Abstract
Despite of a multitude of excellent studies, the regulatory role of natural killer (NK) cells in the pathogenesis of inflammatory cardiac disease is greatly underappreciated. Clinical abnormalities in the numbers and functions of NK cells are observed in myocarditis and inflammatory dilated cardiomyopathy (DCMi) as well as in cardiac transplant rejection [1–6]. Because treatment of these disorders remains largely symptomatic in nature, patients have little options for targeted therapies [7, 8]. However, blockade of NK cells and their receptors can protect against inflammation and damage in animal models of cardiac injury and inflammation. In these models, NK cells suppress the maturation and trafficking of inflammatory cells, alter the local cytokine and chemokine environments, and induce apoptosis in nearby resident and hematopoietic cells [1, 9, 10]. This review will dissect each protective mechanism employed by NK cells and explore how their properties might be exploited for their therapeutic potential.
1. Introduction
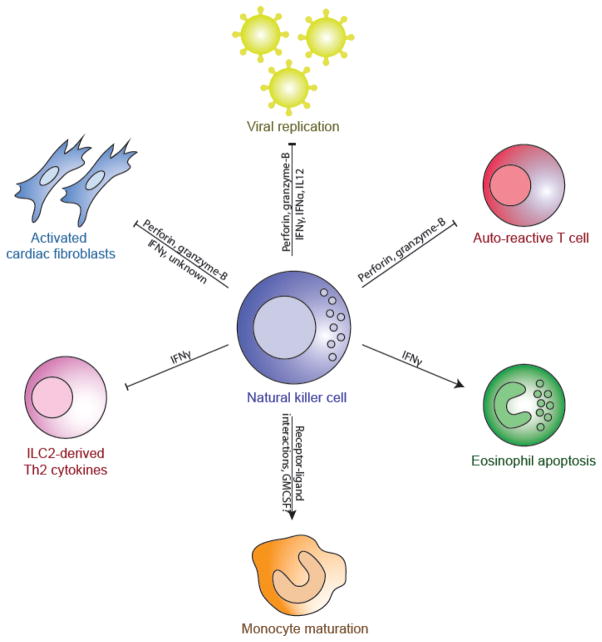
Inflammatory heart disease comprises a spectrum of etiologies and symptoms, but are grouped together for their major characteristic–the infiltration of activated hematopoietic cells into the cardiac tissue. NK cells have been shown to be dysregulated in many of these diseases including transplant rejection, myocarditis, and cardiac fibrosis. In this review, we explore how cardiac inflammation is modulated by natural killer cells (Figure 1).
Figure 1.
Schematic of the mechanisms by which NK cells regulate the inflammatory cardiac environment
NK cells comprise the largest subset of the innate lymphoid cell (ILC) family. ILCs include the IFNγ-producing T-bet+ Type 1 ILCs, the IL4-secreting Th2-associated GATA3+ Type 2, and the IL17A-producing Rorγt+ Type 3 ILCs [11, 12]. ILCs are morphologically lymphocytic in nature, but lack the somatic rearrangement of antigen receptors found in the more classical T-cells and B-cells of the adaptive immune system. ILCs are responsive to multiple immune signals, are required for defense against pathogens, and are necessary for the formation of lymphoid organs [13, 14]. Additionally, ILCs play a major role in both repairing damaged tissue and maintaining tissue homeostasis [15]. Type 1 ILCs, which include both NK cells and non-classical NK ILC1 cells, are characterized by their ability to produce IFNγ and express the transcription factor T-bet. However, recent fate mapping research has indicated that NK cells and conventional ILCs have distinct developmental lineages, a finding underscored by the cytotoxic abilities exclusive to NK cells [16, 17]. In contrast, all other ILCs share a common committed progenitor [18].
Due to their convergent evolution, it can be difficult to draw direct comparisons between specific mouse and human NK subsets [19]. However, both groups have inherited identical functionality despite lacking sequence homology in surface molecules [20–22]. Differences in mammalian origin notwithstanding, all NK cells contain pre-formed cytotoxic granules that are released upon specific activation signals that do not require previous sensitization. Thus, NK cells were originally classified as innate cytotoxic effector lymphocytes due to their stochastically expressed activation and inhibitory receptors and lack of memory formation [23–25]. Since then, they have been recognized for their ability to modulate the immune system well-beyond the innate response, although they are still the first line of defense against many intracellular pathogens [24, 26]. The absence of NK cells results in increased viral titer and dissemination in multiple animal models, including cytomegalovirus, hepatitis, and influenza [27, 28].
Additionally, there is accumulating evidence that NK cells possess some manner of memory ability [29, 30]. Mice deficient in B and T cells have the ability to mount increasingly elevated antigen-specific immune responses against certain haptens and viruses after sensitization [31, 32]. This was recently shown to be true in SHIVSF162P3-infected and SIVmac251-infected rhesus macaques, whereby splenic and hepatic NK cells taken from these animals were specifically able to lyse Gag- and Env-pulsed dendritic cells in an NKG2-dependent fashion [33].
The earliest murine NK precursors are non-stromal bone marrow cells known as pre-NK progenitors (pre-NKP) that express surface makers CD117 and CD244 and the transcription factor Id2 [15, 34–36]. From this stage, the cells acquire CD122, the β-receptor subunit of both IL-2 and IL-15, and CD132, the common-γ chain. IL-15 is required for the development and maturation of fully functional NK cells [37, 38]. It is unknown what signals control the shift from pre-NKPs to NKPs, though transcription factors EOMES and T-bet are required [39]. NKPs are defined as cells that express CD122 and CD132, but lack the functional capacities of mature NK cells and do not express other lineage markers such as CD3, CD19, and CD14. Henceforth, these cells are committed and do not develop into other cell types upon in vitro stimulation [40, 41].
NKP cells develop into immature NK cells (iNK) upon IL-15 stimulation, a process that can be amplified with the addition of other CD122 and CD132 ligands such as IL-3, IL-7, c-kit ligand (KL), and flt-3 ligand (FL). The latter cytokines are not required for NK maturation, but are actively expressed by bone marrow stromal cells [39, 42]. iNK cells express high levels of CXCR4, a receptor for the highly expressed stromal-factor 1 in the bone marrow, and thus are retained until maturation [43]. They also begin to express other NK-associated markers such as NKp46, NK1.1 (in C57B/6 or C57BL/10 mice), CD94, and CD27 [35]. The exact mechanisms of NK maturation have not yet been well-delineated, though licensing of these cells through the ligation of MHCI with NK inhibitory receptors such as the Ly49 and KIR families is required [44]. Without MHCI contact, immature NK cells remain anergic and nonfunctional, explaining why MHCI-deficient mice do not undergo massive inflammation despite having normal numbers of NK cells [44, 45].
Mature mouse NK cells acquire the expression of DX5 and CD11b, along with various organ-specific chemokine receptors that may help egress from the bone marrow to specific sites in the body. These mature NK cells present fluid organ-specific phenotypes through the differential expression of chemokine receptors, cytokine secretions profiles, and inherent cytotoxic abilities [46, 47]. Transferring of murine NK cells into another organ results in the conversion of the transferred cells to the tissue-specific NK phenotype [48]. Mature mouse NK cells also acquire their canonical cytotoxicity functions, such as preformed vesicles containing granzyme-B and perforin and expression of the cytotoxicity mediators Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL). Depending on the activation signals received, mature NK cells may also secrete various cytokines and inflammatory mediators, among which the type I interferons, tumor necrosis factor alpha (TNFα), IFNγ, and IL-13 are the most well-known [49, 50].
Similar to positive selection of T cells in the thymus, an NK cell is “licensed” and considered mature with the successful ligation of an MHCI molecule. Human NK cells express a series of killer inhibitory receptors (KIRs) that recognize their host’s specific MHCI HLA haplotype [51]. The triggering of KIRs with unrecognized HLA molecules result in alloreactivity. In transplantation, this mismatch between donor and recipient HLA haplotypes results in the expansion of activated alloreactive NK cells, thus instigating the process of transplant rejection [52].
Similarly, both mouse and human NK cells recognize their targets by their abnormal presentation of MHCI such as in tumor or virally infected cells. MHCI on healthy cells results in the ligation of an inhibition receptor on the surface of NK cells, preventing the release of perforin and granzyme-B. However, NK cells have hundreds of invariant receptors that are stochastically expressed on each cell’s surface during development that recognize a wide variety of self and foreign antigens [53, 54]. For example, the viral proteins influenza hemagglutin (HA) and Sendai HA-neuraminadase (HN), directly ligate the mouse and human NK activation receptors NKp44 and NKp46, respectively [28]. Altogether, NK cells comprise an immunologically distinct niche that is not yet well characterized. They have the ability to impact the immune system in a wide variety of ways–from direct cytotoxicity to releasing soluble factors that can alter both innate and adaptive immune response.
2. Heart Disease Accompanied by Inflammation
Inflammation is a common thread underlying the pathogenesis of multiple cardiac conditions including myocarditis, cardiac transplant rejection, and myocardial infarction. Clinically, deficits in NK cell levels may contribute to low grade cardiac inflammation [55–57]. In this review, we examine the ability of NK cells to modulate the inflammatory response accompanying several heart diseases and discuss how these cells may provide a target for future biological therapies.
2.1. Coronary Artery and Ischemic Heart Disease
Coronary artery and ischemic heart diseases are leading causes of death in the developed world [58]. A wide variety of risk factors can be cited as contributing to disease including atherosclerosis, familial inheritance, and arterial hypertension [59]. Infections and pathogen burdens such as cytomegalovirus (CMV) and Chlamydia pneumoniaeare are also major factors in the determination of heart function [60–62]. Thus, coronary artery and ischemic heart disease can be considered diseases that are a combination of etiological origin and a prolonged inflammatory state.
Multiple clinical studies have shown that coronary artery and ischemic heart disease patients have a decreased NK presence either through total numbers and/or phenotypic ability [63–65]. One study reported lower percentages and total numbers of CD3-CD56bright and CD3-CD56dim circulating NK cells with lower cytotoxic and IFNγ production ability in patients [64]. Another study reported decreased NK cells in patients at the time of diagnosis. Furthermore, a 12-month follow-up showed that a continued deficient in NK cells was correlated with low-grade cardiac inflammation, whereas patients that had restored circulating NK cells had little to no cardiac inflammation [65, 66].
In conclusion, a deficit of NK cells has been well-established in coronary artery and ischemic heart disease. What remains to be determined, however, is whether this absence is causative or merely symptomatic remains to be seen.
2.2. Myocardial Infarction
The detrimental immunological cascade that takes place after the ischemic event has resolved by thrombolytic therapy or primary percutaneous coronary intervention (PPCI) often promotes scar formation and fibrosis [67]. The restoration of blood flow to previously infarcted heart tissues, known as ischemia-reperfusion injury (IRI) results in greater damage to the local cardiomyocytes than the initial infarction caused. The injured cardiomyocytes release a combination of alarmins, chemokines, and cytokines that ultimately call for the infiltration of inflammatory cell populations dominated largely by neutrophils and monocytes [67]. Both these inflammatory populations and overload of reactive oxygen species and calcium and magnesium ions cause tissue damage and begin a cascade of events ultimately leading to tissue necrosis and collagen deposition [68]. This may help explain why, despite successful reperfusion after an acute myocardial infarction, death rates range from 7–15% post-procedure [69–71]. Much of myocardial infarction research is aimed at controlling this inflammatory surge in order to improve long-term patient outcomes.
The role of NK cells in myocardial reperfusion injury has not been well-described. Most of the research has been focused on NK-dependent monocytic maturation and activation. Crosstalk between monocytes and NK cells infiltrating the infarcted tissue can drive a T-bet/IFNγ/IL-12 signaling loop that results in the activation of both cellular populations and the increased recruitment of other inflammatory cells. Furthermore, this crosstalk was required for angiotensin-II induced vascular injury [10, 72].
Other studies have demonstrated that the worsened cardiac outcomes seen in basophil and mast cell-deficient c-kit−/− mice after MI could be rescued by the transfer of hematopoietic stem cells (HSC), which leads to increased neovascularization and recruitment of cardiac progenitor cells. The effect of this transfer was limited in the absence of NK cells [1]. NK cells have also been shown to interact with cardiac endothelial cells post-MI to increase vascularization and angiogenesis [73, 74]. However, attempts by other groups to repeat these findings were unsuccessful, with most groups finding that the majority of HSCs differentiated into neutrophils with limited establishment in the heart as resident cells [75–77]. Overall, the role of NK cells in the pathogenesis of MI is yet to be determined.
2.3. Transplant Rejection
As NK cells are crucial in distinguishing foreign from self, they play an essential role in the progression of transplant rejection and graft versus host disease (GVHD) through both innate immunity and the establishment of a tissue-specific adaptive immune response. In terms of the innate response, MHCI (HLAI) mismatch between donor and recipient tissues results in transplant rejection due to cytotoxicity from failed ligation of killer inhibitor receptors (KIRs) expressed on NK cells. On the adaptive side, the immune response generated by NK cells results in a cascade of events leading to an antigen-specific response against donor tissue. NK cells infiltrate cardiac grafts and transplants and accumulate in lesions found in donor tissue, contributing to acute rejection in both animal and human studies [78–80]. Clinically, cyclosporine A has been used to great success in suppressing rejection by limiting both the expansion of T and NK cells. Human NK cells treated with cyclosporine resulted in reduced proliferation in the CD56bright inflammatory subsets in response to IL-2 and IL-15 [81].
However, several non-cardiac transplant models have pointed to the ability of NK cells to promote tolerance. The depletion of T cells prior to clinical bone marrow transplantation results in a large expansion of the NK component and leads to graft establishment. Li et al found that in a model of skin transplantation, NK cells can effectively kill antigen-presenting cells (APCs) trafficking to donor tissue, helping to prevent the establishment of an adaptive immune response [82]. Other studies have shown that perforin from NK cells is vital for tolerance to allografts and that the transfer of perforin-competent NK cells is sufficient for transplantation tolerance in perforin-deficient recipients [83]. In other models, the transfer of alloreactive NK cells prior to HLA-mismatched tissue transplantation reduced the need for high-intensity conditioning and limited GVHD.
In mouse models of cardiac transplantation, NK cells are not required for acute allographic rejection in the presence of CD4+ cells. However, in the event of suboptimal T-cell responses, NK cells are necessary for the establishment of graft rejection [84, 85]. In Rag1−/−γc−/− mice, cold ischemic cardiac grafts resulted in chronic allography vasculopathy (CAV) only after the transfer of NK cells. This rejection was IL-6-mediated, as IL-6−/− grafts did not result in CAV in the presence of NK cells in IL6 competent recipients. Similarly, the combined use of anti-NKG2D and anti-CTLA-4 antibodies lead to increased cardiac allograft survival through reducing CAV by limiting alloantibody- and IL-6-production and increasing T-regulatory cell function [6, 86].
NK cells play a major role in determining the short and long-term clinical outcomes of cardiac transplant patients. They are the initiators in the immune response following the recognition of foreign antigens and, therefore, represent a major therapeutic target for improving patient outcomes after transplantation and engraftment. Their balancing effect of the immune response following transplantation provides an ideal target for fine-tuning the patient’s immune system in favor of tolerance.
2.4. Myocarditis and Inflammatory Dilated Cardiomyopathy
Both idiopathic and viral myocarditis patients have severely low levels of NK cells and NK cytotoxicity, pointing towards defects in both their frequency and function [56, 57]. The same was also found in DCM patients, though it remains unclear whether this is the result of primary myocarditis [87]. Perforin-caused pores on the surface of virally-infected myocytes have been found in both clinical myocarditis patients and in animal viral myocarditis models [88]. In contrast, heart biopsies of 18 early myocarditis patients did not reveal any NK cell infiltration, though this may be due to the sampling limitations [89].
NK cells play an essential role in the defense against acute viral pathogens associated with clinical myocarditis. We and others have reported NK cells to be protective against both coxsackievirus B (CBV) and murine cytomegalovirus (MCMV)-induced myocarditis, albeit these studies depleted both NK and NKT populations simultaneously [90, 91]. The direct mediators of viral recognition are unknown in CBV and MCMV, though ligation of the activating receptor Ly49H by the viral glycoprotein m157 and destabilization of inhibitory receptor Ly49C by abnormal MHCI expression is necessary in limiting MCMV infection [92, 93].
C57BL/6 and BALB.B6-Cmv1r mice, which express NK1.1 on their NK cells are susceptible to acute viral myocarditis, but resistant to developing chronic inflammation and DCM. NK1.1 antibody treatment in these strains, resulted in a disease that mirrored the natural progression of susceptible BALB/c MCMV- and CBV-induced viral myocarditis [90]. This indicates that NK cells play an important role in the determination of strain-dependent resistance to experimental myocarditis. We have recently showed that NK cells can protect against myocarditis even in the absence of virus, using a mouse model of experimental autoimmune myocarditis (EAM) [9]. NK cells controlled eosinophil numbers through the induction of eosinophil apoptosis, a mechanism also shown in other human and mouse studies in vitro [94, 95]. These data indicate that NK cells may be the missing mediator needed to control myocarditis and, thus the ensuing DCMi, by limiting both viral replication and the inflammatory response in this disease [96, 97].
3. Mechanisms of Protection
There are a variety of mechanisms by which NK cells are proposed to alter both the cardiac environment and the extrinsic factors that play a role in cardiac disease progression, such as viral pathogens. Additionally, many cardiac diseases can be broken into two general phases–the first being the acute etiological origin and the second being an inflammatory resolution or lack thereof. In both of these, NK cells can exert a varied number of effects from limiting inflammation to responding to a hypoxic environment.
3.1. Viral Replication
Perhaps one of the best studied aspects of NK biology is their relationship to viral infection [28]. NK cells are critical in suppressing viral replication by recognizing and lysing infected host cells. NK cells are responsible for the first burst of type I interferons that begins the anti-viral inflammatory cascade by releasing IFN-α and IFN-β [24, 98]. NK cells also release a multitude of pro-inflammatory cytokines that mediate the innate and adaptive immune response such as IL-12, IFNγ, and IFNλ [99–101]. Additionally, viral infection often results in altered transcription and protein expression causing infected cells to dysregulate the levels of MHCI molecules and/or express viral proteins on their cell membranes [102]. The former results in inefficient ligation of inhibitory receptors on NK cells with MHCI molecules on the infected cell, while the latter leads to the direct triggering of NK activation through the activation receptors that recognize various viral proteins such as NKp44 and NKp46 [103, 104].
The ability of NK cells to limit viral infections is important in cardiac biology [105–108]. NK cells are required for protection against mouse models of CBV and MCMV-mediated myocarditis in an IFNγ-dependent manner [90, 109, 110]. Clinically, rare individuals with NK cell deficiencies have an increased susceptibility to viral infections, though not specifically cardiac infections [111–114].
3.2. Inflammation
NK cells have been shown to modulate the immune system in a wide variety of ways. From monocyte maturation to T-cell anergy, NK cells can alter immune cell physiology either directly through receptor-ligand interactions or indirectly through cytokine secretion. Furthermore, the mechanisms by which NK cells alter disease are not limited to cytotoxicity. Cytokine release resulting in the alteration of Th subtypes, direct contact-mediated lysis of auto-aggressive T cells, and accelerated maturation of monocytes and dendritic cells are all ways by which NK cells modulate the innate and adaptive immune system [96, 97, 115].
In terms of mechanisms related to autoimmune disease and their inflammatory cascades, NK cells have been found to accumulate in the joints of rheumatoid arthritis (RA) patients, in the skin lesions of psoriasis, and in the brain lesions of multiple sclerosis [116, 117]. RA patient joints contained higher proportions of activated CD56bright and IFNγ production compared to periphery NK cells in the same individuals [117, 118]. When isolated and co-cultured with patient monocytes, these activated NK cells were able to induce differentiation of monocytes into dendritic cells, signifying an active role in the immune environment [119]. Furthermore, individuals with NK-lymphoproliferative disease of granular lymphocytes, a genetic condition resulting in the chronic accumulation of circulating NK cells, have increased risk of developing autoimmune manifestations [120, 121]. Thus, NK cells may have the ability to directly control autoimmune inflammation of the heart, such as in myocarditis or DCMi.
Recently, studies have shown that NK cells may directly limit the presence of eosinophils in the cardiac environment [9, 94, 95]. We showed that NK cells can protect against the pathogenesis of myocarditis in the mouse model by limiting eosinophilic accumulation in the cardiac environment. Awad et al showed that NK cells isolated from healthy human PBMCs induced the activation and apoptosis of eosinophils as measured by CD62L and CD69 when co-cultured together in vitro [95]. Apoptosis was initiated due to increases in reactive oxygen species (ROS) in eosinophils and cell death could be mitigated through the use of mitochondrial inhibitors rotenone and antimycin [95]. Furthermore, IFNγ from activated NK cells could downregulate the release of Th2 cytokines from ILC2 populations. ILC2 stimulate the production of eotaxins from cardiac fibroblasts, and NK-derived IFNγ could effectively limit the ability of eosinophils to traffic to the heart by indirectly decreasing local eotaxin levels [122].
Barnig et al demonstrated that asthmatic patients have a higher number NK cells that are highly activated in the blood than those from healthy donors. These NK cells also expressed increased levels of CD69, NKG2D, and ALX/FPR2, a receptor for lipoxin A4 (LXA4), which is known to be elevated in the lungs of asthmatics and administration limits bronchoconstriction [94, 123]. Furthermore, LXA4 decreased eosinophilic trafficking and also limits NK cytotoxicity. The addition of LXA4 to co-cultures of NK cells and eosinophils isolated from the blood of both healthy and asthmatic donors limited the level of eosinophilic apoptosis [94]. Depletion of NK cells from animals of atherosclerosis resulted in the attenuation of lesion size independent of lipid levels. Transfer of NK cells back into NK-deficient mice resulted in increased disease in a perforin and granzyme-B dependent mechanism.
3.3. Hypoxia
The effects of oxidative stress on NK cells has been an active field of study due to interest in NK cells as a cancer therapy and the hypoxic nature of tumor microenvironments. While NK cells are effective at lysing tumor cells in vitro, in vivo conditions have significantly less available oxygen due to the overwhelming nutrient and oxygen demands of the rapidly dividing cancer cells [124]. In humans, NK cells adapt to these hypoxic conditions through the upregulation of hypoxia inducible factor 1α (HIF1α) [125]. However, this comes at the cost of downregulating multiple activation markers, leaving the cells hypo-responsive to external stimuli. NK-mediated lysis of tumor cells is impaired in response to granzyme-B deficiency due to increased autophagy [126]. Much of NK-based tumor research has been to reverse this NK-inactivation that occurs upon migration into the tumor tissue.
With regards to hypoxia as it relates to cardiac inflammatory disease, much of the damage due to myocardial infarction is attributable to the inflammatory response seen in hypoxic cardiac tissue following the initial event. NK cells traffic to the tissue after an ischemic injury in multiple animal models in response to chemotactic signals from local resident cells [127–129]. In rats, NK cell infiltration reaches a peak 7 days after a cardiac ischemic injury [129]. Their activation in neuronal ischemic tissue appears to be contact-mediated by the ligation of activation markers such as NKG2D and their accumulation in brain infarction models are shown to the detrimental to disease outcomes [127]. Conversely, NK cells are protective in hepatic ischemia-reperfusion injury models through ligation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [130].
3.4. Fibrosis
In terms of fibrosis, NK cells are well-studied in both mice and humans for their ability to resolve and prevent liver damage and fibrosis [131]. Clinically, it has been observed that levels of NK cells are negatively correlated with liver damage in patients with chronic hepatitis C infection [132, 133]. The transfer of NK cells into mice undergoing carbon tetrachloride-induced fibrosis limited the severity of disease and the production and deposition of collagen [134–136]. NK cells are known to directly lyse activated fibroblasts in multiple murine liver fibrosis models, including schistosomiasis, 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) and carbon tetrachloride [132, 134, 137, 138]. Overall, strong evidence exists for a protective and possibly therapeutic role for NK cells in liver fibrosis. However, it is likely that the mechanism of liver fibrosis is not true in the cardiac environment.
Our group has shown that NK cells are protective against the development of cardiac fibrosis by both directly limiting collagen formation in cardiac fibroblasts and by preventing the accumulation of certain inflammatory populations in the heart [9]. Unlike the co-culture of NK cells and liver fibroblasts, the addition of NK cells to activated primary cardiac fibroblasts did not induce fibroblast cell death. Instead, IFNγ and other mediators, produced by the NK cells, induced an anti-inflammatory chemokine environment, protecting against fibrosis in the heart through negative regulation of pro-fibrotic cell types such as eosinophils [9]. Other groups have shown in mice that the expansion of NK cells from c-kit+ bone marrow cell transfers to the heart after MI was responsible for a reduction in cardiac myocyte apoptosis and collagen formation, along with an increase in neovascularization [1, 139]. Given these studies, in addition to the ability of NK cells to limit the mechanism of collagen deposition in liver fibrosis models and the protective effects of NK cells post-MI, it is highly likely that NK cells are protective against the development of cardiac fibrosis.
4. NK-Based Immunotherapies
NK-based immunotherapies are heavily investigated in both academic and commercial settings not for their possibilities for cardiac disease therapy, but for their anti-cancer potential [140–143]. Tumors evade the immune response by specifically inducing defects in NK activation and the transfer of ex vivo activated autologous NK cells into metastatic renal cell carcinoma (RCC), malignant glioma, and breast cancer patients limited tumor progression [141, 144–148]. Other NK-based therapies employ cytokines to stimulate endogenous NK populations or transgenic primary or cell-line derived NK cells that highly express certain cytokines or receptors. Thus, this pre-existing infrastructure could be redirected and adapted to improving cardiac transplantation outcomes and other cardiac inflammatory diseases.
The use of IL-2 and other cytokines to activate endogenous NK populations for anti-tumor therapies has met with limited success. IL-2 alone or in combination with IL-12, IL-15, I3L-18, IL-21 and type I interferons induced NK activation and tumor lysis in vitro, but failed to provide patients with positive outcomes, due to IL-2-dependent expansion of Treg populations [149–153]. Other studies have looked to bolstering NK transfers with low doses of IL-2. While this increased numbers of circulating NK cells in the bloodstream, the effect was short-lived and failed to maximize cytotoxicity as determined by in vitro assays [153, 154]. Furthermore, clinical trials have shown that while ex-vivo IL-2 stimulated NK cell transfer therapy can be safely performed without the toxicity seen in direct cytokine infusion, patients showed no improvement in clinical outcomes when compared to matched controls [155]. However, in terms of cardiac biology, the use of IL-2 to stimulate NK cell populations show promise. Systemic injections of IL-2 post-MI improved left ventricular fractional shortening, improved neovascularization, and reduced collagen deposition via NK-dependent mechanisms [73].
Whole NK cells can be isolated from peripheral or cord blood or generated en mass from CD34+ hematopoietic cells from autologous or allogeneic human donors [156]. NK cell lines may also be utilized, with a particularly cytotoxic line NK-92 having recently been safely used in patients with advanced cancers with some anti-tumor success [157]. Thus, there is a large, readily available, and easily characterized source of NK cells unique to each patient that can be exploited.
Furthermore, these NK cells may be genetically manipulated to express both homing chemokine receptors and/or chimeric antigen receptors (CARs) to increase their chances of achieving their immunological aims though the latter will have limited obvious applicability outside the fields of cancer and autoimmunity [158–161]. The transfer of NK cells in animal models of cardiac transplantation has shown the most promise in certain tolerogenic conditions and would be a promising avenue of transplant rejection therapy [162]. Furthermore, as NK cells can control both viral load and inflammation in myocarditis and subsequent dilated cardiomyopathy, they could be an excellent candidate for targeted biologic therapy. Patients would no longer have to be stratified by the presence of virus in the heart as they are currently for immunosuppressive or IFN therapy, in order to determine their course of treatment as NK cells would be able to target both mechanisms, viral and autoimmune, simultaneously.
5. Conclusions
In conclusion, NK cells are distinct controllers of disease and dysregulation as their biology can dramatically shift clinical outcomes. More clinical research is needed to determine exactly how these cells and their mechanisms might be exploited for therapeutic gains. It would be greatly advantageous to turn already commercially available cancer-based NK therapies towards the many holes in current inflammatory cardiac disease treatments.
Highlights.
Blockade of NK cells and their receptors protect against disease severity and pathogenesis in animal models of inflammatory cardiac disease.
NK cells suppress immune cell maturation and activation, alter local inflammatory environments, and induce apoptosis in both resident and hematopoietic cells.
As NK cells control both viral load and inflammation in myocarditis and subsequent dilated cardiomyopathy, they are an excellent candidate for targeted biologic therapy.
Clinical NK therapies employ cytokine therapy to stimulate endogenous NK cells, NK transfers, and transgenic modification of NK cells that highly express certain cytokines or cell surface receptors.
This pre-existing infrastructure could be redirected and adapted to include therapies for improving cardiac transplantation outcomes and other cardiac inflammatory diseases.
Acknowledgments
This work was supported by NIH/NHLBI grants R01HL118183 (Daniela Čiháková), and R01HL113008 (Daniela Čiháková) and Catalyst Award, JHU (Daniela Čiháková). SuFey Ong was the recipient NIH/NHLBI grant National Research Service Award F31 HL112665-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ayach BB, et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103(7):2304–9. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuroki S, Miyahara K, Uematsu T. Immunological aspects in patients with acute myocardial infarction. Jpn Circ J. 1993;57(1):37–46. doi: 10.1253/jcj.57.37. [DOI] [PubMed] [Google Scholar]
- 3.Klarlund K, et al. Depressed natural killer cell activity in acute myocardial infarction. Clin Exp Immunol. 1987;70(1):209–16. [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JL, Carlquist JF, Hammond EH. Deficient natural killer cell activity in patients with idiopathic dilated cardiomyopathy. Lancet. 1982;2(8308):1124–7. doi: 10.1016/s0140-6736(82)92786-6. [DOI] [PubMed] [Google Scholar]
- 5.Galati D, et al. Peripheral depletion of NK cells and imbalance of the Treg/Th17 axis in idiopathic pulmonary fibrosis patients. Cytokine. 2014;66(2):119–26. doi: 10.1016/j.cyto.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZX, et al. Natural killer cells play a critical role in cardiac allograft vasculopathy in an interleukin-6--dependent manner. Transplantation. 2014;98(10):1029–39. doi: 10.1097/TP.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 7.Shah AM, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7(5):740–51. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipshultz SE, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348(17):1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 9.Ong S, et al. Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration. Am J Pathol. 2015;185(3):847–61. doi: 10.1016/j.ajpath.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knorr M, Munzel T, Wenzel P. Interplay of NK cells and monocytes in vascular inflammation and myocardial infarction. Front Physiol. 2014;5:295. doi: 10.3389/fphys.2014.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 12.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–75. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 13.Bordon Y. Innate lymphoid cells: on the origin of ILCs. Nat Rev Immunol. 2014;14(3):133. doi: 10.1038/nri3629. [DOI] [PubMed] [Google Scholar]
- 14.Bordon Y. Mucosal immunology: ILCs broker peace deals in the gut. Nat Rev Immunol. 2013;13(7):473. doi: 10.1038/nri3480. [DOI] [PubMed] [Google Scholar]
- 15.Vosshenrich CA, Di Santo JP. Developmental programming of natural killer and innate lymphoid cells. Curr Opin Immunol. 2013;25(2):130–8. doi: 10.1016/j.coi.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, et al. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat Immunol. 2015;16(10):1044–50. doi: 10.1038/ni.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klose CS, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157(2):340–56. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Constantinides MG, et al. A committed precursor to innate lymphoid cells. Nature. 2014;508(7496):397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barten R, et al. Divergent and convergent evolution of NK-cell receptors. Trends Immunol. 2001;22(1):52–7. doi: 10.1016/s1471-4906(00)01802-0. [DOI] [PubMed] [Google Scholar]
- 20.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13(2):133–44. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Older Aguilar AM, et al. Coevolution of killer cell Ig-like receptors with HLA-C to become the major variable regulators of human NK cells. J Immunol. 2010;185(7):4238–51. doi: 10.4049/jimmunol.1001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parham P, et al. Variable NK cell receptors exemplified by human KIR3DL1/S1. J Immunol. 2011;187(1):11–9. doi: 10.4049/jimmunol.0902332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujisaki H, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–7. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vivier E, et al. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 25.Lanier LL. The origin and functions of natural killer cells. Clin Immunol. 2000;95(1 Pt 2):S14–8. doi: 10.1006/clim.1999.4816. [DOI] [PubMed] [Google Scholar]
- 26.Golden-Mason L, et al. Hepatitis C viral infection is associated with activated cytolytic natural killer cells expressing high levels of T cell immunoglobulin- and mucin-domain-containing molecule-3. Clin Immunol. 2015;158(1):114–25. doi: 10.1016/j.clim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–94. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 29.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12(6):500–8. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 30.Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16(2):112–23. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 31.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11(12):1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Leary JG, et al. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 33.Reeves RK, et al. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015;16(9):927–32. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3(6):523–8. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 35.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011;11(10):645–57. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–86. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 39.Daussy C, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. 2014;211(3):563–77. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang X, et al. Single line or parallel lines: NK cell differentiation driven by T-bet and Eomes. Cell Mol Immunol. 2012;9(3):193–4. doi: 10.1038/cmi.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fathman JW, et al. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood. 2011;118(20):5439–47. doi: 10.1182/blood-2011-04-348912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minagawa M, et al. Enforced expression of Bcl-2 restores the number of NK cells, but does not rescue the impaired development of NKT cells or intraepithelial lymphocytes, in IL-2/IL-15 receptor beta-chain-deficient mice. J Immunol. 2002;169(8):4153–60. doi: 10.4049/jimmunol.169.8.4153. [DOI] [PubMed] [Google Scholar]
- 43.Beider K, et al. Involvement of CXCR4 and IL-2 in the homing and retention of human NK and NK T cells to the bone marrow and spleen of NOD/SCID mice. Blood. 2003;102(6):1951–8. doi: 10.1182/blood-2002-10-3293. [DOI] [PubMed] [Google Scholar]
- 44.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 45.Sungur CM, et al. Murine NK-cell licensing is reflective of donor MHC-I following allogeneic hematopoietic stem cell transplantation in murine cytomegalovirus responses. Blood. 2013;122(8):1518–21. doi: 10.1182/blood-2013-02-483503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi FD, et al. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11(10):658–71. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma R, Das A. Organ-specific phenotypic and functional features of NK cells in humans. Immunol Res. 2014;58(1):125–31. doi: 10.1007/s12026-013-8477-9. [DOI] [PubMed] [Google Scholar]
- 48.Lassen MG, et al. Intrahepatic IL-10 maintains NKG2A+Ly49- liver NK cells in a functionally hyporesponsive state. J Immunol. 2010;184(5):2693–701. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fauriat C, et al. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115(11):2167–76. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–9. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kroemer A, et al. The innate NK cells, allograft rejection, and a key role for IL-15. J Immunol. 2008;180(12):7818–26. doi: 10.4049/jimmunol.180.12.7818. [DOI] [PubMed] [Google Scholar]
- 52.Kroemer A, Edtinger K, Li XC. The innate natural killer cells in transplant rejection and tolerance induction. Curr Opin Organ Transplant. 2008;13(4):339–43. doi: 10.1097/MOT.0b013e3283061115. [DOI] [PubMed] [Google Scholar]
- 53.Arnon TI, Markel G, Mandelboim O. Tumor and viral recognition by natural killer cells receptors. Semin Cancer Biol. 2006;16(5):348–58. doi: 10.1016/j.semcancer.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gauntt CJ, et al. Role of natural killer cells in experimental murine myocarditis. Springer Semin Immunopathol. 1989;11(1):51–9. doi: 10.1007/BF00197084. [DOI] [PubMed] [Google Scholar]
- 56.Kanda T, et al. Idiopathic myocarditis associated with T-cell subset changes and depressed natural killer activity. Jpn Heart J. 1990;31(5):741–4. doi: 10.1536/ihj.31.741. [DOI] [PubMed] [Google Scholar]
- 57.Yang YZ, Jin PY, Wang QD. Natural killer cell activity and induction of alpha and gamma interferon in patients with Coxsackie B viral myocarditis. Zhonghua Xin Xue Guan Bing Za Zhi. 1988;16(6):337–9. 382. [PubMed] [Google Scholar]
- 58.Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med. 2013;369(10):954–64. doi: 10.1056/NEJMra1203528. [DOI] [PubMed] [Google Scholar]
- 59.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366(1):54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 60.Hsich E, et al. Cytomegalovirus infection increases development of atherosclerosis in Apolipoprotein-E knockout mice. Atherosclerosis. 2001;156(1):23–8. doi: 10.1016/s0021-9150(00)00608-0. [DOI] [PubMed] [Google Scholar]
- 61.Campbell LA, Kuo CC. Chlamydia pneumoniae--an infectious risk factor for atherosclerosis? Nat Rev Microbiol. 2004;2(1):23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- 62.Chen S, et al. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185(9):5619–27. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou N, et al. Increased expression of T cell immunoglobulin- and mucin domain-containing molecule-3 on natural killer cells in atherogenesis. Atherosclerosis. 2012;222(1):67–73. doi: 10.1016/j.atherosclerosis.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Hak L, et al. NK cell compartment in patients with coronary heart disease. Immun Ageing. 2007;4:3. doi: 10.1186/1742-4933-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jonasson L, Backteman K, Ernerudh J. Loss of natural killer cell activity in patients with coronary artery disease. Atherosclerosis. 2005;183(2):316–21. doi: 10.1016/j.atherosclerosis.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Backteman K, Ernerudh J, Jonasson L. Natural killer (NK) cell deficit in coronary artery disease: no aberrations in phenotype but sustained reduction of NK cells is associated with low-grade inflammation. Clin Exp Immunol. 2014;175(1):104–12. doi: 10.1111/cei.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 68.Ueha S, Shand FH, Matsushima K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front Immunol. 2012;3:71. doi: 10.3389/fimmu.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 70.Weaver WD, et al. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review. JAMA. 1997;278(23):2093–8. [PubMed] [Google Scholar]
- 71.Michels KB, Yusuf S. Does PTCA in acute myocardial infarction affect mortality and reinfarction rates? A quantitative overview (meta-analysis) of the randomized clinical trials. Circulation. 1995;91(2):476–85. doi: 10.1161/01.cir.91.2.476. [DOI] [PubMed] [Google Scholar]
- 72.Kossmann S, et al. Angiotensin II-induced vascular dysfunction depends on interferon-gamma-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol. 2013;33(6):1313–9. doi: 10.1161/ATVBAHA.113.301437. [DOI] [PubMed] [Google Scholar]
- 73.Bouchentouf M, et al. Interleukin-2 enhances angiogenesis and preserves cardiac function following myocardial infarction. Cytokine. 2011;56(3):732–8. doi: 10.1016/j.cyto.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 74.Bouchentouf M, et al. Induction of cardiac angiogenesis requires killer cell lectin-like receptor 1 and alpha4beta7 integrin expression by NK cells. J Immunol. 2010;185(11):7014–25. doi: 10.4049/jimmunol.1001888. [DOI] [PubMed] [Google Scholar]
- 75.Murry CE, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 76.Nygren JM, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10(5):494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 77.Balsam LB, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 78.Ankersmit HJ, et al. Death-inducing receptors and apoptotic changes in lymphocytes of patients with heart transplant vasculopathy. Clin Exp Immunol. 2002;127(1):183–9. doi: 10.1046/j.1365-2249.2002.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, et al. The activating immunoreceptor NKG2D and its ligands are involved in allograft transplant rejection. J Immunol. 2007;179(10):6416–20. doi: 10.4049/jimmunol.179.10.6416. [DOI] [PubMed] [Google Scholar]
- 80.Hsieh CL, et al. NK cells and transplantation. Transpl Immunol. 2002;9(2–4):111–4. doi: 10.1016/s0966-3274(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, et al. The unexpected effect of cyclosporin A on CD56+CD16- and CD56+CD16+ natural killer cell subpopulations. Blood. 2007;110(5):1530–9. doi: 10.1182/blood-2006-10-048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu G, et al. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203(8):1851–8. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beilke JN, et al. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med. 2005;11(10):1059–65. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 84.McNerney ME, et al. Role of natural killer cell subsets in cardiac allograft rejection. Am J Transplant. 2006;6(3):505–13. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 85.Hirohashi T, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12(2):313–21. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weis M, von Scheidt W. Cardiac allograft vasculopathy: a review. Circulation. 1997;96(6):2069–77. doi: 10.1161/01.cir.96.6.2069. [DOI] [PubMed] [Google Scholar]
- 87.Molofsky AB, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210(3):535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–8. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chow LH, et al. Phenotypic analysis of infiltrating cells in human myocarditis. An immunohistochemical study in paraffin-embedded tissue. Arch Pathol Lab Med. 1989;113(12):1357–62. [PubMed] [Google Scholar]
- 90.Fairweather D, et al. From infection to autoimmunity. J Autoimmun. 2001;16(3):175–86. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- 91.Godeny EK, Gauntt CJ. Involvement of natural killer cells in coxsackievirus B3-induced murine myocarditis. J Immunol. 1986;137(5):1695–702. [PubMed] [Google Scholar]
- 92.Zhang W, et al. The pro-inflammatory cytokine IL-22 up-regulates keratin 17 expression in keratinocytes via STAT3 and ERK1/2. PLoS One. 2012;7(7):e40797. doi: 10.1371/journal.pone.0040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sakabe JI, et al. Calcipotriol Increases hCAP18 mRNA Expression but Inhibits Extracellular LL37 Peptide Production in IL-17/IL-22-stimulated Normal Human Epidermal Keratinocytes. Acta Derm Venereol. 2014 doi: 10.2340/00015555-1775. [DOI] [PubMed] [Google Scholar]
- 94.Barnig C, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5(174):174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Awad A, et al. Natural killer cells induce eosinophil activation and apoptosis. PLoS One. 2014;9(4):e94492. doi: 10.1371/journal.pone.0094492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johansson S, et al. NK cells: elusive players in autoimmunity. Trends Immunol. 2005;26(11):613–8. doi: 10.1016/j.it.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 97.Perricone R, et al. NK cells in autoimmunity: a two-edg’d weapon of the immune system. Autoimmun Rev. 2008;7(5):384–90. doi: 10.1016/j.autrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 98.Fernandez NC, et al. Dendritic cells (DC) promote natural killer (NK) cell functions: dynamics of the human DC/NK cell cross talk. Eur Cytokine Netw. 2002;13(1):17–27. [PubMed] [Google Scholar]
- 99.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 100.Tillmann HL, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139(5):1586–92. 1592 e1. doi: 10.1053/j.gastro.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 101.Cooper MA, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 102.Jost S, Altfeld M. Evasion from NK cell-mediated immune responses by HIV-1. Microbes Infect. 2012;14(11):904–15. doi: 10.1016/j.micinf.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arnon TI, et al. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31(9):2680–9. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 104.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–60. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 105.Wheeler DS, Kooy NW. A formidable challenge: the diagnosis and treatment of viral myocarditis in children. Crit Care Clin. 2003;19(3):365–91. doi: 10.1016/s0749-0704(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 106.Schultz JC, et al. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84(11):1001–9. doi: 10.1016/S0025-6196(11)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pollack A, et al. Viral myocarditis-diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015 doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 108.Levi D, Alejos J. Diagnosis and treatment of pediatric viral myocarditis. Curr Opin Cardiol. 2001;16(2):77–83. doi: 10.1097/00001573-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 109.Kanda T, et al. Effect of interleukin-18 on viral myocarditis: enhancement of interferon-gamma and natural killer cell activity. J Mol Cell Cardiol. 2000;32(12):2163–71. doi: 10.1006/jmcc.2000.1242. [DOI] [PubMed] [Google Scholar]
- 110.Barin JG, et al. Fatal eosinophilic myocarditis develops in the absence of IFN-gamma and IL-17A. J Immunol. 2013;191(8):4038–47. doi: 10.4049/jimmunol.1301282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Haas M, et al. A triallelic Fc gamma receptor type IIIA polymorphism influences the binding of human IgG by NK cell Fc gamma RIIIa. J Immunol. 1996;156(8):2948–55. [PubMed] [Google Scholar]
- 112.Jawahar S, et al. Natural Killer (NK) cell deficiency associated with an epitope-deficient Fc receptor type IIIA (CD16-II) Clin Exp Immunol. 1996;103(3):408–13. doi: 10.1111/j.1365-2249.1996.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Vries E, et al. Identification of an unusual Fc gamma receptor IIIa (CD16) on natural killer cells in a patient with recurrent infections. Blood. 1996;88(8):3022–7. [PubMed] [Google Scholar]
- 114.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4(15):1545–58. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 115.Pazmany L. Do NK cells regulate human autoimmunity? Cytokine. 2005;32(2):76–80. doi: 10.1016/j.cyto.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 116.Santiago-Schwarz F, et al. Rheumatoid arthritis serum or synovial fluid and interleukin 2 abnormally expand natural killer-like cells that are potent stimulators of IgM rheumatoid factor. J Rheumatol. 1992;19(2):223–8. [PubMed] [Google Scholar]
- 117.Pridgeon C, et al. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright,CD94bright,CD158negative phenotype. Rheumatology (Oxford) 2003;42(7):870–8. doi: 10.1093/rheumatology/keg240. [DOI] [PubMed] [Google Scholar]
- 118.Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum. 2002;46(7):1763–72. doi: 10.1002/art.10410. [DOI] [PubMed] [Google Scholar]
- 119.Dalbeth N, et al. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173(10):6418–26. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 120.Zambello R, Semenzato G. Large granular lymphocyte disorders: new etiopathogenetic clues as a rationale for innovative therapeutic approaches. Haematologica. 2009;94(10):1341–5. doi: 10.3324/haematol.2009.012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zambello R, Semenzato G. Natural killer receptors in patients with lymphoproliferative diseases of granular lymphocytes. Semin Hematol. 2003;40(3):201–12. doi: 10.1016/s0037-1963(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 122.Molofsky AB, et al. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015;43(1):161–74. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnig C, Levy BD. Lipoxin A4: a new direction in asthma therapy? Expert Rev Clin Immunol. 2013;9(6):491–3. doi: 10.1586/eci.13.36. [DOI] [PubMed] [Google Scholar]
- 124.Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72(16):3906–11. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 125.Balsamo M, et al. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol. 2013;43(10):2756–64. doi: 10.1002/eji.201343448. [DOI] [PubMed] [Google Scholar]
- 126.Baginska J, et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc Natl Acad Sci U S A. 2013;110(43):17450–5. doi: 10.1073/pnas.1304790110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gan Y, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci U S A. 2014;111(7):2704–9. doi: 10.1073/pnas.1315943111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim HJ, et al. TLR2 signaling in tubular epithelial cells regulates NK cell recruitment in kidney ischemia-reperfusion injury. J Immunol. 2013;191(5):2657–64. doi: 10.4049/jimmunol.1300358. [DOI] [PubMed] [Google Scholar]
- 129.Yan X, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 130.Fahrner R, et al. Tumor necrosis factor-related apoptosis-inducing ligand on NK cells protects from hepatic ischemia-reperfusion injury. Transplantation. 2014;97(11):1102–9. doi: 10.1097/TP.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 131.Notas G, Kisseleva T, Brenner D. NK and NKT cells in liver injury and fibrosis. Clin Immunol. 2009;130(1):16–26. doi: 10.1016/j.clim.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 132.Gao B, Radaeva S, Jeong WI. Activation of natural killer cells inhibits liver fibrosis: a novel strategy to treat liver fibrosis. Expert Rev Gastroenterol Hepatol. 2007;1(1):173–80. doi: 10.1586/17474124.1.1.173. [DOI] [PubMed] [Google Scholar]
- 133.Blockade of NKG2D synergized with CTLA4-Ig in promoting long-term graft survival in murine models of cardiac transplantation: retraction. Transplantation. 2013;96(3):e20. doi: 10.1097/TP.0b013e3182a35979. [DOI] [PubMed] [Google Scholar]
- 134.Muhanna N, et al. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011;60(1):90–8. doi: 10.1136/gut.2010.211136. [DOI] [PubMed] [Google Scholar]
- 135.Hintermann E, et al. CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J Autoimmun. 2010;35(4):424–35. doi: 10.1016/j.jaut.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yi HS, et al. Alcohol dehydrogenase III exacerbates liver fibrosis by enhancing stellate cell activation and suppressing natural killer cells in mice. Hepatology. 2014;60(3):1044–53. doi: 10.1002/hep.27137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. 2013;1832(7):1061–9. doi: 10.1016/j.bbadis.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Radaeva S, et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130(2):435–52. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 139.Boukouaci W, et al. Natural killer cell crosstalk with allogeneic human cardiac-derived stem/progenitor cells controls persistence. Cardiovasc Res. 2014;104(2):290–302. doi: 10.1093/cvr/cvu208. [DOI] [PubMed] [Google Scholar]
- 140.Eguizabal C, et al. Natural killer cells for cancer immunotherapy: pluripotent stem cells-derived NK cells as an immunotherapeutic perspective. Front Immunol. 2014;5:439. doi: 10.3389/fimmu.2014.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng M, et al. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230–52. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Keener AB. Natural killers: cataloging immune cells for immunotherapy. Nat Med. 2015;21(3):207–8. doi: 10.1038/nm0315-207. [DOI] [PubMed] [Google Scholar]
- 143.Krasnova Y, et al. Bench to bedside: NK cells and control of metastasis. Clin Immunol. 2015 doi: 10.1016/j.clim.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 144.Porgador A, et al. Natural killer cell lines kill autologous beta2-microglobulin-deficient melanoma cells: implications for cancer immunotherapy. Proc Natl Acad Sci U S A. 1997;94(24):13140–5. doi: 10.1073/pnas.94.24.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baggio L, et al. Natural killer cell adoptive immunotherapy: Coming of age. Clin Immunol. 2016 doi: 10.1016/j.clim.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 146.deMagalhaes-Silverman M, et al. Posttransplant adoptive immunotherapy with activated natural killer cells in patients with metastatic breast cancer. J Immunother. 2000;23(1):154–60. doi: 10.1097/00002371-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 147.Ishikawa E, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24(3b):1861–71. [PubMed] [Google Scholar]
- 148.Escudier B, et al. Immunotherapy with interleukin-2 (IL2) and lymphokine-activated natural killer cells: improvement of clinical responses in metastatic renal cell carcinoma patients previously treated with IL2. Eur J Cancer. 1994;30A(8):1078–83. doi: 10.1016/0959-8049(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 149.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–39. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 150.Smyth MJ, et al. Cytokines in cancer immunity and immunotherapy. Immunol Rev. 2004;202:275–93. doi: 10.1111/j.0105-2896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 151.Farag SS, Caligiuri MA. Cytokine modulation of the innate immune system in the treatment of leukemia and lymphoma. Adv Pharmacol. 2004;51:295–318. doi: 10.1016/S1054-3589(04)51013-X. [DOI] [PubMed] [Google Scholar]
- 152.Rosenberg SA. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J Sci Am. 2000;6(Suppl 1):S2–7. [PubMed] [Google Scholar]
- 153.Ma C, Armstrong AW. Severe adverse events from the treatment of advanced melanoma: a systematic review of severe side effects associated with ipilimumab, vemurafenib, interferon alfa-2b, dacarbazine and interleukin-2. J Dermatolog Treat. 2014;25(5):401–8. doi: 10.3109/09546634.2013.813897. [DOI] [PubMed] [Google Scholar]
- 154.Rosenberg SA, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313(23):1485–92. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 155.Burns LJ, et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32(2):177–86. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 156.Spanholtz J, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One. 2011;6(6):e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tonn T, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15(12):1563–70. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 158.Burga RA, et al. Improving efficacy of cancer immunotherapy by genetic modification of natural killer cells. Cytotherapy. 2016 doi: 10.1016/j.jcyt.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 159.Tassev DV, Cheng M, Cheung NK. Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer Gene Ther. 2012;19(2):84–100. doi: 10.1038/cgt.2011.66. [DOI] [PubMed] [Google Scholar]
- 160.Altvater B, et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15(15):4857–66. doi: 10.1158/1078-0432.CCR-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chang YH, et al. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73(6):1777–86. doi: 10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- 162.van der Touw W, Bromberg JS. Natural killer cells and the immune response in solid organ transplantation. Am J Transplant. 2010;10(6):1354–8. doi: 10.1111/j.1600-6143.2010.03086.x. [DOI] [PubMed] [Google Scholar]