Abstract
The parasite Trypanosoma cruzi causes a persistent infection, Chagas disease, affecting millions of persons in endemic areas of Latin America. As a result of immigration, this disease has now been diagnosed in non-endemic areas worldwide. Although, the heart and gastrointestinal tract are the most studied, the insulin secreting β cell of the endocrine pancreas is also a target of infection. In this review, we summarize available clinical and laboratory evidence to determine whether T. cruzi-infection-mediated changes of β cell function is likely to contribute to the development of hyperglycemia and diabetes. Our literature survey indicates that T. cruzi infection of humans and of experimental animals relates to altered secretory behavior of β cells. The mechanistic basis of these observations appears to be a change in stimulus-secretion pathway function rather than the loss of insulin producing β cells. Whether this attenuated insulin release ultimately contributes to the pathogenesis of diabetes in human Chagas disease however, remains to be determined. Since the etiologies of diabetes are multifactorial including genetic and life-style factors, the use of cell- and animal-based investigations, allowing direct manipulation of these factors, are important tools in testing if reduced insulin secretion has a causal influence on diabetes in the setting of Chagas disease. Long-term clinical investigations will be required to investigate this link in humans.
Keywords: Chagas disease, Trypanosoma cruzi, pancreas, β cell, insulin, diabetes
Introduction
Chagas disease, caused by infection with the protozoan parasite Trypanosoma cruzi affects millions of individuals in endemic areas of Latin America. As a result of immigration to non-endemic areas, this neglected tropical disease is becoming a global health concern (Conners et al. 2016; Tanowitz et al. 2011). In the United States, it is estimated that over 300,000 individuals are currently infected with T. cruzi (Bern et al. 2011).
Comorbidity of a variety of infections and diabetes mellitus (DM) has recently been reported (Dooley and Chaisson 2009; van Crevel et al. 2016). Moreover, the soaring rise in DM worldwide but especially in areas where infectious disease is highly endemic, has become a major concern, particularly in determining the management of patients with both diseases (Kumar et al. 2016; Siddiqui et al. 2016; van Crevel et al. 2016). In regard to Chagas disease and to minimize adverse outcomes due to inadequate simultaneous control of infection and diabetes, it is essential to know if a link between T cruzi infection and DM exists. The present review of existing studies documents associations of T cruzi parasitism and altered β cell function. Whether Chagas disease might induce glucose intolerance or worsen glycemic control in people with DM is either not known or considerably less clear.
Although modes of T cruzi parasite transmission include blood transfusion, organ donation, oral consumption of the parasite (e.g. contaminated sugar cane juice) and mother-to-child vertical transmission (Bern 2015), the majority of cases are vector-borne, which has also occurred within the continental United States (Bern 2015; Bern et al. 2011; Garcia et al. 2015). Infection with T. cruzi results in acute, indeterminate and at times, clinically relevant chronic disease. While the parasite may be seen on a blood smear during the early acute stage, acute infection is often undetected (Bern 2015) as the symptoms may be non-specific. However, some patients may display fever, hepatosplenomegaly and anorexia during acute infection (Bern 2015). During the indeterminate phase there are no clinical signs or symptoms and parasites are not observed on routine examination of blood films. However, antibodies to T. cruzi are detected in the serum of infected patients. Approximately 30% of individuals who are infected will eventually progress to a clinically evident chronic stage of infection that is associated with the classic manifestations of chronic Chagas disease, i.e. cardiomyopathy and mega-syndromes (e.g. megaesophagus and megacolon) (Bern 2015). The common causes of morbidity and mortality hereto attributed to Chagas disease relate to the cardiac and gastrointestinal tract and research has mainly focused on these organ systems. T. cruzi, however, can infect any nucleated cell of its host and in recent years there has been increased interest in the consequences of this infection on endocrine and exocrine functions of the pancreas (Long et al. 1980a; Mott Cde et al. 1988a; Mott Cde et al. 1988b; Nagajyothi et al. 2013). T. cruzi-induced hypoinsulinemia has been demonstrated and partially characterized in a murine Chagas disease model (Nagajyothi et al. 2013). In addition, there is evidence of reduced insulin secretion post glucose challenge, increased hyperglycemia and diabetes among human patients that have Chagas disease (dos Santos et al. 1999a). Despite a growing body of literature, from animal and human studies which indicates that T. cruzi infection is associated with alterations in insulin release (Guariento et al. 1993; Nagajyothi et al. 2013; Oliveira et al. 1993; Tanowitz et al. 1988; Vieira et al. 1970), the mechanistic basis remains incompletely understood.
The synthesis, storage and secretion of insulin are complex processes. Insulin is synthesized by the pancreatic β-cell, one of several cell types that comprise the pancreatic islet of Langerhans (Pisania et al. 2010). Insulin synthesis is initiated by the production of its precursor, preproinsulin (Steiner et al. 2009). Upon translocation into the lumen of the endoplasmic reticulum and signal peptide cleavage, preproinsulin enters the secretory pathway as proinsulin (Steiner et al. 2009). Advancing to the Golgi, then entering secretory granules, results in further processing of proinsulin to yield equimolar quantities of insulin and C-peptide (Fu et al. 2013; Steiner et al. 2009).
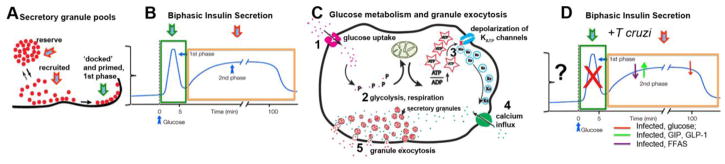
Because healthy β-cells maintain blood glucose levels within narrow limits, they must secrete insulin in such a way as to exactly meet demand (Figure 1). In order to accomplish this task, insulin is stored in secretory granules whose release can be activated by the primary stimulant glucose, and further refined by additional metabolic and hormonal signals (Hutton et al. 1990; Juhl and Hutton 2004). For example, fatty acids and amino acids, while only modest stimulators alone, synergize with glucose stimulation amplifying insulin release (Hutton et al. 1990; Juhl and Hutton 2004). Incretins such as glucagon-like peptide-1 (GLP-1), gastric inhibitory polypeptide (GIP), as well as neurotransmitters, modulate insulin release (Juhl and Hutton 2004). In addition, the healthy pancreatic β cell releases insulin in a time-dependent manner. Based on the timing, the nature of the stimulus and the release competence of secretory granule pools, this response is classically divided into “first” and “second” phases of insulin secretion (Bratanova-Tochkova et al. 2002; Levy et al. 1976; Wang and Thurmond 2009).
Figure 1. Models of insulin secretion.
According to current models of insulin release, rapid mobilization of docked and primed granules (green arrow, A), elicits the 1st phase of insulin secretion (B). Although elevated glucose is the physiological stimulus, plasma membrane depolarization and changes in Ca2+ concentrations can be enough to stimulate hormone output at this point. Continued glucose metabolism (C), essential for sustained recruitment of granules (red arrows, A) contributes to prolonged, 2nd phase of insulin release (red arrow, B, D). ‘Incretins’ (for example, GIP, GLP-1, FFAs), as ligands for G protein coupled receptors (GPCRs) can further amplify the secretory response. The details of how infection impacts the full spectrum of GPCR-related signaling pathways are incompletely understood, but available evidence suggests that T cruzi parasite attenuates glucose and FFA stimulated insulin secretion and impairs the mobilization of the docked granule pool (D).
At the cellular level, the fundamental observations on how stimulation results in insulin granule exocytosis were made more than 30 years ago and have remained largely unchanged: glucose entry and metabolism increases the intracellular ATP to ADP ratio resulting in the closure of ATP-sensitive potassium (KATP) ion channels, depolarization of the β-cell membrane, activation of voltage-gated Ca2+ channels and a rise in intracellular Ca2+ causing secretory granules to fuse with the plasma membrane, releasing contents into the blood stream (Henquin et al. 2009; Hutton 1986; Lacy 1975; Steiner and James 1992). More recently, details on mitochondrial reactions have added to our mechanistic insights on how glycolysis, the Krebs cycle and the electron transport chain work in concert to generate signals important for granule exocytosis (Maechler 2002; Maechler 2013). Disruption in any step of this process can initiate dysregulation of glucose homeostasis, resulting in hyperglycemia, and over a period of time can lead to diabetes mellitus.
Evidence from Human Studies
One serious limitation in investigating glucose metabolism and insulin release in Chagasic patients has been the small sample size. Not surprisingly, these studies have yielded varying results. For example, Saldanha (1997) examined 17 patients with Chagas disease (8 with either megaesophagus or megacolon, 9 with congestive heart failure) and 8 non-Chagasic controls. The post mortem inspection of the pancreas in infected patients (chronic stage of infection) demonstrated inflammation and fibrosis. No amastigotes were observed in the islets. Representing an even smaller number of individuals than the work carried out by Saldanha and focusing exclusively on women over 40 years of age (6 with Chagas disease, 4 with cardiomyopathy, 2 with a mega syndrome compared to 4 controls), histopathological examination of post mortem tissue samples by dos Santos et al. (1999b) showed no significant differences in regard to pancreatic inflammation and fibrosis.
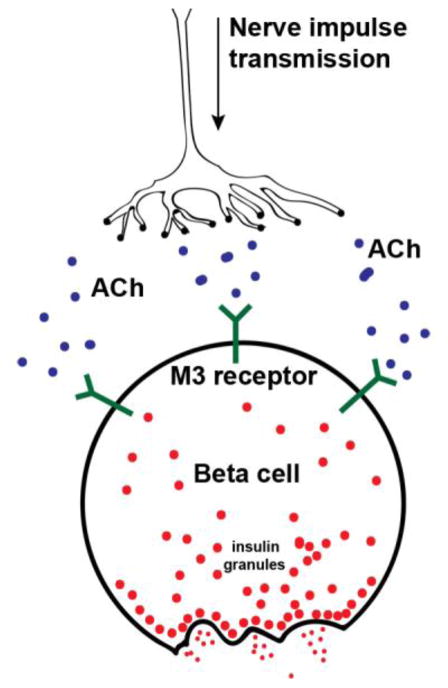
T. cruzi infection is associated with autonomic dysfunction which may result in disruption of parasympathetic innervation of β-cells, an important stimulus for insulin secretion (Figure 2). Thus, in considering potential mechanisms for reduced insulin secretion in the setting of T. cruzi infection, pancreatic nerve cells have been examined (dos Santos et al. 1999a; dos Santos et al. 2004; Guariento et al. 1994; Guariento et al. 1993; Oliveira et al. 1993). In fact, it was suggested that neuritis and autonomic dysfunction were etiologic factors for pancreatic disease in this setting (dos Santos et al. 2004). The Rocha group (1998) compared pancreatic sections obtained from 14 individuals with chronic Chagas disease to 14 controls and found the total pancreatic neuron counts to be significantly lower in the samples from infected individuals. Ganglionitis was not observed in any of the examined pancreata, indicating that the denervation had likely occurred long before the death of the patient (Rocha et al. 1998). The authors argued that nerve dysfunction is likely to contribute to decreased insulin secretion in patients with Chagas disease.
Figure 2. Parasympathetic islet innervation and insulin release.
Parasympathetic innervation and release of acetylcholine (Ach) stimulates granule exocytosis through muscarinic (M3) receptor signaling located at the cell surface of β-cells. Cholinergic regulation is also of great importance to glucagon secretion. Select gut derived polypeptides regulate the insulin/glucagon ratio by inhibiting insulin secretion while augmenting glucagon release induced by vagal stimulation. How T. cruzi- associated denervation affects these cholinergically mediated secretory responses, is currently not known. In a mouse model of Chagas however, glucagon secretion remains insensitive to infection (8).
Health characteristics and socioeconomic factors of immigrants with Chagas disease living in Switzerland were examined by Jackson et al. (2012), and this population consisted predominately of women (84.7%) from Bolivia (94.2%). Metabolic characteristics such as fasting plasma blood glucose (single measurement) as well as BMI and cholesterol levels were evaluated in this population. Of the 137 eligible participants who had a full evaluation, 111 (81%) patients were in the indeterminate phase, 25 (18.3%) patients presented with Chagasic cardiomyopathy, and 1 (0.7%) with mega syndrome (digestive tract involvement). Of these 137 patients, 32 (23.4%) had impaired fasting glucose levels and 4 (2.9%) had diabetes. The participants, in general, had a low income and multiple other medical problems (e.g. hypertension and hypercholesterolemia). Although the lack of an adequate control group and small population size were serious limitations of this study, results suggest that with human infection, endocrine pancreatic dysfunction associated with impaired glucose homeostasis and even hyperglycemia can be demonstrated in early stages of the disease.
To explore the possibility of a causal relationship, rates of hyperglycemia and diabetes in women with and without Chagas disease were compared (dos Santos et al. 1999a). There were 647 patients, 362 with Chagas disease and 285 controls. The diagnosis of Chagas disease was confirmed by three serological tests. In order to increase the chance of finding glycemic disturbances and minimize other variables, the study population was limited to women who were at least 40 years old. Chagasic patients were classified into three groups: (i) asymptomatic, (ii) with ‘mega’ (megaesophagus or megacolon) syndromes, or (iii) with cardiac involvement. Diabetes was defined by two or more fasting blood glucose level above 140 mg/dL or prior diagnosis by a healthcare professional. Hyperglycemia was defined as a fasting blood glucose levels above 110 mg/dL. Overall, no significant differences in the rates of either diabetes or hyperglycemia were found; subgroup analysis however, revealed that Chagasic patients with cardiac manifestations had significantly higher rates of diabetes and hyperglycemia, 37.4% vs. 26.7% and 15.1% vs. 7.4%, respectively. A limitation of this study is that for many patients only a single glucose level was obtained. Nevertheless, this study shows that examining subpopulations may improve the sensitivity of detecting a possible relationship between the cardiac form of Chagas disease and glycemic dysfunction.
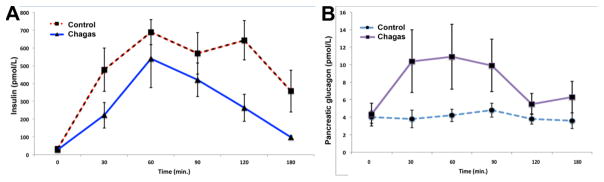
To determine the impact of Chagas disease on pancreatic function, Long et al. (1980b) performed clinical studies on eight patients: with either Chagasic megaesophagus and/or megacolon and compared them with 6 controls. Stimulus secretion coupling was tested using three approaches: patients underwent an oral glucose tolerance test (OGTT) to (i) stimulate release of gut hormones (incretins) in addition to raising glucose; intravenous glucose tolerance test (IVGTT) to (ii) measure glucose stimulation alone, and an insulin-induced hypoglycemia test to (iii) stimulate alpha cells to release glucagon. Blood was obtained at different time-points and analyzed for glucose, insulin and glucagon levels. The blood glucose concentrations were initially higher in the Chagasic patients than in the controls and the insulin response was reduced (OGTT). Release of gut hormones motilin, gastrin, and gastric inhibitory polypeptide (GIP) was the same for both groups (OGTT). Consistent with reduced insulin secretion, pancreatic glucagon concentrations were higher in the Chagasic patients under both basal conditions and during OGTT. In the IVGTT, Chagasic patients had consistently lower (although not statistically significant) insulin release. The insulin-induced hypoglycemia test showed significantly lower plasma glucagon at two time-points (180 and 210 minutes) as well as at the mean peak response. The percent rise in pancreatic polypeptide, another hormone measured in the insulin-induced hypoglycemia test, was significantly lower at several time-points, as was the maximal rise achieved. Overall, this study, using several endpoints to test endocrine pancreatic function, indicates a wider involvement of islet cell types impaired with Chagas disease than has been previously considered (Figure 3).
Figure 3. Insulin and glucagon secretion in Chagasic patients compared to healthy controls.
Comparison of plasma insulin levels (+/− SEM) in Chagasic and non-Chagasic patients after a 100 g oral D-glucose challenge (graphical representation of data from Long RG et al (42) reveals that in parallel to the report in mouse models (8), infection attenuates both early and prolonged phases of insulin output in humans (A). Glucose regulates pancreatic islet α and β cells in opposite directions: it is stimulatory for insulin release and inhibitory for glucagon secretion. Despite similar plasma concentrations of the incretin GIP secreted from the intestine (not shown), pancreatic glucagon release in individuals with Chagas disease is exaggerated (B).
Glucose tolerance in predominantly male patients with indeterminate Chagas disease has also been tested (Guariento et al. 1993). In that study sixteen patients with Chagas disease underwent an OGTT and the results were compared to 28 controls. Following an overnight fast, an oral glucose load (100g) was administered and venous blood samples drawn at six time points between -30 and 120 min post-ingestion. Basal and peak levels of both blood glucose and insulin, and the total area under the curve (AUC) for blood insulin were assessed. Whereas compared with controls, Chagasic patients had significantly lower blood insulin levels (both peak and basal), blood glucose concentrations between the two groups were not significantly different.
Glucose tolerance in a cohort of alcoholic patients with chronic Chagas disease has been examined as well (Oliveira et al. 1993). Four groups were included in this study: alcoholics with Chagas disease, non-alcoholics with Chagas disease, alcoholics without Chagas disease and non-alcoholics without Chagas disease. The four groups were comprised of male patients without a history of diabetes. All patients had a 12-h fast followed by an IVGTT (500mg/kg glucose). Venous blood was drawn pre-IVGTT as well as 5 and 10 min post-glucose infusion and blood glucose and insulin levels were measured at all time-points. The results revealed significant differences between blood insulin levels among these groups, but no differences in blood glucose concentrations. When compared to non-alcoholic, non-Chagasic patients, both groups of Chagasic patients (alcoholic and non-alcoholic) had lower insulin levels. These differences were found at both 5 and 10-min post IVGTT as well as in the total integrated response for insulin (AUC).
In a study designed to determine the effects of Chagas disease on cardiovascular responses to a glucose load, decreased insulin response in a subgroup of patients was observed (Guariento et al. 1994). Patients with the indeterminate form of the disease were subjected to an OGTT and changes in heart rate, blood pressure as well as the “double cross index” (systolic blood pressure multiplied by heart rate) were measured. In addition to these cardiovascular parameters, blood glucose and insulin levels were obtained. A 100g oral glucose load was given following a 12–14 h fast and 16 male patients with Chagas disease were compared with 28 controls. Blood samples were obtained at 30-min intervals from -30 to 120 min post-OGTT (6 total). Blood pressure and heart rate readings were determined at 10-min intervals from the onset of the OGTT until 4 h after initiation of the test. Eight of 16 patients had a ‘hypoinsulinemic’ cardiovascular response to the OGTT. These 8 patients had statistically lower blood insulin levels, measured as AUC, when compared to controls. There were however, no significant differences in amounts of blood glucose between Chagasic patients and controls, even after subgroup analysis. Both Chagas disease groups (hypoinsulinemic and normoinsulinemic) had less variance in systolic blood pressure when compared to the control group. The authors attributed these differences in cardiovascular variation following the OGTT to lower insulin levels, as insulin can increase heart rate and blood pressure by sympathetic activation, sodium reabsorption and direct myocardial action. This study underscores the notion that some Chagasic patients display a reduction in insulin secretion and connects it to a functional outcome of such decreased insulin levels: decreased variance in cardiovascular measures.
In summary, the existing observations demonstrating similar plasma glucose accompanied by lower insulin concentrations are consistent with (i) inadequate insulin response or (ii) heightened sensitivity to the effects of insulin. Such differences should be explored in future studies and tested in a larger study population. In addition, inconsistent results and small sample sizes make it difficult to unequivocally determine whether infection-mediated alterations in blood glucose and insulin availability result in diabetes; the clinical data however is consistent with the notion that infection in humans is having effects on endocrine pancreatic function.
Evidence From Preclinical Studies that T. cruzi infection results in reduced insulin release
The mechanistic details on how T. cruzi infection may contribute to hypoinsulinemia or hyperglycemia and diabetes observed in some reports in patients with Chagas disease are currently sparse, underscoring the importance of animal studies.
Several studies have examined the consequences of T. cruzi infection on pancreatic function in rodents. Lenzi et al. (1996) infected BALB/c mice with 105 of the CL strain of T. cruzi and mice were sacrificed 15 days post-infection (dpi). There was diffuse interstitial pancreatitis associated with edema, infiltration of monocytes and macrophages, especially around intralobular ducts and lobular atrophy suggesting acinar cell loss. The Albuquerque group (1990) on the other hand, found amastigote nests in islets as well as pancreatic ducts and acini. Islets were disorganized with smaller and ‘pale’ α and β cells, in which the nuclei were smaller in volume. Presence of amastigotes in both the endocrine and exocrine pancreas were confirmed in infected mice in a separate study (Corbett et al. 2002). These authors noted fat necrosis in the exocrine pancreas, which they attributed to duct and acinar cell destruction secondary to lysis of T. cruzi pseudocysts.
Immunosuppressive therapy is often involved in clinical situations when treating Chagasic patients. To examine potential consequences of this therapeutic approach in immunocompromised mouse models, Calabrese et al. (1994) studied T. cruzi infection of OF1 mice treated with either cyclophosphamide, indomethacin, corticosteroids or receiving no treatment. Subsequent to drug exposure, all mice were infected with either Y or CL strains of the parasite. Mortality was monitored up to 30 days post infection (dpi), with specimens being taken for post mortem examination at 8, 12, and 15 dpi. Not surprisingly, mortality was higher in nearly all experimental groups versus controls. Endocrine function was not examined but in athymic nude mice, amastigote nests in pancreatic islets could be detected ‘without inflammation’. When compared to littermates with an intact thymus, inflammation with a prominent presence of mononuclear cells were demonstrated in this tissue but parasite nests were not found. The authors concluded that further study was needed but that unless further compromised by immunosuppression, the immune system continued to protect several organ systems, including the endocrine pancreas during the acute stages of infection.
The dos Santos group (2004) employing a hamster model, investigated the consequence of T. cruzi infection on pancreatic β cells. Blood glucose and insulin levels in circulation were sampled in the animals during the acute phase (every 15 days until 60 dpi) as well as in the chronic phase (4 times between 105 and 375 dpi). Plasma insulin concentrations were significantly lower in 6 out of 8 infected animals during both acute and chronic infection compared to uninfected control animals so that overall, infected animals displayed lower insulin levels compared to controls. Nevertheless, infected hamsters had significantly higher blood glucose concentrations at only one time-point, 60 dpi, and significantly lower levels at 30 and 375 dpi. The authors believed that the erratic blood glucose levels observed may have been due to physiologic characteristics of the hamsters since hamsters have oral pouches in which they store food in addition to having highly variable gastric emptying. Additionally, with the exception of two time-points, immunohistochemical evaluation revealed no significant decrease in pancreatic β-cell mass, indicating that the hypoinsulinemia observed was functional rather than structural. Neuritis was also observed in infected hamsters, leading the investigators to hypothesize that denervation of pancreatic islets may have led to decreased insulin secretion.
To understand the molecular basis for reduced functional capacity and exercise tolerance observed in patients with Chagas disease, glucose and insulin utilization was examined in T. cruzi-infected rats (Novaes et al. 2012). These experimental parameters were chosen because exercise depends on increased rate of glycolysis and insulin stimulated glucose uptake into contracting skeletal muscles (Goodyear and Kahn 1998). Responsiveness to glucose and insulin was tested in male rats infected the Y-strain of T. cruzi and then subjected to an oral glucose tolerance test (OGTT) or an insulin tolerance test (ITT) during exercise. Following a 16-h fast, 3g/kg of glucose was given orally and blood glucose concentrations were measured at 0, 30, 60, 90, 120 min following glucose administration (OGTT). Following a 2 h fast, insulin at 0.5 units/kg was administered subcutaneously and blood glucose levels measured for the time points already indicated (ITT). Consistent with reduced availability of insulin, infected rats displayed significantly higher fasting blood glucose as well as higher glucose concentrations during the OGTT. The area under the curve (AUC) was found to be significantly higher in infected rats compared to controls. The results of the ITT revealed that at 30 min post-injection and at all subsequent time periods, infected animals had significantly higher blood glucose levels when compared to controls. The glucose decay rate as another indicator on insulin sensitivity however, was similar in both groups. Based on occurrence of anorexia and weight loss with infection (Schebeleski-Soares et al. 2009), evidence showing that modifications in body composition can alter the insulin response (Creutzfeldt et al. 1983) and the similarities of glucose decay rates, the authors favored the view that infection related insulin resistance was not a likely explanation for the difference observed with ITT. Moreover, nine weeks post-infection histopathological examination demonstrated minimal changes in the endocrine pancreas with marked morphological changes in acinar cells and no significant differences in the number of β-cells between infected and control rats. Overall, the unaltered number of β-cells by T. cruzi infection with significant alterations in glucose homeostasis in infected animals led to the conclusion that infection reduced glucose-stimulated release of insulin.
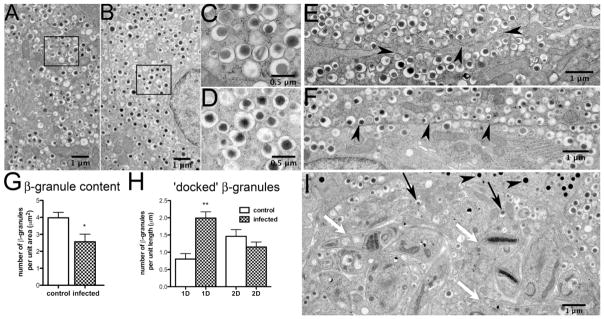
Our laboratory examined the consequences of T. cruzi infection (Brazil strain) in CD-1 mice during both acute (up to 30 dpi) and chronic (90 dpi) infection (Nagajyothi et al. 2013). Histopathology of the pancreas showed intracellular amastigotes, intense inflammation, destruction of pancreatic acinar cells, inflammation of blood vessels and inflammation of pancreatic ducts. Immunohistochemistry revealed marked disruption of pancreatic islet morphology, which was prominent during acute infection but appeared to recover by 100 dpi. Electron microscopy (Figure 4) revealed details of granule morphology and amastigotes within insulin producing β-cells. In infected mice 30 dpi, the number of insulin granules tended to be lower relative to uninfected controls, but this did not achieve statistical significance (Figure 4). However, a build-up of insulin granules at the β-cell membrane could be clearly demonstrated. Furthermore, insulin secretion studies confirmed that at the earliest time-point when parasites could first be detected in the blood (15 dpi), the insulin rise in response to elevated glucose was blunted in infected mice. This reduced glucose sensitivity continued to the end of the study at 100 dpi (chronic stage of infection).
Figure 4. Granule morphology and cellular distribution in control and infected mouse pancreatic islets.
Electron micrographs of T. cruzi-infected or uninfected islets at 30 days post-infection were analyzed for changes in granule number or distribution. During acute infection, amastigotes were observed within the pancreatic β-cells (Figure 4 I, white arrows). However, no parasites were detected in α-cells and the glucagon granules appeared to be intact (Figure 4 I, black arrowheads). The integrity of insulin granules also appeared morphologically normal (Figure 4 A–D, Figure 4 I, black arrows). Compared to tissues from uninfected mice, the total number of insulin granules was reduced in pancreata obtained from mice 30 days post-infection (Figure 4 G). Consistent with decreased 1st phase secretion, in the infected β cells there was a significant increase in the number of docked insulin granules, i.e. granules localized within one granule diameter from the plasma membrane (Nagajyothi et al. 2013). Figure was reprinted with permission of the American Journal of Pathology and Elsevier.
The β-3-adrenergic receptor is found on the surface of the adipocyte and it is thought that when this receptor is stimulated it causes the release of free fatty acids (FAs) that in turn stimulate the release of insulin from the pancreatic β-cell; however, this has not been directly demonstrated. When a β-3-receptor agonist CL 316, 243 was administered to mice, FA stimulated insulin levels were significantly reduced in infected mice compared to uninfected mice (Nagajyothi et al. 2013). In T. cruzi infected mice, injection of the depolarizing agent L-arginine, which should cause the release of ‘docked’ granules already positioned at the plasma membrane, did not elicit the expected increase of insulin levels (Nagajyothi et al. 2013). This reduced insulin response with L-arginine stimulation occurred during both acute and chronic infection suggesting an impairment of 1st phase insulin secretion as a consequence of T. cruzi parasitism. Overall, these data suggest that there are several points along the biphasic insulin release pathway that are related to alterations in β-cell function observed during T. cruzi infection (Figure 1). Moreover, the observation of decreased gluconeogenesis with preserved glucagon production and secretion gave important clues as to why in the presence of hypoinsulinemia, hyperglycemia could not be demonstrated in this model. Thus, this study provided for the first time, a likely mechanistic basis that explained how lower insulin levels could occur in infection in the absence of the typical hyperglycemia associated with diabetes (Nagajyothi et al. 2013).
T. cruzi infection also significantly alters host lipid profiles (Li et al. 2016). Surprisingly, a high fat diet (HFD), which affects the host lipid homeostasis was protective and decreased mortality in infected CD-1 mice (acute infection). Treatment of T. cruzi infected CD-1 mice with the anti-diabetic drug metformin, a drug whose therapeutic effects are partly mediated by activation of AMP-activated protein kinase (AMPK) and modulation of mitochondrial dynamics, resulted in further improved survival compared to HFD mice during acute infection (Brima et al. 2015; Sosnicki et al. 2016). Although the mechanisms of action of metformin in the survival of T. cruzi infected mice is not yet understood and insulin secretion was not examined, it is worth noting that both the HFD and metformin, which are known to differentially attenuate host metabolism, resulted in decreased mortality in T. cruzi-infected CD-1 mice, illustrating links between metabolism and the pathogenesis of this infection.
Discussion
As noted, trypomastigotes infect almost any nucleated cell (Lenzi et al. 1996) but as a comparison of studies on pancreatic infection reveals, utilizing the Brazil or Bolivian strains led to detection of T. cruzi specific infection of pancreatic β-cells cells (Albuquerque et al. 1990; Calabrese et al. 1994; Nagajyothi et al. 2013). By contrast, the studies that relied on the CL strain of the parasite or did not specifically identify the strain, could not find amastigotes within β-cells (Lenzi et al. 1996; Saldanha et al. 2001). These findings imply cell tropism among different T. cruzi strains, an experimental variable that not only deserves to be systematically analyzed in future animal studies but should be considered in the design of more specific tests that detect T cruzi strains in infected humans. Improvements on the use of currently available test to diagnose Chagas disease in humans are also essential. According to a recent review of commercially available laboratory tests (enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) to detect Chagas disease, sensitivity, specificity or both were highly variable (Brasil et al. 2016). In most of the studies, sample description, details regarding the recommended decision threshold, and how the decision thresholds were estimated were often missing (Brasil et al. 2016). The reference standard was highly heterogeneous among and twenty-one percent of all of the investigations did not apply the same reference standard to classify those with and without Chagas disease (Brasil et al. 2016). In brief, the heterogeneity in outcomes observed in the human studies may well have been a reflection of the differences in sample analysis and better controlled investigations would strengthen our ability to determine whether or not a link between Chagas and diabetes can be made.
When β-cells, and islets in general, were identified as infected, qualitative changes were observed (Lenzi et al. 1996; Novaes et al. 2012). Despite infection, the overall number of β-cells was not reduced (dos Santos et al. 1999a). Beta cells, however, are part of an organ system, the islet. For a more comprehensive account of how infection targets islet function, other islet cell types await better characterization. Nevertheless, the existing observations support the notion that defects in pancreatic endocrine function include a functional impairment of pancreatic β-cells.
The finding of hypoinsulinemia in animal models was reliably documented in several studies and findings were consistent among species (dos Santos et al. 2004; Nagajyothi et al. 2013). Review of the current human studies as to whether T. cruzi infection leads to hyperglycemia and diabetes on the other hand, is inconclusive. For one, findings of hypoinsulinemia were variable, found in all Chagasic patient groups (Nagajyothi et al. 2009; Oliveira et al. 1993) or only among a sub-group of infected patients (Guariento et al. 1993). In addition, decreased insulin secretion did not consistently correlate with increased prevalence of hyperglycemia. In some of the human studies on hyperglycemia in Chagasic patients, infected patients had unusually high rates of glucose intolerance (i.e. a pre-diabetic or metabolic syndrome state) (Jackson et al. 2012), a variable that needs to be more consistently evaluated. Analysis of hyperglycemia and diabetes according to the different sub-groups of infected patients especially among a sub-group of patients with Chagasic heart disease however, appears to be a promising approach to achieve greater statistical power.
While there are strong indications in epidemiological and cross-sectional studies, the uncertainty in the clinical studies as to blood glucose levels among Chagasic patients may have been introduced by inconsistent use of screening methods designed to detect diabetes. One study carried out an OGTT, one an IVGTT and the other subjected patients to both tests (Guariento et al. 1993; Long et al. 1980b; Oliveira et al. 1993). The published studies, to date, have only utilized fasting and/or random glucose tests, and often with single measurements. To this end, it would be useful to follow patients with chronic Chagas disease prospectively, along with an appropriate control group over the course of several years to compare rates of the development of diabetes as well as changes over time in serial measurements of hemoglobin-A1C and other metabolic markers. Many studies were carried out with relatively small sample sizes. To address the question of whether or not Chagas disease leads to diabetes, large-scale, prospective studies must be carried out, include both sexes and standardization of biochemical testing. Ideally, these new inquiries will incorporate the latest advances that stratify DM characterization into a range of diabetic subgroups (Tuomi et al. 2014). The recent discovery that combining the 1 hour post glucose load OGTT with HbA1c identifies subsets of individuals at high risk for both DM and cardiovascular disease (Bergman et al. 2016; Fiorentino et al. 2016) may be another particularly suitable approach for development in the setting of Chagas disease.
The prevailing focus on diabetes as a disorder characterized by increasing hyperglycemia may simply not readily apply to Chagas disease patients. An important parallel to what changes in strategy may have to be implemented to detect a version of diabetes as a result of infection is perhaps best drawn from the clinical experiences with Cystic-fibrosis (CF) patients. A subset of CF patients can exhibit decreased insulin secretion with virtually normal or moderate hyperglycemia, relative preservation of fasting glucose and an absence of complications generally characterized by diabetes (Dobson et al. 2004; Handwerger et al. 1969; Konrad et al. 2013). What later was labeled as Cystic-fibrosis-related diabetes (CFRD) was first described in 1955 by Shwachman et al. and went unnoticed until in 1962 when Rosan and colleagues reviewed a series of 1300 CF patients and found 10 had DM. Although the number of affected individuals was small, the findings challenged the conventional measures of glycemia and insulin resistance adopted from the non-CF population as entirely valid in CF patients (Dobson et al. 2004; Rosan et al. 1962). Subsequent studies focusing on the global effects of insulin deficiency rather than primarily concentrating on its role in glucose homeostasis resulted in changed guidelines on appropriate diagnosis and treatment for CFRD (Dobson et al. 2004; Hameed et al. 2011). Progressive increase in detection of CFRD, led to the recognition of CFDR as an end-stage of progressive insulin deficiency (Hameed et al. 2011). Altogether this series of events combined with additional research into this important field resulted in improved patient care options currently missing in the management of individuals with Chagas disease.
Finally, whether the lack of elevated glucose observed in some studies simply reflects changes in insulin resistance/insulin sensitivity needs to better described. To date, a relationship between T. cruzi infection and insulin resistance particularly as it applies to adipocyte function has been noted (Cabalen et al. 2016; Nagajyothi et al. 2009) but outcomes on the performance of insulin secreting β cells, not extensively studied. This concept however, has been widely examined in bacterial sepsis. In bacterial sepsis, glucose metabolism is markedly altered and hyperglycemia in the face of hyperinsulinemia is described in the setting of stress, such as systemic infection and shock. During bacterial sepsis, changes in the quantity and affinity of insulin receptors lead to a significant decrease in insulin responsiveness by adipocytes contributing to marked differences in insulin stimulated uptake of glucose and result in hyperglycemia (Igarashi et al. 1992). The hyperglycemic state is proinflammatory (Nedrebo et al. 2004; Nishikawa et al. 2000) eliciting pro-inflammatory responses in adipocytes (Lin et al. 2005). Here, in some critically ill patients, exogenous insulin administration affects survival (Dandona et al. 2003; van den Berghe et al. 2001). Moreover, some have suggested that insulin resistance is an essential part of an adaptive response of the host to fight infection (Dhar and Castillo 2011). Whether similar mechanisms are found in the setting of Chagas disease is not known, but deserves additional studies.
Efforts to determine mechanistic basis for hypoinsulinemia and to a lesser extent hyperglycemia with T. cruzi infection do exist, but clear understanding of cellular details remains incomplete. It is well recognized that T. cruzi infects the brain, but whether this might impair insulin secretion is not known. Signaling and the role of the central nervous system (Figure 2) in stimulating hormone output have been considered and several authors have speculated that a dysfunction of pancreatic neurons may be a cause. Neuronal loss or inflammation of neurons and “neuritis” of the β cell has been proposed as a potential means for attenuating insulin secretion (dos Santos et al. 1999a; Guariento et al. 1994; Guariento et al. 1993; Oliveira et al. 1993). Only one study examined this hypothesis directly and found that there were indeed fewer neurons closely associated with the endocrine pancreata infected with T. cruzi (Rocha et al. 1998). While the results obtained from the Rocha study support the hypothesis of neuronal dysfunction as a possible cause of hypoinsulinemia, the data obtained were exclusively based on morphological analysis of tissue sections. No clinical data from the patients, such as blood insulin or glucose levels, were included. It is therefore impossible to establish causality of hypoinsulinemia due to such neuronal losses.
Observations from our laboratory (Nagajyothi et al. 2013) in infected mice demonstrated increased numbers of insulin granules docked at the β-cell membrane that were not released in response to stimulation. To provide a possible mechanism for the observation of hypoinsulinemia without hyperglycemia, non-glucose reagents (arginine and free fatty acids, Figure 4), both known to promote granule release, were used to attempt to restore normal insulin levels. Both, however, failed to do so, providing further evidence for the hypothesis that the defect lies at the step of stimulus-secretion coupling to release hormone. We found that glucagon levels were not decreased, and could actually be elevated during infection. The lack of hyperglycemia was thus not attributable to infection and the subsequent disruption of α-cell function. Hepatic gluconeogenesis however, was decreased in infected animals (Nagajyothi et al. 2013). Therefore, while decreased insulin in the circulation would lead to impaired blood glucose utilization by peripheral tissues, the reduced gluconeogenesis would lead to a net zero effect on glycemic levels.
Analysis of the extant literature, as presented in this review, clearly supports the notion that infection with T. cruzi leads to a hypoinsulinemic state in the infected host. This hypoinsulinemic state likely lies not within insulin production but rather in secretion of the hormone (Nagajyothi et al. 2013). As the primary determinant of insulin secretion is glucose metabolism by the β-cell, this is an appropriate pathway that may extend the findings of Nagayothi et al (2013). Other researchers have shown that the T. cruzi parasite attenuates certain enzymes involved in the Krebs cycle in infected cardiac tissue (Garg et al. 2004). These same enzymes are crucial for glucose metabolism in pancreatic β-cells, and thus warrant investigation as to their role in the parasite mediated inhibition of insulin secretion.
There will still be many challenges to definitively determining whether or not Chagas disease leads to an increased incidence of diabetes. Both are diseases of poverty and potential confounders are numerous. Many of the same risk factors for developing diabetes, such as access to healthcare, socioeconomic status and ethnicity are common among Chagasic individuals. Nevertheless, answers to the many questions our review of the literature raised are expected to address significant limitations in our current understanding on how an impaired function of insulin secreting β cells contributes to the severity of chronic T cruzi infection. Infection clearly manipulates the pathways responsible for adjusting insulin supply to meet demand and mechanistic insight into the details could provide clues for possible treatments.
SUMMARY
The role of T cruzi in regulating insulin secretion during infection of β cells has not been studied extensively in either humans or animal models. In the case of glucose stimulated insulin secretion, infection appears to attenuate the early phase of rapid granule exocytosis as well as the response to select stimulus. Thus far, most of our knowledge on hormone output in Chagas disease patients comes from relatively few clinical studies, many with small sample sizes. Much more in regard to essential contributions from the parasite or host in modulating pancreatic islet cell biology through signaling, metabolic or transcriptional alterations has yet to be clearly defined. We need to also understand to what extent the regulation and actions of T cruzi are either redundant or unique in different target organs and address the question if selective control in different tissues is involved that needs to be coordinated on a more global level. Considering for example, the possibility of insulin abundance/sensitivity as mediators of broad-range host/parasite interactions would open up possibilities for new strategies in parasitic control.
Acknowledgments
This study was supported by NIH Grants R01-DK55758, R01-DK099110 and P01-DK088761 as well as a grant from the Cancer Prevention and Research Institute of Texas (CPRIT RP140412) to P.E.S., NIH Grants R21-AI-124000 to H.B.T. and HL122866 and HL-112099 to J.N. and the Einstein Research Fellowship. Office of Medical Student Research, Office of Medical Education, Albert Einstein College of Medicine and the Medical Scholars Program, IDSA Education and Research Foundation both to Q.D. Figure 1 (C) represents a modified version based on a diagram by Jean-Philippe Cartailler (Vanderbilt University/β Cell Biology Consortium) and his permission for its use is gratefully acknowledged.
References
- Albuquerque RH, Ribeiro RD, Lopes RA, Lamano Carvalho TL, Paula-Lopes OV. Tissue tropism of different Trypanosoma cruzi strains. IX. Alterations in A and B Langerhans islet cells produced by the slender and broad forms of the Bolivian strain. Mem Inst Oswaldo Cruz. 1990;85:8. [Google Scholar]
- Bergman M, Chetrit A, Roth J, Jagannathan R, Sevick M, Dankner R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia: Observations from the 24 year follow-up of the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabetes Research and Clinical Practice. 2016;120:221–228. doi: 10.1016/j.diabres.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Bern C. Chagas’ Disease. N Engl J Med. 2015;373:1882. doi: 10.1056/NEJMc1510996. [DOI] [PubMed] [Google Scholar]
- Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ Disease in the United States. Clin Microbiol Rev. 2011;24:655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil PE, Castro R, Castro L. Commercial enzyme-linked immunosorbent assay versuspolymerase chain reaction for the diagnosis of chronic Chagas disease: a systematic review and meta-analysis. Mem Inst Oswaldo Cruz. 2016;111:1–19. doi: 10.1590/0074-02760150296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratanova-Tochkova TK, Cheng H, Daniel S, Gunawardana S, Liu YJ, Mulvaney-Musa J, Schermerhorn T, Straub SG, Yajima H, Sharp GW. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes. 2002;51(Suppl 1):S83–90. doi: 10.2337/diabetes.51.2007.s83. [DOI] [PubMed] [Google Scholar]
- Brima W, Eden DJ, Mehdi SF, Bravo M, Wiese MM, Stein J, Almonte V, Zhao D, Kurland I, Pessin JE, Zima T, Tanowitz HB, Weiss LM, Roth J, Nagajyothi F. The brighter (and evolutionarily older) face of the metabolic syndrome: evidence from Trypanosoma cruzi infection in CD-1 mice. Diabetes Metab Res Rev. 2015;31:346–359. doi: 10.1002/dmrr.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabalen ME, Cabral MF, Sanmarco LM, Andrada MC, Onofrio LI, Ponce NE, Aoki MP, Gea S, Cano RC. Chronic Trypanosoma cruzi infection potentiates adipose tissue macrophage polarization toward an anti-inflammatory M2 phenotype and contributes to diabetes progression in a diet-induced obesity model. Oncotarget. 2016;7:13400–13415. doi: 10.18632/oncotarget.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese KS, Lagrange PH, da Costa SC. Trypanosoma cruzi: histopathology of endocrine system in immunocompromised mice. Int J Exp Pathol. 1994;75:453–462. [PMC free article] [PubMed] [Google Scholar]
- Conners EE, Vinetz JM, Weeks JR, Brouwer KC. A global systematic review of Chagas disease prevalence among migrants. Acta Tropica. 2016;156:68–78. doi: 10.1016/j.actatropica.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett CE, Scremin LH, Lombardi RA, Gama-Rodrigues JJ, Okumura M. Pancreatic lesions in acute experimental Chagas’ disease. Rev Hosp Clin Fac Med Sao Paulo. 2002;57:63–66. doi: 10.1590/s0041-87812002000200003. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W, Ebert R, Nauck M, Stockmann F. Disturbances of the entero-insular axis. Scand J Gastroenterol Suppl. 1983;82:111–119. [PubMed] [Google Scholar]
- Dandona P, Aljada A, Dhindsa S, Garg R. Insulin as an anti-inflammatory and antiatherosclerotic hormone. Clin Cornerstone Suppl. 2003;4:S13–20. doi: 10.1016/s1098-3597(03)90062-7. [DOI] [PubMed] [Google Scholar]
- Dhar A, Castillo L. Insulin resistance in critical illness. Curr Opin Pediatr. 2011;23:269–274. doi: 10.1097/MOP.0b013e3283464b3e. [DOI] [PubMed] [Google Scholar]
- Dobson L, Sheldon CD, Hattersley AT. Conventional measures underestimate glycaemia in cystic fibrosis patients. Diabet Med. 2004;21:691–696. doi: 10.1111/j.1464-5491.2004.01219.x. [DOI] [PubMed] [Google Scholar]
- Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9(12):737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos VM, da Cunha SF, de Teixeira VP, Monteiro JP, dos Santos JA, dos Santos TA, dos Santos LA, da Cunha DF. Frequency of diabetes mellitus and hyperglycemia in chagasic and non-chagasic women. Rev Soc Bras Med Trop. 1999a;32:489–496. doi: 10.1590/s0037-86821999000500004. [DOI] [PubMed] [Google Scholar]
- dos Santos VM, de Lima MA, Cabrine-Santos M, de Stefani Marquez D, de Araújo Pereira G, Lages-Silva E, Ramírez LE. Functional and histopathological study of the pancreas in hamsters (Mesocricetus auratus) infected and reinfected with Trypanosoma cruzi. Parasitol Res. 2004;94:125–133. doi: 10.1007/s00436-004-1183-8. [DOI] [PubMed] [Google Scholar]
- dos Santos VM, de Teixeira VP, da Cunha DF, da Cunha SF, Monteiro JP, dos Santos JA, dos Santos TA, dos Santos LA. Pancreatic anatomopathologic changes in chronic chagasic women. Preliminary data. Arq Gastroenterol. 1999b;36:127–132. [PubMed] [Google Scholar]
- Fiorentino TV, Andreozzi F, Mannino GC, Pedace E, Perticone M, Sciacqua A, Perticone F, Sesti G. One-hour post-load hyperglycemia combined with HbA1c identifies pre-diabetic individuals with a higher cardio-metabolic risk burden. Atherosclerosis. 2016;253:61–69. doi: 10.1016/j.atherosclerosis.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic B-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9:25–53. [PMC free article] [PubMed] [Google Scholar]
- Garcia MN, Aguilar D, Gorchakov R, Rossmann SN, Montgomery SP, Rivera H, Woc-Colburn L, Hotez PJ, Murray KO. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg. 2015;92:325–330. doi: 10.4269/ajtmh.14-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg N, Gerstner A, Bhatia V, DeFord J, Papaconstantinou J. Gene expression analysis in mitochondria from chagasic mice: alterations in specific metabolic pathways. Biochem J. 2004;381(Pt 3):743–752. doi: 10.1042/BJ20040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- Guariento ME, Olga E, Muscelli A, Gontijo JA. Chronotropic and blood pressure response to oral glucose load in Chagas’ disease. Sao Paulo Med J. 1994;112:602–606. doi: 10.1590/s1516-31801994000300006. [DOI] [PubMed] [Google Scholar]
- Guariento ME, Saad MJ, Muscelli EO, Gontijo JA. Heterogenous insulin response to an oral glucose load by patients with the indeterminate clinical form of Chagas’ disease. Braz J Med Biol Res. 1993;26:491–495. [PubMed] [Google Scholar]
- Hameed S, Jaffe A, Verge CF. Cystic fibrosis related diabetes (CFRD)--the end stage of progressive insulin deficiency. Pediatr Pulmonol. 2011;46:747–760. doi: 10.1002/ppul.21495. [DOI] [PubMed] [Google Scholar]
- Handwerger S, Roth J, Gorden P, Di Sant’ Agnese P, Carpenter DF, Peter G. Glucose intolerance in cystic fibrosis. N Engl J Med. 1969;281:451–461. doi: 10.1056/NEJM196908282810901. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Nenquin M, Ravier MA, Szollosi A. Shortcomings of current models of glucose-induced insulin secretion. Diabetes Obes Metab. 2009;11(Suppl 4):168–179. doi: 10.1111/j.1463-1326.2009.01109.x. [DOI] [PubMed] [Google Scholar]
- Hutton JC. Calcium-binding proteins and secretion. Cell Calcium. 1986;7:339–352. doi: 10.1016/0143-4160(86)90037-0. [DOI] [PubMed] [Google Scholar]
- Hutton JC, Bailyes EM, Rhodes CJ, Rutherford NG, Arden SD, Guest PC. Biosynthesis and storage of insulin. Biochem Soc Trans. 1990;18:122–124. doi: 10.1042/bst0180122. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Yamatani K, Fukase N, Daimon M, Ohnuma H, Takahashi H, Manaka H, Tominaga M, Sasaki H. Sepsis inhibits insulin-stimulated glucose transport in isolated rat adipocytes. Diabetes Res Clin Pract. 1992;15:213–218. doi: 10.1016/0168-8227(92)90027-o. [DOI] [PubMed] [Google Scholar]
- Jackson Y, Castillo S, Hammond P, Besson M, Brawand-Bron A, Urzola D, Gaspoz JM, Chappuis F. Metabolic, mental health, behavioural and socioeconomic characteristics of migrants with Chagas disease in a non-endemic country. Trop Med Int Health. 2012;17:595–603. doi: 10.1111/j.1365-3156.2012.02965.x. [DOI] [PubMed] [Google Scholar]
- Juhl K, Hutton J. Stimulus-secretion coupling in the pancreatic β-cell. Adv Exp Med Biol. 2004;552:66–90. [PubMed] [Google Scholar]
- Konrad K, Thon A, Fritsch M, Fröhlich-Reiterer E, Lilienthal E, Wudy SA, Holl RW. Comparison of cystic fibrosis-related diabetes with type 1 diabetes based on a German/Austrian Pediatric Diabetes Registry. Diabetes Care. 2013;36:879–886. doi: 10.2337/dc12-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AK, Chandrasekaran V, Kannan T, Murali AL, Lavanya J, Sudha V, Swaminathan S1, Ramachandran G. Anti-tuberculosis drug concentrations in tuberculosis patients with and without diabetes mellitus. Eur J Clin Pharmacol. 2016 doi: 10.1007/s00228-016-2132-z. In Press. [DOI] [PubMed] [Google Scholar]
- Lacy PE. Endocrine secretory mechanisms. Am J Pathol. 1975;79:170–188. [PMC free article] [PubMed] [Google Scholar]
- Lenzi HL, Oliveira DN, Lima MT, Gattass CR. Trypanosoma cruzi: paninfectivity of CL strain during murine acute infection. Exp Parasitol. 1996;84:16–27. doi: 10.1006/expr.1996.0086. [DOI] [PubMed] [Google Scholar]
- Levy J, Herchuelz A, Sener A, Malaisse WJ. The stimulus-secretion coupling of glucose-induced insulin release. XX. Fasting: A model for altered glucose recognition by the B-cell. Metabolism. 1976;25:583–591. doi: 10.1016/0026-0495(76)90012-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Shah-Simpson S, Okrah K, Belew AT, Choi J, Caradonna KL, Padmanabhan P, Ndegwa DM, Temanni MR, Corrada Bravo H, El-Sayed NM, Burleigh BA. Transcriptome Remodeling in Trypanosoma cruzi and Human Cells during Intracellular Infection. PLoS Pathog. 2016;12(4):e1005511. doi: 10.1371/journal.ppat.1005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem. 2005;280:4617–4626. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- Long RG, Albuquerque RH, Bishop AE, Polak JM, Bloom SR. The peptidergic system in Chagas’s disease [proceedings] Trans R Soc Trop Med Hyg. 1980a;74:273–274. [PubMed] [Google Scholar]
- Long RG, Albuquerque RH, Prata A, Barnes AJ, Adrian TE, Christofides ND, Bloom SR. Response of plasma pancreatic and gastrointestinal hormones and growth hormone to oral and intravenous glucose and insulin hypoglycaemia in Chagas’s disease. Gut. 1980b;21:772–777. doi: 10.1136/gut.21.9.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler P. Mitochondria as the conductor of metabolic signals for insulin exocytosis in pancreatic β-cells. Cell Mol Life Sci. 2002;59:1803–1818. doi: 10.1007/PL00012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler P. Mitochondrial function and insulin secretion. Mol Cell Endocrinol. 2013;379:12–18. doi: 10.1016/j.mce.2013.06.019. [DOI] [PubMed] [Google Scholar]
- de Mott CB, Guarita DR, Sipahi AM, Bettarello A. Functional evaluation of the exocrine pancreas in patients with chronic Chagas’ disease. Rev Hosp Clin Fac Med Sao Paulo. 1988a;43:279–287. [PubMed] [Google Scholar]
- de Mott CM, Guarita DR, Bettarello A. The exocrine pancreas and Chagas’ disease. Rev Hosp Clin Fac Med Sao Paulo. 1988b;43:177–179. [PubMed] [Google Scholar]
- Nagajyothi F, Desruisseaux MS, Weiss LM, Chua S, Albanese C, Machado FS, Esper L, Lisanti MP, Teixeira MM, Scherer PE, Tanowitz HB. Chagas disease, adipose tissue and the metabolic syndrome. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):219–225. doi: 10.1590/s0074-02762009000900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajyothi F, Kuliawat R, Kusminski CM, Machado FS, Desruisseaux MS, Zhao D, Schwartz GJ, Huang H, Albanese C, Lisanti MP, Singh R, Li F, Weiss LM, Factor SM, Pessin JE, Scherer PE, Tanowitz HB. Alterations in glucose homeostasis in a murine model of Chagas disease. Am J Pathol. 2013;182:886–894. doi: 10.1016/j.ajpath.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedrebo T, Karlsen TV, Salvesen GS, Reed RK. A novel function of insulin in rat dermis. J Physiol. 2004;559(Pt 2):583–591. doi: 10.1113/jphysiol.2004.067751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Novaes RD, Gonçalves RV, Penitente AR, Talvani A, Neves CA, Natali AJ, Maldonado IR. Trypanosoma cruzi infection alters glucose metabolism at rest and during exercise without modifying the morphology of pancreatic islets in rats. Pathol Res Pract. 2012;208:480–488. doi: 10.1016/j.prp.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Oliveira LC, Juliano Y, Novo NF, Neves MM. Blood glucose and insulin response to intravenous glucose by patients with chronic Chagas’ disease and alcoholism. Braz J Med Biol Res. 1993;26:1187–1190. [PubMed] [Google Scholar]
- Pisania A, Weir GC, O’Neil JJ, Omer A, Tchipashvili V, Lei J, Colton CK, Bonner-Weir S. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest. 2010;90:1661–1675. doi: 10.1038/labinvest.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, de Oliveira LC, Alves RS, Lopes ER. Pancreatic neuronal loss in chronic Chagas’ disease patients. Rev Soc Bras Med Trop. 1998;31:43–49. doi: 10.1590/s0037-86821998000100006. [DOI] [PubMed] [Google Scholar]
- Rosan RC, Shwachman H, Kulczvcki LI. Diabetes mellitus and cystic fibrosis of the pancreas. Laboratory and clinical observations. Am J Dis Child. 1962;104:625–634. doi: 10.1001/archpedi.1962.02080030625007. [DOI] [PubMed] [Google Scholar]
- Saldanha JC. Avaliação morfológica e morfométrica das ilhotas pancreáticas na fase crônica da doença de Chagas. Revista da Sociedade Brasileira de Medicina Tropical. 1997;30:165–166. [Google Scholar]
- Saldanha JC, dos Santos VM, dos Reis MA, da Cunha DF, Antunes Teixeira VP. Morphologic and morphometric evaluation of pancreatic islets in chronic Chagas’ disease. Rev Hosp Clin Fac Med Sao Paulo. 2001;56:131–138. doi: 10.1590/s0041-87812001000500001. [DOI] [PubMed] [Google Scholar]
- Schebeleski-Soares C, Occhi-Soares RC, Franzói-de-Moraes SM, de Oliveira Dalálio MM, Almeida FN, de Ornelas Toledo MJ, de Araújo SM. Preinfection aerobic treadmill training improves resistance against Trypanosoma cruzi infection in mice. Appl Physiol Nutr Metab. 2009;34:659–665. doi: 10.1139/H09-053. [DOI] [PubMed] [Google Scholar]
- Shwachman H, Leubner H, Catzel P. Mucoviscidosis. Adv Ped. 1955;7:249–323. [PubMed] [Google Scholar]
- Siddiqui AN, Khayyam KU, Sharma M. Effect of diabetes mellitus on tuberculosis treatment outcome and adverse reactions in patients receiving directly observed treatment strategy in India: A prospective study. Biomed Res Int. 2016 doi: 10.1155/2016/7273935. Article ID 72739357273935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnicki S, Kapral M, Weglarz L. Molecular targets of metformin antitumor action. Pharmacol Rep. 2016;68:918–925. doi: 10.1016/j.pharep.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Steiner DF, James DE. Cellular and molecular biology of the beta cell. Diabetologia. 1992;35(Suppl 2):S41–8. doi: 10.1007/BF00586278. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Park SY, Stoy J, Philipson LH, Bell GI. A brief perspective on insulin production. Diabetes Obes Met Suppl. 2009;4:189–196. doi: 10.1111/j.1463-1326.2009.01106.x. [DOI] [PubMed] [Google Scholar]
- Tanowitz HB, Amole B, Hewlett D, Wittner M. Trypanosoma cruzi infection in diabetic mice. Trans R Soc Trop Med Hyg. 1988;82:90–93. [PubMed] [Google Scholar]
- Tanowitz HB, Weiss LM, Montgomery SP. Chagas disease has now gone global. PLoS Negl Trop Dis. 2011;5(4):e1136. doi: 10.1371/journal.pntd.0001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet. 2014;383:1084–1094. doi: 10.1016/S0140-6736(13)62219-9. [DOI] [PubMed] [Google Scholar]
- van Crevel R, van de Vijver S, Moore DA. The global diabetes epidemic: what does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol. 2016 doi: 10.1016/S2213-8587(16)30081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Vieira CB, Soubihe NV, Ferriolli Filho F. Peculiarities of insulin hypoglycemia in the chronic stage of Chagas’ disease. II. Experimental study in dogs and mice infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1970;12:179–184. [PubMed] [Google Scholar]
- Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122(Pt 7):893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]