesting a wide array of potential applications and a need for further study into S1PR regulations to have the next generation of more selective S1P modulators [60,61,62] (Table 1).
5. Conclusion
In summary, despite the fact that there are a number of treatment options for RRMS, there is no effective treatment for SPMS. S1PR modulators prevent recirculation of the auto-reactive lymphocytes from the lymphatic tissues and might be promising disease modifying agents for SPMS. Not only are these S1PR modulators being investigated in SPMS, they also represent the potential to be effective in an array of inflammatory conditions such as RA, SLE, PM, and DM. Although their efficacy and safety in these conditions remain under investigation, S1PR modulators might provide potential treatments for several autoimmune diseases.
Acknowledgments
David Fox is currently supported by grants from NIH NIAID Autoimmune Center of Excellence: UM1-AI110557 and has nothing to disclose. Elena Schiopu has received research support from Novartis. Yang Mao-Draayer has served as a consultant and/or received grant support from: Acorda, Bayer Pharmaceutical, Biogen Idec, EMD Serono, Genzyme, Novartis, Questor, Chugai, and Teva Neuroscience and is currently supported by grants from NIH NIAID Autoimmune Center of Excellence: UM1-AI110557; NIH NINDS R01-NS080821 and the University of Michigan Neurology Department.
The authors thank Ali Mirza for assistance in creating Fig. 1.
Footnotes
Disclosure
Jeffrey Sarazin has nothing to disclose.
References
- 1.Magliozzi R, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. http://dx.doi.org/10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 2.Weiner HL. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J. Neurol. 2008;255(Suppl. 1):3–11. doi: 10.1007/s00415-008-1002-8. http://dx.doi.org/10.1007/s00415-008-1002-8. [DOI] [PubMed] [Google Scholar]
- 3.Quintana FJ, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. http://dx.doi.org/10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stadelmann C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr. Opin. Neurol. 2011;24:224–229. doi: 10.1097/WCO.0b013e328346056f. http://dx.doi.org/10.1097/WCO.0b013e328346056f. [DOI] [PubMed] [Google Scholar]
- 5.Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog. Neurobiol. 2011;93:1–12. doi: 10.1016/j.pneurobio.2010.09.005. http://dx.doi.org/10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter SF, et al. The direct effects of fingolimod in the central nervous system: implications for relapsing multiple sclerosis. CNS Drugs. 2016;30:135–147. doi: 10.1007/s40263-015-0297-0. http://dx.doi.org/10.1007/s40263-015-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 2013;328(0):9–18. doi: 10.1016/j.jns.2013.02.011. http://dx.doi.org/10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am. J. Manag. Care. 2013;(Suppl. 2):S15–S20. [PubMed] [Google Scholar]
- 9.Goodin DS, et al. Disease modifying therapies in multiple sclerosis: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58:169–178. doi: 10.1212/wnl.58.2.169. http://dx.doi.org/10.1212/WNL.58.2.169: 1526-632X. [DOI] [PubMed] [Google Scholar]
- 10.Kappos L, et al. Final analysis of the European multicenter trial on IFNbeta-1b in secondary-progressive MS. Neurology. 2001;57:1969–1975. doi: 10.1212/wnl.57.11.1969. http://dx.doi.org/10.1212/WNL.57.11.1969:1526-632X. [DOI] [PubMed] [Google Scholar]
- 11.Panitch H, Miller A, Paty D, Weinshenker B. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63:1788–1795. doi: 10.1212/01.wnl.0000146958.77317.3e. http://dx.doi.org/10.1212/01.WNL.0000146958.77317.3E:1526-632X. [DOI] [PubMed] [Google Scholar]
- 12.Cohen JA, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl J. Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. http://dx.doi.org/10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 13.Kappos L, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl J. Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. http://dx.doi.org/10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 14.Kappos L, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl J. Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. http://dx.doi.org/10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 15.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. http://dx.doi.org/10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. http://dx.doi.org/10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 17.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. http://dx.doi.org/10.1016/S0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 18.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. http://dx.doi.org/10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 19.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 2005;5:560–570. doi: 10.1038/nri1650. http://dx.doi.org/10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 20.Walzer T, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1- phosphate receptor. Nat. Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. http://dx.doi.org/10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 21.Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1- phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 2013;328:9–18. doi: 10.1016/j.jns.2013.02.011. http://dx.doi.org/10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-Or A. The immunology of multiple sclerosis. Semin. Neurol. 2008;28:29–45. doi: 10.1055/s-2007-1019124. http://dx.doi.org/10.1055/s-2007-1019124. [DOI] [PubMed] [Google Scholar]
- 23.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. http://dx.doi.org/10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 24.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. http://dx.doi.org/10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 25.Chiba K, et al. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol. 1998;160:5037–5044. (ISSN: 1550–6606) [PubMed] [Google Scholar]
- 26.Cohen JA, et al. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016 Apr;15(4):373–381. doi: 10.1016/S1474-4422(16)00018-1. http://dx.doi.org/10.1016/S1474-4422(16)00018-1. [DOI] [PubMed] [Google Scholar]
- 27.Kappos L, Arnold DL, Bar-Or A, Camm J, Derfuss T, Kieseier BC, Sprenger T, Greenough K, Ni P, Harada T. Safety and efficacy of amiselimod in relapsing multiple sclerosis (MOMENTUM): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016 Oct;15(11):1148–1159. doi: 10.1016/S1474-4422(16)30192-2. http://dx.doi.org/10.1016/S1474-4422(16)30192-2. [DOI] [PubMed] [Google Scholar]
- 28.Gergely P, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br. J. Pharmacol. 2012;167:1035–1047. doi: 10.1111/j.1476-5381.2012.02061.x. http://dx.doi.org/10.1111/j.1476-5381.2012.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis ND, et al. Circulating monocytes are reduced by sphingosine-1-phosphate receptor modulators independently of S1P3. J. Immunol. 2013;190:3533–3540. doi: 10.4049/jimmunol.1201810. http://dx.doi.org/10.4049/jimmunol.1201810. [DOI] [PubMed] [Google Scholar]
- 30.Chiba K, Adachi K. Sphingosine 1-phosphate receptor 1 as a useful target for treatment of multiple sclerosis. Pharmaceuticals. 2012;5(5):514–528. doi: 10.3390/ph5050514. http://dx.doi.org/10.3390/ph5050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Sullivan C, et al. The dual S1PR1/S1PR5 drug BAF312 (siponimod) attenuates demyelination in organotypic slice cultures. J. Neuroinflammation. 2016;13:31. doi: 10.1186/s12974-016-0494-x. http://dx.doi.org/10.1186/s12974-016-0494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terai K, et al. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116:1053–1062. doi: 10.1016/s0306-4522(02)00791-1. http://dx.doi.org/10.1016/S0306-4522(02)00791-1. [DOI] [PubMed] [Google Scholar]
- 33.Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, Gudz TI. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 2007;21:1503–1514. doi: 10.1096/fj.06-7420com. http://dx.doi.org/10.1096/fj.06-7420com. [DOI] [PubMed] [Google Scholar]
- 34.Jaillard C, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J. Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. http://dx.doi.org/10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung CG, et al. Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia. 2007;55:1656–1667. doi: 10.1002/glia.20576. http://dx.doi.org/10.1002/glia.20576. [DOI] [PubMed] [Google Scholar]
- 36.Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17–27. doi: 10.1002/glia.10297. http://dx.doi.org/10.1002/glia.10297. [DOI] [PubMed] [Google Scholar]
- 37.McGiffert C, Contos JJ, Friedman B, Chun J. Embryonic brain expression analysis of lysophospholipid receptor genes suggests roles for s1p(1) in neurogenesis and s1p(1–3) in angiogenesis. FEBS Lett. 2002;531:103–108. doi: 10.1016/s0014-5793(02)03404-x. http://dx.doi.org/10.1016/S0014-5793(02)03404-X. [DOI] [PubMed] [Google Scholar]
- 38.Harada J, Foley M, Moskowitz MA, Waeber C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J. Neurochem. 2004;88:1026–1039. doi: 10.1046/j.1471-4159.2003.02219.x. http://dx.doi.org/10.1046/j.1471-4159.2003.02219.x. [DOI] [PubMed] [Google Scholar]
- 39.Kajimoto T, et al. Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol. Cell. Biol. 2007;27:3429–3440. doi: 10.1128/MCB.01465-06. http://dx.doi.org/10.1128/MCB.01465-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizugishi K, et al. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. http://dx.doi.org/10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J. Neurosci. 1997;17:6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toman RE, et al. Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J. Cell Biol. 2004;166:381–392. doi: 10.1083/jcb.200402016. http://dx.doi.org/10.1083/jcb.200402016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiMenna L, et al. Fingolimod protects cultured cortical neurons against excitotoxic death. Pharmacol. Res. 2013;67:1–9. doi: 10.1016/j.phrs.2012.10.004. http://dx.doi.org/10.1016/j.phrs.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J. Physiol. 2006;575:101–113. doi: 10.1113/jphysiol.2006.111575. http://dx.doi.org/10.1113/jphysiol.2006.111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kappos L, et al. Efficacy and safety of siponimod in secondary progressive multiple sclerosis - Results of the placebo controlled, double-blind, Phase III EXPAND study. Oral presentation presented at: 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis; September 2016; London, UK. pp. 14–17. [Google Scholar]
- 46.Tsunemi S, Iwasaki T, Kitano S, Imado T, Miyazawa K, Sano H. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin. Immunol. 2010;136:197–204. doi: 10.1016/j.clim.2010.03.428. http://dx.doi.org/10.1016/j.clim.2010.03.428. [DOI] [PubMed] [Google Scholar]
- 47.Danko K, Vencovsky J, Lundberg IE, Amato AA, Oddis CV, Molnar M, Moher AM, Colin L, Muellershausen F, Lee D, Gergely P. The selective sphingosine-1- phosphate receptor 1/5 modulator siponimod (BAF312) shows beneficial effects in patients with active, treatment refractory polymyositis and dermatomyositis: a phase IIa proof-of-concept, double-blind, randomized trial. Arthritis Rheum. 2014;910(Supp 10):S403. http://dx.doi.org/10.1002/art.38914. [Google Scholar]
- 48.McInnes IB, O'Dell JR. State-of-the-art: rheumatoid arthritis. Ann. Rheum. Dis. 2010;69:1898–1906. doi: 10.1136/ard.2010.134684. http://dx.doi.org/10.1136/ard.2010.134684. [DOI] [PubMed] [Google Scholar]
- 49.Zhao C, Fernandes MJ, Turgeon M, Tancrede S, Di Battista J, Poubelle PE, Bourgoin SG. Specific and overlapping sphingosine-1-phosphate receptor functions in human synoviocytes: impact of TNF-alpha. J. Lipid Res. 2008;49:2323–2337. doi: 10.1194/jlr.M800143-JLR200. http://dx.doi.org/10.1194/jlr.M800143-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. http://dx.doi.org/10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 51.Fleischmann RM, et al. The oral S1P lyase inhibitor LX3305 (LX2931) demonstrates favorable safety and potential clinical benefit at 12-weeks in a phase 2 proof-of-concept trial in patients with active rheumatoid arthritis on stable methotrexate therapy. Arthritis Rheum. 2011;910(Supp 10):S1018. http://dx.doi.org/10.1002/art.33310. [Google Scholar]
- 52.Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R, Paulus H, Strand V, Tugwell P, Weinblatt M, James Williams H, Wolfe F, Kieszak S. American college of rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–735. doi: 10.1002/art.1780380602. http://dx.doi.org/10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 53.Clark DN, Markham JL, Sloan CS, Poole BD. Cytokine inhibition as a strategy for treating systemic lupus erythematosus. Clin. Immunol. 2013 Sep;148(3):335–343. doi: 10.1016/j.clim.2012.11.001. http://dx.doi.org/10.1016/j.clim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Gottschalk TA, Tsantikos E, Hibbs ML. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Front. Immunol. 2015;6:550. doi: 10.3389/fimmu.2015.00550. http://dx.doi.org/10.3389/fimmu.2015.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moulton VR, Tsokos GC. Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis Res. Ther. 2011;13(2):207. doi: 10.1186/ar3251. http://dx.doi.org/10.1186/ar3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wardemann H, Nussenzweig MC. Advances in Immunology. Vol. 95. Academic Press; 2007. B-cell self-tolerance in humans; pp. 83–110. [DOI] [PubMed] [Google Scholar]
- 57.Milisenda JC, Selva-O'Callaghan A, Grau JM. The diagnosis and classification of polymyositis. J. Autoimmun. 2014 Feb-Mar;48–49:118–121. doi: 10.1016/j.jaut.2014.01.025. http://dx.doi.org/10.1016/j.jaut.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003 Sep 20;362(9388):971–982. doi: 10.1016/S0140-6736(03)14368-1. http://dx.doi.org/10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 59.Mammen AL. Dermatomyositis and polymyositis. Ann. N. Y. Acad. Sci. 2010;1184:134–153. doi: 10.1111/j.1749-6632.2009.05119.x. http://dx.doi.org/10.1111/j.1749-6632.2009.05119.x. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez-Cabrera PJ, et al. S1P signaling: new therapies and opportunities. F1000 Prime Reports. 2014;6:109. doi: 10.12703/P6-109. http://dx.doi.org/10.12703/P6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Z, Zhao C. Sphingosine-1-phosphate and rheumatoid arthritis: pathological implications and potential therapeutic targets. In: Matsuno H, editor. Innovative Rheumatology. 2013. InTech, http://dx.doi.org/10.5772/53308. [Google Scholar]
- 62.Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, Brinkmann V, Nadler JL, Lynch KR. The immune modulator FYT720 prevents autoimmune diabetes in nonobese diabetic mice☆. Clin. Immunol. 2003 Apr;107(1):30–35. doi: 10.1016/s1521-6616(02)00054-2. http://dx.doi.org/10.1016/S1521-6616(02)00054-2. [DOI] [PubMed] [Google Scholar]