Abstract
Atypical femur fracture (AFF), a serious complication of long-term bisphosphonate therapy, is usually preceded by an incomplete fracture appearing on the lateral femur. AFF is most likely the result of severely suppressed bone turnover (SSBT). However, the differences in bone structure and turnover between patients with incomplete and complete AFF remain unknown. We examined trans-iliac bone biopsies from 12 white postmenopausal women with AFF (incomplete = 5; complete = 7) on BP therapy of >5 years, and 43 healthy white premenopausal women. Histomorphometric measurements were performed separately in cancellous, intra-cortical and endosteal envelopes. Of the 43 histomorphometric measurements on 3 difference bone surfaces (cancellous, intracortical and endosteal), only 2 bone resorption variables (Oc.S/BS & Oc.S/NOS) on the endosteal surface, were significantly lower in patients with complete AFF than those with incomplete AFF. Compared to healthy premenopausal women, the trabecular bone volume, thickness and number were all significantly lower in patients with AFF. The dynamic bone formation variables in patients with AFF were significantly reduced on all bone surfaces. The likelihood of a biopsy with no tetracycline labeling was significantly higher in AFF patients than in healthy premenopausal women. Based on these results, we conclude that there are no significant differences in bone turnover between patients with incomplete and complete AFF, suggesting that the suppression of bone turnover had already existed in the femur with incomplete AFF. Compared to healthy premenopausal women, bone turnover is similarly suppressed in patients with either type of AFF.
Keywords: Atypical femur fracture, Prodromal bone deterioration, Severely suppressed bone turnover, Bisphosphonates, Iliac bone biopsy
INTRODUCTION
Bisphosphonates (BPs) are commonly used for prevention and treatment of osteoporosis [1]. The anti-fracture efficacy of BPs is well established during the first 3–5 years of treatment [2, 3], but their risk benefit ratio beyond 5 years is less clear [4] because of the growing concern about low energy femoral shaft and sub-trochanteric fractures, collectively referred to as atypical femur fractures (AFF) [5–7]. AFFs include incomplete and complete patterns seen on plain radiographs [8]. Incomplete AFF is characterized by cortical thickening associated with a beaking or bump at the lateral cortex of the femur, in which a transverse fracture line is often visible [8]. However, several recent reports indicate that fracture line is not always seen in the bumps at lateral cortex of the femur [9–14]. Complete AFF, progressing from an incomplete fracture, extends through both cortices and usually has a medial spike, the fracture is in transverse or short-oblique orientation, and mostly not comminuted [8].
BPs reduce bone turnover by inhibiting osteoclastic bone resorption [15], a necessary requirement, for their therapeutic efficacy in patients with osteopenia and osteoporosis [16]. However, prolonged BP therapy, especially in higher doses, may suppress bone turnover to an extremely low level, impairing normal bone renewal [5]. This severely suppressed bone turnover (SSBT) could attenuate microdamage repair and, consequently, compromise bone mechanical and physical properties [8, 17, 18]. Recently, Iwata et al [19] reported a case with 9-year BP treatment having large amount of microdamage in the thickened cortex at the site near AFF as well as SSBT in the iliac bone biopsy, suggesting that SSBT is the most likely potential link between prolonged BP use and the development of AFF.
Whether there is a difference in bone turnover between patients with incomplete and complete AFF remains unclear. Regardless, bone turnover is essential to replace old and damaged bone with new bone to maintain bone quality. When bone turnover is severely suppressed, it is very likely to compromise bone material properties. Since not all incomplete AFFs will progress to complete AFF [20], we speculate that bone turnover would be more severely suppressed in patients with complete AFF than those who present with incomplete AFF. Additionally, we postulate that the bone turnover rate in patients with either incomplete or complete AFF is significantly below the normal level. The reference values used to verify the suppression of bone turnover in AFF patients have been derived from postmenopausal women [5]. However, the reference values for normal bone turnover should ideally be obtained from premenopausal women [21, 22], because the rate of bone turnover is significantly increased after menopause, which is a major contributor to postmenopausal osteoporosis [23]. Thus, if the bone turnover rate in postmenopausal women declines to the premenopausal level following BP treatment, it cannot be inferred as SSBT. In other words, SSBT is confirmed only when the bone turnover rate falls below the premenopausal level; this is somewhat analogous to calculating T-sore for bone mineral density. In the present study, we investigated the effect of SSBT on the development of AFF.
We examined bone turnover status on the cancellous, intracortical and endosteal surfaces in trans-iliac bone biopsies from patients with incomplete and complete AFF after long-term BP treatment (> 5 years) as well as from normal healthy premenopausal women. We decided a priori to pool data from the 2 patient groups, only if there were no significant differences in bone turnover indices between the groups, to compare with the premenopausal women. Otherwise, each group would be compared separately with data from the normal premenopausal women.
MATERIALS AND METHODS
Subjects
Twelve patients with AFF (7 complete and 5 incomplete) were recruited through our routine clinical practice from 2004 to 2014. All were white postmenopausal women on long-term BP treatment and all were treated with alendronate at a dose of 10 mg/day or 70 mg/week for >5 years, in addition to 1000 mg calcium and 400–800 IU vitamin D supplements daily. No patient was on corticosteroids or other medications known to inhibit bone turnover during BP treatment. Antero-posterior (AP) radiographs of the femurs were obtained in each patient. All patients were recruited consecutively without any ascertainment bias. The time interval between AFF (complete or incomplete) diagnosis and bone biopsy was < 6 months in all patients.
Forty-three white premenopausal women were recruited between 1981–1993 as part of a larger study of the effect of age & menopause on bone structure and remodeling, the details of which have been published [24, 25]. All subjects were skeletally healthy according to the prevailing standard criteria [24], and served as the comparator group for the patients with AFF.
An informed consent was obtained from each subject. The study was approved by the Institutional Review Board of Henry Ford Hospital.
The Radiographic Diagnosis of AFF and Group Assignment
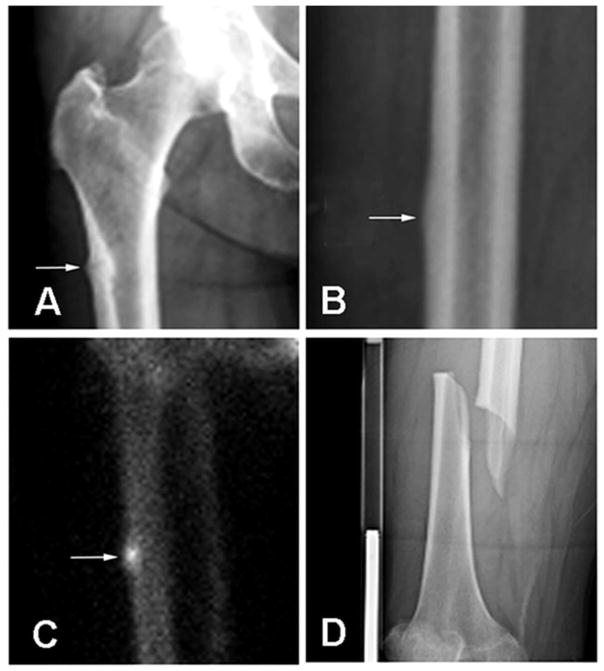
Incomplete AFF was determined directly when a thin fracture line was seen in the thickened cortex and/or bump(s) at the lateral femur on the initial radiograph (Fig 1A). Isotope bone scan was performed only when a fracture line was not seen (Fig 1B); incomplete AFF was confirmed by the focal accumulation of radioactive materials at the site of thickened cortex (Fig 1C). Complete AFF were located either in the sub-trochanteric or diaphyseal region of the femur. In addition to the lateral cortical thickening, the fracture line was in transverse or short oblique orientation passing through the whole femur and often associated with medial cortical spike (Fig 1D).
Fig 1.
Characteristics of incomplete and complete atypical femoral fracture (AFF). A) incomplete AFF can be diagnosed when an anterior-posterior radiograph of the femur shows a transverse fracture line appearing in a bump at the thickened lateral cortex (arrow); B) incomplete AFF is suspected when the radiograph shows thickened lateral cortex but no obvious fracture (arrow); C) the suspected incomplete AFF in Fig B is confirmed by positive isotope bone scan (arrow); D) complete AFF is a complete transverse fracture located in the femoral diaphysis.
Based on the radiographic features in both femurs, the patients were assigned to incomplete or complete AFF groups [26]. The patients in incomplete AFF group had unilateral or bilateral incomplete fracture. Similarly, the patients in complete AFF group had unilateral or bilateral complete fracture. Patients were also assigned to complete AFF group if they had incomplete and complete fractures concomitantly in different femurs.
Bone histomorphometry
Before biopsy, all subjects received in vivo double tetracycline labeling with an inter-label time of 14 days. A cylindrical trans-iliac bone biopsy core with intact cortices, was obtained using a 7.5 mm trephine, and processed, embedded, sectioned, stained and examined as previously reported [24, 25]. All bone histomorphometric variables were designated in accordance with the nomenclature recommended by the American Society for Bone and Mineral Research (ASBMR) [27]. The static histomorphometric indices were measured in sections stained with modified toluidine blue method, and the dynamic remodeling indices were measured in unstained sections [24].
The parameters related to bone structures included total bone volume per tissue volume (BV/TV, %), trabecular thickness (Tb.Th, μm) and number (Tb.N, #/mm2), and cortical thickness (Ct.Th, μm). Tb.Th and Tb.N were calculated indirectly from the bone surface to volume ratio and BV/TV. Static and remodeling indices were measured separately on the cancellous, intracortical and endosteal surfaces. The static indices included osteoid and eroded surfaces as a fraction of bone surface (OS/BS, % and ES/BS, %); wall thickness (W.Th, μm; the average distance between the cement line and the quiescent bone surface); and osteoid thickness (O.Th, μm), measured directly on the bone surface with osteoid. The surface lengths covered by osteoblasts and osteoclasts (Ob.S and Oc.S) were measured separately and expressed as a fraction of bone surface (Ob.S/BS, % and Oc.S/BS, %), as well as a fraction of osteoid surface for Ob.S (Ob.S/OS, %) and as a fraction of non-osteoid surface for Oc.S (Oc.S/NOS).
The dynamic remodeling indices were also measured separately on the cancellous, intra-cortical and endosteal surfaces. The double and single tetracycline labeled surfaces represented the extent of bone surface where mineralization was in progress (mineralizing surface, MS) during the period of tetracycline administration, from which the MS as a fraction of total bone and of osteoid surfaces (MS/BS, % and MS/OS, %) were calculated. Mineral apposition rate (MAR, μm/day) was obtained from the average distance between the two tetracycline labels divided by the interval of administration (14 days in our study). Bone formation rate at the surface level (BFR/BS, μm3/μm2/year) were calculated as MAR x (MS/BS). Activation frequency (Ac.f, #/year), the annual probability of activation of a new remodeling site at any given locus on the bone surfaces, was derived from BFR/BS)/W.Th. For the surface containing only a single label, a minimum value of 0.1 μm/day was assigned to MAR.[28] If no label was present, MAR was treated as a missing value, and MS/BS and BFR/BS were assigned a zero [25].
Statistics
The difference for each variable in cancellous, intra-cortical and endosteal envelopes was compared between patients with incomplete and complete AFF, and between the pooled incomplete and complete AFF patients and healthy premenopausal women using student t tests. Mann-Whitney test was used when the variables were not normally distributed. Bonferroni correction was applied for multiple comparisons, and only the p values after Bonferroni correction at the relevant level were considered statistically significant. The proportional data of different bone surfaces with no tetracycline labeling were compared between AFF patients and healthy premenopausal women using Fisher’s Exact test. Differences were considered statistically significant at p <0.05 (or after Bonferroni correction as indicated in the tables) on a two-tailed test.
The low frequency of complete (n = 7) and incomplete (n = 5) AFF constrained the available sample size. With a sample size of 12 AFF Vs. 43 normal, and a Bonferroni adjusted α of 0.003125, the effect size detectable with 80% power is 1.3, and for comparison of complete vs incomplete AFF, the detectable effect size is a very large 2.9.
RESULTS
Demographic characteristics of patients with incomplete and complete AFF
The demographic characteristics of patients with incomplete and complete AFF are shown in Table 1. There was no difference in the duration of BP treatment between patients with incomplete and complete AFF (9.40 ± 4.04 Vs. 7.71 ± 4.31 years). Patients with incomplete AFF were older than patients with complete AFF, but the difference was not significant (72.4 ± 5.32 Vs, 61.0 ± 12.0 years, p = 0.077). Of the 12 patients, 6 suffered from bilateral femur abnormalities (2 patients each with bilateral incomplete AFF, bilateral complete AFF, and mixed incomplete and complete AFF). In total, 18 femurs were affected; 9 each with incomplete and complete AFF. Thigh pain, the predominant symptom, occurred in all femurs with complete AFF (9/9; 100%), but in only one femur with incomplete AFF (1/9; 11%). Of the 9 femurs with incomplete AFF, only 2 (22%) showed fracture line within the thickened lateral cortex, the remaining 7 without a fracture line were confirmed by isotope bone scan.
Table 1.
Demographic characteristics of patients with incomplete and complete AFF, and normal premenopausal women
| Incomplete AFF | Complete AFF | Premenopausal | |
|---|---|---|---|
| Number of patients | 5 | 7 | 43 |
| Gender | All female | All female | All female |
| Race | All white | All white | All white |
| Age (years, mean(SD)) | 72.4 (5.32) | 61.0 (12.0) | 37.4 (7.84) |
| Type of BP | Alendronate | Alendronate | - |
| Duration of BP use (years, mean(SD)) | 9.40 (4.04) | 7.71 (4.31) | - |
| Affected femurs | 9 | 9 | - |
| Affected femurs with prodromal symptoms | 1/9 (11%) | 9/9 (100%) | - |
| Incomplete fracture with fracture line | 2/9 (22%) | - | - |
| Patients with bilateral femur Involvement | 2 | 4* | - |
Two patients had bilateral AFF and other 2 mixed AFF and PBD
Bone histomorphometry
Comparison between patients with incomplete and complete AFF
Except for the 2 bone resorption indices (Oc.S/BS & Oc.S/NOS) on the endosteal surface, which were nominally significantly (i.e., before the Bonferroni correction) lower in patients with complete AFF (Table 4), all other variables in biopsy did not show significant differences between these 2 groups. (Tables 2–4). Accordingly, the data from the two groups were pooled (and henceforth referred to as AFF patients) for the comparison with the data from healthy premenopausal women.
Table 4.
Comparison of bone histomorphometric measurements for iliac endosteal bone between patients with imcomplete and complete AFF, as well as between AFF patients and premenopausal women
| Incomplete AFF n = 5 |
Complete AFF n = 7 |
p-value* | Normal n = 43 |
Pooled AFF n = 12 |
p-value* | |
|---|---|---|---|---|---|---|
| Static | ||||||
| W.Th (μm) | 31.7 (2.42) | 35.6 (8.61) | 0.418 | 42.4 (8.08) | 34.0 (6.86) | 0.004 |
| OS/BS (%) | 3.44 (2.34) | 3.72 (5.48) | 0.343 | 16.2 (10.7) | 3.60 (4.29) | <0.001 |
| O.Th (μm) | 4.10 (1.74) | 3.38 (2.17) | 0.538 | 8.63 (2.78) | 3.68 (1.95) | <0.001 |
| Ob.S/BS (%) | 1.22 (2.06) | 0.915 (1.35) | 0.755 | 4.40 (3.89) | 1.04 (1.60) | 0.002 |
| Ob.S/OS (%) | 23.6 (26.5) | 16.4 (16.0) | 0.800 | 27.8 (19.2) | 19.4 (20.2) | 0.192 |
| ES/BS (%) | 4.44 (2.07) | 3.06 (5.27) | 0.596 | 9.62 (5.87) | 3.64 (4.15) | <0.001 |
| Oc.S/BS (%) | 1.58 (1.39) | 0.297 (0.420) | 0.041# | 0.486 (0.745) | 0.833 (1.11) | 0.173 |
| Oc.S/NOS (%) | 1.66 (1.46) | 0.329 (0.493) | 0.047# | 0.615 (0.938) | 0.881 (1.17) | 0.287 |
| Dynamic | ||||||
| MS/BS (%) | 1.96 (2.68) | 0.361 (0.955) | 0.268 | 7.02 (6.19) | 1.03 (1.95) | <0.001 |
| MAR (μm) | 0.170 (0.213) | 0.070 (0.185) | 0.268 | 0.492 (0.265) | 0.112 (0.194) | <0.001 |
| BFRs (μm3/μm2/year) | 2.86 (5.17) | 0.645 (1.71) | 0.268 | 15.1 (14.4) | 1.57 (3.55) | <0.001 |
| Ac.f (/year) | 0.018 (0.027) | 0.015 (0.039) | 0.527 | 0.374 (0.364) | 0.016 (0.033) | <0.001 |
Data expressed as mean (SD)
p-values significant after a Bonferroni adjustment for 12 tests, i.e. p ≤ 0.05/12 = 0.00417 are shown in bold
p-values not significant after a Bonferroni correction for 12 tests, i.e. p ≤ 0.05/12 = 0.00417.
Table 2.
Comparison of bone histomorphometric measurements for iliac cancellous bone between patients with incomplete and complete AFF, as well as between AFF patients and premenopausal women
| Variables | Incomplete AFF n = 5 |
Complete AFF n = 7 |
p-value* | Normal n = 43 |
Pooled AFF n = 12 |
p-value* |
|---|---|---|---|---|---|---|
| Structural | ||||||
| BV/TV (%) | 16.0 (5.33) | 16.4 (6.86) | 0.910 | 24.5 (7.21) | 16.2 (6.00) | <0.001 |
| Tb.Th (μm) | 122 (18.4) | 123 (30.9) | 0.953 | 140 (23.3) | 122 (25.4) | 0.025 |
| Tb.N (/mm2) | 1.29 (0.316) | 1.30 (0.246) | 0.982 | 1.74 (0.368) | 1.30 (0.264) | <0.001 |
| Static | ||||||
| W.Th (μm) | 30.2 (3.86) | 26.6 (4.20) | 0.219 | 37.1 (3.81) | 28.2 (4.26) | <0.001 |
| OV/BV (%) | 0.135 (0.146) | 0.146 (0.355) | 0.432 | 1.91 (1.32) | 0.142 (0.276) | <0.001 |
| OS/BS (%) | 1.45 (1.25) | 1.36 (2.80) | 0.343 | 13.9 (8.24) | 1.40 (2.20) | <0.001 |
| O.Th (μm) | 4.41 (1.94) | 4.11 (3.48) | 0.432 | 9.35 (2.06) | 4.24 (2.83) | <0.001 |
| Ob.S/BS (%) | 0.319 (0.425) | 0.114 (0.257) | 0.343 | 4.25 (2.93) | 0.199 (0.336) | <0.001 |
| Ob.S/OS (%) | 12.8 (15.1) | 2.58 (4.41) | 0.117 | 30.6 (13.2) | 6.83 (11.0) | <0.001 |
| ES/BS (%) | 5.20 (4.19) | 2.99 (3.68) | 0.202 | 6.56 (3.03) | 3.91 (3.88) | 0.005 |
| Oc.S/BS (%) | 0.976 (0.707) | 0.289 (0.278) | 0.073 | 0.682 (0.718) | 0.575 (0.591) | 0.654 |
| Oc.S/NOS (%) | 0.997 (0.731) | 0.299 (0.299) | 0.073 | 0.820 (0.864) | 0.590 (0.610) | 0.501 |
| Dynamic | ||||||
| MS/BS (%) | 1.60 (2.17) | 0.256 (0.307) | 0.343 | 6.13 (3.53) | 0.814 (1.50) | <0.001 |
| MAR (μm) | 0.133 (0.125) | 0.109 (0.126)) | 0.758 | 0.578 (0.167) | 0.119 (0.120) | <0.001 |
| BFRs (μm3/μm2/year) | 1.20 (1.50) | 0.183 (0.227) | 0.303 | 13.1 (7.24) | 0.606 (1.06) | <0.001 |
| Ac.f (/year) | 0.021 (0.025) | 0.008 (0.009) | 0.610 | 0.353 (0.197) | 0.013 (0.017) | <0.001 |
Data expressed as mean (SD)
p-values significant after a Bonferroni adjustment for 16 tests, i.e. p ≤ 0.05/16 = 0.003125 are shown in bold
Comparison between AFF patients and healthy premenopausal women
As expected, there was a significant difference in cancellous bone structure between AFF patients and premenopausal women (Tables 2 and 3). BV/TV, Tb.Th and Tb.N were all significantly decreased in patients with AFF.
Table 3.
Comparison of bone histomorphometric measurements for iliac cortical bone between patients with incomplete and complete AFF, as well as between AFF patients and premenopausal women
| Incomplete AFF n = 5 |
Complete AFF n = 7 |
p-value* | Normal n = 43 |
Pooled AFF n = 12 |
p-value* | |
|---|---|---|---|---|---|---|
| Structural | ||||||
| BV/TV (%) | 96.2 (0.979) | 96.0 (1.16) | 0.828 | 95.6 (2.15) | 96.1 (1.04) | 0.548 |
| Ct.Th (mm) | 1.20 (0.149) | 1.40 (0.408) | 0.530 | 1.35 (0.397) | 1.32 (0.329) | 0.895 |
| Static | ||||||
| W.Th (μm) | 37.4 (1.12) | 41.3 (8.01) | 0.610 | 44.0 (11.9) | 39.8 (6.34) | 0.547 |
| OV/BV (%) | 0.059 (0.049) | 0.086 (0.070) | 0.479 | 0.324 (0.267) | 0.075 (0.061) | <0.001 |
| OS/BS (%) | 4.77 (3.28) | 5.59 (3.80) | 0.708 | 12.7 (7.66) | 5.25 (3.46) | <0.001 |
| O.Th (μm) | 4.97 (2.03) | 5.00 (1.54) | 0.979 | 9.07 (2.34) | 4.99 (1.67) | <0.001 |
| Ob.S/BS (%) | 0.735 (1.10) | 0.884 (0.785) | 0.755 | 3.87 (3.13) | 0.822 (0.822) | 0.002 |
| Ob.S/OS (%) | 11.7 (9.05) | 12.6 (11.1) | 0.882 | 28.8 (19.3) | 12.3 (9.86) | 0.010 |
| ES/BS (%) | 2.80 (2.04) | 3.73 (3.22) | 0.580 | 4.45 (3.61) | 3.34 (2.72) | 0.323 |
| Oc.S/BS (%) | 0.477 (0.488) | 0.663 (0.971) | 1.000 | 0.434 (0.509) | 0.585 (0.781) | 0.454 |
| Oc.S/NOS (%) | 0.514 (0.542) | 0.724 (1.08) | 1.000 | 0.498 (0.580) | 0.636 (0.867) | 0.505 |
| Dynamic | ||||||
| MS/BS (%) | 3.58 (4.20) | 1.59 (2.23) | 0.432 | 7.95 (5.78) | 2.42 (3.19) | <0.001 |
| MAR (μm) | 0.160 (0.114) | 0.123 (0.164) | 0.530 | 0.589 (0.217) | 0.139 (0.140) | <0.001 |
| BFRs (μm3/μm2/year) | 2.28 (2.47) | 1.75 (2.81) | 0.530 | 19.2 (16.1) | 1.97 (2.57) | <0.001 |
| Ac.f (/year) | 0.051 (0.070) | 0.048 (0.086) | 0.788 | 0.455 (0.373) | 0.049 (0.077) | <0.001 |
Data expressed as mean (SD)
p-values significant after a Bonferroni adjustment for 15 tests, i.e. p ≤ 0.05/15 = 0.003 are shown in bold
Except for W.Th on the intracortical surface, and Ob.S/OS on the endosteal surface, most of the static bone formation variables were significantly reduced in AFF patients as compared with premenopausal women (Tables 2–4). Bone resorption variable, ES/BS, was significantly reduced on the cancellous and endosteal surfaces, but non-significantly reduced on the intracortical surface in AFF patients (Tables 2–4). However, after Bonferroni correction, the difference in ES/BS was significant only on the endosteal surface. There was no significant difference in osteoclast variables (Oc.S/BS & Oc.S/NOS) between AFF patients and premenopausal subjects on any of the bone surfaces (Tables 2–4).
Compared to the premenopausal women, the dynamic bone formation variables in AFF patients were significantly lower on all the bone surfaces (Tables 2–4). The mean MS/BS was reduced by 86.7%, 69.6% and 85.3% on the cancellous, intra-cortical and endosteal surfaces respectively. The mean values of MAR, BFR/BS and Ac.f were decreased, respectively, by 64.7%, 95.4% and 96.3% on the cancellous surface; by 60.6%, 89.7% and 89.2% on the intracortical surface and by 34.5%, 89.6% and 95.7% on the endosteal surface. All the differences in dynamic bone formation variables remained significant even after Bonferroni correction for multiple comparisons between AFF patients and premenopausal women (Tables 2–4). Of the 12 patients with AFF, 5 had no tetracycline labeling in cancellous bone, whereas 3 of them had no label at all in the whole biopsy. The likelihood of missing labels on each bone surface was significantly higher in AFF patients than in premenopausal women (Table 5)
Table 5.
Comparison of bone surfaces without tetracycline labeling between AFF patients and premenopausal women
| Bone Surface | With Label | Without Label | p |
|---|---|---|---|
| Cancellous | |||
| Normal | 43 (100) | 0 (0) | <0.001 |
| AFF | 7 (58.3) | 5 (41.7) | |
| Intracortical | |||
| Normal | 42 (97.7) | 1 (2.33) | 0.001 |
| AFF | 7 (58.3) | 5 (41.7) | |
| Endosteal | |||
| Normal | 39 (90.7) | 4 (9.30) | <0.001 |
| AFF | 4 (33.3) | 8 (66.7) | |
| All Surfaces | |||
| Normal | 43 (100) | 0 (0) | 0.008 |
| AFF | 9 (75.0) | 3 (25.0) | |
Data expressed as number (percent)
DISCUSSION
It is now well established that long term BP treatment can result in AFF, the risk of which ranged from 0.023% to 0.13% [29–31]. However, there is no systematic study regarding the development of AFF. There are at least 3 different reported radiologic phenotypes of AFFs in patients on long term BP therapy: complete AFF, incomplete AFF with a discernable fracture line, and incomplete AFF without a fracture line [7, 8, 26]. The first 2 types conform to the current definition of the ASBMR-Task Force, but not the last [7, 8]. Our results showed that 7/9 (78%) incomplete AFFs were not associated with a discernable fracture line in the bump on lateral femoral cortex. Such incomplete AFFs can be easily confirmed by a positive isotope bone scan or an MRI [12, 14, 32]. In a study of Koh et al [20], all 4 patients with complete AFF had a fracture line across the thickened cortex seen in pre-AFF radiographs. In contrast, of the 12 femurs with incomplete AFF that did not progress to complete AFF, only one (8.3%) femur had such a fracture line. In a very recent and rather elegant semi-quantitative study, Min et al [26] found that about 60% (10/17) of the incomplete AFFs progressed to complete AFF within 6 months when there was a fracture line, but only 10% (3/29) did so when there was no fracture line [20]. These data suggest that the risk of progressing to complete AFF is significantly increased in patients with a fracture line across the thickened cortex [20, 26], and imply that the nature and scope of bone injury may be different in incomplete AFFs with and without a fracture line.
AFFs most certainly represent stress fractures in an insufficient bone [32]. Although local thickening or visible callus or both are almost always present at the site of a stress fractures, a fracture line cannot always be demonstrated by standard x-rays [20, 32, 33]. Using radiological and histological examinations, Uhthoff et al [33] provided sufficient evidence that the periosteal reaction occurred at a site of increased stress in the absence of an actual fracture. It is highly likely that in patients on long-term BP treatment such stage-wise scenario often occurs in AFFs as they progress from incomplete AFF without a fracture line to incomplete AFF with a fracture line, and finally to a complete AFF [20, 26]. This also may explain the wide variability in clinical presentation (with or without preceding pain). Such progressive stages are probably related to the deterioration of bone material properties, including alteration of collagen cross-linking, hypermineralization with reduced heterogeneity and decreased vascularity, etc. [8], all of which increase bone fragility resulting in microdamage in bone matrix. Accumulation of microdamage would stimulate periosteal reaction to form external callus [20, 34], but bone continuity remains intact (i.e., no discernable fracture line). We suggested that these changes be designated as “prodromal bone deterioration (PBD)” instead of incomplete AFF, since the damage to bone is at the nano/micro-scale without necessarily breaking the bone continuity. Synthesizing from our data and literature review, we believe that most of the so-called incomplete AFFs may actually be PBDs [20].
In this study, we did not find significant differences in either bone structure or turnover between patients with incomplete and complete AFF, suggesting that the abnormality of bone structure and suppression of bone turnover had already existed in the femur prior to incomplete fracture. The histomorphometric results showed that compared to premenopausal women the trabecular bone volume, number and thickness were all significantly decreased in AFF patients, implying that the cancellous bone deficit, particularly in women with osteoporosis, was not restored to the premenopausal level even after >5 years of BP treatment. In contrast, there was no significant difference in cortical bone volume and thickness between AFF patients and premenopausal women, consistent with predominantly cancellous bone loss in postmenopausal women [24]. The major anti-fracture effect of BPs is through inhibition of osteoclastic bone resorption rather than stimulation of osteoblastic bone formation [15]; this scenario can prevent further bone loss but slightly increase the pre-existing bone mass [33]. Accordingly, long-term use of BPs for osteoporosis could preserve cortical bone but cannot restore cancellous bone to the premenopausal level.
We found that bone turnover in AFF patients was significantly below the premenopausal level, with mean reductions of 70–87% in MS/BS, and of 90–95% in BFR/BS and Ac.f on different bone surfaces. Double-labels were present in 67% of the AFF patients, and 3 patients (25%) had no tetracycline labels in their entire bone biopsy specimens. Recker et al [22] reviewed several studies and concluded that bone turnover, as measured by Ac.f, in postmenopausal osteoporosis patients declined to the premenopausal level after 3–>5 years of BP treatment. Double-labels were present in 99% of the biopsy samples overall (range 94–100%; including double-labels in the cortical and endosteal envelopes) [22]. These findings differ markedly from the results in AFF patients on long-term BP treatment. Odvina et al [5] reported that double-labels were missing in all the 9 patients with AFF while on alendronate therapy for 3–8 years, in whom 5 biopsy samples revealed occasional single tetracycline labels. Miller et al [34] reported that 7 (47%) of the 15 AFF patients had undetectable labeling in iliac cancellous bone. These data suggest that patients with SSBT are more likely to develop AFF after prolonged BP treatment.
The major effect of BPs is to inhibit osteoclastic bone resorption [15]. The static histomorphometric data showed that bone resorption, as measured by ES/BS, was reduced on cancellous and endosteal surfaces in AFF patients. A combination of decreased ES/BS and MS/BS suggests that the replacement of old or damaged bone with new bone was significantly reduced in AFF patients. Interestingly, the osteoclast surface, represented by Oc.S/BS and Oc.S/NOS, was not decreased in patients with AFF. This appears paradoxical to the known actions of BPs to inhibit osteoclastogenesis and promote osteoclast apoptosis [35, 36]. Weinstein et al [37] reported that the number of osteoclasts was increased after 3 years of alendronate treatment, but approximately one third of these osteoclasts were giant, hyper-nucleated and detached from the bone surface. Jobke et al [38] found that the bone erosion surface and depth were significantly reduced after BP treatment, but the osteoclast surface remained unchanged, similar to our results. These findings suggest that decreased bone resorption after BP treatment is mainly due to reduced (or abrogated) osteoclast function rather their numbers.
There were several limitations in the present study. First, this was a retrospective study with small sample size, which might have contributed our inability to detect differences between the PBD and AFF groups. For instance, 3–5 fold differences were observed in Oc.S/BS (%) and Oc.S/NOS % in Tables 2 and 4, respectively, which could not be declared statistically significant. Second, we do not have bone biopsies from patients treated with BP but without AFF. Third, due to lack of a control group, we are unable to determine the association between BP treatment adherence and atypical fractures. Fourth, biopsies were not obtained from the site of AFF. Although the pharmacological intervention may produce different effects on bones with and without load-bearing [39], the iliac bone biopsy is still preferred for studies on bone turnover in patients with AFF [5, 40, 41]. In addition, bone turnover at the site of AFF would be stimulated during fracture healing [5, 42]; this focal increase in bone turnover may cause misjudgment of the changes of overall bone turnover elsewhere in the skeleton in patients with BP treatment. Finally, biopsy of the femur bone is impractical (perhaps may even be harmful) to obtain from patients with PBD and AFF.
In conclusion, we found no significant differences in bone structure and turnover between patients with incomplete and complete AFF, suggesting that the suppression of bone turnover had already existed in the femur with incomplete AFF. Compared to normal premenopausal women, bone turnover was severely suppressed in AFF patients.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR062103. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank Ajaykumar D. Rao, MD, M.MSc, FACP, Assistant Professor, Department of Endocrinology, Temple University, Philadelphia for helpful discussions during the preparation of the manuscript and critical review, Clarita V Odvina, MD, formerly of the Department of Mineral Metabolism, South Western Medical School, for her astute original clinical observation that led to the concept of “severe suppression of bone turnover (SSBT)” and atypical fractures, late Dr. A.M. Parfitt for many helpful discussions during the initiation of this study, and suggesting the term SSBT to distinguish it from adynamic bone disease seen in dialysis patients, and Ms. Elizabeth Warner, Research Coordinator for the Pathogenesis of Atypical Femur Fracture Study.
Footnotes
Conflicts of Interest: Shijing Qiu, George Divine, Saroj Palnitkar, Pooja Kulkarni, Trent S Guthrie, Mahalakshmi Honasoge, and Sudhaker D Rao declare that they have no conflict of interest.
References
- 1.Marcus R, Bouxsein ML. Bisphosphonates: Pharmacology and use in the treatment of osteoporosis. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Osteoporosis. Elsevier; Amsterdam. Boston. Heidelberg. London. New York. Oxford. Paris. San Diego. San Francisco. Singapore. Sydney. Tokyo: 2008. pp. 1725–1742. [Google Scholar]
- 2.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 3.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 4.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: A systematic review and meta-analysis. J Bone Miner Res. 2013;28:1729–1737. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 6.Kharazmi M, Michaelsson K, Hallberg P. Prodromal Symptoms in Patients with Bisphosphonate-Associated Atypical Fractures of the Femur. J Bone Miner Metab. 2014;33:516–522. doi: 10.1007/s00774-014-0611-9. [DOI] [PubMed] [Google Scholar]
- 7.Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Howe TS, van der Meulen MC, Weinstein RS, Whyte MP. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American society for bone and mineral research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 8.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster D, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Koval K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Sen HT, van der Meulen MC, Weinstein RS, Whyte M. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 9.Ahlman MA, Rissing MS, Gordon L. Evolution of bisphosphonate related atypical fracture retrospectively observed with DXA scanning. J Bone Miner Res. 2012;27:496–498. doi: 10.1002/jbmr.543. [DOI] [PubMed] [Google Scholar]
- 10.Porrino JA, Jr, Kohl CA, Taljanovic M, Rogers LF. Diagnosis of proximal femoral insufficiency fractures in patients receiving bisphosphonate therapy. AJR Am J Roentgenol. 2010;194:1061–1064. doi: 10.2214/AJR.09.3383. [DOI] [PubMed] [Google Scholar]
- 11.Harborne K, Hazlehurst JM, Shanmugaratnam H, Pearson S, Doyle A, Gittoes NJ, Choudhary S, Crowley RK. Compliance with established guidelines for the radiological reporting of atypical femoral fractures. Br J Radiol. 2015;89:20150443. doi: 10.1259/bjr.20150443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajino Y, Kabata T, Watanabe K, Tsuchiya H. Histological finding of atypical subtrochanteric fracture after long-term alendronate therapy. J Orthop Sci. 2012;17:313–318. doi: 10.1007/s00776-011-0085-8. [DOI] [PubMed] [Google Scholar]
- 13.Somford MP, Draijer FW, Thomassen BJ, Chavassieux PM, Boivin G, Papapoulos SE. Bilateral fractures of the femur diaphysis in a patient with rheumatoid arthritis on long-term treatment with alendronate: clues to the mechanism of increased bone fragility. J Bone Miner Res. 2009;24:1736–1740. doi: 10.1359/jbmr.090408. [DOI] [PubMed] [Google Scholar]
- 14.La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long-term bisphosphonate therapy. AJR Am J Roentgenol. 2012;198:1144–1151. doi: 10.2214/AJR.11.7442. [DOI] [PubMed] [Google Scholar]
- 15.Russell RG. Bisphosphonates: The first 40years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson JM, Little DG. Bisphosphonates in orthopedic applications. Bone. 2011;49:95–102. doi: 10.1016/j.bone.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 18.Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/s8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 19.Iwata K, Mashiba T, Hitora T, Yamagami Y, Yamamoto T. A large amount of microdamages in the cortical bone around fracture site in a patient of atypical femoral fracture after long-term bisphosphonate therapy. Bone. 2014;64:183–186. doi: 10.1016/j.bone.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma. 2010;24:75–81. doi: 10.1097/BOT.0b013e3181b6499b. [DOI] [PubMed] [Google Scholar]
- 21.Recker R, Lappe J, Davies KM, Heaney R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res. 2004;19:1628–1633. doi: 10.1359/JBMR.040710. [DOI] [PubMed] [Google Scholar]
- 22.Recker RR, Ste-Marie LG, Chavassieux P, McClung MR, Lundy MW. Bone safety with risedronate: histomorphometric studies at different dose levels and exposure. Osteoporos Int. 2015;26:327–337. doi: 10.1007/s00198-014-2850-y. [DOI] [PubMed] [Google Scholar]
- 23.Seeman E. Invited Review: Pathogenesis of osteoporosis. J Appl Physiol. 2003;95:2142–2151. doi: 10.1152/japplphysiol.00564.2003. [DOI] [PubMed] [Google Scholar]
- 24.Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effect of ethnicity and age or menopause on the structure and geometry of iliac bone. J Bone Miner Res. 1996;11:1967–1975. doi: 10.1002/jbmr.5650111219. [DOI] [PubMed] [Google Scholar]
- 25.Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res. 1997;12:498–508. doi: 10.1359/jbmr.1997.12.4.498. [DOI] [PubMed] [Google Scholar]
- 26.Min BW, Koo KH, Park YS, Oh CW, Lim SJ, Kim JW, Lee KJ, Lee YK. Novel Scoring System for Identifying Impending Complete Fractures in Incomplete Atypical Femoral Fractures. J Clin Endocrinol Metab. 2016:jc20162787. doi: 10.1210/jc.2016-2787. [DOI] [PubMed] [Google Scholar]
- 27.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauge E, Mosekilde L, Melsen F. Missing observations in bone histomorphometry on osteoporosis: implications and suggestions for an approach. Bone. 1999;25:389–395. doi: 10.1016/s8756-3282(99)00194-5. [DOI] [PubMed] [Google Scholar]
- 29.Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362:1761–1771. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 30.Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ, Laupacis A. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305:783–789. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 31.Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011;364:1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 32.Tripto-Shkolnik L. Atypical femoral fractures and their relation to bisphosphonate use. Isr Med Assoc J. 2013;15:447–450. [PubMed] [Google Scholar]
- 33.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–1768. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 34.Miller PD, McCarthy EF. Bisphosphonate-associated atypical sub-trochanteric femur fractures: Paired bone biopsy quantitative histomorphometry before and after teriparatide administration. Semin Arthritis Rheum. 2015;44:477–482. doi: 10.1016/j.semarthrit.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Russell RG, Croucher PI, Rogers MJ. Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int. 1999;9(Suppl 2):S66–80. doi: 10.1007/pl00004164. [DOI] [PubMed] [Google Scholar]
- 36.D’Amelio P, Grimaldi A, Di Bella S, Tamone C, Brianza SZ, Ravazzoli MG, Bernabei P, Cristofaro MA, Pescarmona GP, Isaia G. Risedronate reduces osteoclast precursors and cytokine production in postmenopausal osteoporotic women. J Bone Miner Res. 2008;23:373–379. doi: 10.1359/jbmr.071031. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jobke B, Milovanovic P, Amling M, Busse B. Bisphosphonate-osteoclasts: changes in osteoclast morphology and function induced by antiresorptive nitrogen-containing bisphosphonate treatment in osteoporosis patients. Bone. 2014;59:37–43. doi: 10.1016/j.bone.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Portero-Muzy NR, Chavassieux PM, Bouxsein ML, Gineyts E, Garnero P, Chapurlat RD. Early effects of zoledronic acid and teriparatide on bone microarchitecture, remodeling and collagen crosslinks: comparison between iliac crest and lumbar vertebra in ewes. Bone. 2012;51:714–719. doi: 10.1016/j.bone.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Tamminen IS, Yli-Kyyny T, Isaksson H, Turunen MJ, Tong X, Jurvelin JS, Kroger H. Incidence and bone biopsy findings of atypical femoral fractures. J Bone Miner Metab. 2013;31:585–594. doi: 10.1007/s00774-013-0448-7. [DOI] [PubMed] [Google Scholar]
- 41.Armamento-Villareal R, Napoli N, Diemer K, Watkins M, Civitelli R, Teitelbaum S, Novack D. Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: a case series. Calcif Tissue Int. 2009;85:37–44. doi: 10.1007/s00223-009-9263-5. [DOI] [PubMed] [Google Scholar]
- 42.Jamal SA, Dion N, Ste-Marie LG. Atypical femoral fractures and bone turnover. N Engl J Med. 2011;365:1261–1262. doi: 10.1056/NEJMc1107029. [DOI] [PubMed] [Google Scholar]